Abstract
A novel approach to DNA probe hybridization and heteroduplex analysis, termed directed heteroduplex analysis (DHDA) is presented here to illustrate its utility in simplification of human lymphocyte antigen (HLA)-typing. By strategic labeling of single-stranded probe sequences, DHDA allows the identification of specific heteroduplex structures that contribute to the differentiation of DQA1 and DQB1 alleles. Because of the high degree of polymorphism among major histocompatibility complex class II second exon sequences, this analysis of 50 different heteroduplex molecules provides evidence of the importance of unpaired bases and mismatched base pairs and their effect on heteroduplex electrophoretic-mobility differences. This strategy is further used to genotype accurately a family for DQA1 which was previously analyzed by sequence specific oligonucleotide (SSO) probe hybridization. To differentiate by SSO-typing among the DQA1 and DQB1 alleles analyzed in this study requires the use of 23 different probes. Equivalent results are obtained by DHDA using only three probes. Therefore, this study suggests that accurate HLA-typing can be simplified by DHDA. Additionally, DHDA may be useful for differentiation of DNA sequence polymorphisms in other genetic systems.
Full text
PDF



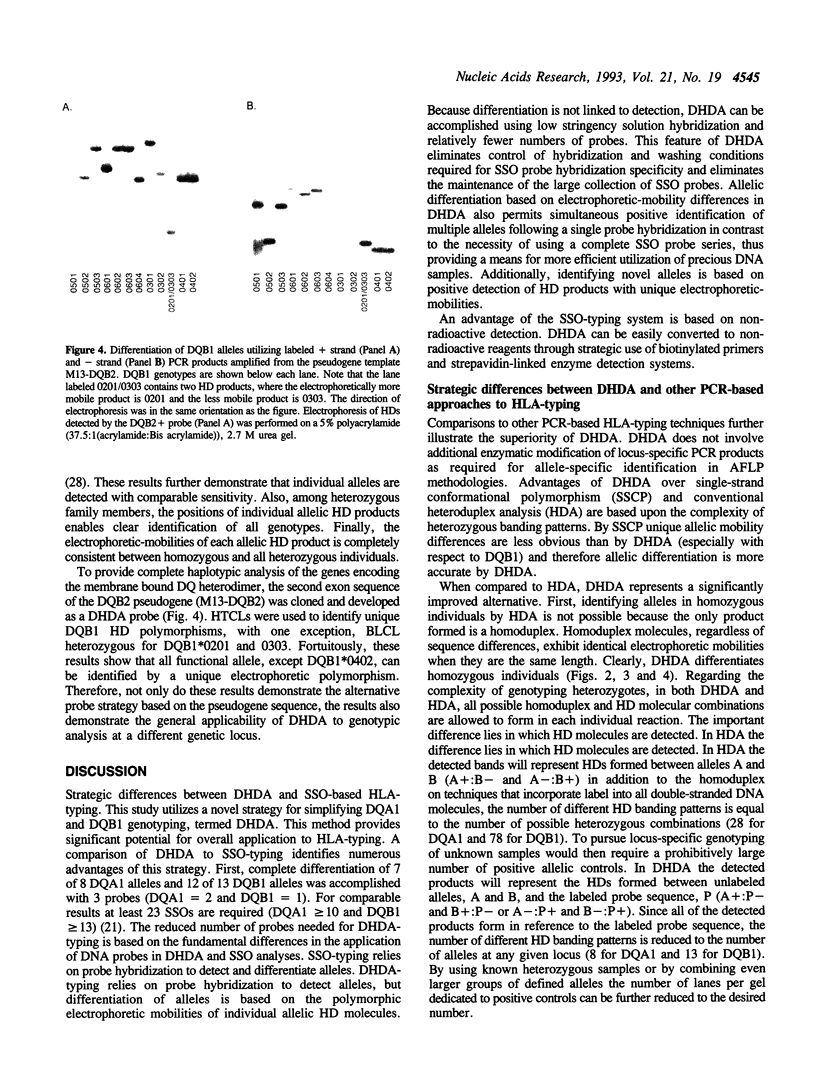


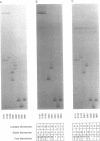
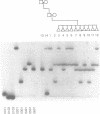
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begovich A. B., McClure G. R., Suraj V. C., Helmuth R. C., Fildes N., Bugawan T. L., Erlich H. A., Klitz W. Polymorphism, recombination, and linkage disequilibrium within the HLA class II region. J Immunol. 1992 Jan 1;148(1):249–258. [PubMed] [Google Scholar]
- Bhattacharyya A., Lilley D. M. The contrasting structures of mismatched DNA sequences containing looped-out bases (bulges) and multiple mismatches (bubbles). Nucleic Acids Res. 1989 Sep 12;17(17):6821–6840. doi: 10.1093/nar/17.17.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington M., White M. B., Dean M., Mann D., Ward F. E. The use of DNA heteroduplex patterns to map recombination within the HLA class II region. Hum Immunol. 1992 Feb;33(2):114–121. doi: 10.1016/0198-8859(92)90061-q. [DOI] [PubMed] [Google Scholar]
- Colvin R. B. Cellular and molecular mechanisms of allograft rejection. Annu Rev Med. 1990;41:361–375. doi: 10.1146/annurev.me.41.020190.002045. [DOI] [PubMed] [Google Scholar]
- Duquesnoy R. J., Trager J. D., Zeevi A. Propagation and characterization of lymphocytes from transplant biopsies. Crit Rev Immunol. 1991;10(6):455–480. [PubMed] [Google Scholar]
- Erlich H., Bugawan T., Begovich A. B., Scharf S., Griffith R., Saiki R., Higuchi R., Walsh P. S. HLA-DR, DQ and DP typing using PCR amplification and immobilized probes. Eur J Immunogenet. 1991 Feb-Apr;18(1-2):33–55. doi: 10.1111/j.1744-313x.1991.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Hill A. V., Allsopp C. E., Kwiatkowski D., Anstey N. M., Twumasi P., Rowe P. A., Bennett S., Brewster D., McMichael A. J., Greenwood B. M. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991 Aug 15;352(6336):595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- Hoshino S., Kimura A., Fukuda Y., Dohi K., Sasazuki T. Polymerase chain reaction--single-strand conformation polymorphism analysis of polymorphism in DPA1 and DPB1 genes: a simple, economical, and rapid method for histocompatibility testing. Hum Immunol. 1992 Feb;33(2):98–107. doi: 10.1016/0198-8859(92)90059-v. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. L., Reay P. A., Ehrich E. W., Davis M. M. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- Kimura A., Dong R. P., Harada H., Sasazuki T. DNA typing of HLA class II genes in B-lymphoblastoid cell lines homozygous for HLA. Tissue Antigens. 1992 Jul;40(1):5–12. doi: 10.1111/j.1399-0039.1992.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mabey D. C., Bailey R. L., Dunn D., Jones D., Williams J. H., Whittle H. C., Ward M. E. Expression of MHC class II antigens by conjunctival epithelial cells in trachoma: implications concerning the pathogenesis of blinding disease. J Clin Pathol. 1991 Apr;44(4):285–289. doi: 10.1136/jcp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S. G., Bodmer J. G. HLA class II nucleotide sequences, 1992. Tissue Antigens. 1992 Nov;40(5):229–243. doi: 10.1111/j.1399-0039.1992.tb02050.x. [DOI] [PubMed] [Google Scholar]
- Meyer C. G., Tannich E., Harders J., Henco K., Horstmann R. D. Direct sequencing of variable HLA gene segments after in vitro amplification and allele separation by temperature-gradient gel electrophoresis. J Immunol Methods. 1991 Sep 13;142(2):251–256. doi: 10.1016/0022-1759(91)90113-t. [DOI] [PubMed] [Google Scholar]
- Nepom G. T., Erlich H. MHC class-II molecules and autoimmunity. Annu Rev Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- Parkman R. Graft-versus-host disease. Annu Rev Med. 1991;42:189–197. doi: 10.1146/annurev.me.42.020191.001201. [DOI] [PubMed] [Google Scholar]
- Petzl-Erler M. L., Belich M. P., Queiroz-Telles F. Association of mucosal leishmaniasis with HLA. Hum Immunol. 1991 Dec;32(4):254–260. doi: 10.1016/0198-8859(91)90088-q. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Olerup O., Troye-Blomberg M. The immune recognition of malaria antigens. Parasitol Today. 1991 Jan;7(1):5–11. doi: 10.1016/0169-4758(91)90076-z. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Sorrentino R., Cascino I., Tosi R. Subgrouping of DR4 alleles by DNA heteroduplex analysis. Hum Immunol. 1992 Jan;33(1):18–23. doi: 10.1016/0198-8859(92)90047-q. [DOI] [PubMed] [Google Scholar]
- Sorrentino R., Potolicchio I., Ferrara G. B., Tosi R. A new approach to HLA-DPB1 typing combining DNA heteroduplex analysis with allele-specific amplification and enzyme restriction. Immunogenetics. 1992;36(4):248–254. doi: 10.1007/BF00215055. [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Barker P., Griffith J. Visualization of diagnostic heteroduplex DNAs from cystic fibrosis deletion heterozygotes provides an estimate of the kinking of DNA by bulged bases. J Biol Chem. 1992 Mar 5;267(7):4911–4915. [PubMed] [Google Scholar]
- White M. B., Carvalho M., Derse D., O'Brien S. J., Dean M. Detecting single base substitutions as heteroduplex polymorphisms. Genomics. 1992 Feb;12(2):301–306. doi: 10.1016/0888-7543(92)90377-5. [DOI] [PubMed] [Google Scholar]
- Yunis I., Salazar M., Yunis E. J. HLA-DR generic typing by AFLP. Tissue Antigens. 1991 Aug;38(2):78–88. doi: 10.1111/j.1399-0039.1991.tb01884.x. [DOI] [PubMed] [Google Scholar]