Abstract
Low-dose hydrocortisone therapy modulates inflammatory responses in adults and improves outcomes in some septic adults and neonates, but its immunologic effects have not been evaluated in neonates. The objective of this study was to evaluate effects of low-dose hydrocortisone (LDHC) therapy on ex vivo immune function in neonatal horses (foals). We hypothesized that LDHC treatment would dampen pro-inflammatory responses without impairing neutrophil function. Hydrocortisone (1.3 mg/kg/day i.v.) was administered to foals in a tapering 3.5 day course. Peripheral blood leukocytes were collected from foals before, during and after hydrocortisone treatment. A separate group of age-matched untreated foals served as controls. Endotoxin-induced peripheral blood mononuclear cell gene expression of inflammatory cytokines was measured by real time quantitative RT-PCR. Neutrophils were incubated with labeled, killed S. aureus or E. coli for assessment of phagocytosis, and with phorbol myristate acetate, zymosan, or endotoxin for measurement of reactive oxygen species (ROS) production. Neutrophil phagocytosis and ROS production were similar in both groups. Foals receiving hydrocortisone had significantly decreased endotoxin-induced expression of TNF-α, IL-6, IL-8, and IL-1β. These data suggest that this LDHC treatment regimen ameliorates endotoxin-induced pro-inflammatory cytokine expression in neonatal foals without impairing innate immune responses needed to combat bacterial infection.
INTRODUCTION
Cortisol is vital for the stress response to critical illness, and plays an essential role in regulation of the inflammatory response (1). In some patients, though, the cortisol response to illness is inadequate, a condition called relative adrenal insufficiency (RAI) or critical illness-related corticosteroid insufficiency (CIRCI) (2-4). The physiologic consequences of RAI/CIRCI can include cardiovascular collapse and an excessive, unregulated systemic inflammatory response (3, 4). A number of studies have documented a significantly higher incidence of multiple organ failure and death in septic patients with RAI/CIRCI as compared to patients with intact hypothalamic-pituitary-adrenal (HPA) axis function (4-6). An exaggerated systemic inflammatory response characterized by imbalance in pro- and anti-inflammatory cytokine production is also documented in septic people and animals, and is also predictive of increased disease severity and death (1, 7-12). Cortisol insufficiency during sepsis is theorized to contribute to such an overwhelming inflammatory response (1, 10, 12).
Thus, glucocorticoids have been administered to septic patients in varying doses in attempts to quell inflammation. While high-dose corticosteroid therapy is associated with adverse effects and decreased survival in septic patients (13-15), a number of studies have described improved shock reversal and survival in septic patients with RAI/CIRCI when supplemented with low-dose hydrocortisone (LDHC) (4, 13-17).
As fetal HPA axis function does not mature until the peripartum period, cortisol insufficiency may be of particular significance in the neonate (3, 18). A number of studies have documented cortisol insufficiency in septic adult and pediatric patients and in critically ill full-term and pre-term infants (4, 6, 19-22). However, to the authors’ knowledge, the effects of LDHC therapy on immune function in neonates of any species are not described. Given the numerous differences in endocrine and immune function and in hydrocortisone pharmacokinetics between adults and neonates (3, 18, 23), specific evaluation of LDHC therapy in the neonate is critical to optimize therapeutic recommendations for this population.
Like infants, neonatal horses (foals) exhibit substantial HPA axis immaturity in the perinatal period (24-27) and are highly susceptible to the development of bacterial sepsis (28). In addition, naturally-occurring RAI/CIRCI is described in approximately 40% of septic neonatal foals and is correlated with increased incidence of shock, multiple organ failure, and death (29), as documented in septic adults, children, and infants (4, 6, 19-22). Furthermore, septic foals also exhibit similar inflammatory dysregulation to septic infants, characterized by increased expression of pro-inflammatory cytokine genes such as TNF-α and IL-6 (7, 8, 30).
Thus, the primary objective of this study was to examine the effects of LDHC therapy on measures of immune function in neonatal foals in an ex vivo model. In addition, effects of LDHC therapy on HPA axis function were examined. We hypothesized that LDHC treatment: 1) would not significantly impair the ability of peripheral blood granulocytes to produce reactive oxygen species (ROS) or phagocytose bacteria; 2) would significantly decrease endotoxin-induced expression of pro-inflammatory cytokine genes and significantly increase endotoxin-induced expression of anti-inflammatory cytokine genes in peripheral blood mononuclear cells (PBMCs); and 3) would not significantly suppress HPA axis function in vivo after discontinuation of hydrocortisone administration.
MATERIALS AND METHODS
Animals
Thirty-nine healthy 2-to-7-day-old full-term foals (gestational age ≥ 330 days, weight 40-60 kg) from university research herds were used. Foals were housed with the dam and were determined to be healthy during the study period by daily physical examination. Eleven foals (4 females, 7 males) were treated with LDHC (HYDRO group), and 28 age-matched foals (15 females, 13 males) were untreated (CONTROL group). Seventeen CONTROL foals were used to provide age-matched comparisons with HYDRO foals for immune function testing, and 11 CONTROL foals provided age-matched HPA axis function comparisons with HYDRO foals. Group assignments were randomized by order of foaling and were known to the investigators.
Study methods were approved by the University of Georgia's and Clemson University's Institutional Animal Care and Use Committee, and mare/foal pairs were cared for according to the guidelines stated in an Animal Use Protocol determined and approved by each university's Department of Animal Resources.
LDHC Treatment and Sampling Protocol
Sampling protocols for HYDRO and CONTROL foals are shown in Figure 1. Between 36-60 hours of age, all HYDRO foals had an intravenous jugular catheter placed under brief standing restraint. Beginning 12-18 hours after catheter placement, HYDRO foals received a tapering 3.5 day course of hydrocortisone sodium succinate (Pfizer, New York, NY) as follows: 1.3 mg/kg/day for 48 hours, 0.65 mg/kg/day for 24 hours, and 0.33 mg/kg/day for 12 hours. This total daily dose was determined by multiplying the daily endogenous cortisol production rate in healthy neonatal foals (31) by a factor of 2 to approximate an appropriate cortisol response to stress as described in other species (2, 32, 33). This total daily dose was divided into 6 doses administered as an i.v. bolus every 4 hours.
Figure 1.
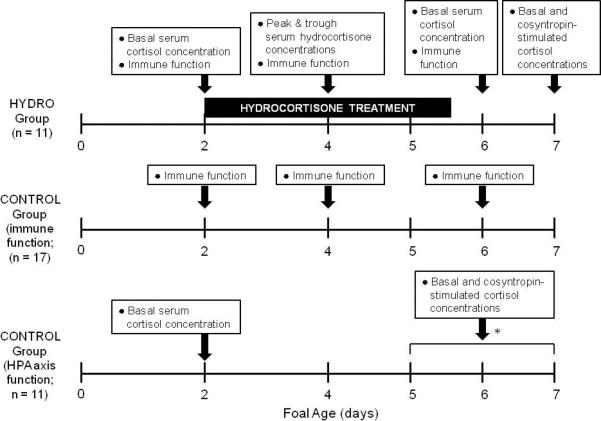
Foal groups and sampling protocols. *CONTROL foals used for HPA axis function comparisons with HYDRO foals were sampled on day 2 of age and once between days 5 and 7 of age.
Sixty-five milliliters of blood was collected just before the first dose of hydrocortisone (PRE-TREATMENT), after 48 hours of therapy before the initial dose was halved (DURING TREATMENT), and 12 hours after cessation of hydrocortisone administration (POST-TREATMENT). 5 mL blood was allowed to clot, centrifuged, and serum stored at -80°C until analysis for cortisol/hydrocortisone concentrations. The remaining 60 mL was anti-coagulated with 2 mL 100 μM EDTA for isolation of peripheral blood leukocytes. At the DURING TREATMENT sample, blood was also collected 5 minutes after hydrocortisone administration for measurement of peak serum hydrocortisone concentration.
In the 17 CONTROL foals used for immune function comparisons, blood was collected similarly on day 2, day 4, and day 6 of life to permit age-matched comparisons with the HYDRO group.
Twenty-four hours after discontinuation of hydrocortisone therapy, post-treatment HPA axis function and responsiveness was assessed in 8/11 HYDRO foals. Blood was collected for measurement of basal cortisol concentrations and a paired low-dose (10 μg) / high dose (100 μg) cosyntropin (synthetic ACTH, α 1-24 corticotropin, Amphastar Pharmaceuticals, Rancho Cucamonga, CA) stimulation test (24) was performed (Figure 2). 10 μg cosyntropin, rather than the standard 1 μg dose used in people, was used for the low-dose cosyntropin stimulation test because lack of a measurable cortisol response to 1 μg cosyntropin in neonatal foals has been previously demonstrated(34).
Figure 2.
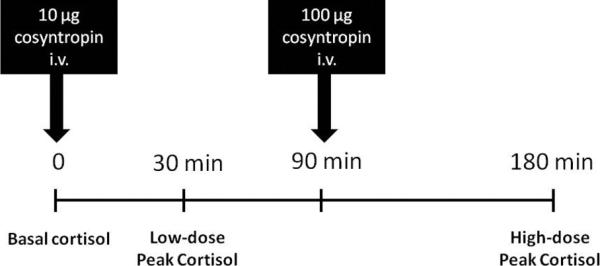
Paired low-dose / high-dose cosyntropin stimulation test design. At time 0, blood was collected for measurement of basal cortisol concentration, followed by administration of 10 μg cosyntropin i.v. Blood was collected 30 minutes later to assess the cortisol response to 10 μg cosyntropin (Low-dose Peak Cortisol). At 90 minutes, 100 μg cosyntropin was administered i.v., and blood was collected 90 minutes later to assess the cortisol response to 100 μg cosyntropin (High-dose Peak Cortisol).
In the 11 CONTROL foals used for HPA axis function comparisons, blood was collected on day 2 and between days 5 and 7 of age for measurement of basal cortisol concentrations for comparisons with HYDRO foals. A paired cosyntropin stimulation test was also performed between day 5 and 7 of age in these CONTROL foals for comparison with HYDRO foals’ POST-TREATMENT cosyntropin stimulation responses. Some findings in this group have been published previously (24).
Cortisol and Hydrocortisone Assays
Serum total cortisol and hydrocortisone concentrations were determined on an automated analyzer using a chemiluminescent immunoassay (Immulite™, Diagnostics Product Corporation, Los Angeles, CA) validated for use in the horse {Reimers, 1996 #174;Singh, 1997 #169}.
Ex vivo Immune Function Testing
Peripheral blood granulocytes and PBMCs were isolated within 120 minutes of collection by density-gradient centrifugation over Histopaque-1077 (Sigma Aldrich, St. Louis, MO) as previously described (37, 38). Viability of both cell types was greater than 95% as assessed by trypan blue exclusion.
Neutrophil ROS production in response to endotoxin (100 ng/ml; E. coli 055:B5 LPS; List Biological Inc., Campbell, CA), zymosan (1000 ng/ml; Molecular Probes, Eugene, OR), and phorbol myristate acetate (PMA, 10-7 M; Molecular Probes, Eugene, OR) was measured using a fluorometric assay as previously described (37). Assessment of ROS production in response to these three stimulants permitted assessment of the cells’ Toll-like receptor 4 (TLR4)-mediated ROS production (endotoxin), dectin-1-mediated ROS production (zymosan), and overall capacity to produce ROS in response to protein kinase C activation (PMA).
To determine phagocytic function, isolated neutrophils were re-suspended to a final concentration of 3 × 106 cells/ml in RPMI 1640 without phenol red supplemented with 50 μg gentamicin sulfate, 2 mM L-glutamine, 1 mM sodium pyruvate, and 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT). Bodipy-labeled, inactivated Escherichia coli and Staphylococcus aureus (Invitrogen, Carlsbad, CA) were diluted to a final concentration of 2 × 106/ml. 200 μl of resuspended cells were incubated in duplicate with 50 μl E. coli or S. aureus or with media only for 60 minutes at 37°C and 5% CO2. Cells were then washed with FACS buffer and fixed in 1% formalin. Flow cytometric analysis was conducted within 7 days of fixation (Accuri C6 Cytometer, Accuri Cytometers Inc., Ann Arbor, MI). Extracellular bacterial fluorescence was quenched with 0.4% trypan blue (Sigma Chemical, St. Louis, MO), and samples were then assessed for the percent of fluorescent-positive cells using commercial software (CFlowPlus, Accuri Cytometers Inc., Ann Arbor, MI).
After isolation, PBMCs were resuspended in RPMI-1640 supplemented with 100 IU/ml penicillin, 100 mg/ml streptomycin, and 10% equine serum (Hyclone, Logan, UT). The cells were equally divided among 6 sterile 60 × 15 mm Petri dishes and incubated for 30 minutes at 37°C in 5% CO2. Plates were then treated with 1 ng/ml endotoxin (E. coli 011:B4 LPS; List Biological Inc., Campbell, CA) or an equivalent volume of media and incubated at 37°C and 5% CO2 for 1, 4, and 20 hours. Cells were scraped from the plates in cold phosphate buffered saline, pelleted via brief centrifugation at 14,000 × g, and lysed with RNA cell lysis solution (Qiagen, Valencia, CA) in combination with 10 μl/ml 2-mercaptoethanol (Sigma Chemical, St. Louis, MO) and stored at -80°C until RNA extraction. Total RNA was extracted from cell lysates using the RNeasy mini RNA extraction kit (Qiagen, Valencia, CA) according to the manufacturer's protocol and treated with DNase I at 25°C for 30 minutes. Only samples having 260:280 nm absorbance ratios between 2.0 and 2.2 as measured on a NanoDrop spectrophotometer (ThermoFisher Scientific, Wilmington, DE) were processed for cDNA synthesis with the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) using 500 ng RNA as template.
Expression of TLR-4, TNF-α, IL-1β, IL-6, IL-10, IL-4, IL-8 and TGF-β were quantified using validated two-step real time quantitative RT-PCR assays with SYBR Green detection in an Applied Biosystems 7900HT sequence detection system(Foster City, CA), with 18S ribosomal RNA used as an endogenous housekeeping control as previously reported (39, 40). Changes in gene expression were calculated by relative quantification against 18s rRNA using the ΔΔCT method with plain media controls used as the calibrator. Fold changes in gene expression between endotoxin-stimulated and unstimulated cells from the same foal for each incubation period (1, 4, and 20 hours) at each sampling time point were calculated as 2-ΔΔCT.
Data Analysis
Between-group comparisons between age-matched HYDRO and CONTROL foals were conducted using Student's t tests for parametrically distributed data and Mann Whitney U tests for non-parametric data, after normality was assessed with the Kolmogorov-Smirnov test. For inflammatory mediator gene expression, LPS-induced fold changes in gene expression ≤ 3-fold were not considered relevant gene induction. Thus, if both CONTROL and HYDRO foals failed to exhibit an endotoxin-induced change in expression of >3-fold for a specific gene, further between-group comparisons for that gene at that day and incubation time point were not conducted. Statistical analysis was performed using commercial statistical software (Prism Version 4, GraphPad Software, Inc., San Diego, CA) and statistical significance was set at P < 0.05.
RESULTS
No severe adverse effects were noted in any HYDRO foals. Two foals developed a partial thrombus at the jugular catheter site during treatment and one foal developed mild diarrhea 72 hours into hydrocortisone treatment, all of which resolved without specific therapy.
ROS Production
Neutrophil ROS production in response to endotoxin, zymosan, and PMA in HYDRO and CONTROL foals is shown in Table 1. No significant differences in ROS production in response to any stimulants were found between HYDRO and CONTROL foals before, during or after hydrocortisone treatment.
Table 1.
Neutrophil ROS production in HYDRO foals before, during, and after hydrocortisone treatment and in age-matched CONTROL foals after ex vivo stimulation with endotoxin (100 ng/ml), zymosan (100 ng/ml), and PMA (10-7 M). ROS production is expressed as corrected arbitrary fluorescence units (AFUs), which were calculated by subtracting background fluorescence in unstimulated control cells from fluorescence in stimulated cells.
| Response to endotoxin (corrected AFUs) | Response to zymosan (corrected AFUs) | Response to PMA (corrected AFUs) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PRE-TREATMENT | DURING TREATMENT | POST-TREATMENT | PRE-TREATMENT | DURING TREATMENT | POST-TREATMENT | PRE-TREATMENT | DURING TREATMENT | POST-TREATMENT | |
| HYDRO Foals (n=11) | 6.4 ± 4.6 (0.4 – 15.1) | 4.2 ± 5.9 (-1.7 – 18.2) | 4.3 ± 5.7 (-1.9 – 16.6) | 16.2 ± 3.0 (9.1 – 19.4) | 15.8 ± 9.8 (-3.3 – 33.7) | 19.5 ± 17.8 (-3.7 – 56.3) | 51.6 ± 16.2 (29.8 – 75.3) | 71.2 ± 22.8 (43.7 – 16.8) | 100.6 ± 57.9 (41.5-227.4) |
| CONTROL Foals (n=13) | 2.9 ± 4.1 (-2.7 – 10.6) | 0.7 ± 5.0 (-6.4 – 7.4) | 0.4 ± 3.3 (-4.7 – 4.9) | 13.7 ± 7.2 (2.3 – 25.8) | 9.3 ± 5.9 (0.4 – 18.9) | 11.3 ± 2.9 (8.3 – 18.0) | 65.2 ± 17.4 (41.2 – 93.1) | 71.6 ± 21.9 (40.0-110.6) | 89.6 ± 34.7 (45.5-176.6) |
Data is expressed as mean ± standard deviation (range). No significant differences (P < 0.05) were found between HYDRO and CONTROL foals.
Phagocytic Function
Neutrophil phagocytosis of E. coli and S. aureus in HYDRO and CONTROL foals is shown in Table 2. No significant differences in phagocytic function were found between HYDRO and CONTROL foals before, during, or after hydrocortisone treatment.
Table 2.
Phagocytosis of killed, bodipy-labeled E. coli and S. aureus by isolated neuotrophils from HYDRO foals before, during, and after hydrocortisone treatment and from age-matched CONTROL foals. Phagocytosis is expressed as % positive cells detected by flow cytometry after quenching of extracellular fluorescence with 0.4% trypan blue.
| Phagocytosis of E. coli (% positive cells) | Phagocytosis of S. aureus (% positive cells) | |||||
|---|---|---|---|---|---|---|
| PRE-TREATMENT | DURING TREATMENT | POST-TREATMENT | PRE-TREATMENT | DURING TREATMENT | POST-TREATMENT | |
| HYDRO Foals (n=11) | 16.7 ± 13.2 (2.0 – 44.6) | 19.4 ± 12.7 (3.3 – 38.2) | 16.7 ± 10.6 (0.0 – 33.5) | 22.0 ± 9.2 (5.0 – 34.8) | 17.6 ± 8.9 (6.3 – 34.0) | 19.2 ± 11.1 (5.2 – 46.8) |
| CONTROL Foals (n=9) | 11.9 ± 9.9 (1.0 – 28.2) | 14.3 ± 13.9 (2.7 – 45.1) | 11.8 ± 8.3 (1.5 – 22.3) | 19.6 ± 14.0 (1.3 – 41.3) | 14.9 ± 10.4 (6.2 – 39.8) | 11.1 ± 6.8 (2.2 – 22.3) |
Data is expressed as mean ± standard deviation (range). No significant differences (P < 0.05) were found between HYDRO and CONTROL foals.
Inflammatory Molecule Gene Expression
Both HYDRO and CONTROL foals exhibited a > 3-fold endotoxin-induced change in gene expression for TNF-α, IL-6, IL-1β, IL-8, and IL-10 for at least one incubation time on each sampling day, permitting between-group comparisons. Endotoxin-induced changes in gene expression for TLR-4, IL-4, and TGF-β were < 3-fold for both groups and at all incubation times on all sampling days, so between-group analysis was not conducted for these genes.
No significant differences in endotoxin-induced gene expression were found between HYDRO and CONTROL foals at the PRE-TREATMENT sample for any genes except IL-8, for which HYDRO foals had significantly lower expression than CONTROL foals (P = 0.027) after 4 hours of incubation with endotoxin.
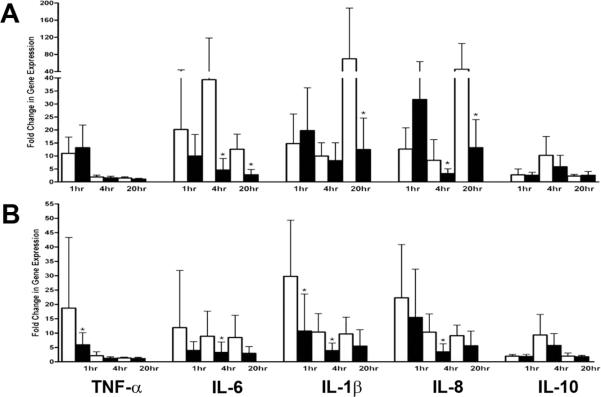
Endotoxin-induced changes in gene expression for TNF-α, IL-6, IL-1β, IL-8, and IL-10 in HYDRO foals during and after hydrocortisone treatment and in age-matched CONTROL foals are shown in Figure 3. At the DURING TREATMENT sample, HYDRO foals exhibited significantly lower endotoxin-induced expression of IL-6 (P < 0.001), IL-1β (P < 0.036), and IL-8 (P = 0.004 to P = 0.019) than CONTROL foals. In addition, HYDRO foals also exhibited lower expression of IL-1β and IL-10 after 4 hrs of endotoxin incubation that approached statistical significance (P = 0.053, P = 0.050 respectively).
Figure 3.
Mean fold change in mRNA expression of TNF-α, IL-6, IL-1β, IL-8, and IL-10 in PBMCs from HYDRO foals (n=11; ■) and age-matched CONTROL foals (n= 15; □) incubated with endotoxin (1 ng/ml) for 1, 4, and 20 hours. Fold change in mRNA expression is relative to unstimulated PBMCs from the same animal. Expression during and after hydrocortisone treatment is shown in parts (A) and (B) respectively. *Significant (P < 0.05) difference between HYDRO and CONTROL foals.
At the POST-TREATMENT sample, endotoxin-induced expression of IL-6 (P = 0.023), IL-1β (P < 0.001 to P = 0.021), and IL-8 (P = 0.002) remained significantly lower in HYDRO foals than CONTROL foals. Endotoxin-induced expression of TNF-α was also significantly lower in HYDRO foals (P = 0.047) at this sample. Endotoxin-induced expression of IL-1β and IL-8 after 20 hrs of incubation also was lower in HYDRO foals, and appproached statistical significance (P = 0.054, P = 0.054 respectively).
Serum Cortisol and Hydrocortisone Concentrations
Serum cortisol and hydrocortisone concentrations in HYDRO foals before, during, and after LDHC treatment are shown in Table 3. Paired cosyntropin stimulation test results in HYDRO and CONTROL foals are shown in Table 4. There were no significant differences between HYDRO and age-matched CONTROL foals in HPA axis function as assessed by basal cortisol concentration before or after hydrocortisone treatment, or by peak or delta cortisol (peak – basal cortisol) responses to low- (10 μg) or high- (100 μg) dose cosyntropin after hydrocortisone treatment.
Table 3.
Serum cortisol/hydrocortisone concentrations in HYDRO and age-matched CONTROL foals before, during and after low-dose hydrocortisone treatment.
| Serum Cortisol/Hydrocortisone* Concentration (μg/dl) PRE-TREATMENT (Day 2 of age) | Serum Cortisol/Hydrocortisone* Concentration (μg/dl) DURING TREATMENT** (Day 4 of age) | Serum Cortisol/Hydrocortisone* Concentration (μg/dl) POST-TREATMENT (Day 6 of age) | ||
|---|---|---|---|---|
| Trough | Peak | |||
| HYDRO Foals (n=11) | 2.6 ± 1.0 (1.6 – 4.7) | 2.1 ± 1.4 (0.4 – 4.9) | 22.3 ± 9.5 (15.0 – 44.9) | 2.5 ± 1.0 (1.2 – 4.6) |
| CONTROL Foals (n=11) | 2.4 ± 1.0 (1.3 – 4.4) | n/a | n/a | 2.0 ± 0.8 (1.0 – 3.6) |
Data are expressed as mean ± standard deviation (range). No significant differences (P < 0.05) were found between HYDRO and CONTROL foals.
Cortisol and hydrocortisone are chemically indistinguishable.
Samples for trough and peak cortisol/hydrocortisone concentrations DURING TREATMENT were collected immediately before and 5 minutes after i.v. bolus administration of hydrocortisone respectively.
Table 4.
Basal serum Cortisol concentrations and serum cortisol responses to a paired low-dose (10μg) / high-dose (100 μg) cosyntropin stimulation test in HYDRO and age-matched CONTROL foals.
| Basal Cortisol (μg/dl) | Low-dose Peak* Cortisol (μg/dl) | High-dose Peak** Cortisol (μg/dl) | Low-dose Delta† Cortisol (μg/dl) | High-dose Delta† Cortisol (μg/dl) | |
|---|---|---|---|---|---|
| HYDRO Foals (n=8) | 1.8 ± 0.9 (0.7 – 3.6) | 3.1 ± 1.0 (1.9 – 4.6) | 5.0 ± 1.7 (3.4 – 7.9) | 1.4 ± 0.7 (0.6 – 2.8) | 3.2 ± 1.4 (1.8 – 6.1) |
| CONTROL Foals (n=11) | 2.0 ± 0.8 (1.0 – 3.6) | 3.3 ± 0.8 (2.3 – 4.4) | 5.5 ± 1.1 (3.5 – 7.4) | 1.3 ± 0.6 (0.6 – 2.6) | 3.5 ± 1.3 (1.7 – 5.9) |
Data are expressed as mean ± standard deviation (range). No significant differences (P < 0.05) were found between HYDRO and CONTROL foals.
Low-dose Peak Cortisol concentrations were obtained 30 minutes after i.v. administration of 10 μg cosyntropin.
High-dose Peak Cortisol concentrations were obtained 90 minutes after i.v. administration of 100 μg cosyntropin.
Delta cortisol concentrations were calculated by subtracting the basal cortisol concentration from the peak cortisol concentration reached after administration of 10 μg (Low-dose Delta Cortisol) or 100 μg cosyntropin (High-dose Delta Cortisol).
DISCUSSION
The results herein provide support for our hypotheses, and illustrate that LDHC dampens the pro-inflammatory cytokine response to ex vivo endotoxin exposure without significantly suppressing neutrophil function in neonatal foals. Furthermore, HPA axis function after discontinuation of LDHC treatment was not significantly suppressed in HYDRO foals. In sum, these findings suggest that a similar LDHC regimen may ameliorate a detrimental pro-inflammatory response in septic neonates without impairing innate immune and endocrine responses to the inciting infection.
The finding that LDHC did not impair ROS production or phagocytic function in isolated foal neutrophils is consistent with previous reports from adult animals and humans (41-43). The results herein support these previous studies and suggest that innate immune mechanisms also remain intact in neonates receiving LDHC.
The significant reduction in endotoxin-induced gene expression of the pro-inflammatory cytokines TNF-α, IL-6, IL-1β, and IL-8 in HYDRO foals was also consistent with previous reports of decreased pro-inflammatory mediators in septic adult humans receiving LDHC (32, 42-44). However, to the authors’ knowledge this is the first study to show persistence of anti-inflammatory effects following discontinuation of hydrocortisone therapy, as evidenced by a significant decrease in TNF-α, IL-6, and IL-1β expression in HYDRO foals 12 hours after discontinuation of hydrocortisone. As transcriptional effects of corticosteroids can involve modification of regulatory molecule production, it is not surprising that some functional genomic effects might persist after steroid administration ceases. Though further study is needed, a short course of LDHC may also result in prolonged anti-inflammatory effects in clinical patients.
While increased expression of the anti-inflammatory cytokines TGF-β and IL-4 was expected in HYDRO foals, a relevant > 3-fold endotoxin-induced change in expression was not observed for these genes and no further between-group comparisons were possible. Expression of these cytokines may not be induced until later in the inflammatory response, and differences might have been observed if longer endotoxin exposure was conducted. Alternatively, it is possible that expression of TGF-β and IL-4 is not regulated via TLR-4-mediated pathways in foal PBMCs.
Expression of the anti-inflammatory cytokine IL-10 was significantly increased after endotoxin exposure in comparison to unstimulated cells in both groups of foals, but expression in HYDRO foals was comparable to CONTROL foals. Corticosteroid treatment has been shown to increase production of IL-10 from isolated human PBMCs (10), but other studies have documented suppression of IL-10 production with LDHC therapy in septic adult humans (42). These contradictory effects may reflect temporal changes in cytokine production related to the duration of the inflammatory response in clinical sepsis versus experimental models. Furthermore, as this study examined IL-10 gene expression rather than protein production, post-transcriptional changes in IL-10 production may have been missed.
It is important to note that the LDHC regime used in this study employed a lower daily dose and shorter course of therapy than current recommendations in septic adult humans (16). The hydrocortisone dose and q. 4 hour dosing protocol used in our study was based on daily endogenous cortisol production rates and secretion patterns in healthy foals(31), and was derived similarly to LDHC recommendations in adult humans (2, 32, 33). This hydrocortisone regimen did suppress pro-inflammatory responses in leukocytes from healthy foals to a degree comparable to that achieved with higher doses in septic and endotoxemic adult animals and people (10, 32, 41, 42). A similar daily hydrocortisone dose of 1-2 mg/kg divided into intermittent boluses has been recently recommended for use in human neonates (3) with CIRCI. The appropriate duration of hydrocortisone treatment in both neonates and adults, however, remains unclear, and is best governed by the underlying disease process being managed (i.e. septic shock vs. prematurity) and the clinical response of the individual patient. A shorter LDHC course than the standard 7-day protocol (16) was evaluated here because the authors questioned if shorter treatment duration would provide still adequate anti-inflammatory effects, and thus might have potential clinical utility in patients (such as neonates) in which adverse effects of steroid treatment are particularly undesirable. Further study to evaluate the effects of shorter-duration LDHC regimens in adults and neonates in a clinical setting is needed.
An important limitation of this study is the use of an ex vivo model of infection with cells obtained from a small number of healthy animals. Infection in the live animal or person stimulates complex host-pathogen interactions with important endocrine and immunological consequences that could impact the response to LDHC in clinical patients. For instance, the inflammatory response in naturally-occurring sepsis is likely present for a longer duration and to a greater degree than in this experimental model, so altered steroid dosing might be required in clinical patients. The anti-inflammatory effects of the LDHC protocol used in this report must be confirmed in larger-scale in vivo studies with experimental and naturally-occurring infection.
In conclusion, the LDHC therapy protocol employed in the study herein dampened the ex vivo pro-inflammatory response to endotoxin in neonatal foals without significantly impairing ex vivo neutrophil function or endogenous HPA axis activity. These anti-inflammatory effects occurred with a lower dose and shorter course than is currently recommended in adults with RAI/CIRCI, and some anti-inflammatory effects persisted after discontinuation of hydrocortisone. Further study is needed to evaluate the immunologic and clinical effects of a similar protocol in clinical patients.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Lorelei Jones, Kari Anderson, Dr. Gary Heusner, Dr. Greg Queen, and the students who assisted with sampling; Londa Berghaus, Shay Bush, Kate Hurley, Natalie Norton, Denise Pope, and Caroline Salter for technical assistance; and Dr. Roy Berghaus for statistical consultation.
This study was supported by grants from the National Institutes of Health (GM086003), the Grayson Jockey Club Research Foundation, and the American Quarter Horse Foundation.
ABBREVIATIONS
- CIRCI
critical illness-related corticosteroid insufficiency
- HPA
hypothalamic-pituitary-adrenal
- LDHC
low-dose hydrocortisone
- PBMC
peripheral blood mononuclear cell
- PMA
phorbol myristate acetate
- RAI
relative adrenal insufficiency
- ROS
reactive oxygen species
Footnotes
Portions of this work were presented in abstract form at the American College of Veterinary Internal Medicine Annual Forum, Anaheim, CA, June 9-12, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Marik PE. Mechanisms and clinical consequences of critical illness associated adrenal insufficiency. Curr Opin Crit Care. 2007;13:363–369. doi: 10.1097/MCC.0b013e32818a6d74. [DOI] [PubMed] [Google Scholar]
- 2.Aneja R, Carcillo J. What is the rationale for hydrocortisone treatment in children with infection-related adrenal insufficiency and septic shock. Arch Dis Child. 2007;92:165–169. doi: 10.1136/adc.2005.088450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez EF, Watterberg KL. Relative adrenal insufficiency in the preterm and term infant. J Perinatol. 2009;29:S44–S49. doi: 10.1038/jp.2009.24. [DOI] [PubMed] [Google Scholar]
- 4.Marik PE. Critical illness-related corticosteroid insufficiency. Chest. 2009;135:181–193. doi: 10.1378/chest.08-1149. [DOI] [PubMed] [Google Scholar]
- 5.Annane D, Maxime V, Ibrahim F, Alvarez J, Abe E, Boudou P. Diagnosis of adrenal insufficiency in severe sepsis and septic shock. Am J Respir Crit Care Med. 2006;174:1319–1326. doi: 10.1164/rccm.200509-1369OC. [DOI] [PubMed] [Google Scholar]
- 6.Pizarro CF, Troster EJ, Damiani D, Carcillo JA. Absolute and relative adrenal insufficiency in children with septic shock. Crit Care Med. 2005;33:855–859. doi: 10.1097/01.ccm.0000159854.23324.84. [DOI] [PubMed] [Google Scholar]
- 7.Gold JR, Perkins GA, Erb HN, Ainsworth DM. Cytokine profiles of peripheral blood mononuclear cells isolated from septic and healthy neonatal foals. J Vet Intern Med. 2007;21:482–488. doi: 10.1892/0891-6640(2007)21[482:cpopbm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Harris MC, D'Angio CT, Gallagher PR, Kaufman D, Evans J, Kilpatrick L. Cytokine elaboration in critically ill infants with bacterial sepsis, necrotizing enterocolitis, or sepsis syndrome: correlation with clinical parameters of inflammation and mortality. J Pediatr. 2005;147:462–468. doi: 10.1016/j.jpeds.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26:711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Haddad JJ, Saadé NE, Safieh-Garabedian B. Cytokines and neuro-immune-endocrine interactions: a role for the hypothalamic-pituitary-adrenal revolving axis. J Neuroimmunol. 2002;133:1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 11.Ng PC, Li K, Wong RP, Chui K, Wong E, Li G, Fok TF. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Child Fetal Neonatal Ed. 2003;88:F209–F213. doi: 10.1136/fn.88.3.F209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soni A, Pepper G, Wyrwinski P, Ramirez N, Simon R, Pina T, Gruenspan H, Vaca C. Adrenal insufficiency occurring during septic shock: Incidence, outcome, and relationship to peripheral cytokine levels. Am J Med. 1995;98:266–271. doi: 10.1016/S0002-9343(99)80373-8. [DOI] [PubMed] [Google Scholar]
- 13.Annane D, Bellissant E, Bollaert P, Briegel J, Confalonieri M, De Gaudio R, Keh D, Kupfer Y, Oppert M, Meduri G. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301:2362–2375. doi: 10.1001/jama.2009.815. [DOI] [PubMed] [Google Scholar]
- 14.Keh D, Sprung C. Use of corticosteroid therapy in patients with sepsis and septic shock: an evidence based review. Crit Care Med. 2004;32:S527–S533. doi: 10.1097/01.ccm.0000142983.15421.11. [DOI] [PubMed] [Google Scholar]
- 15.Minneci PC, Deans KJ, Eichacker PQ, Natanson C. The effects of steroids during sepsis depends on dose and severity of illness: an updated meta-analysis. Clin Microbiol Infect. 2009;15:308–318. doi: 10.1111/j.1469-0691.2009.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, Keh D, Briegel J, Beishuizen A, Dimopoulou I, Tsagarakis S, Singer M, Chrousos GP, Zaloga G, Bokhari F, Vogeser M, American College of Critical Care Medicine Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 17.Watterberg KL, Gerdes JS, Cole CH, Aucott SW, Thilo EH, Mammel MC, Couser RJ, Garland JS, Rozycki HH, Leach CL, Backstrom C, Shaffer ML. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics. 2004;114:1649–1657. doi: 10.1542/peds.2004-1159. [DOI] [PubMed] [Google Scholar]
- 18.Quintos JB, Boney CM. Transient adrenal insufficiency in the premature newborn. Curr Opin Endocrinol Diabetes Obes. 2010;17:8–12. doi: 10.1097/MED.0b013e32833363cc. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez EF, Montman R, Watterberg KL. ACTH and cortisol response to critical illness in term and late preterm newborns. J Perinatol. 2008;28:797–802. doi: 10.1038/jp.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng PC, Lam CW, Fok TF, Lee CH, Ma KC, Chan IH, Wong E. Refractory hypotension in preterm infants with adrenocortical insufficiency. Arch Dis Child Fetal Neonatal Ed. 2001;84:F122–F124. doi: 10.1136/fn.84.2.F122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soliman AT, Taman KH, Rizk MM, Nasr IS, Alrimawy H, Hamido MS. Circulating adrenocorticotropic hormone (ACTH) and cortisol concentration in normal, appropriate-for-gestational-age newborns versus those with sepsis and respiratory distress: cortisol response to low-dose and standard-dose ACTH tests. Metabolism. 2004;53:209–214. doi: 10.1016/j.metabol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Tantivit P, Subramanian N, Garg M, Ramanathan R, deLemos RA. Low serum cortisol in term newborns with refractory hypotension. J Perinatol. 1999;19:352–357. doi: 10.1038/sj.jp.7200202. [DOI] [PubMed] [Google Scholar]
- 23.Watterberg KL. Adrenocortical function and dysfunction in the fetus and neonate. Semin Neonatol. 2004;9:13–21. doi: 10.1016/j.siny.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Hart KA, Heusner GL, Norton NA, Barton MH. Hypothalamic-pituitary-adrenal axis assessment in healthy term neonatal foals utilizing a paired low dose/high dose ACTH stimulation test. J Vet Intern Med. 2009;23:344–351. doi: 10.1111/j.1939-1676.2008.00271.x. [DOI] [PubMed] [Google Scholar]
- 25.Silver M, Fowden A. Prepartum adrenocortical maturation in the fetal foal: responses to ACTH. J Endocrinol. 1994;142:417–425. doi: 10.1677/joe.0.1420417. [DOI] [PubMed] [Google Scholar]
- 26.Silver M, Fowden A, Know J, Ousey J, Franco R, Rossdale P. Sympathoadrenal and other responses to hypoglycaemia in the young foal. J Reprod Fertil Suppl. 1987;35:607–614. [PubMed] [Google Scholar]
- 27.Silver M, Ousey J, Dudan F, Fowden A, Know J, Cash R, Rossdale P. Studies on equine prematurity 2: Post natal adrenocortical activity in relation to plasma adrenocorticotrophic hormone and catecholamine levels in term and premature foals. Equine Vet J. 1984;16:278–286. doi: 10.1111/j.2042-3306.1984.tb01927.x. [DOI] [PubMed] [Google Scholar]
- 28.Cohen ND. Causes of and farm management factors associated with disease and death in foals. J Am Vet Med Assoc. 1994;204:1644–1651. [PubMed] [Google Scholar]
- 29.Hart KA, Slovis NM, Barton MH. Hypothalamic-pituitary-adrenal axis dysfunction in hospitalized neonatal foals. J Vet Intern Med. 2009;23:901–912. doi: 10.1111/j.1939-1676.2009.0323.x. [DOI] [PubMed] [Google Scholar]
- 30.Morris DD, Moore JN. Tumor necrosis factor activity in serum from neonatal foals with presumed septicemia. J Am Vet Med Assoc. 1991;199:1584–1589. [PubMed] [Google Scholar]
- 31.Hart KA, Dirikolu L, Ferguson D, Norton N. Barton M Daily endogenous cortisol production and hydrocortisone pharmacokinetics in adult horses and neonatal foals. Am J Vet Res. doi: 10.2460/ajvr.73.1.68. In Press. [DOI] [PubMed] [Google Scholar]
- 32.Briegel J, Kellermann W, Forst H, Haller M, Bittl M, Hoffmann G, Buchler M, Uhl W, Peter K. Low-dose hydrocortisone infusion attentuates the systemic inflammatory response syndrome. The Phospholipase A2 Study Group. Clin Investig. 1994;72:782–787. doi: 10.1007/BF00180547. [DOI] [PubMed] [Google Scholar]
- 33.Lamberts SW, Bruining HA, de Jong H. Corticosteroid therapy in severe sepsis. N Engl J Med. 1997;337:1285–1292. doi: 10.1056/NEJM199710303371807. [DOI] [PubMed] [Google Scholar]
- 34.Hart KA, Ferguson DC, Heusner GL, Barton MH. Synthetic adrenocorticotropic hormone stimulation tests in healthy neonatal foals. J Vet Intern Med. 2007;21:314–321. doi: 10.1892/0891-6640(2007)21[314:sahsti]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Reimers TJ, Salerno VJ, Lamb SV. Validation and application of solid-phase chemiluminescent immunoassays for diagnosis of endocrine diseases in animals. Comp Haematol Int. 1996;6:170–175. [Google Scholar]
- 36.Singh AK, Jiang Y, White T, Spassova D. Validation of nonradioactive chemiluminescent immunoassay methods for the analysis of thyroxine and cortisol in blood samples obtained from dogs, cats, and horses. J Vet Diagn Invest. 1997;9:261–268. doi: 10.1177/104063879700900307. [DOI] [PubMed] [Google Scholar]
- 37.Donovan DC, Jackson CA, Colahan PT, Norton NN, Clapper JL, Moore JN, Hurley DJ. Assessment of exercise-induced alterations in neutrophil function in horses. Am J Vet Res. 2007;68:1198–1204. doi: 10.2460/ajvr.68.11.1198. [DOI] [PubMed] [Google Scholar]
- 38.Sun WC, Moore JN, Hurley DJ, Vandenplas ML, Linden J, Cao Z, Murray TF. Adenosine A2A receptor agonists inhibit lipopolysaccharide-induced production of tumor necrosis factor-alpha by equine monocytes. Vet Immunol Immunopathol. 2008;121:91–100. doi: 10.1016/j.vetimm.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Figueiredo MD, Salter CE, Andrietti AL, Vandenplas ML, Hurley DJ, Moore JN. Validation of a reliable set of primer pairs for measuring gene expression by real-time quantitative RT-PCR in equine leukocytes. Vet Immunol Immunopathol. 2009;131:65–72. doi: 10.1016/j.vetimm.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Figueiredo MD, Vandenplas ML, Hurley DJ, Moore JN. Differential induction of MyD88- and TRIF-dependent pathways in equine monocytes by Toll-like receptor agonists. Vet Immunol Immunopathol. 2009;127:125–134. doi: 10.1016/j.vetimm.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 41.Heller AR, Heller SC, Borkenstein A, Stehr SN, Koch T. Modulation of host defense by hydrocortisone in stress dose during endotoxemia. Intensive Care Med. 2003;29:1456–1463. doi: 10.1007/s00134-003-1831-y. [DOI] [PubMed] [Google Scholar]
- 42.Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, Volk H- D, Doecke WD, Falke K, Gerlach H. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock. Am J Respir Crit Care Med. 2003;167:512–520. doi: 10.1164/rccm.200205-446OC. [DOI] [PubMed] [Google Scholar]
- 43.Kaufmann I, Briegel J, Schliephake F, Hoelzl A, Chouker A, Hummel T, Schelling G, Thiel M. Stress doses of hydrocortisone in septic shock: beneficial effects on opsonization-dependent neutrophil functions. Intensive Care Med. 2008;34:344–349. doi: 10.1007/s00134-007-0868-8. [DOI] [PubMed] [Google Scholar]
- 44.Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, Hemmer B, Hummel T, Lenhart A, Heyduck M, Stoll C, Peter K. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized double-blind single center study. Crit Care Med. 1999;27:723–732. doi: 10.1097/00003246-199904000-00025. [DOI] [PubMed] [Google Scholar]