Abstract
Traumatic brain injury (TBI) is a major public health issue affecting 1.4 million Americans each year, of which approximately 50,000 die annually. High-fat sucrose (HFS) diets are another public health issue which can lead to obesity, hypertension, and many other debilitating disorders. These two disorders combined can lead to more complicated issues. It has recently been shown that HFS diets can reduce levels of brain-derived neurotrophic factor (BDNF) leading to reductions in neuronal and behavioral plasticity. This reduction in BDNF is suspected of increasing the susceptibility of the brain to injury. To test the effects of a HFS diet on recovery of function post-TBI, male Sprague-Dawley rats were used in this study. Eight weeks prior to TBI, rats were placed on a special HFS diet (n=14) or a standard rodent diet (n=14). Following this eight week period, rats were prepared with bilateral frontal cortical contusion injuries (CCI) or sham procedures. Beginning two days post-TBI, animals were tested on a battery of behavioral tests to assess somatosensory dysfunction and spatial memory in the Morris water maze, with a reference memory and a working memory task. Following testing, animals were sacrificed and their brains processed for lesion analysis. The HFS diet worsened performance on the bilateral tactile adhesive removal test in sham animals. Injured animals on the Standard diet had a greater improvement in somatosensory performance in the adhesive removal test and had better performance on the working memory task compared to animals on the HFS diet. The HFS diet also resulted in significantly greater loss of cortical tissue post-CCI than in the Standard diet group. This study may aid in determining how nutritional characteristics or habits interact with damage to the brain.
Keywords: American Diet, Brain Injury, Recovery of Function, Nutrition, Behavior, TBI
1. Introduction
The National Institutes of Health (NIH) has stated that TBI is among the leading causes of acute and chronic disability in the United States. Each year, 1.7 million Americans endure a TBI and 50,000 die [2]. Brain injury in the US, therefore, warrants investigation because it is both a health concern that lacks an effective treatment and is an increasingly larger expenditure to the American public. Additionally, individuals more than 65 years of age have higher rates of hospitalization and mortality than any other age group [31]. The overall incidence for TBI per 100,000 individuals is 60.6; however, the rate for individuals more than 65 years of age is 155.9 [4]. Since there are no currently approved treatments the NIH stresses the importance of prevention as its primary defense against TBI. However, of equal importance are factors that may exacerbate the injury, such as age at the time of injury, dietary Mg2+ status, or other nutritional factors [12, 18].
Recent research has shown that nutritionally based therapies are critically important in terms of TBI outcome. Therapeutic administration of nicotinamide (Vitamin B3) following TBI has been shown to be effective at improving behavioral and histological outcome in various models of TBI [7, 11, 15–17, 20–21, 23, 30]. Other nutrient compounds administered following injury, such as riboflavin (Vitamin B2), pyridoxine (Vitamin B6) and magnesium (Mg2+) have also been shown to improve functional recovery in rodent models of TBI [13–14, 19, 24]. It has recently been shown that proper caloric intake following TBI can lead to greater survival rates among patients with TBI. In a recent clinical study, severe TBI patients that were not fed within 5 to 7 days following TBI had a 2 to 4 fold increased likelihood of death. Also, every 10 kcal/kg decrease in caloric intake was associated with a 30–40% increase in mortality rates [10]. Though it appears that proper caloric intake following TBI may lead to greater recovery rates; however, there is little data outlining the outcome of the effects of poor nutrition prior to TBI.
A recent review article has chronicled the effects of many nutrients on brain function, specifically focused on cognition and synaptic plasticity, and provided many interesting avenues for investigation [8]. Of most relevance to the current study is the role of a high-fat sucrose (HFS) diet on synaptic plasticity and recovery of function following TBI. Several studies have shown that a HFS diet impairs neural plasticity and learning [9, 26, 34] but only one study has shown that administration of a HFS diet prior to fluid percussion injury (FPI) resulted in abnormal neural plasticity and impaired cognitive outcome post-injury [33]. A recent study has shown that a 5 week HFS diet prior to 6-hydroxydopamine (6-OHDA) lesioning of the medial forebrain bundle resulted in greater neurodegeneration and reduced dopamine levels compared to animals on the control diet [27].
Given the typical American diet (high fat, high sucrose content) it should come as no surprise that many of these 1.4 million TBI recipients suffer from poor nutritional levels prior to injury. The standard diet that the typical American consumes each day can lead to many debilitating health problems, including obesity, hypercholesterolemia, and cardiovascular disease. Trends toward obesity from HFS diets continue to rise. In a study conducted from 1999–2000, about 66% of American adults were considered overweight or obese and the prevalence of being overweight rose to 15% in adolescents and children [5, 28]. Thus, this potential risk factor is worthy of continued examination in TBI.
Although the effect of an HFS diet on neural plasticity and behavioral function in the uninjured rat is well known, few studies have examined this relationship in the injured brain [8, 26, 33–34]. Thus, the purpose of this study was to examine the effects of a pre-injury HFS diet on the behavioral outcome following traumatic brain injury. Eight weeks prior to TBI, rats were placed on either a standard rodent diet or a HFS diet. Following TBI, all rats were placed back on the standard rodent diet, tested on a battery of behavioral tests, and their brains harvested for histological analyses.
2. Methods
2.1 Subjects
Twenty-eight, 14–15 week old, male Sprague-Dawley rats were used in this experiment. The experimental procedures conducted within this study were reviewed and approved by the Institutional Animal Care and Use Committee and the study was conducted in a facility certified by the American Association for the Accreditation of Laboratory Animal Care. Rats were maintained on a standard 12 hour light/dark cycle with food and water available ad libitum.
2.2 Dietary Manipulation
Eight weeks prior to CCI, rats were placed on either a high-fat sucrose diet (#D12451, Research Diets Inc., New Brunswick, NJ) or remained on a standard rodent laboratory rodent control diet (#D12450B, Research Diets Inc., New Brunswick, NJ). The HFS diet contained 45% fat, 70% carbohydrate, and 20% protein compared to its control diet that contained 10% fat, 35% carbohydrate, and 20% protein. All rats received food and H2O ad libitum and their weights and health were monitored daily. Following CCI, all animals were placed back on the standard rodent laboratory control diet.
2.3 Surgery
Aseptic procedures and conditions were maintained for all animals during the surgical procedure in accordance with previously detailed methods [21–22]. Animals were anesthetized using a mixture of Isoflurane (2–4%) and oxygen (0.8 L/min) without intubation and then prepared for surgery. When the rat was unresponsive (no ocular or pedal reflexes) its head was shaved and the animal was placed into a stereotaxic device. The head was then scrubbed with 70% alcohol, followed by betadine, and a midline incision was made in the skin and underlying fascia. A circular craniotomy (6.0 mm diameter) was made using a surgical drill and a specially designed drill bit that prevented damage to the meninges and cortex. The craniotomy was bilateral and centered over the frontal cortex (3.0 mm anterior to bregma). The contusion injury was created using the Benchmark™ Stereotaxic Impactor, an electromagnetic contusion device (www.myneurolab.com, St. Louis, MO) using a sterile, aluminum impactor tip (5.0 mm diameter) that was activated at a velocity of 2.75 m/sec. The impactor tip was positioned above the cortex and upon activation of the piston, the impactor tip made contact with the cortex for 0.5 sec, which resulted in a 2.0 mm compression of the cortex. Following the contusion, any bleeding was controlled and then the incision was closed with absorbable suture material. To maintain normal body temperature (37°C) during surgery and recovery the rats were placed on a warm water recycling bed and pump system (EZ Anesthesia, Palmer, PA). Sham animals underwent the same procedures, including craniotomy, but did not receive CCI.
2.4 Bilateral Tactile Adhesive Removal Test
The bilateral tactile adhesive removal test is used to assess somatosensory dysfunction following TBI, in which the latency to remove a small round adhesive patch (113 mm2) from the radial aspect of each forelimb was recorded [11, 20]. Two trials per day were administered 5 min apart to minimize habituation effects. Each trial was terminated either when both patches had been removed or if 2 min had elapsed. Baseline latencies to remove the patches were recorded prior to injury. Animals were tested on days 2, 4, 6, 10, 12, 14, and 18 post-CCI. The dependent measure of interest was the total latency to remove both patches.
2.5 Cognitive Assessment
The MWM has been widely utilized to assess cognitive performance following brain injury [17, 25]. A blue fiberglass tank, 150 cm in diameter and 76 cm deep, was filled to a depth of 32 cm at 24ºC. An acrylic platform (10 cm × 10 cm) was submerged 2.0 cm below the surface of the water. The latency to escape was recorded by the SMART video tracking system software (San Diego Instruments, San Diego, CA). All animals were assessed on the acquisition of a reference memory task on days 11–14 post-CCI [11–13, 17, 19, 25, 30]. Each rat was tested for 4 trials each day, starting from each of the four randomly chosen release points. The inter-trial interval (ITI) was 15 mins. The trial was terminated when the animal reached the submerged platform located in the center of the northeast quadrant or when 90 sec had elapsed. Each rat was allowed to remain on the platform for 15 sec, after which it was placed in a warm holding cage until the next trial.
Working memory performance was tested on days 15–17 post-CCI using previously established methods [11, 13–14, 17, 22]. The platform was submerged at the center of a new randomly chosen quadrant (southwest, northwest, and southeast) each day. Each animal was given 4 trials per day, starting from one of four randomly chosen release points (ITI = 15 min). The first trial of each of these three days was considered an information trial and was not included in subsequent analyses. Each trial ended when the animal located the platform or when 90 sec had elapsed.
2.6 Histology
At 21 days post-CCI, rats were anesthetized with urethane (3.0 g/kg, i.p.) and were transcardially perfused with 0.9% phosphate buffered saline (PBS), followed by 10% phosphate buffered formalin (PBF). The brains were then post-fixed in PBF following removal from the cranium. A 30% sucrose solution was used to cryopreserve the brains for 3 days prior to frozen sectioning. Serial sections (40 μm thick) were sliced using a sliding microtome and electronic freezing stage and collected into phosphate buffer (PB).
2.7 Lesion Analysis
Following frozen sectioning, a series of coronal sections were brush mounted onto gelatin-subbed microscope slides, stained with cresyl violet, dehydrated and cover-slipped. The extent of the lesion was analyzed using an Olympus BX-51 microscope and DP-70 camera. Images throughout the extent of the injury were captured using the digital capturing system, and measurement of the lesioned tissue was performed using University of Texas Health Science Center San Antonio (UTHSCSA) ImageTool software. The Calvalieri method was used to calculate the volume of the remaining frontal cortex [3]. The number of sections and section thickness (40 μm) were multiplied by the mean area of the lesion cavity (calculated at four stereotaxic coordinates surrounding the lesion: 3.70, 2.70, 1.70, and 0.70 relative to bregma) [29]. The extent of cortical injury was measured by calculating the volume of remaining cortex and compared across groups [11, 13–14].
2.8 Statistical Analysis
Analysis of variance (ANOVA) tests were performed using procedures for general linear models (SPSS 15.0 for Windows; SPSS, Inc. Chicago, IL) with operations for repeated measures, where appropriate, for all behavioral measures. The between group factors were Injury and Diet group and the within group factor was day of testing. This resulted in the following experimental groups: CCI-HFS diet [n = 7], CCI-standard diet [n = 7], sham-HFS diet [n = 7], and sham-standard diet [n = 7]. Huynh-Feldt probabilities (HFP) were used to correct for Type-1 error associated with repeated measures and post hoc means comparisons, respectively [11, 13–14]. Anatomical data were analyzed with one-way ANOVA procedures and post-hoc t-tests. A significance level of p < 0.05 was used for all statistical analyses.
3.0 Results
3.1 Bodyweight Analysis
Body weights at the time of injury were analyzed with an ANOVA using a single factor (Group) in the analysis. As expected, the diets caused a significant difference in body weights between groups. The main effect of group [F(3,24) = 4.57, p = 0.0] was statistically significant indicating that the rats fed the HFS diet had a significantly increased body weight at the time of injury. Following diet exposure and at the time of injury the body weights were (CCI-HFS diet [mean = 431.4 g; SEM = 9.4 g], CCI-standard diet [mean = 407.0 g; SEM = 9.2 g], sham-HFS diet [mean = 442.4 g; SEM = 12.4 g], and sham-standard diet [mean = 396.9 g: SEM = 8.0 g]).
3.2 Bilateral Tactile Adhesive Removal Test
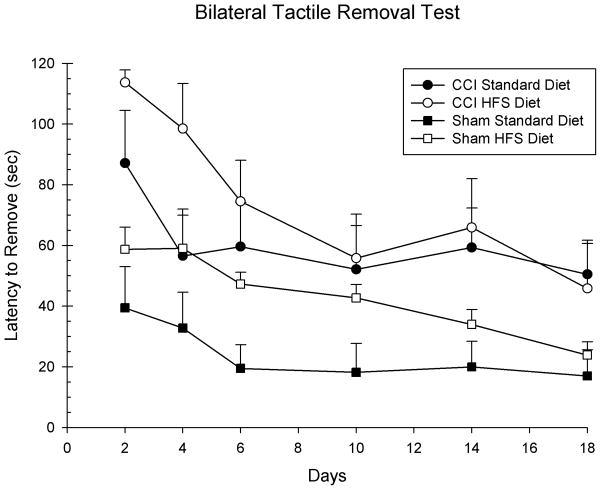
Latencies to remove the tactile adhesives were analyzed with a 3-way ANOVA with 2 between factors (Injury and Diet) and 1 within factor (Day) as the factors in the analysis. The main effects of Day [F(5,120) = 10.47, p = 0.001], Injury [F(1,24) = 17.71, p = 0.001], and Diet [F(1,24) = 4.61, p = 0.04] were statistically significant. All of the subsequent interactions were not significant (p > 0.05). Of special note is the effect of HFS diet in the sham injury conditions. As can be seen in Figure 1, the HFS diet impaired performance in the sham condition compared to the standard control diet [F(1,12) = 8.39, p = 0.01].
Figure 1.
The HFS diet altered recovery of sensorimotor performance in the injured animals compared to the standard diet. The graph shows plotted mean (+S.E.M.) latencies to remove the tactile stimuli applied to the forelimbs. The HFS diet did delay the recovery of function on the first two test days; however, over the entire length of the testing period it did not significantly impair recovery compared to the standard diet. The HFS diet also significantly impaired performance in the sham animals compared to the standard diet.
3.32 MWM: Reference Memory
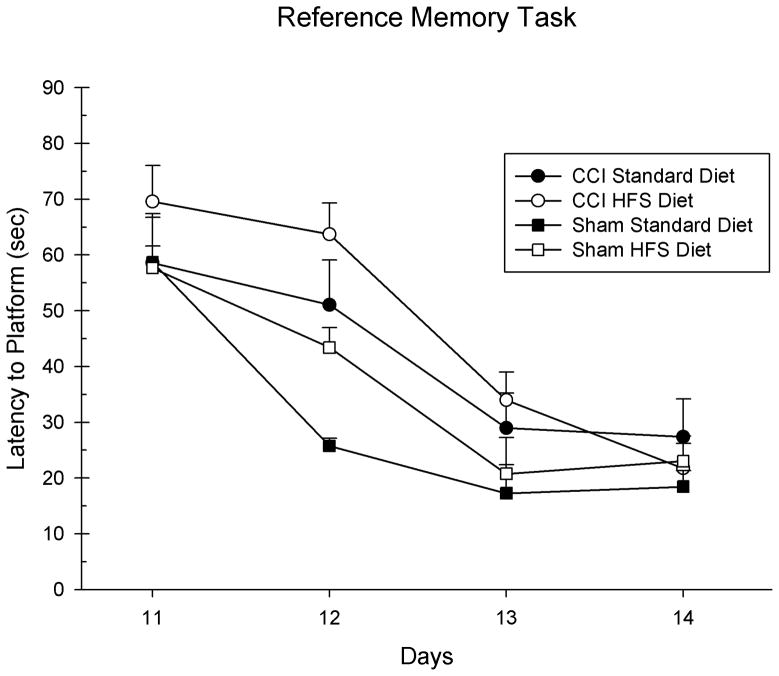
Post-CCI performance on the acquisition of a reference memory task was analyzed with a 3-way ANOVA with 2 between factors (Injury and Diet) and 1 within factor (Day) as the factors in the analysis. The main effects of Day [F(3,72) = 63.77, p = 0.001] and Injury [F(1,24) = 6.84, p = 0.01] were statistically significant; however, the main effect of Diet was not [F(1,24) = 1.92, p = 0.18]. See Figure 2. There was a significant interaction between Day x Injury, [F(3,72) = 3.51, p = 0.02]. All of the other subsequent interactions were not significant (p > 0.05).
Figure 2.
The HFS diet only marginally altered the acquisition of a reference memory task in the MWM compared to the standard diet in the injured animals. The graph shows plotted mean (+S.E.M.) swim latencies to the hidden platform in the MWM. The HFS diet did not significantly impair learning acquisition on this task compared to the standard diet post-CCI.
3.4 MWM: Working Memory Performance
Post-CCI performance on the working memory task was analyzed with a 3-way ANOVA with 2 between factors (Injury and Diet) and 1 within factor (Day) as the factors in the analysis. The main effects of Day was not significant [F(2,48) = 2.23, p = 0.12]; however, the main effects of Injury [F(1,24) = 11.45, p = 0.002] and Diet [F(1,24) = 4.84, p = 0.04] were statistically significant. The interaction between Injury x Diet just failed to reach significance [F(3,72) = 3.51, p = 0.07]. See Figure 3. There was a significant interaction between Day x Injury, [F(1,24) = 3.54, p = 0.02]. All of the other subsequent interactions were not significant (p > 0.05).
Figure 3.
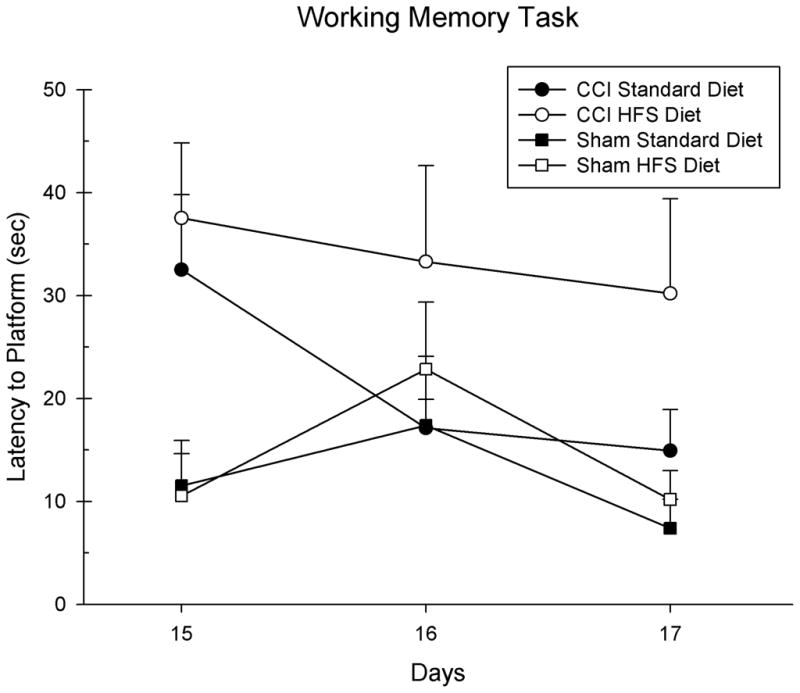
The HFS diet significantly impaired the acquisition of a working memory task in the MWM compared to the standard diet in the injured animals. The graph shows plotted mean (+S.E.M.) swim latencies to the hidden platform that has been relocated to a new location each testing day in the MWM. The HFS diet significantly impaired learning acquisition on this task compared to the standard diet post-CCI.
3.5 Lesion Analysis
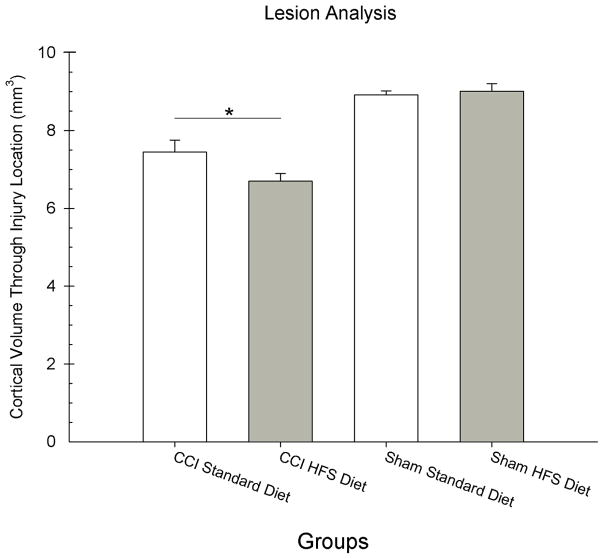
The reduction in cortical tissue volume throughout the injury site was analyzed with an ANOVA with 2 between factors (Injury and Diet) in the analysis. A significant difference in injury volume between groups was found. The main effect of Injury [F(1,24) = 91.06, p = 0.001] was statistically significant, as well as, the interaction between Injury x Diet [F(1,24) = 4.60, p = 0.04] (See Figure 4). The main effect of Diet was not statistically significant [F(1,24) = 2.73, p = 0.11]. Of special interest was the difference between the Standard Diet and HFS Diet injured groups so a post hoc t-test was performed that showed that the injured HFS Diet group [mean = 6.70; SEM = 0.21] had significantly larger injury cavities compared to the injured standard diet [mean = 7.45; SEM = 0.27] group [t(12) = 2.23, p = 0.04]. Representative histology is presented in Figure 5.
Figure 4.
The HFS diet increased the size of the injury cavity compared to the standard diet. Analysis of the remaining frontal cortical volume is shown for each group. The graph shows plotted mean (+S.E.M.) cortical volumes for each group. The HFS diet resulted in less remaining cortical tissue (i.e., larger injury cavity) compared to the standard diet (* = p < 0.01).
Figure 5.
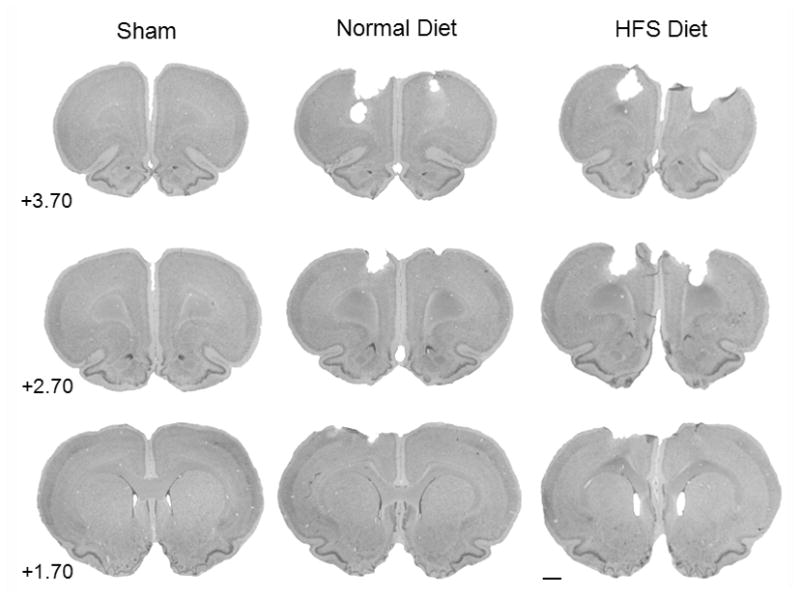
Histology plate. Shown are representative cresyl violet images (40 μm) of sections from all three experimental groups at coordinates 3.70 mm, 2.70 mm, and 1.70 mm relative to bregma [29] (0.44×, scale bar = 2.0 mm).
4. Discussion
The main experimental objective of this study was to examine the role of a HFS diet on functional recovery following TBI. Following the HFS diet period uninjured animals did not differ in their performance on the cognitive assessments; however, it was found that the HFS diet significantly altered sensorimotor performance on the tactile removal task. The present data indicated that eight weeks of a HFS diet prior to induction of TBI altered normal recovery patterns of sensorimotor and cognitive tasks following injury compared to the standard diet. Following injury the HFS diet worsened the magnitude of the tactile removal deficit but there were no significant differences in recovery. The HFS diet also did not significantly impair the acquisition of a reference memory task following injury compared to the standard diet. However, on the working memory task the HFS diet did significantly worsen performance. Thus, it appears that the HFS diet did alter recovery patterns following injury and that this effect was task dependent.
Although the behavioral data on the HFS diet were somewhat varied, it was clear from the lesion analysis that the HFS significantly worsened the extent of the injury, determined by a significantly greater loss of cortical tissue following CCI. In our experimental model we performed 8 weeks of diet manipulation prior to injury and then put the animals back onto the standard diet post-injury. This was done to mimic the medical condition of having a poor diet prior to injury and then being put on a balanced diet while in the hospital and during rehabilitation in a neurorestorative facility. It is very likely that if the HFS diet had been continued post-injury that our behavioral differences would have been much more severe and consistent with previous research [9, 26, 34]. In general, switching back to the standardized diet post-TBI may have provided some degree of neuroprotection and helped to improve recovery. Previous work with the HFS diet prior to FPI has shown that the cognitive outcome following injury was worsened compared to that of a control diet and that a reduction in BDNF levels in the hippocampus may have been the cause [33].
It has recently been shown that HFS diets can reduce levels of brain-derived neurotrophic factor (BDNF) leading to reductions in neuronal and behavioral plasticity [26]. Eight weeks of a HFS diet reduced BDNF levels in the hippocampus, altered hippocampal plasticity and resulted in a significant impairment in the acquisition of a reference memory task in the MWM [26]. A recent study demonstrated that this effect also occurs in the middle-aged rat [9]. BDNF, which has been shown to be important for neuronal vitality and function, appears to be significant in neuronal events underlying learning and memory since animals with reduced expression of BDNF exhibit deficits in learning and memory [1, 26]. The HFS induced reduction in BDNF is suspected of increasing the susceptibility to brain insults following TBI. To evaluate this concept, animals were placed on a HFS diet 4 weeks prior to FPI trauma [33]. It was found that injured animals on the HFS diet had significant reductions in BDNF levels and learning performance compared to those on the standard diet. In a study detailing the effects of TBI on BDNF knockout mice, it appears that the lack of BDNF increases cell death of adult-born immature neurons in the hippocampus following CCI [6]. Thus, it is likely that these same mechanisms are responsible for the significant effects observed in the present data. Although, it has not been previously reported, the finding in the current study that demonstrated significantly impaired sensorimotor behavior in the HFS-sham group helps strengthen the statement that altered BDNF levels can alter behavioral performance.
Interestingly, dietary supplementation with vitamin E has been shown to overcome the deleterious effects of an HFS diet [34]. Animals were placed on HFS diets for 8 weeks either with supplementation of Vitamin E (500 IU/kg/day) or without. It was found that supplementation with Vitamin E prevented the reduction in BDNF and the cognitive impairments seen in animals on the HFS diet alone [34]. Thus, it is clear that the HFS diet alters BDNF levels and behavioral performance, but may be a deficit that can be overcome with supplementation of various neuroprotective compounds.
As expected, eight weeks on the HFS significantly increased the body weight of the animals compared to the standard control diet. Although the HFS diet animals weighed more at the time of injury compared to the controls it is unlikely that this would have had any significant effect on post-CCI performance based on our experience comparing different strains and ages of rats in animal models of TBI [12, 32]. Compared to previous studies, the present data replicated the effects of an HFS diet on cognitive performance following FPI [33] and extended these findings to include the CCI animal model. In addition, it was also found that the deleterious effects of an HFS diet on recovery of function also extended to sensorimotor behavior. In fact, the HFS diet alone significantly worsened sensorimotor performance in the present study on the bilateral tactile adhesive removal task. The present study also demonstrated that the HFS diet produced a significant degree of vulnerability in the brain following injury and resulted in more extensive cortical damage post-CCI. As mentioned previously, the findings from the present study also occurred in the animals after they had been returned to the standard diet post-CCI. Thus, the effect of a short period of intensive HFS diet may be long lasting and would support the BDNF mechanism of action [26, 33–34].
Animal models of TBI have been frequently used for the last 30 years to study all aspects of traumatic injuries to the brain. These studies have examined preclinical efficacy of novel therapies and have started to unravel the pathophysiology associated with these injuries. The neurotrauma research field has gained much knowledge related to TBI through these animal models. The present data has helped demonstrate that that other medical conditions (e.g., poor diet) can have a deleterious outcome on TBI. It is hoped that in the coming decades that new therapies to treat TBI will become standard in the field of neurotrauma.
Acknowledgments
Research support provided by an SIU-C REACH award and the NIH, NS045647. The authors would like to thank Nick Kaufman for his assistance on the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castrén E, Berninger B, Leingärtner A, Lindholm D. Regulation of brain-derived neurotrophic factor mRNA levels in hippocampus by neuronal activity. Prog Brain Res. 1998;117:57–64. doi: 10.1016/s0079-6123(08)64007-8. [DOI] [PubMed] [Google Scholar]
- 2.CDC. Injury Prevention and Control: Traumatic Brain Injury. 2010 Retrieved September 22, 2010 from http://www.cdc.gov/traumaticbraininjury/statistics.html.
- 3.Coggeshall RE. A consideration of neural counting methods. TINS. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- 4.Coronado VG, Thomas KE, Sattin RW, Johnson RL. The CDC traumatic brain injury surveillance system: Characeristics of persons aged 65 years and older hospitalized with a TBI. J Head Trauma Rehabil. 2005:215–28. doi: 10.1097/00001199-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Flegal K, Carroll M, Ogden C, Clifford L. prevalence and trends in obesity amoung US adults. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Chen J. Conditional knockout of brain-derived neurotrophic factor in the hippocampus increases death of adult-born immature neurons following traumatic brain injury. J Neurotrauma. 2010;26:1325–35. doi: 10.1089/neu.2008.0744. [DOI] [PubMed] [Google Scholar]
- 7.Goffus AM, Anderson GD, Hoane MR. Sustained delivery of nicotinamide limits cortical injury and improves functional recovery following traumatic brain injury. Oxid Med Cell Longev. 2010;3:145–52. doi: 10.4161/oxim.3.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gómez-Pinilla F. Brain foods: The effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–78. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granholm A-C, Biomonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. Journal of Alzheimer’s Disease. 2008;14:133–45. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Härtl R, Gerber LM, Ni Q, Ghajar J. Effect of early nutrition on deaths due to severe traumatic brain injury. J Neurosurg. 2008;109:50–6. doi: 10.3171/JNS/2008/109/7/0050. [DOI] [PubMed] [Google Scholar]
- 11.Hoane MR, Akstulewicz SL, Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in the rat. J Neurotrauma. 2003;20:1189–98. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- 12.Hoane MR, Lasley LA, Akstulewicz SL. Middle age increases tissue vulnerability and impairs sensorimotor and cognitive recovery following traumatic brain injury in the rat. Behav Brain Res. 2004;153:189–97. doi: 10.1016/j.bbr.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Hoane MR. Treatment with magnesium improves reference memory but not working memory while reducing GFAP expression following traumatic brain injury. Restor Neurol Neurosci. 2005;23:67–77. [PubMed] [Google Scholar]
- 14.Hoane MR, Wolyniak J, Akstulewicz SL. Administration of riboflavin improves behavioral outcome and reduces edema formation and GFAP expression following traumatic brain injury. J Neurotrauma. 2005:1112–22. doi: 10.1089/neu.2005.22.1112. [DOI] [PubMed] [Google Scholar]
- 15.Hoane MR, Gilbert DR, Holland MA, Pierce JL. Nicotinamide reduces acute cortical neuronal death and edema in the traumatically injured brain. Neurosci Lett. 2006:35–9. doi: 10.1016/j.neulet.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Hoane MR, Kaplan SA, Ellis AL. The effects of nicotinamide on apoptosis and blood-brain barrier breakdown following traumatic brain injury. Brain Res. 2006;1125:185–93. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Hoane MR, Tan AA, Pierce JL, Anderson GD, Smith DC. Nicotinamide treatment reduces behavioral impairments and provides cortical protection after fluid percussion injury in the rat. J Neurotrauma. 2006;23:1535–48. doi: 10.1089/neu.2006.23.1535. [DOI] [PubMed] [Google Scholar]
- 18.Hoane MR. Magnesium dietary manipulation and recovery of function following controlled cortical damage in the rat. Magnes Res. 2007;21:29–37. [PubMed] [Google Scholar]
- 19.Hoane MR. Assessment of cognitive function following magnesium therapy in the traumatically injured brain. Magnes Res. 2007;20:229–36. [PubMed] [Google Scholar]
- 20.Hoane MR, Pierce JL, Holland MA, Anderson GD. Nicotinamide treatment induces behavioral recovery when administered up to 4 hours following cortical contusion injury in the rat. Neuroscience. 2008;154:861–8. doi: 10.1016/j.neuroscience.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoane MR, Pierce JL, Kaufman NA, Beare JE. Variation in chronic nicotinamide treatment after traumatic brain injury can alter components of functional recovery independent of histological damage. Oxid Med Cell Longev. 2008;1:46–53. doi: 10.4161/oxim.1.1.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoane MR, Kaufman NA, Vitek MP, McKenna SE. COG1410 improves cognitive performance and reduces cortical neuronal loss in the traumatically injured brain. J Neurotrauma. 2009;26:1–10. doi: 10.1089/neu.2008.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland MA, Tan AA, Smith DC, Hoane MR. Nicotinamide treatment provides acute neuroprotection and GFAP regulation following fluid percussion injury. J Neurotrauma. 2008;25:140–52. doi: 10.1089/neu.2007.0312. [DOI] [PubMed] [Google Scholar]
- 24.Kuypers NJ, Hoane ME. Pyridoxine administration improves behavioral and anatomical outcome after unilateral contusion injury in the rat. J Neurotrauma. 2010;27:1275–82. doi: 10.1089/neu.2010.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindner MD, Plone MA, Cain CK, Frydel BR, Francis JM, Emerich DF, Sutton RL. Dissociable long-term cognitive deficits after frontal versus sensorimotor cortical contusions. J Neurotrauma. 1998;15:199–216. doi: 10.1089/neu.1998.15.199. [DOI] [PubMed] [Google Scholar]
- 26.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 27.Morris LK, Bomhoff GL, Stanford JA, Geiger PC. Neurodegeneration in an animal model of Parkinson’s disease is exacerbated by a high-fat diet. AM J Physiol Regul Integr Comp Physiol. 2010;299:1082–90. doi: 10.1152/ajpregu.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogden C, Flegal K, Carroll M, Johnson C. prevalence and trends in overweight among US children and adolescents. JAMA. 2002:288. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. New York: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- 30.Quigley A, Tan AA, Hoane MR. The effects of hypertonic saline and nicotinamide on sensorimotor and cognitive function following cortical contusion injury in the rat. Brain Res. 2009;1304:138–48. doi: 10.1016/j.brainres.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006:456–65. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Tan AA, Quigley A, Smith DC, Hoane MR. Strain differences in response to traumatic brain injury in Long-Evans compared to Sprague-Dawley rats. J Neurotrauma. 2009;26:539–48. doi: 10.1089/neu.2008.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu A, Molteni R, Ying Z, Gómez-Pinilla F. A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience. 2003;119:365–75. doi: 10.1016/s0306-4522(03)00154-4. [DOI] [PubMed] [Google Scholar]
- 34.Wu A, Ying Z, Gómez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19:1699–707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]