Abstract
Although long-lasting behavioral memories have long been thought to require equally persistent molecular changes, little is known about the biochemical underpinnings of memory storage and maintenance. Increasing evidence now suggests that long-term behavioral change may be associated with epigenetic regulation of transcription in the central nervous system. In this review, we present evidence that changes in DNA methylation contribute to memory formation and maintenance, consider how DNA methylation affects readout of memory-related genes, and discuss how these changes may be important in the large-scale context of memory circuits. Finally, we discuss potential challenges involved in examining epigenetic changes in the brain and highlight how epigenetic mechanisms may be relevant for other cognitive processes.
Introduction – the need for self-perpetuation in memory
A critical component of behavioral memory is its persistence over time despite the perpetual turn-over of cellular proteins and the formation of new associations and new memories. When considered in the context of molecular mechanisms that control neuronal function in memory-related neural circuits, this capacity is not trivial. In fact, to understand the neural substrates of learning and memory, we must first understand how single molecules or groups of molecules in the brain can store information across time despite the relatively rapid turnover of every protein within a cell (Mammen et al., 1997; Price et al., 2010). At the molecular level, the perpetuation of a specific activation state that contributes to cellular memory requires that individual molecules have the ability to self-perpetuate a given type of activity despite turnover and in the absence of the stimulus which initiated that activation state. This has been hypothesized to occur via a specific type of biochemical reaction in which the active or mnemogenic form of a molecule is able to autoconvert a nascent form of itself into an active form. This type of reaction has long been hypothesized to underlie memory (Crick, 1984; Lisman, 1985; Roberson and Sweatt, 2001; Bailey et al., 2004), making the search for a molecule capable of autoconversion a critical step in elucidating cellular as well as systems-level memory storage.
Searching for self-perpetuating molecular activity: the case of CaMKII
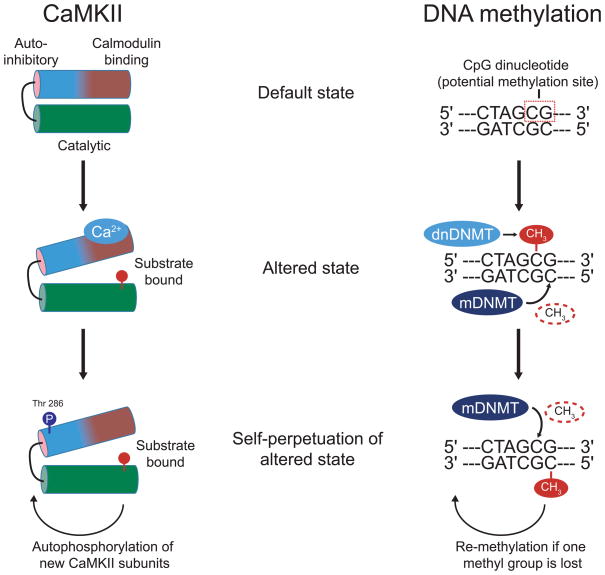
The historical search for a molecular storage device for long term memory is perhaps best encapsulated by the story of Ca2+ /calmodulin (CaM)-dependent protein kinase II (CaMKII). As its name implies, CaMKII is activated by calcium ions as well as calmodulin. When activated, CaMKII alters its physical structure, such that the catalytic domain (which contains the substrate binding site) can become active (see Figure 1). Increased synaptic calcium causes CaMKII to translocate to postsynaptic sites, where it can bind to the NMDA receptor, placing it in an ideal location to regulate synaptic strength (Leonard et al., 1999; Bayer et al., 2001). However, in the case of very strong synaptic stimulation, prolonged activation by calcium also allows a site on the inhibitory domain of CaMKII to undergo phosphorylation at threonine-286 by a nearby subunit (Rich and Schulman, 1998). This phosphorylation prevents the inhibitory subunit from binding to the catalytic domain, effectively increasing the duration of CaMKII activity. Moreover, because this site can continue to be phosphorylated by neighboring subunits within the holoenzyme in the absence of Ca2+ /calmodulin, CaMKII also essentially possesses a unique form of “autonomous” activity in which the catalytic domain can remain in an active state in the absence of the initial stimulus (Lisman et al., 2002). In essence, this auto-activation would conceptually serve as a cellular memory trace by which the previous calcium stimulation is “remembered”. This type of autophosphorylation event has long been proposed to underlie the molecular storage of memory in part because it can outlast the relatively rapid protein turnover within a cell, and thereby perpetuate a biochemical signal long after the initiating stimulus had ceased (Lisman, 1985; Lisman et al., 2002).
Figure 1.
Self-perpetuation mechanisms in learning and memory. Left, CaMKII possesses a catalytic region that is usually bound by the autoinhibitory subunit (first panel), resulting in an inability to act at possible substrates such as the NMDA receptor. When activated by calcium or calmodulin, this autoinhibition gate is opened, allowing the catalytic subunit bind substrates (second panel). Subsequently, in the absence of the original calcium stimulus, autophosphorylation of threonine 286 on the autoinhibitory domain prevents binding to the catalytic domain, preserving catalytic activity (third panel). Right, DNA possesses CpG dinucleotides capable of undergoing methylation (first panel). De novo DNMTs methylate previously unmethylated CpG sites. Maintenance DNMTs recognize hemi-methylated DNA and methylate the complementary strand (second panel). Together, these reactions produce a change in transcriptional processes. Even with spontaneous or enzymatic removal of methyl group at one cytosine residue, maintenance DNMTs remethylate DNA to preserve transcriptional change.
In keeping with this idea, induction of long-term potentiation (LTP) produces an increase in CaMKII activity that is required for the induction of LTP (Malinow et al., 1989; Silva et al., 1992a; Fukunaga et al., 1993; Barria et al., 1997). Concomitant with this LTP deficit, mice that lack CaMKII or have a single mutation at the autophosphorylation site (Thr 286) also exhibit robust memory deficits (Silva et al., 1992b; Giese et al., 1998; Frankland et al., 2001). However, despite these results, doubts remain about the role of CaMKII autonomy in supporting long-term memories and persistent LTP at synapses. One important caveat is that although CaMKII is required for LTP induction, it does not appear to be required for the expression of LTP once it has been induced (Chen et al., 2001; Buard et al., 2010). There is also no evidence which indicates that CaMKII autonomy can be maintained for the lifetime of a behavioral memory, which would be necessary if it was perpetuating the memory trace. Indeed, the observation that CaMKII autonomy does not appear to be necessary for the retrieval or storage of long-lasting memories suggests otherwise (Buard et al., 2010). Together, these results suggest that although CaMKII may certainly be involved in the initiation of LTP and the formation of long-term memories, other mechanisms may support the stabilization and maintenance of lifelong memories.
Other candidates
Nevertheless, molecules that remain active despite the absence of an initiating stimulus remain attractive candidates for long-term memory storage. For example, PKMζ, an isoform of PKC, lacks the regulatory catalytic subunit possessed by other PKC isoforms, and therefore possesses prolonged activity (Sacktor, 2008). This unique arrangement appears to confer PKMζ with an important role in learning and LTP that is markedly different from the role played by other PKC isoforms (Sacktor et al., 1993). Another excellent example is cytoplasmic polyadenylation element binding protein (CPEB), which is increased at Aplysia sensory synapses in response to serotonin stimulation in a translation-dependent but transcription-independent manner (Si et al., 2003b). Importantly, this protein also appears to have prion-like capabilities, with two different conformational forms (Si et al., 2003a; Si et al., 2010). In this case, the active conformation is self-perpetuating, as it is able to convert inactive CPEB into active CPEB. More recent work has revealed that the active conformation of CPEB is necessary for the long-term maintenance of synaptic facilitation at these synapses, providing an interesting corollary to long-term behavioral responses (Si et al., 2010). Analogous proteins have also been discovered in the mouse hippocampus (Theis et al., 2003), suggesting the potential for a conserved role in synaptic plasticity across species. However, future studies will be required to more thoroughly examine a role for CPEB in mammalian memory storage.
A role for epigenetics?
The epigenome sits at the interface of the cellular environment and the genome, enabling epigenetic changes to exert robust control over transcriptional processes. In somatic cells, epigenetic influences are responsible for cellular differentiation and the perpetuation of the cellular phenotype over time and across cell division (Feinberg, 2007; Reik, 2007). Unlike a number of cytoplasmic protein modifications, epigenetic mechanisms possess a number of features that are consistent with a molecular storage device for long-term memory. First, these modifications are believed to be relatively stable in comparison to other alterations. Secondly, this class of modifications is capable of altering gene expression directly, and is therefore able to modulate gene programs known to be involved in learning and memory. As changes in gene transcription are known to be necessary for memory stabilization, these characteristics raise the intriguing possibility that epigenetic mechanisms within non-dividing cells in the CNS are coopted to support long-lasting behavioral changes in response to particular types of experience. Although these epigenetic changes include a number of unique mechanisms (including histone modifications, microRNA activity, and proteins with prion-like activity), for the purposes of this review we will focus our discussion on the potential role of DNA methylation in learning and memory.
DNA Methylation
Perhaps the most intriguing epigenetic modification is the methylation of individual cytosine residues on DNA itself. Indeed, one of the earliest theoretical papers concerning the molecular mechanisms that might underlie long-term memory (Griffith and Mahler, 1969) speculated that DNA methylation might contribute to memory encoding. Although their specific model, “DNA Ticketing”, is untenable in light of our current understanding of the molecular biology of memory, this early paper accurately envisions the potential of a role for chemical modification of DNA in memory formation and storage. As an aside, the paper is also fascinating to read because the writing style and freedom to speculate that are manifest in the manuscript are no longer seen in “high-profile” journals in the modern era.
DNA methylation, which occurs at CpG dinucleotides within DNA, is catalyzed by two different forms of DNA methyltransferases (DNMTs), both of which are expressed in mature neurons (Bird, 1999; Feng et al., 2010). De novo DNMTs are able to methylate previously unmethylated cytosines, whereas maintenance DNMTs recognize hemi-methylated DNA and methylate the unmethylated strand (Figure 1). For both of these reactions, S-adenosyl methionine serves as the methyl donor. The result is an incredibly strong reaction which has the ability to restore itself if lost. For example, even if one methyl group is somehow removed from one strand, maintenance DNMTs will recognize this and re-establish a methylated cytosine. This peculiar arrangement highlights an important feature of DNA methylation, which is that despite ongoing events that may remove the methylation mark, a substrate exists to effectively perpetuate that mark through time (Figure 1). In fact, this reaction has been proposed to serve as one of the mechanisms that underlie lifelong inactivation of one X chromosome in females (Chow and Brown, 2003; Chow et al., 2005). Thus, this reaction has the same basic form as CaMKII phosphorylation, but has been observed to persist across time and despite ongoing cellular stimulation. Therefore, we view this mechanism as a key candidate for the molecular storage of long-term memory.
Interestingly, in addition to undergoing methylation in response to environmental stimuli, it appears that demethylation of cytosine bases in DNA may be as or even more important to cellular function (Wu and Zhang, 2010). The mechanism through which this occurs is presently unclear and controversial (Ooi and Bestor, 2008; Gehring et al., 2009; Dulac, 2010). Given that the methyl group and cytosine base at a methylated CpG are linked via a covalent carbon-carbon bond, it is unlikely that an enzyme directly removes the methyl group. However, a number of alternative methods have been proposed that may account for rapid DNA demethylation (Ma et al., 2009a; Ma et al., 2009b; Wu and Zhang, 2010). One particularly attractive model proposes that a methylated cytosine may undergo deamination, which produces a thymine residue. Subsequently, base excision repair machinery would recognize the T-G mismatch in DNA, and substitute an unmethylated cytosine for thymine (Ma et al., 2009a). This process appears to occur within the brain in response to neuronal activity, and is assisted by growth arrest and DNA damage-inducible protein 45 (GADD45) (Ma et al., 2009b). However, multiple mechanisms have been implicated in demethylation of DNA, including direct demethylation by proteins with methyl-binding domains (e.g., MBD2), oxidative demethylation, complete excision of the methylated cytosine by DNA glycosylases, and deamination by RNA editing enzymes (for a detailed review of the mechanisms, see Wu and Zhang, 2010). Thus, although the exact mechanisms underlying demethylation of DNA remain controversial, it is clear that decreases in methylation can and do play an important role in brain function. How these mechanisms might operate vis-à-vis the self-perpetuating mechanisms described above remains an open question at this time.
Cytosine hydroxymethylation
In addition to simple methylation, cytosine residues can also undergo hydroxymethylation (OHMeC) in the adult brain (Kriaucionis and Heintz, 2009; Tahiliani et al., 2009). Interestingly, this modification severely decreases the binding affinity of methyl-binding domain proteins such as MeCP2 for the cytosine base (Valinluck et al., 2004). Moreover, although the carbon bond between a methyl group and the cytosine base is likely too strong to undergo direct enzymatic removal, this is not the case for OHMeC. Thus, it is possible that OHMeC acts as an intermediate stage that marks individual MeC modifications for demethylation. Therefore, hydroxymethylation could conceptually reverse the effects of DNA methylation, or at least make the original modification ineffective at recruiting binding proteins that alter gene transcription. However, despite the obvious appeal of this mechanism in regulating gene expression, little is known about how and it influences the formation and maintenance of behavioral memories.
DNA methylation as a molecular component of the engram
Memory is a multi-component process consisting of distinct phases that require distinct changes within the CNS. Unlike short-term memory (STM), the formation and maintenance of long-term memory (LTM) requires the synthesis of new protein within the brain (Davis and Squire, 1984), suggesting that it is mediated by a mechanism or group of mechanisms that operate within the nucleus of a cell. Given that DNA methylation plays a major role in transcriptional regulation and is capable of perpetuation of potentially life-long changes in gene expression, we and others became interested in determining whether changes in DNA methylation are required for the formation and storage of behavioral memories.
Dynamic DNA methylation changes in memory formation
Initial studies tested the hypothesis that DNMT activity might regulate the induction of hippocampal long-term potentiation (LTP), and this was indeed found to be the case (Levenson et al 2004). Subsequent studies examined the role of DNA methylation in hippocampal-dependent forms of learning, such as contextual fear conditioning. These studies revealed that DNMT expression is significantly enhanced within the hippocampus shortly after contextual fear conditioning, and blocking DNMT activity in the hippocampus correspondingly disrupts the formation of context-shock associations (Miller and Sweatt, 2007). Likewise, double knockout of DNMT1 and DNMT3a in adult neurons in mouse forebrain resulted in impaired escape latency and memory for platform location on the Morris water maze, as well as deficits in the 24-hr retention (but not immediate recall) of a contextual fear memory (Feng et al., 2010). Moreover, DNA methylation at the reelin gene (which is associated with memory formation) is decreased following fear conditioning, whereas DNA methylation at the PP1 gene (which is viewed as a memory repressor) is enhanced following fear conditioning (see Figure 2). Importantly, both of these changes returned to baseline levels 24 hours after conditioning, suggesting dynamic temporal regulation of both DNA methylation and demethylation in the hippocampus (Miller and Sweatt, 2007).
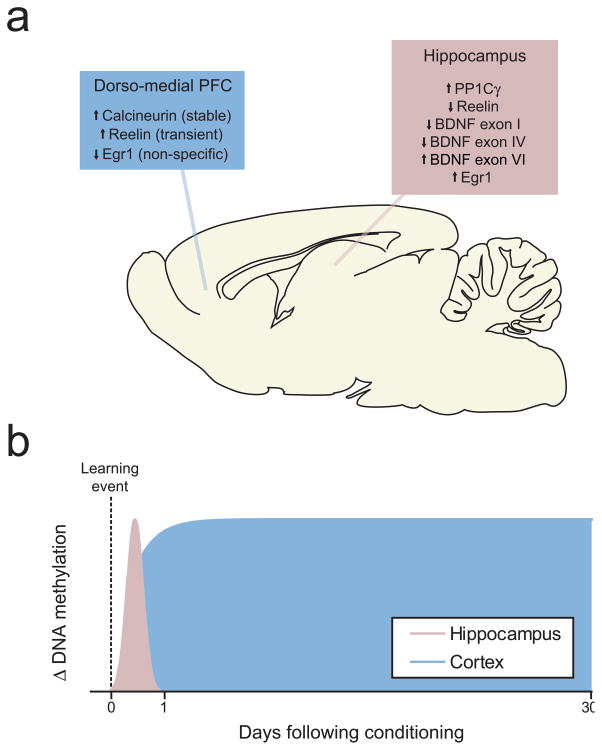
Figure 2.
Unique targets and timelines for DNA methylation/demethylation in the hippocampus and cortex. a, Sagittal section of rat brain representing methylation changes occurring at CpG islands in the promoter regions of selected genes in response to contextual fear conditioning. Both DNA methylation and demethylation are observed at different genes and even within the same gene. Brain section graphic taken from (Paxinos and Watson, 2005). b, Temporal dynamics of DNA methylation changes in response to contextual fear conditioning. Although all of the changes observed in the hippocampus return to basal levels within 24 hours of learning, at least one change (increased calcineurin methylation) is maintained within the dorsomedial PFC. Importantly, blocking DNA methylation in the hippocampus immediately after conditioning disrupts memory consolidation. In contrast, inhibiting DNA methylation in the dmPFC immediately after learning has no effect on retrieval of remote memories 30 days later, but blocking DNA methylation several days immediately prior to remote retrieval significantly impairs performance, indicating a necessity for ongoing DNA methylation in the maintenance of fear memories (Miller et al., 2010).
Additionally, the gene encoding brain-derived neurotrophic factor (BDNF), which has recently been linked to the persistence of fear memories (Alonso et al., 2005; Bekinschtein et al., 2007; Bekinschtein et al., 2008a; Bekinschtein et al., 2008b), also undergoes unique changes in DNA methylation as a result of fear conditioning (Lubin et al., 2008). Specifically, exon IV of the BDNF gene undergoes significant suppression in DNA methylation in its promoter region following contextual fear conditioning, and this corresponds to a substantial increase in exon IV mRNA that returns to baseline levels within 24 hours (Lubin et al., 2008). This change in DNA methylation was reversed by intrahippocampal infusions of the DNMT inhibitor zebularine as well as the NMDA receptor blocker MK-801, which both impaired memory formation (Lubin et al., 2008). In contrast, methylation at BDNF exons I and VI is substantially decreased following context exposure alone, resulting in significantly increased mRNA for these exons.
These findings suggest that changes in DNA methylation, even at the same gene within the same brain structure, are regulated in a highly complex fashion by different stimuli during learning, and that only some of these changes correspond to and are important for memory formation. These observations also reveal a general mechanism by which DNA methylation may contribute to neuronal function. By regulating the expression of specific protein isoforms, DNA methylation could influence the creation of differently spliced versions of the same protein. This so-called alternative splicing can account for the differential regulation of multiple proteins with potentially distinct functions within a cell, essentially expanding the amount of information encoded in the proteome without altering the number of genes within the genome (Nilsen and Graveley, 2010). Alternative splicing can take a number of different forms, such as selection of one exon over another competing exon and exclusion of an entire exon within a gene. These variations can alter the efficiency of the protein in modulation of specific targets, thereby changing protein function. Indeed, other studies have also reported that specific BDNF transcripts are used differently in other brain regions as well (Rattiner et al., 2004; Ou and Gean, 2007). Moreover, DNA methylation status at different exons at other genes clearly contributes to splice expression profiles (Maunakea et al., 2010). Future studies will be required to determine how this rather amazing temporal and genetic selectivity is achieved.
Taken together, these experiments also raise important questions. For example, although most plasticity-permissive genes undergo decreased methylation (and increased expression) during memory formation, compounds that theoretically decrease DNA methylation (DNMT inhibitors) were found to impair memory formation. While this appears to create a paradox, it is important to note that in the case of BDNF, DNMT inhibitors actually prevent (instead of enhance) the learning-associated hypomethylation and subsequent increase in mRNA expression of exon IV (Lubin et al., 2008). This occurs despite the fact that in experimentally naïve animals, DNMT inhibitors decrease BDNF methylation at all gene exons examined. Thus, DNMT inhibitors may produce different results when learning-related signaling mechanisms are activated compared to when they are not activated. In fact, there is evidence that DNMTs are involved in both active methylation and demethylation of DNA, depending on the cellular context (Metivier et al., 2008). One possibility is that DNMTs play a homeostatic role in DNA methylation by adding methyl groups when methylation is too low and removing them when methylation is too high. Another explanation for this discrepancy is that although DNMTs presumably demethylate pro-memory genes such as BDNF and reelin, they also demethylate memory repressor genes such as PP1, producing an increase in the expression of these genes (Miller and Sweatt, 2007). Therefore, DNMT inhibitors could potentially activate genes which serve to counteract or prevent memory formation and maintenance. Thus, the effects produced by DNMT inhibitors may result more from activation of memory suppressor genes, which could in turn down-regulate the expression of memory activator genes (Lubin et al., 2008).
Stable DNA methylation changes in memory maintenance
Although the data discussed above demonstrate that DNA methylation is important for the establishment of hippocampus-dependent memories, all of these changes reverse to baseline within 24 hours of conditioning. Clearly, this rapid removal of altered methylation marks is not consistent with the memory-related biochemical reaction discussed above. However, although the hippocampus has been implicated in the formation and consolidation of memories, its precise role appears to be temporally limited, and it is not believed to be the ultimate storage site for multiple forms of long-term memory (Squire et al., 1993; Bontempi et al., 1999; Frankland and Bontempi, 2005). Rather, the maintenance of long-term remote memories is thought to occur within the cortex. Indeed, a number of studies have now revealed that neural activity in some cortical areas increases in a manner consistent with memory storage, and remote memories can be disrupted by manipulation of these cortical areas (Cui et al., 2004; Frankland et al., 2004; Maviel et al., 2004; Shema et al., 2007).
With this in mind, one might predict that if patterns of DNA methylation in the brain are involved in the regulation of behavioral memories, they should reflect or incorporate this systems consolidation. For example, given that the formation (but not long-term storage) of certain forms of fear-related memories are dependent upon the hippocampus, we may expect that DNA methylation in this region is uniquely conducive to memory formation as compared to methylation patterns in other regions. The findings discussed above indicate that this is the case: DNA methylation is rapidly altered in the hippocampus following the formation of a fear memory, but returns to naïve control levels within 24 hours. Likewise, if cortical areas such as the prefrontal cortex (PFC) underlie the maintenance and/or storage of memories, it is possible that cortical DNA methylation profiles differ from those in the hippocampus.
To determine if this is the case, a recent report examined methylation at the several memory-related genes in the dorsomedial PFC (dmPFC) at different time points following contextual fear conditioning, the same paradigm used in the studies discussed above (Miller et al., 2010). This study found that calcineurin underwent robust methylation in its CpG-rich promoter region within 1 day following learning. Moreover, this effect persisted for up to 30 days following conditioning (this was the longest time point tested), and corresponded to changes in calcineurin mRNA and protein levels. Interestingly, other changes in DNA methylation were also observed, but were either more transient (e.g., increased reelin methylation) or not association-specific (e.g., decreased Egr1 methylation, which also occurred in the context-only group). Thus, fear learning was found to induce long-lasting but gene-specific changes in DNA methylation.
Finally, this study also revealed that DNA methylation in the dmPFC is required for the expression of the contextual fear memory, as infusion of three different DNMT inhibitors into this area prior to a probe test 30 days after contextual fear conditioning all significantly weakened the retrieval of fear memory. Conversely, the same infusions had no effect on 30-day memory retrieval when delivered within 2 days after the initial learning experience. In combination with the reports reviewed above, these findings suggest that DNA methylation changes are used to support both the formation and maintenance of fear memories in a time-dependent, regionally specific manner. Moreover, the difference between DNA methylation changes in the cortex and hippocampus are entirely consistent with the function of these structures in the establishment and long-term consolidation of memory.
DNA methylation in other memory systems
Importantly, the memory deficits induced by DNMT inhibitors do not appear to be specific to the class of memories mentioned above. Indeed, recent studies have elegantly demonstrated that conditioned place preference memory is also regulated by DNA methylation in a spatially and temporally distinct fashion. Thus, microinfusions of the DNMT inhibitor 5-aza-2-deoxycytidine into the hippocampus essentially abolished the formation of a conditioned place preference (CPP) for cocaine reward, but had no effect on the retrieval of a previously established cocaine CPP (Han et al., 2010). Interestingly, this effect was reversed in the prelimbic cortex, where infusions of 5-aza-2-deoxycytidine failed to abolish the formation of cocaine CPP, but eliminated the retrieval of an established CPP (Han et al., 2010). This observation lends credence to the idea that memory formation and storage are supported by unique changes in DNA methylation operating in the hippocampus and cortex, respectively (Figure 2). However, the effect of changes in DNA methylation on the establishment of memory does not appear to be limited to these regions. Thus, another recent report found that DNMT inhibition in the nucleus accumbens (NAc) actually boosted the formation of a cocaine CPP, whereas virally-induced overexpression of DNMT3a in the NAc impaired CPP (LaPlant et al., 2010). Moreover, DNMT methylation also regulates spine density in the NAc (LaPlant et al., 2010), a region which has previously been implicated in reward learning and the formation of addiction-related memories (Berke and Hyman, 2000; Day and Carelli, 2007). Thus, these diverse results indicate that DNA methylation is important if not critical for the formation and storage of many different behavioral memories, but likely plays very unique roles within different neural structures and in relation to different types of memory.
DNA methylation in the context of other epigenetic modifications
We would also note that these data have built on a large literature implicating post-translational modification of histone proteins in learning and memory (Swank and Sweatt, 2001; Levenson et al., 2004; Wood et al., 2006; Vecsey et al., 2007; Graff and Mansuy, 2008; Gupta et al., 2010; Peleg et al., 2010). Thus, we do not feel that DNA methylation represents the only nuclear change capable of modulating memory formation and maintenance. Rather, we believe that changes in DNA methylation may interact with other epigenetic changes to regulate the expression of genes that are critical to memory stabilization. Consistent with this idea, a number of studies have revealed that DNA methylation changes act in concert with histone acetylation and methylation (Jones et al., 1998; Wade et al., 1999; Angrisano et al., 2006; Gupta et al., 2010; Thomson et al., 2010), and that these interaction is important for the consolidation of memory (Miller et al., 2008).
Utilization of DNA methylation marks
Although DNA methylation has been shown to be involved in memory formation and storage, little is known about how such modifications are translated into functional change within a cell. By their very nature, epigenetic changes are not in a position to directly alter neuronal function (e.g., by phosphorylating a membrane receptor, trafficking new receptors to a synapse, or building new synapses). How, then, do epigenetic alterations that occur in relation to learning play an important role within memory-related neural circuits? This section will address some potential mechanisms by which changes in epigenetic status within a cell or at a specific genetic location may alter cellular function and ultimately behavior.
Readout
The most straightforward mechanism by which epigenetic marks such as DNA methylation may influence neuronal function to regulate learning and memory is via suppression or enhancement of gene expression. Indeed, a number of genes associated with synaptic plasticity and memory formation possess large CpG islands in their promoter regions and even within intragenic regions. At these sites, DNA methylation has long been viewed as a transcriptional repressor (although this characterization is not necessarily accurate; see (Meaney and Ferguson-Smith, 2010)). The precise mechanisms underlying this phenomenon are unclear, although several models have been suggested (Sweatt, 2009). It is known that certain proteins with methyl binding domains recognize and bind to MeC, and subsequently attract adapter proteins and histone deacetylases (HDACs). As these enzymes function to remove acetyl groups from histone proteins and lead to chromatin compaction, this type of activity would lead to gene silencing. For example, binding of MeCP2 to methylated DNA is usually associated with transcriptional repression, and is associated with decreased expression of memory-suppressor genes such as PP1 (Anier et al., 2010). Likewise, a decrease in methylation at a gene site would lead to transcriptional activation by reducing HDAC activity at that gene. In the context of this paper, it is important to point out that this type of activity may be especially relevant to memory formation. Indeed, demethylation at specific gene loci is associated with increases in expression of memory-enhancer genes such as BDNF, reelin, and Egr1 (Miller and Sweatt, 2007; Lubin et al., 2008; Miller et al., 2010).
However, DNA methylation has also been reported to lead to increases in gene transcription via interactions of MeCP2 with transcription factors like CREB (Chahrour et al., 2008), which may also be an important target for memory-related molecular machinery. Indeed, it has recently been reported that methylation of intragenic sites is highly prevalent throughout the genome (Maunakea et al., 2010), and that this modification is generally associated with transcriptional activation rather than transcriptional repression (Flanagan and Wild, 2007; Ball et al., 2009). To date, investigations of the role of DNA methylation in memory have focused largely on gene promoter regions, making it unclear what precise effect DNA methylation in intragenic regions has on memory formation or maintenance. Nevertheless, it is clear that the relationship between gene transcription and DNA methylation is not as straightforward as initially believed. Thus, an important future direction will be to examine how methylation at intragenic sites alters transcription of plasticity-related genes in the brain during learning. Furthermore, it will be important to examine CpG methylation on a site-by-site basis to determine how methylation at sites with important regulatory functions alters gene readout.
Imprinting and allelic tagging in the adult CNS
A related concept is that DNA methylation (perhaps working in concert with other epigenetic modifications or regulators) may control the expression of genes in an allele-specific manner. Termed imprinting, the control of the specific expression of one allele over another substantially increases the complexity of genetic readout and has recently been found to occur at high levels within the brain (Gregg et al., 2010a; Gregg et al., 2010b). In genetic terms, “imprinting” refers to an intriguing pattern of inheritance wherein the mother’s copy of a gene is differentially expressed relative to the father’s copy of that same gene. In other words, the two alleles (mother’s and father’s copies) of the same gene are somehow epigenetically tagged and subsequently handled differently during development and in the adult offspring of the mother and father. In traditional cases of genetic imprinting, one copy of the gene (e.g. the copy inherited from the father) is fully silenced, leaving the mother’s copy of the gene the exclusive source of cellular mRNA product of that gene.
These parent-of-origin effects on inheritance have been documented for centuries by animal breeders. For example, breeding a female horse with a male donkey gives one type of offspring (a mule), whereas breeding a male horse with a female donkey gives a very different beast, a hinny. Strange inheritance patterns of this sort can only be explained by differential handling of paternal and maternal copies of somatic genes, at least at some level. Thus, this gave rise to the concept that specific somatic genes could somehow be “imprinted” with a molecular mark that crossed the generations through the germline, that marked a particular copy (allele) of a gene as having originated with the mother versus the father. It is well known that DNA methylation contributes to gene imprinting, but it is unclear how this occurs or if this mechanism differs from the mechanisms involved in the targeted changes in methylation that occur at plasticity genes.
In some cases the maternal and paternal copies of a gene can even be preferentially expressed in a brain subregion-specific fashion. For example, the UBE3A gene is imprinted and the maternal copy is selectively expressed in the cerebellum and forebrain, including the hippocampus; the paternal copy of the same gene is selectively and completely silenced in these tissues through DNA methylation of an imprinting locus (Jiang et al., 1998). UBE3A codes for a ubiquitin ligase, and when the maternal copy of the gene is mutated this results in Angelman Syndrome, with a concomitant profound learning and memory disability. Indeed, studies of Angelman Syndrome patients and resulting genetically engineered mouse models were the first to implicate epigenetic mechanisms in controlling learning, memory, and synaptic plasticity (Jiang et al., 1998).
A recent pair of exciting papers from Catherine Dulac’s laboratory have greatly expanded our view of the importance of gene imprinting in CNS function in the adult nervous system (Gregg et al., 2010a; Gregg et al., 2010b). Dulac and her colleagues used genome-wide mRNA expression methodologies to investigate whether the adult CNS exhibits limited versus widespread parent-of-origin effects on gene transcription. Their work demonstrated that over 1300 gene loci in the adult CNS manifest effects on their transcription due to parent-of-origin effects, that is, differential read-out of the paternal versus maternal allele of the gene. Many of these imprinted genes also exhibited brain subregion-selective expression as well. Overall these findings document an impressive contribution of epigenetic marking on genomic regulation, one which carries over from past development into the ongoing function of the mature CNS. One important implication of these studies is that epigenetic control of the expression of parent-specific alleles is a driving factor for regulating gene transcription broadly in the brain. These findings also identify parental expression bias as a major mode of epigenetic regulation in the adult CNS (Table 1). The overall functional role of this mechanism of transcriptional regulation in the ongoing function of the adult CNS is not clear at this point, but the discovery represents an important new type of mechanism to further investigate.
Table 1.
Why Cognitive Epigenetics?
|
We speculate that one function of this genetic parent-of-origin effect may be “allelic marking” of specific copies of a gene within a cell. Thus, we propose that in the case of some genes within the CNS evolution may have selected for a mechanism whereby one allele of a gene (e.g. the paternal copy) can be tagged and identified so that it can be regulated differentially from another copy of the same gene in the same cell. This powerful mechanism would allow a single cell to in effect have two copies of an identical gene (i.e. protein-encoding DNA sequence), so that it could selectively regulate one copy (allele) in one way and the other copy (the other allele) another way. For example, one copy might be used selectively during development, epigenetically marked as appropriate for regulating cellular phenotype during differentiation, and then silenced in the adult CNS. The second copy could then be used in the adult as a fresh template of the gene that would be available for epigenetic and transcriptional regulation uniquely needed for ongoing function in the adult. A second example might be that the two alleles of the same gene within a single cell might be differentially regulated through selective regulation of alternative splicing of products produced from each copy of the gene – this would allow a cell, using epigenetic mechanisms, to produce one specific pattern of exon expression from one allele and a different pattern of exon readout from the other allele.
This concept is motivated by the recent emergence of understanding the overall importance of epigenetic transcription-regulating mechanisms in the function of the adult CNS. Epigenetic mechanisms must of necessity operate directly upon the nucleotides encoding a gene, through either chemical covalent modification or regulation of its 3-dimensional structure. Moreover, a single allele of a gene lasts the entire lifetime of a cell. Thus, in any cell a given gene is available with at most two copies for the cell to use. Treating both copies identically would greatly limit the options that the cell has available to it in terms of regulating the readout of that gene product. After all, a gene is not like an mRNA or a protein that can have thousands of individual copies of itself present. A single allele of a gene is the only chemical copy of that allele that is present in the cell, and epigenetic molecular mechanisms operate directly on that single copy. Treating the two alleles of a gene differentially then would provide two different gene templates that could be differentially regulated by epigenetic mechanisms. Epigenetic marking of the parental versus maternal alleles would be a prerequisite for this sort of differential epigenetic handling.
While speculative, this model is consistent with Dulac’s finding that the “imprinted” genes were not completely silenced in most cases, but were instead differentially regulated in a relative but not all-or-none fashion as would be the case with typical imprinting. These ideas are also consistent with the observation that parent-of-origin effects appear to be particularly prominent in the CNS, where active epigenetic transcriptional regulation appears to be particularly important for mental function. We emphasize that this idea is completely speculative at present, nevertheless we feel it is an interesting possibility and that the existence of these mechanism would greatly increase the capacity of ongoing epigenetic molecular mechanisms in the CNS to regulate cognitive function.
Specificity and integration of DNA methylation
Taken together, these findings pose several important questions. First, how are specific gene loci targeted over others for changes in methylation during the formation and/or storage of a memory? Indeed, although a number of studies have reported changes in DNMT levels that are presumably cell-wide phenomena, the observation that DNA methylation can both increase and decrease at different genes following the same behavioral time point is initially perplexing. In fact, very little is known about how changes in DNA methylation can target specific genes or even specific exons within a gene. One possibility is that specificity in the sequence of DNA itself confers this trait via interactions with transcription factors that target different sets of DNMTs or DNA binding proteins to MeC sites. Additionally, gene selectivity in DNA methylation may be related to the fact that transcription-factor dependent transcription can potentially establish other epigenetic marks (such as histone methylation or acetylation) that in turn interact with subsequent DNA methylation profiles. For example, Cfp1, and zinc-finger binding domain protein, is associated with lysine 4 trimethylation on histone 3 as well as paucity of methylation on the corresponding DNA (Thomson et al., 2010). However, the implication of this gene (and within-gene) selectivity indicates that methylated DNA sites can rapidly and selectively control transcriptional function in a way that other mechanisms may not be able to.
A second question concerns the functional role of methyl binding proteins in transcriptional regulation. Although methylation of DNA was long thought inhibit transcription by association with transcriptional repressors like MeCP2, it is now clear that this simple description is not adequate. In fact, MeCP2 binding can be associated with both transcriptional activation and transcriptional repression, depending on its association with different transcription factors (Chahrour et al., 2008). Thus, recent conceptualizations have argued that single modifications and binding proteins should be viewed within a complex epigenetic landscape that operates to integrate multiple cellular signals that combine to control gene transcription (Meaney and Ferguson-Smith, 2010). Nevertheless, future studies will be required to determine under what circumstances methylated DNA binding proteins result in transcriptional repression or activation, and whether these circumstances differ from gene to gene within the central nervous system.
Circuit dynamics in relation to memory systems
DNA methylation in relation to synaptic and circuit plasticity
Memories are formed and stored in unique neural circuits that involve several brain structures, many different types of neurons, and a multitude of distinct plasticity mechanisms. In fact, it is plasticity at synapses (such as long-term potentiation and long-term depression) that is believed to underlie memory formation and maintenance. LTP and memory formation share many important features, including associativity, input specificity, and a requirement for a sufficiently strong stimulus. Moreover, like the formation of a long-term memory, long-lasting LTP cannot be maintained in the presence of protein synthesis inhibitors (Frey et al., 1988), indicating that protein synthesis is required for persistent LTP.
Therefore, if DNA methylation represents a molecular mechanism of memory storage, it is important to demonstrate a link between synaptic plasticity and changes in methylation. This important issue how now been addressed in several different ways. As mentioned above, in an early study, DNMT inhibitors were used to block DNA methylation in hippocampal slices during the delivery of a theta-burst stimulation of Schaeffer Collateral synapses projecting from area CA3 to CA1 of the hippocampus (Levenson et al., 2006). In vehicle treated slices, this stimulation protocol resulted in long-lasting LTP within the CA1 region of the hippocampus. However, in slices treated with the DNMT inhibitors zebularine or 5-aza-2-deoxycytidine, LTP was abolished within 1–2 hours of induction. Moreover, these drugs produced a robust demethylation at the reelin and BDNF genes, which are known to play a role in synaptic plasticity and were subsequently shown to undergo similar methylation changes during fear conditioning (Miller and Sweatt, 2007; Lubin et al., 2008). Importantly, DNMT inhibition produced no effect on baseline synaptic transmission in slices treated with DNMT inhibitors, indicating that the LTP deficit induced was specific and experience-dependent (Levenson et al., 2006). Moreover, a follow-up to this report demonstrated that the disruption of long-lasting LTP induced by DNMT inhibitors could be reversed by pre-treatment with the histone deacetylase (HDAC) inhibitor trichostatin-A, suggesting a potential cross-talk between histone acetylation and DNA methylation (Miller et al., 2008).
Subsequent genetic approaches have provided an important confirmation of these findings. Given that prodrugs like zebularine or 5-aza-2-deoxycytidine are only thought to be effective after being chemically incorporated into DNA (where they can trap DNMTs), it is also important to demonstrate a more direct link to DNMT activity and synaptic plasticity. In keeping with this, a recent paper examined hippocampal LTP and LTD in mice with a double knock-out of both DNMT isoforms (DNMT1 and DNMT3a) in post-mitotic neurons in the forebrain (Feng et al., 2010). This report revealed that double KO mice (unlike single KO mice) exhibit a significant deficit in hippocampal LTP that mimics the deficit observed after DNMT inhibition in the aforementioned studies. Moreover, these mice also exhibit an enhancement in hippocampal LTD, but little or no change in basal synaptic transmission. These data indicate that DNMT1 and DNMT3a may have overlapping roles in adult neurons, and that at least one form is required to maintain normal hippocampal LTP (Feng et al., 2010).
How might DNA methylation changes, which are presumably cell-wide, contribute to seemingly synapse-specific changes that accompany learning and memory? Given the role of DNA methylation in transcriptional regulation, a parsimonious explanation for these effects on LTP is that persistent changes in the methylation status of plasticity-related genes subserves input-specific changes in synaptic strength. However, it is also possible that DNA methylation changes occurring during learning globally alter the excitability of a cell, making it more or less likely to participate in a memory trace. Alternatively, it is possible that changes in DNA methylation serve to stabilize the inputs that a given cell receives after a plastic change has occurred, in effect removing the cell from further synaptic competition and rendering it aplastic. This explanation seems plausible given that memory stabilization should require that a cell maintain its response to a given set of inputs across weeks or years despite the occurrence of new and different stimulation patterns in the intervening period. Importantly, it is likely that these mechanisms could occur in different brain regions and at different times, meaning that they are not mutually exclusive. Clearly, future experiments will be required to elucidate the precise role or roles of DNA methylation in altered synaptic function.
Cortex specific epigenetic regulation?
Given that the cortex is involved in the long-term storage of remote memories, it is especially important to understand how DNA methylation changes may contribute to the circuit-level phenomena occurring in regions like the PFC. The PFC receives inputs from a number of sensory and limbic systems and projects to areas implicated in motivation and action selection, making it the ideal storage site for specific memories (Groenewegen et al., 1990; Groenewegen and Uylings, 2000). Neuronal processing in the PFC can be extremely selective, with individual cells responding selectively to unique events, sensory and spatial properties of different stimuli, specific outcomes, and expectations (Funahashi et al., 1989; Goldman-Rakic, 1990; Goldman-Rakic et al., 1992; Rolls and Baylis, 1994; Watanabe, 1996; Pasternak and Greenlee, 2005). Thus, one possibility is that DNA methylation provides a regulatory mechanism prior to a learning event in order to generate cell-specific responses. For example, DNA methylation could modulate cell excitability both positively and negatively, thereby determining which neurons are eligible for participation in a given memory trace. This mechanism would ensure that only a small fraction of neurons are capable of undergoing plastic changes at any given time. Such a function would be reminiscent of the role played by CREB during the formation of fear memories in the lateral amygdala (Han et al., 2007; Han et al., 2009; Silva et al., 2009; Zhou et al., 2009). Alternatively, the inherent connectivity of cortical circuits could engender selectivity in responsiveness, while DNA methylation serves to stamp in a specific distribution of synaptic weights after learning in order to preserve certain memories. Finally, given that behavioral representations of a memory likely depend on multiple interacting circuits, another possibility is that DNA methylation may silence an entire node within a circuit to produce a given behavioral outcome. Critically, each of these hypotheses could potentially explain the remote memory deficits induced by DNMT inhibitors.
Regardless of the potential circuit-level roles played by DNA methylation, it is possible (or even likely) that unique epigenetic mechanisms exist in the cortex to support the long-term stabilization and maintenance of specific memories. Indeed, it is already evident that epigenetic changes in the hippocampus tend to be rapid in both onset and offset (Miller and Sweatt, 2007; Lubin et al., 2008; Miller et al., 2008), whereas changes within the cortex may be particularly stable over time (Roth et al., 2009; Miller et al., 2010). Further, there are differences in both the genes selected for methylation changes and the direction of these changes following the same behavioral experience (Figure 2). However, the mechanisms that may support such differential function are unclear. One possible explanation for the temporal differences in DNA methylation changes could lie in region-selective expression of certain DNA modifying enzymes or related transcriptional machinery. For example, DNMT expression is known to vary within different subregions of the hippocampus, and is correlated with the degree of methylation in those regions (Brown et al., 2008). Nevertheless, at this time very little is known about regional differences in the expression of DNMTs or the demethylation apparatus throughout the brain.
Summary and conclusions – cognitive epigenetics
Although the epigenetic changes reviewed above demonstrate dynamic epigenetic regulation of transcription, it is worth noting that none of these changes could be included in the traditional definition of epigenetics, which requires that a change be heritable across cell division (Bird, 2007). As adult neurons are post-mitotic, the epigenetic changes established during learning and maintained across days and months are impressive, but not heritable. In response to this discrepancy, recent conceptualizations have sought to redefine the field of epigenetics so as to include all adaptations to chromatin which mark and perpetuate altered activity states (Bird, 2007). In the same spirit, we argue that the findings discussed above highlight the emergence of “cognitive epigenetics” as a subfield of traditional epigenetics. We emphasize this subfield not to exclude or escape the rigor associated with traditional epigenetics, but rather to recognize the unique challenges and phenomena associated with epigenetic changes in neuronal systems (see Table 1). For example, the dynamic changes in DNA methylation observed in neurons indicate that unique mechanisms may regulate this epigenetic mark. Further, given that neurons do not divide, the epigenetic changes that occur in neurons likely target many different genes that do not undergo epigenetic alteration in somatic cells, and vice-versa. Indeed, the set of epigenetic alterations that regulate cellular differentiation after division may be completely partitioned from the epigenetic changes that dynamically regulate synaptic and cellular plasticity, learning, and memory maintenance. Thus, epigenetic changes in somatic cells may differ in both degree and scope from changes in non-dividing neuronal cells.
The study of epigenetics in neural systems is also associated with a number of unique challenges. One particular difficulty arises from the complexity of neuronal subtypes, even within the same brain region. It would not be surprising if the various types of interneurons and projection neurons exhibit drastically different epigenetic profiles, even at the same moment in time. Thus, an important challenge will be to unfold the many layers of epigenetic regulation that occur within separate cell types, and link each of these layers to overall neuronal and systems function in a meaningful way. A second and related challenge will be to understand how different neural circuits use epigenetic mechanisms in different ways for behavioral adaptation. This will be important not only for understanding the molecular mechanisms underlying the function of a single brain region, but also to understand how multiple regions integrate and store information at different speeds. Finally, it will be necessary to fully understand how the genes that undergo epigenetic modification following learning in turn influence synaptic and circuit plasticity, and understand how and why this may differ in discrete brain regions.
As highlighted above, epigenetic mechanisms in non-dividing CNS cells are subject to sometimes unique regulation that is consistent with a molecular component of memory storage. However, a number of studies now indicate that epigenetic mechanisms (and specifically DNA methylation) are involved in a wide range of behavioral outcomes other than memory formation, including responses to drugs of abuse (Anier et al., 2010; Im et al., 2010; LaPlant et al., 2010), psychiatric disorders and syndromes (Sutcliffe et al., 1992; Amir et al., 1999; Grayson et al., 2005; Petronis, 2010), and the long-lasting effects of early life experiences (Weaver et al., 2004; Weaver et al., 2005; Roth et al., 2009). Thus, similar to memory formation, many types of experience result in long-term changes in the epigenetic environment that contributes to subsequent behavioral output. Therefore, understanding the mechanisms discussed here will help to elucidate not only basic molecular principles of learning and memory, but will also provide key insights into the molecular nature of numerous behaviors and behavioral disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso M, Bekinschtein P, Cammarota M, Vianna MR, Izquierdo I, Medina JH. Endogenous BDNF is required for long-term memory formation in the rat parietal cortex. Learn Mem. 2005;12:504–510. doi: 10.1101/lm.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Angrisano T, Lembo F, Pero R, Natale F, Fusco A, Avvedimento VE, Bruni CB, Chiariotti L. TACC3 mediates the association of MBD2 with histone acetyltransferases and relieves transcriptional repression of methylated promoters. Nucleic Acids Res. 2006;34:364–372. doi: 10.1093/nar/gkj400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anier K, Malinovskaja K, Aonurm-Helm A, Zharkovsky A, Kalda A. DNA Methylation Regulates Cocaine-Induced Behavioral Sensitization in Mice. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron. 2004;44:49–57. doi: 10.1016/j.neuron.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Ball MP, Li JB, Gao Y, Lee JH, LeProust EM, Park IH, Xie B, Daley GQ, Church GM. Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells. Nat Biotechnol. 2009;27:361–368. doi: 10.1038/nbt.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008a;14:147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci U S A. 2008b;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bird A. DNA methylation de novo. Science. 1999;286:2287–2288. doi: 10.1126/science.286.5448.2287. [DOI] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- Brown SE, Weaver IC, Meaney MJ, Szyf M. Regional-specific global cytosine methylation and DNA methyltransferase expression in the adult rat hippocampus. Neurosci Lett. 2008;440:49–53. doi: 10.1016/j.neulet.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Buard I, Coultrap SJ, Freund RK, Lee YS, Dell’Acqua ML, Silva AJ, Bayer KU. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J Neurosci. 2010;30:8214–8220. doi: 10.1523/JNEUROSCI.1469-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HX, Otmakhov N, Strack S, Colbran RJ, Lisman JE. Is persistent activity of calcium/calmodulin-dependent kinase required for the maintenance of LTP? J Neurophysiol. 2001;85:1368–1376. doi: 10.1152/jn.2001.85.4.1368. [DOI] [PubMed] [Google Scholar]
- Chow JC, Brown CJ. Forming facultative heterochromatin: silencing of an X chromosome in mammalian females. Cell Mol Life Sci. 2003;60:2586–2603. doi: 10.1007/s00018-003-3121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JC, Yen Z, Ziesche SM, Brown CJ. Silencing of the mammalian X chromosome. Annu Rev Genomics Hum Genet. 2005;6:69–92. doi: 10.1146/annurev.genom.6.080604.162350. [DOI] [PubMed] [Google Scholar]
- Crick F. Memory and molecular turnover. Nature. 1984;312:101. doi: 10.1038/312101a0. [DOI] [PubMed] [Google Scholar]
- Cui Z, Wang H, Tan Y, Zaia KA, Zhang S, Tsien JZ. Inducible and reversible NR1 knockout reveals crucial role of the NMDA receptor in preserving remote memories in the brain. Neuron. 2004;41:781–793. doi: 10.1016/s0896-6273(04)00072-8. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Day JJ, Carelli RM. The nucleus accumbens and Pavlovian reward learning. Neuroscientist. 2007;13:148–159. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C. Brain function and chromatin plasticity. Nature. 2010;465:728–735. doi: 10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JM, Wild L. An epigenetic role for noncoding RNAs and intragenic DNA methylation. Genome Biol. 2007;8:307. doi: 10.1186/gb-2007-8-6-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, O’Brien C, Ohno M, Kirkwood A, Silva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Stoppini L, Miyamoto E, Muller D. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1993;268:7863–7867. [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Gehring M, Reik W, Henikoff S. DNA demethylation by DNA repair. Trends Genet. 2009;25:82–90. doi: 10.1016/j.tig.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325–335. doi: 10.1016/s0079-6123(08)62688-6. discussion 335–326. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Bates JF, Chafee MV. The prefrontal cortex and internally generated motor acts. Curr Opin Neurobiol. 1992;2:830–835. doi: 10.1016/0959-4388(92)90141-7. [DOI] [PubMed] [Google Scholar]
- Graff J, Mansuy IM. Epigenetic codes in cognition and behaviour. Behav Brain Res. 2008;192:70–87. doi: 10.1016/j.bbr.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, Costa E. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Butler JE, Haig D, Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010a;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, Dulac C. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010b;329:643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JS, Mahler HR. DNA ticketing theory of memory. Nature. 1969;223:580–582. doi: 10.1038/223580a0. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. discussion 116–118. [DOI] [PubMed] [Google Scholar]
- Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD. Histone methylation regulates memory formation. J Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Li Y, Wang D, Wei C, Yang X, Sui N. Effect of 5-aza-2-deoxycytidine microinjecting into hippocampus and prelimbic cortex on acquisition and retrieval of cocaine-induced place preference in C57BL/6 mice. Eur J Pharmacol. 2010;642:93–98. doi: 10.1016/j.ejphar.2010.05.050. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, Sweatt JD, Beaudet AL. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iniguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolanos CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1999;96:3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, Malone LM, Sweatt JD. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lisman JE. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci U S A. 1985;82:3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Guo JU, Ming GL, Song H. DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation. Cell Cycle. 2009a;8:1526–1531. doi: 10.4161/cc.8.10.8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009b;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Huganir RL, O’Brien RJ. Redistribution and stabilization of cell surface glutamate receptors during synapse formation. J Neurosci. 1997;17:7351–7358. doi: 10.1523/JNEUROSCI.17-19-07351.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D’Souza C, Fouse SD, Johnson BE, Hong C, Nielsen C, Zhao Y, Turecki G, Delaney A, Varhol R, Thiessen N, Shchors K, Heine VM, Rowitch DH, Xing X, Fiore C, Schillebeeckx M, Jones SJ, Haussler D, Marra MA, Hirst M, Wang T, Costello JF. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–257. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Ferguson-Smith AC. Epigenetic regulation of the neural transcriptome: the meaning of the marks. Nat Neurosci. 2010;13:1313–1318. doi: 10.1038/nn1110-1313. [DOI] [PubMed] [Google Scholar]
- Metivier R, Gallais R, Tiffoche C, Le Peron C, Jurkowska RZ, Carmouche RP, Ibberson D, Barath P, Demay F, Reid G, Benes V, Jeltsch A, Gannon F, Salbert G. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Gavin CF, White JA, Parrish RR, Honasoge A, Yancey CR, Rivera IM, Rubio MD, Rumbaugh G, Sweatt JD. Cortical DNA methylation maintains remote memory. Nat Neurosci. 2010;13:664–666. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–463. doi: 10.1038/nature08909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi SK, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:1145–1148. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory. Mol Pharmacol. 2007;72:350–358. doi: 10.1124/mol.107.034934. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Greenlee MW. Working memory in primate sensory systems. Nat Rev Neurosci. 2005;6:97–107. doi: 10.1038/nrn1603. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. New York: El Sevier; 2005. [DOI] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Petronis A. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature. 2010;465:721–727. doi: 10.1038/nature09230. [DOI] [PubMed] [Google Scholar]
- Price JC, Guan S, Burlingame A, Prusiner SB, Ghaemmaghami S. Analysis of proteome dynamics in the mouse brain. Proc Natl Acad Sci U S A. 2010;107:14508–14513. doi: 10.1073/pnas.1006551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learn Mem. 2004;11:727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Rich RC, Schulman H. Substrate-directed function of calmodulin in autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1998;273:28424–28429. doi: 10.1074/jbc.273.43.28424. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Sweatt JD. Memory-forming chemical reactions. Rev Neurosci. 2001;12:41–50. doi: 10.1515/revneuro.2001.12.1.41. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J Neurosci. 1994;14:5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor TC. PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog Brain Res. 2008;169:27–40. doi: 10.1016/S0079-6123(07)00002-7. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci U S A. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003a;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell. 2010;140:421–435. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell. 2003b;115:893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992a;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992b;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J. Molecular and cellular approaches to memory allocation in neural circuits. Science. 2009;326:391–395. doi: 10.1126/science.1174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annu Rev Psychol. 1993;44:453–495. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, Warren ST. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1:397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- Swank MW, Sweatt JD. Increased histone acetyltransferase and lysine acetyltransferase activity and biphasic activation of the ERK/RSK cascade in insular cortex during novel taste learning. J Neurosci. 2001;21:3383–3391. doi: 10.1523/JNEUROSCI.21-10-03383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M, Si K, Kandel ER. Two previously undescribed members of the mouse CPEB family of genes and their inducible expression in the principal cell layers of the hippocampus. Proc Natl Acad Sci U S A. 2003;100:9602–9607. doi: 10.1073/pnas.1133424100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JP, Skene PJ, Selfridge J, Clouaire T, Guy J, Webb S, Kerr AR, Deaton A, Andrews R, James KD, Turner DJ, Illingworth R, Bird A. CpG islands influence chromatin structure via the CpG-binding protein Cfp1. Nature. 2010;464:1082–1086. doi: 10.1038/nature08924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valinluck V, Tsai HH, Rogstad DK, Burdzy A, Bird A, Sowers LC. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2) Nucleic Acids Res. 2004;32:4100–4108. doi: 10.1093/nar/gkh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PA, Gegonne A, Jones PL, Ballestar E, Aubry F, Wolffe AP. Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nat Genet. 1999;23:62–66. doi: 10.1038/12664. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wood MA, Hawk JD, Abel T. Combinatorial chromatin modifications and memory storage: a code for memory? Learn Mem. 2006;13:241–244. doi: 10.1101/lm.278206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SC, Zhang Y. Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol. 2010;11:607–620. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]