Abstract
The purpose of this study was to estimate dose reduction after implementation of asymmetrical cone beam processing using exposure differences measured in a water phantom and a small cohort of clinical coronary CTA patients. Two separate 320 × 0.5 mm detector row scans of a water phantom used identical cardiac acquisition parameters before and after software modifications from symmetric to asymmetric cone beam acquisition and processing. Exposure was measured at the phantom surface with Optically Stimulated Luminescence (OSL) dosimeters at 12 equally spaced angular locations. Mean HU and standard deviation (SD) for both approaches were compared using ROI measurements obtained at the center plus four peripheral locations in the water phantom. To assess image quality, mean HU and standard deviation (SD) for both approaches were compared using ROI measurements obtained at five points within the water phantom. Retrospective evaluation of 64 patients (37 symmetric; 27 asymmetric acquisition) included clinical data, scanning parameters, quantitative plus qualitative image assessment, and estimated radiation dose. In the water phantom, the asymmetric cone beam processing reduces exposure by approximately 20% with no change in image quality. The clinical coronary CTA patient groups had comparable demographics. The estimated dose reduction after implementation of the asymmetric approach was roughly 24% with no significant difference between the symmetric and asymmetric approach with respect to objective measures of image quality or subjective assessment using a four point scale. When compared to a symmetric approach, the decreased exposure, subsequent lower patient radiation dose, and similar image quality from asymmetric cone beam processing supports its routine clinical use.
Keywords: Coronary imaging, Computed tomography, Angiography, Image reconstruction, Radiation dose
Introduction
Prospectively ECG-gated sixty-four detector row cardiac CT methods require several heart beats using slabs from each gantry rotation to generate a full volume. Inherent cardiac banding artifacts and discontinuities are eliminated with axial single heart beat 320 × 0.5 mm detector row CT acquisition. Because patient radiation exposure limits more widespread use of coronary CT angiography, current wide area detector cardiac acquisitions parallel the axial prospective approach, using a phase window and a stationary patient table. The phase window can be narrowed to a single phase or expanded to the entire R- R interval, i.e., retrospective ECG gating. Following initial work using a relatively wide phase window [1], a 10% phase window width centered at 75% of the R-R interval typically achieves an acceptable compromise between high image quality and radiation dose [2].
We introduce and study further dose reduction by limiting the total beam-on time for 320 × 0.5 mm detector row cardiac CT data acquisition. One-hundred eighty degrees of gantry rotation data is required for “half-scan” reconstruction. However, additional data is required for cone-beam processing. In the current symmetrical algorithm (Fig. 1a) extra data is evenly acquired around the half scan data. Thus, for a 10% phase window width acquisition, a half-rotation is acquired before and after the desired phases. With the new asymmetrical approach, additional data for cone beam processing does not equally surround the half scan data (Fig. 1b). This strategy reduces the overall beam-on time via more efficient projection data acquisition. The purpose of this study is to evaluate the asymmetrical approach for cone-beam processing and to measure exposure differences versus an earlier symmetric reconstruction approach using a water phantom imaged with standard coronary angiography scan parameters. A small clinical cohort is used to validate the phantom measurements.
Fig. 1.
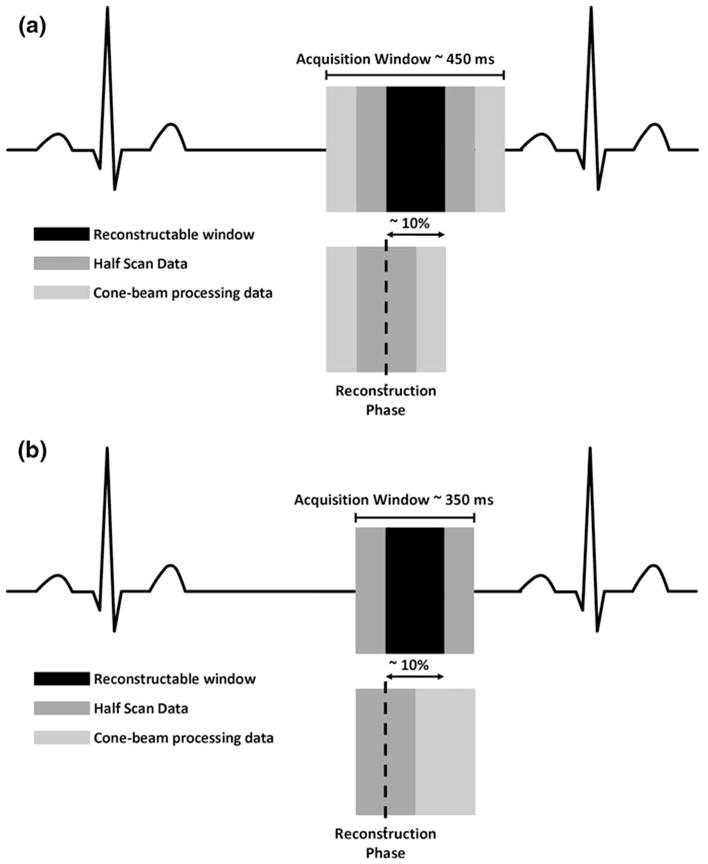
a Symmetric, data for cone beam correction must be acquired equally on each side of the desired phase. b Asymmetric, data for cone beam correction is acquired anywhere in the exposure window. Minimum exposure is 350 ms
Methods
Phantom study
Acquisition
Two separate acquisitions were made using a water phantom. The acquisition parameters (artificial ECG trace, 60 beats per minute, 10% phase window centered in mid-diastole, 120 kV, 400 mA, 350 ms rotation) using axial 320 × 0.5 mm detector row CT were identical. The first data set was acquired before the software modification, and the second data set was acquired after the implementation of the asymmetric cone beam acquisition and processing.
Exposure measurements
A 32 cm diameter water phantom was used to estimate the decrease in exposure from the asymmetric approach using the standard symmetric approach as a reference. Exposure was measured at the surface of the phantom with Optically Stimulated Luminescence (OSL) dosimeters at 12 angular locations, equally spaced by 30 degrees. For each experiment, dosimeters were placed in central axial plane of a 16 cm craniocaudal acquisition.
Image quality measurements
To assess image quality, mean HU and standard deviation (SD) for both approaches were compared using region of interest (ROI) measurements obtained at five points within the water phantom: isocenter, 12, 3, 6, and 9 o’clock positions, 1 cm in from the phantom edge.
Patient study
Demographics
The study was approved by the institution Human Research Committee, and written informed consent was not required for this retrospective evaluation. Sixty-four patients with a clinical indication for coronary CTA were identified: 37 consecutive patients who were imaged when the symmetric acquisition was implemented, plus 27 consecutive patients who were imaged after the scanner software was changed to the asymmetric acquisition and reconstruction approach. Baseline characteristics (Table 1) were obtained from the hospital electronic medical record.
Table 1.
Baseline characteristics for two patient cohorts
| Symmetric | Asymmetric | P value | |
|---|---|---|---|
| Number of patients | 37 | 27 | |
| Indication | 0.313 | ||
| Chest pain | 17 | 12 | |
| Other symptoms | 8 | 4 | |
| Abnormal prior imaging test | 7 | 4 | |
| Pre-surgical | 3 | 7 | |
| Othersa | 2 | 0 | |
| Age (year) | 54.1 ± 11.0 | 53.9 ± 15.4 | 0.954 |
| Sex (M:F) | 15:22 | 17:10 | 0.076 |
| Weight (lb) | 170.7 ± 37.0 | 177.8 ± 35.7 | 0.460 |
| BMI (kg/m2) | 27.8 ± 4.8 | 27.3 ± 4.1 | 0.679 |
| Cardiac risk factors | |||
| Hypertension | 23 | 12 | 0.206 |
| Dyslipidemia | 18 | 17 | 0.314 |
| Diabetes | 4 | 1 | 0.387 |
| Family history of CAD | 11 | 14 | 0.119 |
| Smoking | 2 | 5 | 0.122 |
| CAD history | 1 | 2 | 0.568 |
| Heart rate at CT scan (bpm) | 57.8 ± 5.8 | 54.9 ± 4.9 | 0.036 |
| Beta-blockade | 0.095 | ||
| None | 2 | 7 | |
| Orally | 5 | 5 | |
| 5 mg iv | 14 | 5 | |
| 6–10 mg iv | 8 | 3 | |
| >10 mg iv | 8 | 7 | |
Others include patients’ request, screenings due to strong risk factors, and follow-up after intervention
CT scan parameters
All patients were imaged with prospective ECG gating with a 10–25% phase window width centered at 75% of the R-R interval. The gantry rotation time was 350 ms. All patients were scanned using 320 × 0.5 mm collimation except for two patients in the symmetric group, one of whom was scanned using 240 × 0.5 mm and the other using 280 × 0.5 mm. For the symmetric group, 26 patients (70%) were imaged at 120 kV and 11 patients (30%) were imaged at 100 kV. For the asymmetric group, 21 patients (78%) were imaged with 120 kV, five patients (18%) were imaged with 100 kV, and one patient (4%) was imaged with 80 kV. The decision to use a lower kV setting was made by the attending cardiovascular imager and was largely determined by patient body habitus. The tube current was also chosen by the attending cardiovascular imager. There was a higher mean tube current for the asymmetric group (495 vs. 451 mA). All 0.5 mm slice thickness data was reconstructed using a standard kernel (FC03) and with no noise reduction post-processing.
Image quality and dose estimation
To compare attenuation and image noise between the two patient groups, ROI measurements of mean and SD HU were obtained in the ascending aorta, the left main (LM) coronary artery, the trachea, and the pectoral muscle. Signal-to-noise ratio (SNR) was calculated by dividing the mean HU within the ROI by its SD. To compare contrast-to-noise (CNR) between the symmetric and asymmetric groups, two contrasts were measured. The first was between the ascending aorta and the pectoral muscle. The second was between the left main coronary artery and the pectoral muscle. The CNR was calculated by dividing the difference in contrast by the SD of the aorta and left main, respectively. The effective radiation dose to each patient was estimated using the DLP as provided by the manufacturer reported by the scanner [3].
Qualitative analysis
To evaluate potential differences in image quality between images in the symmetric versus asymmetric groups, all image data sets were anonymized, randomized, and transferred to an image post-processing workstation (Vitrea fX, Vital Images, Min-netonka, MN, USA). An experienced cardiovascular imager blinded to the acquisition and reconstruction approach evaluated image quality using a 4-point scale: 4- excellent, no artifact; 3- good, mild artifact; 2-acceptable, moderate artifact present but images still interpretable, 1- unevaluable, severe artifact renders interpretation not possible. Agreement between the two patient groups was assessed using a Wilcoxon rank-sum test.
Results
Phantom study
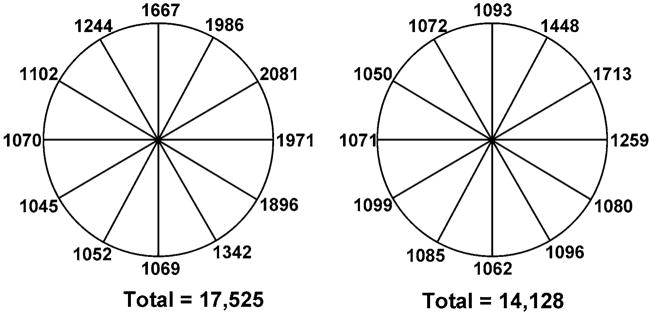
For each algorithm, the acquisition is greater than one gantry rotation. The absolute location (i.e., 3 o’clock vs. 6 o’clock, etc.) does not influence the results since the gantry is continuously rotating and the acquisition is triggered by the ECG signal. The asymmetric reconstruction algorithm had an approximately 20% reduction (14,128 vs. 17,525 millirads) in integral OSL exposure with respect to the standard symmetric algorithm (Fig. 2). The OSLs exposed once had levels near 1,050–1,100 millirads. Using the symmetrical acquisition, seven of the twelve dosimeters received greater exposure, indicating that the gantry rotation well exceeded 360° for a 10% phase window width. This number was reduced to three of the twelve dosimeters for the asymmetric strategy. With respect to image quality obtained from the ROI data, there was no significant difference between the asymmetric (HU = −0.3 ± 1.1 SD = 80.8 ± 10.4) and symmetric (HU = −1.6 ± 1.3 SD = 82.9 ± 9.3) reconstructions. Furthermore, the radial distribution of the noise was similar between the two reconstructions (Fig. 3). The noise follows the sinusoidal pattern associated with all half-scan reconstructions due to the uneven angular sampling of the imaged object.
Fig. 2.
Schematic of water phantom showing position of exposure measurements for the symmetric and asymmetric cone beam processing
Fig. 3.
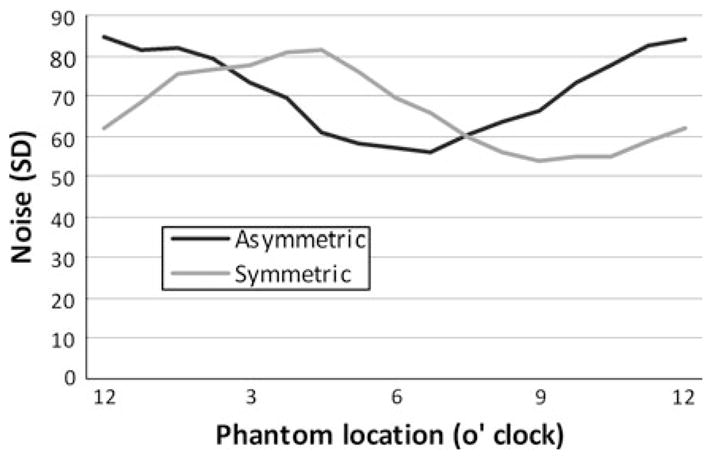
Radial distribution of noise within the water phantom images acquired with the symmetric versus asymmetric algorithms
Patient study
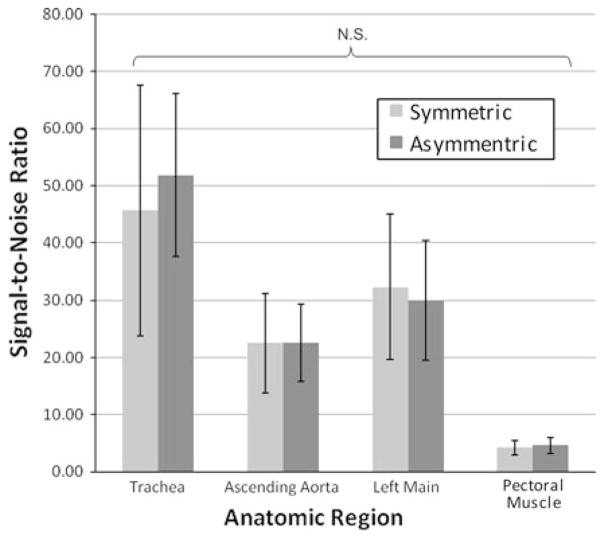
With the exception of heart rate, the patient characteristics (Table 1) did not differ significantly between the two populations. While the difference in heart rate reached statistical significance (symmetric = 57.8 ± 5.8, asymmetric = 54.9 ± 4.9, P = 0.036), the small absolute heart rate difference of about 5% is not expected to have a significant impact upon the delivered radiation dose. If anything, the faster heart rate would cause a slightly shorter scan time giving a radiation dose “advantage” to the symmetrical reconstruction data. The mean effective radiation dose was 24% lower for those patients imaged with the asymmetric approach (4.05 ± 1.3 mSv) when compared to patients imaged with the standard symmetric approach (5.33 ± 2.0 mSv). There was no significant difference in the SNR between the symmetric and asymmetric groups for any of the four anatomical regions (Fig. 4). There was no significant difference in the CNR comparisons between the aorta versus the pectoral muscle the contrast between the left main coronary artery versus the pectoral muscle (Fig. 5).
Fig. 4.
Mean signal-to-noise ratio measurements between those patients acquired with the symmetric versus asymmetric algorithms for four anatomic regions. N.S. refers to not significant
Fig. 5.
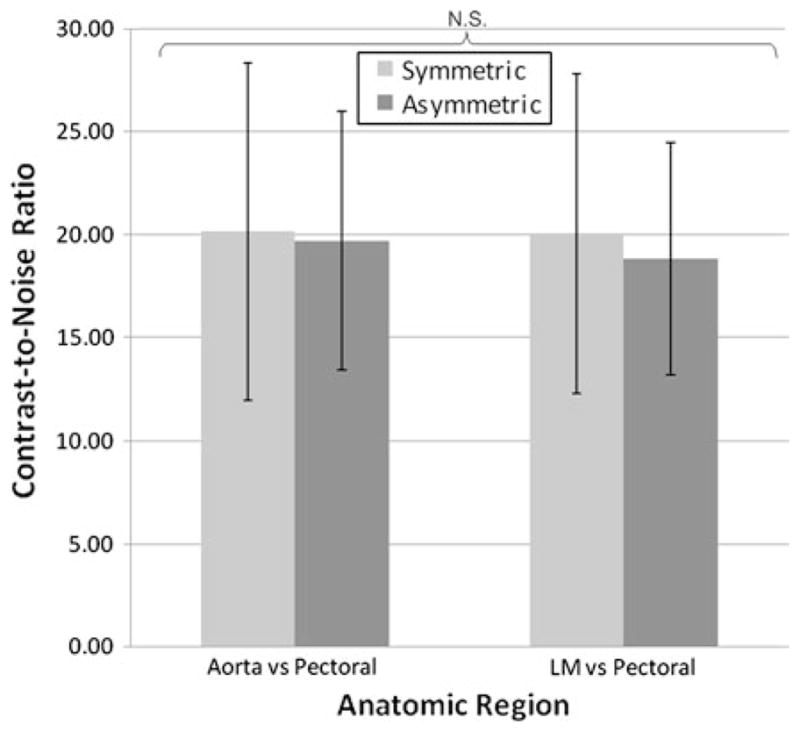
Mean contrast-to-noise ratio measurement differences between those patients acquired with symmetric versus asymmetric algorithms
The subjective assessment of coronary image quality between the two groups (Table 2) shows no significant difference between the symmetric and asymmetric approach with overall scores averaging above 3.5 (maximum 4.0) for both populations.
Table 2.
Subjective assessment of coronary artery image quality between those patients acquired with the symmetric versus asymmetric algorithms
| Mean scores (0.95 confidence interval) |
|||
|---|---|---|---|
| Symmetric | Asymmetric | P* | |
| RCA | 3.46 (3.26–3.65) | 3.52 (3.28–3.52) | 0.711 |
| LAD | 3.68 (3.49–3.86) | 3.67 (3.46–3.88) | 0.950 |
| LCx | 3.49 (3.29–3.68) | 3.52 (3.28–3.76) | 0.841 |
| Overall | 3.54 (3.43–3.65) | 3.57 (3.43–3.70) | 0.758 |
P value by Wilcoxon rank-sum test
Discussion
CT reconstruction requires sufficient data so that at least one cone-beam source point exists for every Radon plane in the field of view [4]. With cone beam acquisitions, this condition is not always met. However, with narrower cone-beam acquisitions such as 64-detector row scans, the resulting shading artifacts are not readily apparent clinically. The conspicuity of these artifacts is greater as the cone-angle increases. The artifacts become obvious with half-scan reconstruction due to the large number of missing Radon planes. With full-scan (360°) reconstruction, the shading artifacts can be avoided by filling in missing Radon planes with projections from the opposite side of the object. However, this approach is undesirable for cardiac imaging because temporal resolution would be compromised, leading to motion artifact.
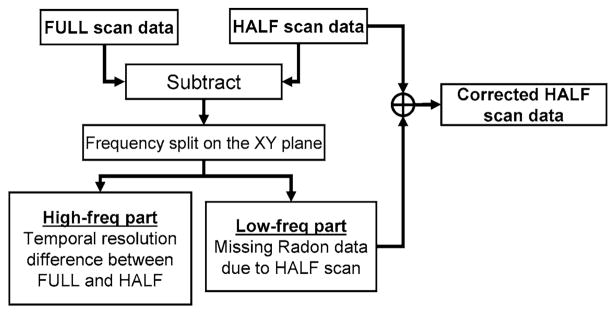
Both symmetric and asymmetric approaches take advantage of the fact that shading artifacts are low frequency data components and temporal resolution is dominated by the high frequency components. Both algorithms use the frequency processed portion of full-scan data to correct shading artifacts present in half-scan data (Fig. 6). First, the half-scan data is subtracted from the full-scan data, and the low frequency components are selected. These are blended with the original half-scan reconstruction to correct for the shading artifacts associated with the missing Radon data in the half-scan reconstruction. Since the temporal resolution is reflected by the high-frequency component of the data, the resulting image data maintains half-scan temporal uniformity.
Fig. 6.
Algorithm for frequency processing half-scan and full-scan data for cardiac cone-beam reconstruction
The algorithms differ in the overall time of the acquisition and the temporal position of the cone-correction data. The symmetric algorithm is based on even distribution of the 180 degrees of cone-correction data. For the asymmetric algorithm, equivalent data is obtained more efficiently from any combination of projections, based on the circular symmetry of the acquisition. For a 10% phase window width, the minimum data collection for the symmetric algorithm is one full rotation plus 10% of the R-R interval and reduced to a full rotation for the asymmetric case. The extended exposure as measured in the phantom experiment is from over-scanning.
Dose optimization [5, 6], particularly for new imaging technologies [7], should follow the As Low As Reasonably Achievable (ALARA) principle [8]. Patient dose is proportional to beam-on time, thus phantom and clinical data support a dose reduction of at least one-fifth using the asymmetric approach. The consistent quantitative image quality, based on both phantom noise measurements and clinical SNR and CNR data, strongly supports the implementation of asymmetric cone beam processing for routine and research-based wide area detector cardiac CT. For a 10% phase window width, the asymmetric approach reduced dose by more than 20%. Based on the discretion of the attending cardiovascular imager, the asymmetric group had a higher mean tube current (495 ± 50 vs. 451 ± 61 mA), even though this group had a slightly lower BMI. This emphasizes the need for clear guidelines in dose optimization; had the imaging parameters been more standardized, the radiation dose reduction for the asymmetric group may have been greater.
Both the symmetric and asymmetric approaches use the same number of projections for the primary image reconstruction, i.e., 180 degrees plus fan angle. Since high frequency information comes from this primary data, the finding of consistent image noise between the symmetric and asymmetric algorithms is expected. Regarding the patient data, both groups had similar body mass index; this factor largely contributes to patient dose. Moreover, both groups showed similar SNR and CNR.
There are several limitations to this study. The phantom measurements were limited in scope, relative, and included only exposure measurements at the surface of a water phantom. Moreover, clinical practice incorporates different scan parameters; our limited water phantom acquisitions used only a single set of standard coronary CTA parameters. We acknowledge that more complex studies using anthropomorphic phantoms or Monte Carlo estimations are necessary to quantitatively assess actual doses. However, the lower cumulative OSL exposure observed in the water phantom data roughly correlated to the lower clinical dose estimates. The patient groups were small and imaging was heterogeneous with respect to tube current, voltage, and craniocaudal coverage; this heterogeneity is expected for a retrospective study positioned before and after a software change, and dose estimates normalized for individual parameters were not reported. The largest difference between the groups was in the tube current. Had we performed normalization with respect to tube current, the dose difference between the symmetric and asymmetric groups would have been 31%. Since a larger potential study would still include clinical heterogeneity, we did not report such normalized calculations in the Results.
In part based on these results, all current 320 × 0.5 mm CT scanners now use the asymmetric approach. This limits the practical impact (i.e., the cardiovascular imager can not choose between the symmetric vs. asymmetric approach), and this we did not include a subjective analysis of image quality. However, the results of equivalent image quality with dose reduction using the asymmetric approach have important implications for more complex studies including cardiac perfusion [9–11] studies of gradients in coronary enhancement [12], and vascular profiling using endothelial shear stress [13] using 320-detector row CT. For clinical validation of both perfusion cardiac CT and coronary CTA contrast gradients, both rest and stress acquisitions will likely prove valuable. Thus, even modest reductions in dose from the asymmetric acquisition become doubled.
In summary, an asymmetric reconstruction algorithm implemented into wide area detector CT has an approximate 20–25% reduction in exposure when compared to the symmetric algorithm currently in practice. Early clinical experience with the asymmetric approach gives a dose reduction with no degradation in image quality and supports its routine use.
Footnotes
Conflict of interest Dr. Rybicki has research agreements with Toshiba Medical Systems Corporation, Bracco Diagnostics, and Vital Images, all of which are unrelated to this project. Dr. Clouse has a research agreement with Toshiba Medical Systems Corporation. Dr. Mather and Ms. Steveson are employees of the parent company Toshiba Medical Systems Corporation.
Contributor Information
Arash Bedayat, Applied Imaging Science Laboratory, Department of Radiology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA.
Frank J. Rybicki, Email: frybicki@partners.org, Applied Imaging Science Laboratory, Department of Radiology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA
Kanako Kumamaru, Applied Imaging Science Laboratory, Department of Radiology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA.
Sara L. Powers, Applied Imaging Science Laboratory, Department of Radiology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA
Jason Signorelli, Applied Imaging Science Laboratory, Department of Radiology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA.
Michael L. Steigner, Applied Imaging Science Laboratory, Department of Radiology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA
Chloe Steveson, Toshiba Medical Systems Corporation, 1385 Shimoishigami, Otawara-shi, Tochigi-ken, Japan.
Shigeyoshi Soga, Applied Imaging Science Laboratory, Department of Radiology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA.
Kimberly Adams, Applied Imaging Science Laboratory, Department of Radiology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA.
Dimitrios Mitsouras, Applied Imaging Science Laboratory, Department of Radiology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA.
Melvin Clouse, Department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Richard T. Mather, Toshiba Medical Research Institute, 706 N Deerpath Dr, Vernon Hills, IL, USA
References
- 1.Rybicki FJ, Otero HJ, Steigner ML, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24:535–546. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- 2.Steigner ML, Otero HJ, Cai T, et al. Narrowing the phase window width in prospectively ECG-gated single heart beat 320-detector row coronary CT angiography. Int J Cardiovasc Imaging. 2009;25:85–90. doi: 10.1007/s10554-008-9347-8. [DOI] [PubMed] [Google Scholar]
- 3.Shrimpton PC, Hillier MC, Lewis MA, et al. National survey of doses from CT in the UK: 2003. Br J Radiol. 2006;79:968–980. doi: 10.1259/bjr/93277434. [DOI] [PubMed] [Google Scholar]
- 4.Tuy HK. An inversion formula for cone-beam reconstruction. Siam J Appl Math. 1983;43:546–552. [Google Scholar]
- 5.Torres FS, Crean AM, Nguyen ET, et al. Strategies for radiation-dose reduction and image-quality optimization in multidetector computed tomographic coronary angiography. Can Assoc Radiol J. 2010;61:271–279. doi: 10.1016/j.carj.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 6.McCollough CH, Primak AN, Braun N, et al. Strategies for reducing radiation dose in CT. Radiol Clin North Am. 2009;47:27–40. doi: 10.1016/j.rcl.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rybicki FJ. Lower radiation dose coronary CT angiography with new imaging technologies. Int J Cardiovasc Imaging. 2009;25:149–151. [Google Scholar]
- 8.Brenner D, Elliston C, Hall E, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 9.George R, Arbab-Zadeh A, Cerci R, et al. Circulation. Chicago: 2010. Combined non-invasive coronary angiography and myocardial perfusion imaging using 320 row detector computed tomography: CT perfusion acquisition, reconstruction, and analysis methods of the CORE-320 multi-center cohort study, AHA. [Google Scholar]
- 10.Zhang C, Zhang Z, Xu L, et al. Preliminary study of myocardial perfusion imaging using 320-row volume CT scan. RSNA; Chicago: 2010. [Google Scholar]
- 11.Kitagawa K, George RT, Arbab-Zadeh A, et al. Characterization and correction of beam-hardening artifacts during dynamic volume CT assessment of myocardial perfusion. Radiology. 2010;256:111–118. doi: 10.1148/radiol.10091399. [DOI] [PubMed] [Google Scholar]
- 12.Steigner ML, Mitsouras D, Whitmore AG, et al. Iodinated contrast opacification gradients in normal coronary arteries imaged with prospectively ECG-gated single heart beat 320-detector row computed tomography. Circ Cardiovasc Imaging. 2010;3:179–186. doi: 10.1161/CIRCIMAGING.109.854307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rybicki FJ, Melchionna S, Mitsouras D, et al. Prediction of coronary artery plaque progression and potential rupture from 320-detector row prospectively ECG-gated single heart beat CT AAngiography: lattice Boltzmann evaluation of endothelial shear stress. Int J Cardiovasc Imaging. 2009;25(Suppl 2):289–299. [Google Scholar]