Abstract
The neural networks supporting encoding of new information are thought to decline with age, although mnemonic techniques such as repetition may enhance performance in older individuals. Accumulation of amyloid-β, one hallmark pathology of Alzheimer’s disease (AD), may contribute to functional alterations in memory networks measured with functional magnetic resonance imaging (fMRI) prior to onset of cognitive impairment. We investigated the effects of age and amyloid burden on fMRI activity in the default network and hippocampus during repetitive encoding.
Older individuals, particularly those with high amyloid burden, demonstrated decreased task-induced deactivation in the posteromedial cortices during initial stimulus presentation and failed to modulate fMRI activity in response to repeated trials, whereas young subjects demonstrated a stepwise decrease in deactivation with repetition. The hippocampus demonstrated similar patterns across the groups, showing task-induced activity that decreased in response to repetition.
These findings demonstrate that age and amyloid have dissociable functional effects on specific nodes within a distributed memory network, and suggest that functional brain changes may begin far in advance of symptomatic AD.
Keywords: episodic memory, functional MRI, hippocampus, amyloid, aging
Introduction
Episodic memory is known to decline with increasing age, and the decline is characterized by a deficit in forming, retaining and recalling memory for unique events (Bäckman et al., 1999; Small, 2001). Current models of memory impairment innormal senescence have proposed that the alterations seen are associated with changes in synaptic function, rather than neuronal loss in the hippocampus and association cortices (e.g. Barnes, 1994; Morrison and Hof, 1997). Recent studies have suggested that the presence of amyloid-β (Aβ), a major histopathological finding in Alzheimer’s disease (AD), might contribute to the deleterious effects occurring in synaptic processes, leading to impaired memory consolidation and failure to form new memories (Walsh and Selkoe, 2007; Freir et al., 2010). As a substantial proportion of cognitively normal older individuals are thought to harbor occult Aβ pathology, both age and amyloid-related alterations in neural activity and/or synaptic connections between distributed networks of brain regions linked to memory function might underlie late-life memory deficits. One important challenge in the field of aging research is to understand how these neurobiological changes impact functional brain activity and how they lead to the cognitive changes that arise during normal aging. Current available neuroimaging tools now provide the opportunity to quantify amyloid deposition in vivo in conjunction with measurement of functional activity during complex cognitive processes such as episodic memory tasks.
Since the advent of functional magnetic resonance imaging (fMRI), the neural substrates of successful memory processes have been shown to consist of multiple sub-processes, requiring coordinated patterns of activity among several large-scale neural networks. There is strong consensus that the hippocampus and adjacent medial temporal lobe (MTL) structures are critical for intact episodic memory (Scoville and Milner, 1957; Squire et al., 1992; Cohen and Eichenbaum, 1993). Functional neuroimaging studies additionally suggest that activity in a set of brain regions, including temporoparietal (medial and inferior) and posterior midline cortical regions (especially posterior cingulate and retrosplenial cortex), is suppressed during successful memory encoding (e.g. Otten and Rugg, 2001; Daselaar et al., 2004; Miller et al., 2008). The consistency with which these regions have appeared in functional neuroimaging studies across multiple cognitive tasks has lead to their description as a “default mode network”, whose function has been shown to be engaged during rest and suppressed during performance of focused cognitive processing (Shulman et al., 1997; Mazoyer et al., 2001; Raichle et al., 2001; McKiernan et al., 2003; Greicius and Menon, 2004; Buckner et al., 2008). Accordingly, the deactivations relative to control conditions observed during such experiments have been interpreted to represent the reallocation of neuronal resources needed for efficient cognitive processing and successful task performance. During aging, failure to suppress activation in regions of the default network, particularly the posteromedial cortices, has been associated with failed encoding and subsequently poor memory performance (Miller et al., 2008), a finding also demonstrated in patients with early AD (Lustig et al., 2003; Celone et al., 2006; Petrella et al., 2007), and in individuals with genetic risk of AD (Fleisher et al., 2009; Pihlajamäki et al., 2009).
Those regions in the default mode network that demonstrate evidence of altered functional activity in the aging brain exhibit overlap with those brain regions most vulnerable to early amyloid deposition. Using positron emission tomography (PET) with Pittsburgh compound B (PiB), a radiotracer that binds with high affinity and specificity to neuritic Aβ plaques (Klunk et al., 2004; Engler et al., 2006), the topographical distribution of amyloid deposition has predominantly been found in posteromedial cortices (including posterior cingulate and precuneus), prefrontal and lateral temporoparietal cortex, and striatum (Klunk et al., 2004; Engler et al., 2006; Kemppainen et al., 2006; Mintun et al., 2006; Edison et al., 2007; Forsberg et al., 2008). This technique has made it possible to demonstrate the presence of amyloid pathology in living patients diagnosed with AD (Klunk et al., 2004; Price et al., 2005) confirming the central role of this pathology in the progression of AD. Moreover, there is evidence that significant amyloid deposition occurs in approximately 20–50% of asymptomatic older adults (Pike et al., 2007; Rowe et al., 2007; Gomperts et al., 2008; Mormino et al., 2009). This finding is consonant with post-mortem reports of Aβ pathology in approximately 25–40% of normal older adults (Davis et al., 1999; Price and Morris, 1999; Knopman et al., 2003; Bennett et al., 2006).
These findings suggest that pathophysiological changes begin years prior to the onset of clinical symptomatology in AD. Several recent studies provide evidence for the hypothesis that amyloid may exert deleterious effects on networks subserving memory function prior to clinical manifestation of overt memory decline (Hedden et al., 2009; Sperling et al., 2009; Sheline et al., 2010). In a study combining PiB PET with fMRI in cognitively normal older and very mildly impaired individuals, increased amyloid deposition in the posteromedial cortex was related to aberrant patterns of default network activity during successful encoding (Sperling et al., 2009). In contrast, no relationship between amyloid and hippocampal activation was observed in the normal older subjects. Two recent studies using resting functional connectivity analyses demonstrated reduced integrity of the default network related to amyloid burden (Hedden et al., 2009; Sheline et al., 2010). Taken together, these results suggest that early amyloid-associated influences on memory networks may be primarily centered in hub regions of the default mode network (Buckner et al., 2009).
Prior studies of age- and amyloid-related influences on functional activity in memory networks have focused on their impact on regional activation magnitude during novel encoding. In order to more fully understand how age and amyloid may impact the function of these networks, it is important to investigate the modulation of neural activity across memory processes. One method for manipulating memory encoding is repeated presentation of stimuli, which enhances memory performance in older adults (Hay and Jacoby, 1999; Jacoby, 1999), in some cases leading to equivalent performance between young and elderly subjects (e.g. Morrow et al., 1999). Previous studies investigating the use of repetition encoding techniques have demonstrated an attenuated neural response to subsequent stimulus presentations, a phenomenon known as “repetition suppression”(see review by Henson and Rugg, 2003), in the hippocampus and adjacent brain regions in the MTL (Gonsalves et al., 2005; Rand-Giovannetti et al., 2006). This suppression of activity, also known as “habituation”, is thought to represent evidence of successful memory encoding or the consolidation process. Repetition suppression may provide a marker of intact neural response insofar as patients with mild cognitive impairment (MCI) (Johnson et al., 2004; 2008) and AD (Golby et al., 2005; Pihlajamäki et al., 2008) fail to display such suppression. The role of habituation within the default mode network, however, remains to be elucidated. To date, only a few studies, investigating priming effects of objects and words in normal subjects, have reported findings of decreased task-induced deactivation with repetition, also known as “repetition enhancement”(Koutstaal et al., 2001; Simons et al., 2003; Orfanidou et al., 2006; Soldan et al., 2008). One recent study investigating repetitive encoding in AD patients found not only impaired repetition suppression in the MTL but also failure of deactivation in the default network (Pihlajamäki et al., 2008). This suggests that assessing hippocampal and posteromedial functioning using repetition as a mnemonic technique to modulate activation during encoding may be helpful in early identification of memory dysfunction seen in aging and AD.
The current study employed such a mnemonic technique in young and cognitively healthy older individuals to examine whether there are regionally specific neural responses within the MTL and default network to repetitive encoding. Based on prior findings of functional alterations in the default network and specifically within the posteromedial cortex among cognitively normal older individuals with occult amyloid pathology, we hypothesized that the greatest amyloid-related differences would be seen in these cortical default network regions rather than in the hippocampus. Consistent with the hypothesis that the default network might exhibit “beneficial deactivation” during successful memory formation (e.g. Daselaar et al., 2004), we predicted that age and amyloid would be related to alterations in the pattern of deactivation within the posteromedial cortex across repeated encoding presentations.
To investigate these hypotheses, we used an adapted version of a face-name associative memory paradigm (Sperling et al., 2003). This task was chosen because difficulty remembering proper names is the most common complaint of older individuals visiting memory clinics (Zelinski and Gilewski, 1988) and the task is dependent on the integrity of the hippocampus as well as the default mode network for successful memory formation (e.g. Sperling et al., 2003; Zeineh et al., 2003; Rand-Giovannetti et al., 2006; Miller et al., 2008; Persson et al., 2010; Vannini et al., 2010). In the current study, we used an event-related paradigm and presented each stimulus in three separate encoding trials to examine repetition-related influences on neural activation for those stimuli that were subsequently correctly remembered.
Materials and Methods
Participants
The study included sixty-seven subjects; 26 young (aged 20–29) and 41 cognitively normal elderly (aged 55–88) individuals. One of the elderly subjects was excluded from the analysis based on an insufficient number of correct responses. Demographic characteristics for the remaining subjects are shown in Table 1. Young subjects were recruited from advertisements posted in the hospital and on the Internet. The data from young subjects comparing initial encoding trials to retrieval activity has been previously published (Vannini et al., 2010). Older subjects were recruited from ongoing longitudinal studies of cognition and aging at Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH). The data from the elderly subjects has not appeared in any other publication. All subjects were right-handed, native English speakers and had normal or corrected-to-normal vision. No subjects had a history of psychiatric or neurological disorders, or were taking medications that significantly affected the central nervous system. All older subjects had a Mini-Mental State Examination (Folstein et al., 1975) score of 28 or above and a Clinical Dementia Rating (CDR) score of 0 (based on a detailed interview with the subject and with a study partner who has daily contact with the subject). All subjects performed within 1.5 SD of age-adjusted norms on all neuropsychological measures. This study was approved by and conducted under the auspices of the Partners Human Research Committee at the BWH and MGH (Boston, MA). Informed written consent was obtained from every subject prior to any experimental procedures.
Table 1.
Demographics and behavioral results
| Young | PiB − | PiB + | |
|---|---|---|---|
| N | 26 | 32 | 8 |
| Gender (F/M) | 13/13 | 23/9 | 4/4 |
| Age (mean years) | 23.3 ± 2.5 | 71.8 ± 1.5 | 80.4 ± 1.7 |
| Education (mean years)* | 15.6 ± 0.2 | 15.9± 0.5 | 15.1 ± 1.0 |
| MMSE (mean score)* | NA | 29.3 ± 0.1 | 29.0 ± 0.3 |
| AMNART (mean score)* | NA | 124.7 ± 1.0 | 120.5 ± 3.1 |
| BNT (mean score)* | NA | 48.6 ± 2.6 | 48.0 ± 5.5 |
| BVFDT (mean score)* | NA | 25.6 ± 1.3 | 26.0 ± 3.7 |
| Face name task accuracy* | |||
| (% Remembered HIT) | 76.5 ± 3.5 | 66.3 ± 2.5 | 53.4 ±7.1 |
| (% Forgotten HIT) | 17.9 ±2.9 | 18.3 ± 2.2 | 27.7 ± 6.6 |
| (% Remember MISS) | 2.6 ± 0.7 | 11.5 ± 1.1 | 8.6 ± 2.2 |
| (% Forgotten MISS) | 2.9 ± 1.0 | 3.8 ±0.8 | 10.3 ± 3.1 |
Values are listed as mean and standard error. Bold text indicates statistical differences between the groups (see text for details). MMSE = Mini Mental State Examination; AMNART = American National Adult Reading Test; BNT = Boston Naming Test; BVFDT = Benton Visual Form Discrimination Test. Missing data indicated by *: 3 PiB neg and 1 Yng for Education and Benton Visual Form Discrimination test; 2 PiB neg for MMSE, AMNART and Boston Naming test; 1 PiB neg for Accuracy and Boston Naming Test.
Face-name experimental task
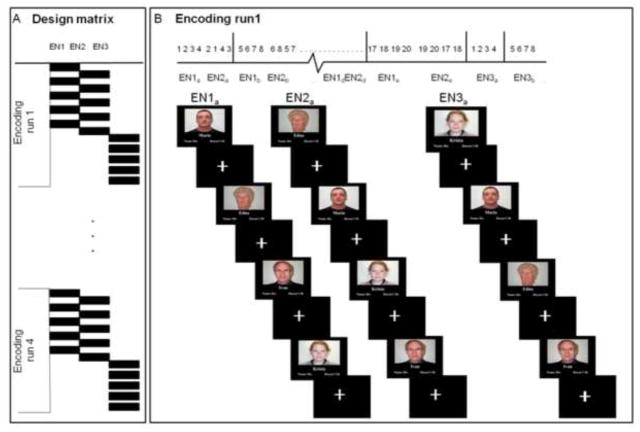
The fMRI experiment consisted of a face-name association encoding and retrieval task as previously described (Vannini et al., 2010). During the task, color photographs of faces were shown against a black background with a fictional first name printed (Times New Roman 36 point font) in white underneath. Subjects were explicitly told to try to remember the name associated with the face. The experiment consisted of 4 encoding runs alternating with 4 retrieval runs. In each encoding run, subjects viewed 20 face-name pairs, each shown for 2.75 seconds (s), presented in pseudorandom order in groups of 4 face-name pairs and interspersed with visual fixation (a white crosshair centred on a black background) periods (see Figure 1B for an example). During fixation, subjects were told to focus their attention on the crosshair. Within a given encoding run, each of the 20 face-name pairs was presented a total of 3 times (see Figure 1A for additional details on the design matrix). To enhance memory encoding, we presented each stimulus twice near the beginning of the run with a mean delay of 15 s between the first and second presentation. At the end of each run, all stimuli were repeated a third time with a mean delay of 2 min from the second presentation. The rationale for this design was to boost encoding success in a manner similar to what might be done in a clinical setting. In total, 80 face-name pairs were presented to the subjects over the 4 encoding runs. To provide a deep encoding task that enhances later memory, subjects were asked to press a button indicating whether the name was a good “fit” for the face or not (Sperling et al., 2003).
Figure 1. fMRI face-name association task.
(A) The design matrix consisted of 4 encoding runs, and in each run 20 face-name pairs were presented to the subjects 3 times (EN1, EN2, EN3). (B) The experimental set up of the first encoding run (EN1a) displays the first group of 4 face-name pairs (five groups of 4 face-name pairs comprised the total of 20 stimuli for each encoding run) and how the stimuli were repeated over the run (EN2 a, EN3a), each time presented in a pseudorandomised order within the group (see numbers on the top of the figure). For each face-name pair, the subjects were asked to press a button indicating a purely subjective decision about whether the name was a good “fit” for the face or not.
To ensure that subjects were attentive and understood the instructions, detailed oral instructions were recited prior to each run. All subjects also completed a practice session both outside and inside of the MR-scanner before the fMRI data were collected.
After each encoding run, a retrieval run was performed consisting of cued-recall followed by forced-choice recognition for each face-name pair learned during the preceding encoding run. In the cued-recall phase, a face was presented for 5.25 s and the subject responded with a button press to indicate whether he or she remembered or forgot the name associated with the face. Subjects were instructed to respond remember only for those faces for which they could explicitly bring to mind the name before seeing the names in the forced-choice recognition phase. During the forced-choice recognition phase, each face was shown (for 3.25s) with two names printed underneath: the correct name that was paired with the face during encoding, and an incorrect name that was previously paired with a different face during encoding. Subjects were instructed to indicate the correct name with a button press. In order to investigate successful encoding, we examined the neural response only for stimuli that the subject indicated were remembered and were also correct on the forced-choice recognition.
The paradigm was designed and generated on an external personal computer using MacStim 2.5 software (WhiteAnt Occasional Publishing, West Melbourne, USA) and projected by means of MR compatible goggle-system (VisuaStim XGA, Resonance Technology Inc., Los Angeles, CA, USA). Subjects responded with an MRI compatible fiber-optical key press device with two buttons held in their right hand and responses (accuracy and reaction time) were recorded by a computer interfaced with the optical switch using MacStim software outside the scanner room.
MR Imaging acquisition
All subjects were scanned using a General Electric 3.0 Tesla MR system (GE Signa), equipped with an eight channel head coil. High-resolution T1-weighted structural images were acquired using a 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MP-RAGE) sequence: 166 sagittal slices, repetition time (TR) = 6.4 msec, echo time (TE) = 2.8 msec, inversion time (TI) = 900 msec, flip angle (FA) = 8°, field of view (FOV) = 260 mm, matrix 256 × 256.
Functional MRI data were acquired using a T2*-weighted gradient-echo EPI sequence sensitive to blood-oxygenation-level-dependent (BOLD) signal [repetition time (TR), 2000 ms; time of echo (TE), 30 ms; flip angle, 90° within a field of view of 220 cm, with a 64 × 64 pixel matrix and a slice thickness of 5 mm (interslice distance, 1 mm)]. Thirty oblique coronal slices, perpendicular to the anterior-posterior commissural (AC-PC) line, were acquired to cover the whole brain. Eight functional runs were acquired for each subject with 145 time points per run. Scanning time for each functional run was 5 min resulting in a total functional scanning time of approximately 40 min. The first five (additional) images in each run were discarded to allow the magnetization to reach equilibrium.
fMRI preprocessing
Functional MRI data were preprocessed on a Linux platform running MATLAB version 7.1 (The Mathworks, Inc., Sherborn, MA, USA) with Statistical Parametric Mapping (SPM 2, Wellcome Department of Cognitive Neurology, London, UK: http://www.fil.ion.ucl.ac.uk/spm2.html). Functional data were motion corrected using sinc interpolation, by aligning (within-subject) each time series to the first image volume using least-square minimization of a six-parameter (rigid-body) spatial transformation. No subject exceeded head movement over 3 mm (in the z axis translation). The data were normalized to the standard SPM2 EPI template and resliced into 3 × 3 × 3-mm3 resolution in Montreal Neurological Institute (MNI) space. Smoothing was accomplished using an isotropic Gaussian kernel of 8 mm full-width half-maximum (FWHM). No scaling was implemented for global effects. The coordinates were later converted to Talairach and Tournoux’s space (Talairach and Tournoux, 1988) using software available online http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach.
fMRI statistical analysis
The face-name stimuli were categorized based on the subjects’ responses during the cued recall (remember vs. forgotten) and forced choice recognition (hit vs. miss) tasks, allowing four possible conditions: remembered hit (RHIT), forgotten hit (FHIT), remembered miss (RMISS), and forgotten miss (FMISS). Given our goal to investigate successful encoding, the current paper focuses on the results using the RHIT variable. For each subject, all runs were concatenated and regressors added, in lieu of global scaling, to account for signal differences between runs.
To identify the regions involved during successful encoding and to confirm that both groups were activating the hippocampus to novel associative stimuli, second level t-statistics (using random effects in SPM2) were separately computed for the young and older subjects, using contrasts created for each individual comparing fMRI activity during the first encoding trial of face-name pairs that were subsequently remembered correctly to a control condition (fixation cross) (RHITenc > Fix). To investigate regions of deactivation during successful encoding and to confirm that both groups were deactivating regions in the default network to novel associative stimuli, the reverse contrast was used (Fix > RHITenc). The resulting whole brain statistical maps were thresholded at p≤0.05 corrected for multiple comparisons using false discovery rate (FDR) and a requirement of 5 contiguous voxels (resampled to 3 × 3 × 3 mm3).
PiB PET acquisition
All older subjects underwent a PiB PET examination at MGH. 11C-PiB was prepared as described by Mathis et al. (2002) and PiB data were acquired at MGH, as previously described (Johnson et al., 2007; Gomperts et al., 2008; Rentz et al., 2010). Following a transmission scan, 10–15 mCi 11C- PiB was injected as a bolus and followed immediately by a 60 min dynamic acquisition. PiB PET data were reconstructed with ordered set expectation maximization, corrected for attenuation, and each frame was evaluated to verify adequate count statistics and absence of head motion. The Logan graphical analysis method, with cerebellar cortex as the reference tissue input function, was used to evaluate specific PiB retention expressed as the distribution volume ratio (DVR) (Price et al., 2005).
PiB PET statistical analysis
In this study, we specifically chose to utilize the individually anatomically-defined regional DVR calculated from the precuneus region, as this region has been shown to exhibit the most consistent elevation of PiB retention in non-demented older subjects (Mintun et al., 2006; Gomperts et al., 2008). The precuneus region was determined from each individual’s high-resolution MP-RAGE image with FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/), using a semi-automated parcellation method based on a probabilistic map (Fischl et al., 2004). Regional PiB DVR was calculated within this region from each individual’s co-registered PET data. The older subjects were classified based on their PiB status, derived from the partial volume corrected PiB DVR values from the precuneus region. Individuals whose DVR values fell within the range of a previous sample of AD subjects (DVR > 1.6; Sperling et al., 2009) were classified as PiB + and individuals that fell below that range was classified as PiB −. The precuneus was selected because of the early and prominent deposition of amyloid in this region, as well as the region’s critical role in memory function (Buckner et al., 2005; Mintun et al., 2006; Sperling et al., 2009; Rentz et al., 2010; Vannini et al.,2010).
Cross-modality statistical analyses
All statistical analyses were calculated using STATISTICA 8 (StatSoft Inc. Tulsa, OK, USA) software. We first performed a whole brain analysis to investigate which areas were activated and deactivated in each group during the first encoding trial (see details above). This analysis was conducted to confirm that the novel encoding task was activating and deactivating the hypothesized areas of interest in both age groups, thus replicating previous studies (e.g. Sperling et al., 2001; 2003; 2009; Rand-Giovannetti et al., 2006; Miller et al., 2008; Pihlajamäki et al., 2008; 2009; Vannini et al., 2010). To further investigate the modulation of functional responses during successful encoding in subsequent trials we performed region of interest (ROI) analyses for the posteromedial cortex (cingulate isthmus) and the hippocampus. Using the FreeSurfer parcellation for each individual, significant voxels (defined from the contrast RHITenc1 > Fix for activation and Fix > RHITenc1 for deactivation) exceeding an uncorrected threshold of p<.05 and residing in the individually-defined anatomical region of the cingulate isthmus and the hippocampus were selected as ROIs. The mean beta weights for each of the three encoding trials were extracted. For some individuals, no above-threshold voxels were present in one of the ROIs, although all subjects demonstrated activation in a subset of the ROIs (the number of subjects included in each analysis is indicated by the degrees of freedom). To examine age-related effects on functional activation during repetitive encoding, we first performed a repeated measure ANOVA with encoding trials as the repeated measure and age-group as the between-subject variable. Post-hoc planned comparisons of least squares means were performed to look at pair-wise differences between specific trials (i.e. trial 1 versus 2, trial 2 versus 3, and trial 1 versus 3) within each group separately and to look at pair-wise differences for each encoding trial (i.e. trial 1, 2, and 3 separately) between the groups when a significant interaction effect was observed between age-group and encoding trials. Within these analyses, we also investigated the linear trends across the 3 trials.
In the old group, age and amyloid were significantly correlated (r=0.54, p<0.001) and hence could confound our ability to examine the independent effects of amyloid. To explore the relationship between amyloid deposition in our cognitively normal elderly individuals and functional activation during repetitive encoding, we therefore performed two analyses: i) a repeated measures ANOVA with encoding trials as the repeated measure and PiB-group as the between-subject variable and ii) a mixed effects model using encoding trials as the repeated measure and PiB-group as the between-subject variable and age as a covariate. Post-hoc planned comparisons of least squares means were performed to look at pair-wise differences between specific trials (i.e. trial 1 versus 2, trial 2 versus 3, and trial 1 versus 3) within each group separately and to look at pair-wise differences for each encoding trial (i.e. trial 1, 2, and 3 separately) between the groups when significant interactions were observed between PiB-group and encoding trials. Within these analyses, we also investigated the linear trends across the 3 trials.
A subsequent analysis was performed to investigate the difference in modulation of functional responses over encoding trials in more detail. First, the mean change in beta weight (Δ beta weight) from the posteromedial cortex and the hippocampus between the first and last encoding trial was calculated for each subject group (RHITenc3 – RHITen1). These data were entered into a one way ANOVA with subject-group (either age or PiB-group) as a between-subjects factor. Last, to further explore the relationship between amyloid and differences in modulation of functional response (as defined above), a partial correlation analysis using age as a covariate was calculated for the older subjects.
Results
Demographic data
Table 1 summarizes the demographic and neuropsychological data for the different subject groups. As can be seen, no significant difference was found for education, MMSE or general IQ (as estimated with the American National Adult Reading Test (AMNART); Grober and Sliwinski, 1991). Also, there were no significant differences in naming on the Boston Naming Test (Kaplan et al., 1983) or visuospatial processing on the Benton Visual Form Discrimination Test (Dee and Benton, 1970), two cognitive processes presumably important for successful completion on the face-name associative memory paradigm. The PiB + individuals were significantly older then the PiB − individuals (t(38) = −2.7, p=0.01). For this reason, all subsequent analyses within the older adult groups included an age covariate.
Task performance
Behavioral results from the fMRI experiment are presented in Table 1. There were a high percentage of correctly remembered face-name stimuli (RHIT) during the task (chance level for RHITs is contingent on the proportion of “remember” responses, and averaged 39.6% for young, 38.9% for PiB −, and 31.0% for PiB +), indicating that the subjects successfully encoded the face-name pairs and suggesting a high attentional engagement during both tasks (Table 1). The percentage of successful hits differed between the young and the PiB + individuals (t(32) = −3.1, p=0.004), but not between the PiB − and young subjects nor between the PiB − and PiB + groups. No significant differences were found between the groups for the percentage of forgotten hits (FHIT). The percentage of remembered misses (RMISS) differed between young and the PiB + individuals (t(32) = 3.7, p<.001) and PiB − individuals (t(55) = 4.0, p<.001), but not between the PiB − and PiB + groups. This suggests that older participants had an increased likelihood of incorrect recognition compared to young despite indicating that they remembered the name. In addition, the percentage of forgotten misses (FMISS) differed between the PiB + individuals and the young subjects (t(32) = 2.9, p=.007) and PiB − individuals (t(37) =−2.7, p=.001), but not between the young and the PiB − individuals. There was a trend toward PiB + subjects responding remember less often (62.0%) than PiB − (77.8%) subjects (t(37) = 1.8, p=.09) or young (77.9%) subjects (t(32) = 2.0, p=.053). Conditional on responding remember (RHIT/(RHIT+RMISS)), recognition was equivalent between PiB − (86.0%), and PiB + (86.7%) subjects, with all older adults having lower recognition than young (96.8%) subjects (t(63) = −4.5, p<.001).
PiB PET amyloid deposition
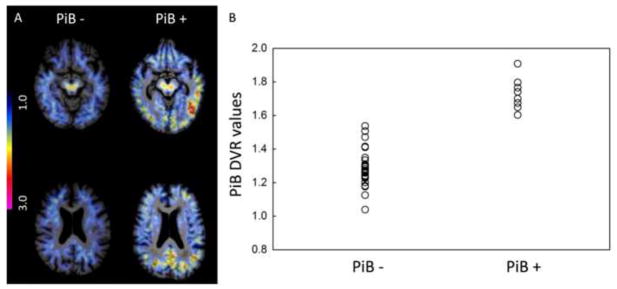
Cortical PiB uptake demonstrated a varied pattern across the older individuals. Figure 2A displays two representative PiB DVR maps, using the cerebellum as reference tissue: an older individual with nonspecific PiB retention (low amyloid burden) and an older individual with significant cortical PiB retention, especially in the posteromedial cortex. Similar to previous PiB PET studies (e.g. Pike et al., 2007; Rowe et al., 2007; Mormino et al., 2009; Sperling et al., 2009; Rentz et al., 2010), we noted that the PiB retention in this sample demonstrated a continuum with values ranging from what could be referred as PiB − to PiB + (Figure 2B) with a small number (n = 8) of subjects demonstrating PiB retention in the range of that detected in AD patients (Sperling et al., 2009). This pattern is in accordance with the hypothesis that amyloid accumulation is a continuous process. There may be a critical threshold of deposition associated with functional and structural imaging abnormalities, similar to those seen in clinical AD, that may increase the likelihood of progression towards clinical symptomatology. In addition to the between-group analysis comparing PiB − and PiB + groups, we also examined accumulation of amyloid as a continuous variable to investigate the impact of fibrillar amyloid burden on the functional response to repetitive encoding.
Figure 2. PiB PET distribution volume ratios.
(A) Distribution volume ratio (DVR) maps of 11-C PiB activity in two representative subjects from the study. Color scale from blue = low (1.0 DVR) to pink = very high (3.0 DVR) levels of PiB retention. (B) Scatter plot of individual DVR values from a cortical region of interest in the precuneus. The cut-off value used to define the groups was set at DVR = 1.6.
Whole brain analyses during initial encoding
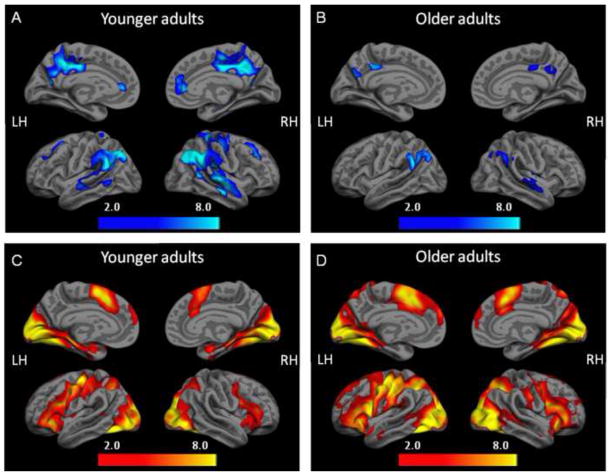
We first examined whole brain analyses for young and old groups separately, comparing event-related fMRI deactivation during fixation to the first encoding trial of face-name pairs that were subsequently correctly remembered (Fix > RHITenc1). Both young and older groups demonstrated significant deactivation in regions associated with the default network (pFDR ≤0.05, 5 voxel extent). Results are shown in Figure 3A and 3B. Significant task-induced deactivation was primarily found in the posteromedial, lateral temporoparietal, and medial prefrontal cortices. As in previous studies, we observed decreased default network activity in the older adults relative to the younger adults, which was most evident in the posteromedial cortex (see ROI results below).
Figure 3. fMRI deactivation and activation patterns during successful repetition encoding.
During the first encoding trial of face-name pairs that were subsequently remembered correctly, significant deactivation in the posteromedial cortex was observed in both (A) young and (B) old groups (SPM2, one-sample t-test using Fix > RHITenc1, p<0.05(FDR corrected), 5 voxels extent threshold) and significant activation in the hippocampus was observed in both (C) young and (D) old groups (SPM2, one-sample t-test using RHITenc1 > Fix, p<0.05(FDR corrected), 5 voxels extent threshold).
Next, we examined whole brain analyses for young and old groups separately, comparing event-related fMRI activation during the first encoding trial of face-name pairs that were subsequently correctly remembered to fixation (RHITenc1 > Fix). Both young and older groups demonstrated significant activation in medial temporal regions, visual cortex, and medial and lateral prefrontal cortex (pFDR≤0.05, 5 voxel extent). Results are shown in Figures 3C and D.
Region of Interest analysis
Repeated encoding in the posteromedial cortex: age-effects
We then performed a repeated measure ANOVA in all the subjects (using encoding trials as the repeated measure and age-group as the between-subject variable) to examine age-related effects on the functional activation during repetitive encoding (see Supplemental Table 1). A significant main effect of age was observed in the right posteromedial cortex (F(1,57) = 4.2, p=0.04) which did not reach a significant threshold in the left hemisphere (p=0.1). There was a significant main effect of encoding trial in both hemispheres (left: F(2, 122) = 47.2, p<0.001; right: F(2, 114) = 61.7, p<0.001) and a significant interaction effect between age-group and encoding trial (left: F(2, 122) = 8.7, p<0.001; right: F(2, 114) = 19.3, p<0.001). The linear contrast for the age-group by encoding trial interaction was significant in both hemispheres (left: F(1, 61) = 16.5, p<0.001; right: F(1, 57) = 31.2, p<0.001), indicating that the older adults had less of a step-wise change in deactivation with repeated encoding than did the younger adults. Planned comparisons revealed that, for the young adults, each stimulus repetition resulted in a significant change in the deactivation response in posteromedial cortex (see Supplemental Table 2 for mean change of beta-weight across encoding trials and detailed statistics). By the third encoding presentation, activity in young subjects was at or above the fixation level. For older adults, a significant stepwise change in deactivation was observed between the first and second encoding trials and between the first and the third encoding trials. However, there was no significant change in deactivation between the second and third encoding trial, indicating that older individuals did not further modulate activation in this region during the third encoding trial (see Supplemental Table 2). Planned comparisons between the age-groups for each encoding trial revealed that older individuals showed less deactivation than did young subjects during the first encoding trial (in the right hemisphere only), and more deactivation during the third encoding trial (in both hemispheres, see Supplemental Table 2). We note that age differences in the level of deactivation on a given encoding trial could be due in part to differences in the implicit baseline exhibited by each group (due to vascular differences, etc.). Of importance, the observed age differences in the degree of change in the deactivation response from the first to the third encoding trial, as evidenced by the above interaction in the linear contrast, cannot be accounted for by such baseline differences.
Repeated encoding in the posteromedial cortex: amyloid-effects
To examine amyloid-related effects on the functional activation during repetitive encoding, we next performed a repeated measure ANOVA for only the old subjects (using encoding trials as the repeated measure and PiB-group as the between-subject variable). The main effect of PiB-group was not significant in either hemisphere (left: p=0.28; right: p=0.18). The main effect of encoding trial was significant in both hemispheres (left: F(2, 70) = 5.0, p<0.01; right: F(2,64) = 8.0, p<0.001), as was the interaction effect between PiB-group and encoding trial (left: F(2,70) = 4.4, p=0.02; right: F(2, 64) = 3.9, p=0.03). The linear contrast for the PiB-group by encoding trial interaction was significant in both hemispheres (left: F(1, 61) = 4.9, p=0.01; right: F(1, 32) = 6.2, p=0.02), indicating that the PiB + individuals had less of a step-wise change in deactivation with repeated encoding than did the PiB − individuals. Planned comparisons within each PiB group revealed that with repeated presentation of stimuli, PiB − individuals displayed a restricted dynamic range of default activity although they followed the general pattern of a stepwise decrease of deactivation seen in the young subjects (Figure 4A). In contrast, the PiB + individuals showed no significant modulation from the first to the third repetition. Note that the PiB + individuals showed a change in deactivation during the second encoding trial but this change was reversed by the third encoding trial – which occurred after a two minute delay (see Supplemental Table 2 for mean change of beta-weight across encoding trials and detailed statistics). Planned comparisons between the PiB-groups for each encoding trial revealed that cognitively normal individuals with high amyloid burden (PiB +) displayed lower deactivation during the first encoding trial (left: F(2, 35) = 6.6, p=0.01; right: F(2, 32) = 4.7, p=0.04) and during the second encoding trial (left: F(2, 35) = 3.2, p=0.08; right: F(2, 32) = 5.2, p=0.03) compared to PiB − individuals. However, no difference was observed between the groups during the third encoding trial. Note that these differences in the initial level of deactivation could be related to differences in the implicit baseline, particularly to the extent that amyloid impacts vascular function (Johnson et al., 2007). However, the observed change in the deactivation response from the first to the third encoding trial associated with amyloid cannot be attributed to such baseline differences.
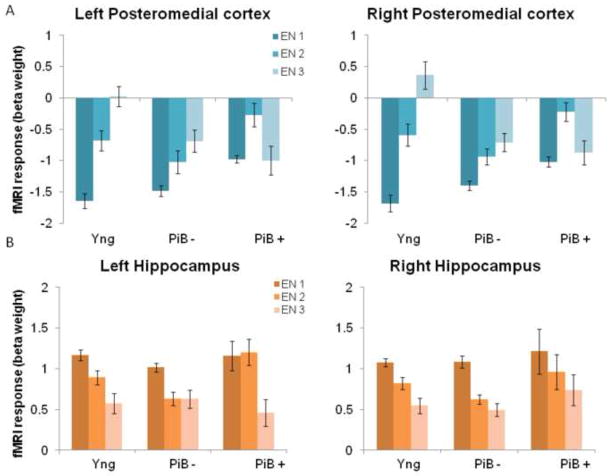
Figure 4. Modulation of response during successful repetition encoding.
(A) Bar graph of the functional response (mean beta weights) for young, individuals with low amyloid burden (PiB −) and individuals with high amyloid burden (PiB +) during the three encoding trials (EN1, EN2, EN3) in left and right posteromedial cortex. See main text for statistically significant differences between the groups. (B) Bar graph of the functional response (mean beta weights) for young, individuals with low amyloid burden (PiB −) and individuals with high amyloid burden (PiB +) during the three encoding trials (EN1, EN2, EN3) in left and right hippocampus. See main text for statistically significant differences between the groups.
Because age and amyloid accumulation were correlated (r = .54), we also performed a subsequent mixed effects model in the older subjects (using encoding trials as the repeated measure and PiB-group as the between-subject variable and controlling for age). This analysis revealed similar results as presented above (see Supplemental Table 1 and 2 for detailed statistics). The main differences when controlling for age was that we now detected a trend-level main effect of encoding trial in each hemisphere (left, p=0.7; right, p=0.77). The interaction-effect between group and encoding trial in the right hemisphere also was reduced to trend-level (p=0.07).
Exploratory Region-of-Interest analysis to investigate the specificity of encoding processes
To investigate if these effects are specific to successful encoding, we performed an additional analysis looking at the “forgotten HITS” (FHITs). That is, stimuli that were in fact correctly recognized but were not subjectively recalled in the cued recall task. Due to the small number of FHITs on average, and high variability among subjects in the percentage of FHITs (10 older subjects and 7 younger subjects had less than 5% FHITs), such analyses have limited power to detect effects with the FHIT stimuli. We found no significant main effects of either age or amyloid, and no significant interaction effects were detected in these analyses, suggesting that the effects presented in the current paper may be specific to successful encoding.
Repeated encoding in the hippocampus: age-effects
Repetition-related changes in fMRI signal in a ROI in the hippocampus was examined by extracting the beta weights from each encoding trial and each individual and a repeated measure ANOVA (using encoding trials as the repeated measure and age-group as the between-subject variable) was performed to examine age-related effects on the functional activation during repetitive encoding. This analysis revealed a similar pattern of repetition suppression across the young and older groups. That is, with repeated presentations, there was no main effect of age ((p=.35) in left or (p=0.47) right hemisphere) and no interaction between age and encoding trial ((p=0.35) in left or (p=0.62) right hemisphere). There was a significant main effect of encoding trial in both hemispheres (left: F(2, 120) = 27.5, p<0.001; right: F(2, 120) =36.6, p<0.001). The linear contrast for the main effect of encoding trial was significant in both hemispheres (left: F(1, 60) = 49.4, p<0.001; right: F(1, 60) = 67.6, p<0.001), indicating that both groups demonstrated a stepwise decrease of activation levels in the hippocampus across repeated presentations (see Supplemental Table 2 for mean change of beta-weight across pairs of trials and detailed statistics).
Repeated encoding in the hippocampus: amyloid-effects
To examine amyloid-related effects on the functional activation during repetitive encoding we performed repeated measures ANOVA (using encoding trials as the repeated measure and PiB-group as the between-subject variable). A significant main effect of PiB-group was observed in the right (F(1, 35) = 5.6, p=0.02, but this effect did not reach a significant threshold in the left hemisphere (p=0.3). There was a significant main effect of encoding trial in both hemispheres (left: F(2, 70) = 11.6, p<0.001; right: F(2, 70) = 11.4, p<0.001), and a significant interaction effect between PiB-group and encoding trial in the left hemisphere (F(2, 70) = 5.2, p<0.001), but not in the right hemisphere (p=0.83). However, the linear contrast for the PiB-group by encoding trial interaction was not significant in either hemisphere (left: p=0.21; right: p=0.76). Planned comparisons revealed a similar pattern of repetition suppression across the groups. That is, with repeated presentation of stimuli, both groups demonstrated a similar pattern of modulation across the trials and hence a similar repetition suppression (see Supplemental Table 2 for mean change of beta-weight across encoding trials and detailed statistics). The only significant pairwise difference observed was during the second encoding trial (left: F(2, 34) = 8.5, p=0.006 and right: F(2, 34) = 4.3, p=0.045) between the PiB − and the PiB + older subjects in the left hippocampus (see Figure 4B), suggesting that individuals with higher amyloid burden had sustained activation during the second encoding trial.
We also performed a subsequent mixed effects model in the older subjects (using encoding trials as the repeated measure and PiB-group as the between-subject variable and controlling for age in the older subjects). This analysis revealed similar results as presented above. The main differences when controlling for age was that we now detected a non significant main effect of encoding trial in each hemisphere (left, p=0.98; right, p=0.29; see Supplemental Table 1 and 2 for detailed statistics).
Regional amyloid deposition is associated with failure of repetition enhancement in the posteromedial cortex, but not repetition suppression in the hippocampus
To examine age-and amyloid related changes in repetition enhancement in the posteromedial cortex and repetition suppression in the hippocampus (calculated as the beta weight extracted from the last encoding trial minus that from the first encoding trial, (RHITenc3 – RHITen1)) in more detail, we performed a between-group analysis for i) age-group and ii) PiB-group. These analyses revealed a significant age-effect in the posteromedial cortex (left: F(1, 61) = 16.5, p<0.001; right: F(1, 57) = 31.2, p<0.001), in that older individuals demonstrated a lower level of repetition enhancement between the first and last encoding trial compared to young subjects. In addition, in the old group only, individuals with high amyloid deposition demonstrated sustained deactivation over encoding trials in the posteromedial cortex, that significantly differed from the PiB − group in the left (F(1, 35) = 5.4, p=0.03) hemisphere, but reached only trend-level significance in the right (F(1, 32) = 2.7, p=0.1) hemisphere (Figure 5, left). In the older individuals, when amyloid burden was treated as a continuous measure and co-varying for age, a significant negative correlation was found in the left (r=−0.39, p=0.019) and right (r=−0.38, p=0.031) posteromedial cortex between repetition enhancement and amyloid burden in the precuneus (Figure 5, right).
Figure 5. Regional amyloid deposition is associated with failure of normal habituation in the default network.
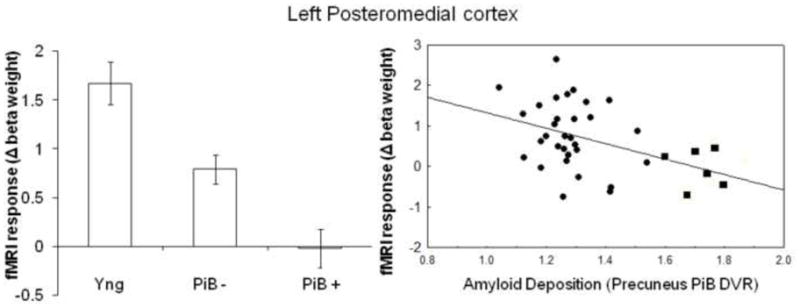
Mean modulation of functional response over encoding trials, calculated as the change in beta weight between the last and first encoding trial in young, individuals with low amyloid burden (PiB −) and individuals with high amyloid burden (PiB +) in left posteromedial cortex. Correlation between the change in the mean beta weight between the first and the last encoding trial and amyloid deposition in precuneus (corrected for age); r=-.39, p=0.019. Individuals with low amyloid burden (circles) and high amyloid burden (squares).
In contrast, no significant age or amyloid related difference was found in either left or right hippocampus for the pattern of repetition suppression between the young and the old (left; p=0.36, and right; p=0.97) or the PiB− and PiB + (left; p=0.23, and right; p=0.73). No relationship was found between amyloid burden in the precuneus and hippocampal repetition suppression response.
Discussion
The results from the current study indicate that repetition-based mnemonic techniques in healthy older subjects generate differential functional responses among the networks subserving memory encoding. In particular, we found that the hippocampus was engaged similarly in young and elderly subjects, whereas posteromedial cortex responses to repetitive encoding differed as a function of age and amyloid burden. Both young and older groups showed evidence of deactivation in the posteromedial cortex during the first encoding trial. However, the older group, in particular those with high amyloid burden, demonstrated less deactivation during the first encoding trial and a tendency to sustain this deactivation across repeated encoding presentations. These results, in combination with previous fMRI studies suggest that age-related differences in successful memory formation are associated with alterations in the posteromedial cortex and that its connectivity to other regions within the default network and the MTL is disrupted by amyloid pathology (Miller et al., 2008; Hedden et al., 2009; Sperling et al., 2009; Sheline et al., 2010). Potential support for the hypothesis that our findings may be specific to successful encoding processes comes from the analysis of forgotten HITs, which did not reveal any significant main effects of either age or amyloid, nor any interaction effects. It must be noted, however, that these analyses likely lacked sufficient power to detect effects. Our findings serve to elucidate the neural underpinnings of memory dysfunction seen in aging and neurodegenerative disease and provide evidence in support of the hypothesis that amyloid pathology, in cognitively intact elderly individuals, is related to disrupted synaptic activity in the networks supporting memory function.
During the initial encoding of face-name associations that were subsequently correctly remembered, a similar pattern of bilateral activation in the hippocampus was observed in young and older subjects. This finding is consistent with previous studies in young (Sperling et al., 2003; Chua et al., 2007) and healthy older subjects (Grön et al., 2003; Rand-Giovannetti et al., 2006; Duverne et al., 2009). Several studies have reported decreased hippocampal activation in older as compared to young subjects (see Hedden and Gabrieli, 2004 for a review). However, the results from these latter studies suggest that the observed age-related decreases in hippocampal activation may be due to decrements in memory performance in the elderly subjects. Because we focused on correctly remembered associations, we may not have been able to observe effects in the hippocampus related to performance. Although no significant differences in performance were detected between the two older groups, we observed a significant difference between the young subjects and the individuals with high amyloid burden. Despite this difference in performance, no significant change in hippocampal activity was detected. These results support prior findings that intact MTL repetition suppression is related to successful encoding processes (Gonsalves et al., 2005; Rand-Giovannetti et al., 2006). In contrast, failure to reduce activation in the MTL memory system to repeated stimuli has been observed in MCI and AD patients (Johnson et al., 2004; Golby et al., 2005; Johnson et al., 2008; Pihlajamäki et al., 2008). We observed that individuals with high amyloid burden demonstrated sustained activation during the second encoding trial in the left hippocampus, but decreased to a similar level as the other groups by the third encoding trial. Finally, we found similar repetition suppression across all groups, suggesting that alterations in the MTL system may be relatively minimal during normal aging (Van Petten, 2004). While our findings are in accordance with previous studies demonstrating similar repetition suppression effects in the MTL system, the role of regions demonstrating deactivation during encoding may be of particular interest.
In contrast to the activation pattern seen in the hippocampus, we observed significant task-induced deactivation during initial encoding for both age groups in regions of the default network, particularly the posteromedial cortex. In this region, the older group and especially those with high amyloid burden demonstrated less deactivation during the first encoding trial compared to the young subjects. The finding of age-related reduction of default network deactivation supports previous observations of this effect during successful encoding (Miller et al., 2008; Duverne et al., 2009), during memory tasks in AD patients (Lustig et al., 2003) and individuals with risk factors for AD (Celone et al., 2006; Petrella et al., 2007; Persson et al., 2008; Pihlajamäki et al., 2008; Fleisher et al., 2009; Pihlajamäki et al., 2009). The finding that cognitively normal older individuals with high amyloid burden demonstrated the most aberrant changes in the default network replicates recent findings and further supports the hypothesis that amyloid deposition may be responsible for dysfunction of brain networks supporting memory in individuals who may be in early (prodromal) stages of AD (Sperling et al., 2009). Further support for this idea comes from two recent studies demonstrating that increased amyloid, including the posteromedial cortex, is related to functional disruption of the default network as measured by intrinsic activity correlations (Hedden et al., 2009; Sheline et al., 2010). The present study adds to these previous results by demonstrating alteration in this network during repetitive encoding as a function of increasing amyloid burden.
The mechanistic underpinning of this finding remains to be elucidated. Recent laboratory studies have demonstrated evidence of hyperactivity and increased calcium influx in neurons surrounding amyloid plaques (Busche et al., 2008; Palop and Mucke, 2010). Thus, our observation of sustained deactivation during repetitive encoding might be indicative of amyloid toxicity in the posteromedial cortices leading to failure of normal repetition enhancement in the default network (see Figure 5A showing a significant negative correlation between amyloid deposition and modulation of response). However, alternative explanations for this phenomenon might also be possible. For instance, we have recently argued that the presence of amyloid deposition might be indicative of the beginning of a pathophysiological cascade eventually leading to clinical AD, but that some individuals are able to resist the cognitive effects of amyloid toxicity for an extended period of time (Sperling et al., 2010), perhaps due to cognitive and brain reserve (Stern, 2006; Rentz et al., 2010). Thus, an alternate hypothesis is that the current finding represents sustained engagement of this network related to continued encoding during subsequent trials. Future work will be needed to disentangle these possibilities.
These results should also be interpreted with regard to the current understanding of the more general role of the default mode network in subserving cognition. The prevailing hypothesis states that the observed deactivation pattern may be the consequence of ongoing information processing and there is also evidence to suggest that the amount of deactivation is related to task difficulty. This latter idea was originally proposed by McKiernan and colleagues, who by using a parametric auditory target detection task in young subjects were able to demonstrate that task induced deactivation increased as task demand increased in a subsequently administered cognitive probe task (McKiernan et al., 2003). With regard to aging, two recent studies have reported that older individuals fail to modulate the default activity with increasing task demand (Persson et al., 2007; Park et al., 2010). Our findings are consonant with these studies, and suggest that failure of the default network to habituate to repetitive stimuli may be a marker of age- and amyloid-related brain dysfunction.
An emerging theory holds that the default mode network and the MTL memory system are coupled in a large distributed neural network, whose close reciprocal relationship is a prerequisite for focused attention and successful memory encoding (Greicius et al., 2003; 2004; Buckner et al., 2005; Daselaar et al., 2006). Recent findings suggest that alterations in this network are observed in MCI and early AD (Lustig et al., 2003; Celone et al., 2006; Petrella et al., 2007) and in cognitively normal older individuals (Miller et al., 2008; Park et al., 2010), the latter demonstrating that age-related memory impairment is associated with a loss of deactivation in the posteromedial cortices but preserved hippocampal activation. Similar to the current findings, AD patients have been shown to exhibit both impaired repetition suppression in the medial temporal lobe along with a failure of parietal deactivation (Pihlajamäki et al., 2008). In light of these data, our findings suggests that amyloid-related disruption in the default mode network during encoding may be an early sign of memory network failure that precedes hippocampal disruption and eventually leads to widespread functional disconnection of the default network and medial temporal lobes accompanied by clinically evident memory impairment associated with AD. These findings suggest that pathophysiological changes begin years prior to the onset of clinical symptomatology in AD. Interestingly, although older individuals with high amyloid burden tended to perform worse on the memory task in the current study, this difference was not significant. Similarly, the current literature is mixed in terms of the relationship between amyloid burden and cognitive performance in clinically normal older individuals. A few studies have found that increased amyloid deposition is related to poorer memory performance (Pike et al., 2007; Rentz et al., 2010; Resnick et al., 2010), but several studies found no relationship (Guillozet et al., 2003; Aizenstein et al., 2008; Jack et al., 2008; Hedden et al., 2009; Mormino et al., 2009; Storandt et al., 2009; Rowe et al., 2010). We hypothesize that amyloid may exert deleterious effects on networks subserving memory function prior to clinical manifestation of overt memory impairment, however, future research will determine whether these cognitively normal older individuals with evidence of amyloid deposition will ultimately develop memory impairment and clinical dementia.
Several limitations of the study should be noted. The current paradigm did not enable us to test memory after each encoding trial and our analysis was restricted to successfully encoded associations after three encoding presentations. We were therefore unable to measure whether additional information was encoded on each subsequent presentation of a given stimulus. Additionally, there were an insufficient number of stimuli that were incorrectly remembered in any of the subject groups to perform a valid comparison between successful and failed memory processes. Future experiments will utilize variants of this paradigm to allow investigation of these limitations. Finally, only eight (20%) of the normal older subjects exhibited elevated PiB retention values in the range of AD patients. Although consistent with previously reported Aβ pathology frequencies in normal older adults of approximately 20–50% in PiB PET studies (Pike et al., 2007; Rowe et al., 2007; Gomperts et al., 2008; Mormino et al., 2009), this small subsample is insufficient to draw firm conclusions using this dichotomous classification. However, we also examined amyloid burden as a continuous variable and found that failures of repetition enhancement in the posteromedial cortex were related to amyloid across the entire sample of older adults. This approach is based on the hypothesis that amyloid accumulates as a continuous process over many years rather than having a sudden onset, and it is not yet clear that there is a specific threshold that represents clear “amyloid positivity”. A related issue is the justification for defining the PiB groups based on the PiB retention in the precuneus region in the current study. Although there is no consensus on the optimal regions for sampling PET amyloid imaging, the most common approach in previous studies has been to use a global index based on the PiB retention in several regions. Because our goal was to study the impact of amyloid pathology on memory processes in particular, and because we were studying normal individuals who may be in very early stages of amyloid accumulation, we choose to sample PiB retention in a region known to be particularly vulnerable to early amyloid deposition (Mintun et al., 2006; Sperling et al., 2009), and that is critical for memory function (e.g. Buckner et al., 2005 and Vannini et al., 2010). Support for this approach also comes from a recent study by Rentz et al., (2010), demonstrating an inverse relationship between precuneus PiB retention and performance on memory neuropsychological measures. Given the high inter-correlation between regional PiB retention, it is likely that other regions or global estimates may show similar patterns, however, it may be advantageous to investigate the relationship between amyloid burden and functional disruption in key nodes of the memory network (Sperling et al., 2009).
In summary, we present evidence of both age- and amyloid-related alterations in the response of posteromedial cortex, a central region of the default mode network, to repetitive encoding, a memory enhancement technique commonly employed in the clinic. Our data demonstrate that young and older subjects activate the hippocampus similarly during initial encoding and exhibit similar repetition suppression in this region, supporting previous findings that the relationship of MTL system to memory function may be largely preserved during normal senescence. In contrast, young subjects demonstrated marked deactivations during initial encoding and repetition enhancement in the posteromedial regions of the default network, whereas the elderly, especially those older individuals with high amyloid burden, demonstrated reduced deactivation during initial encoding and failure of repetition enhancement in the posteromedial cortex. Consistent with previous studies, these results suggest that amyloid deposition is associated with altered functionality in the default network and that Aβ pathology may result in failure of habituation in the posteromedial cortex during memory encoding. These findings serve to elucidate the neural underpinnings of memory dysfunction seen in aging and age-related neuropathology and suggest that functional brain changes related to amyloid deposition may occur far in advance of symptomatic AD.
Supplementary Material
Statistical differences in bold.
Mean and standard error (S.E.) of the beta-weight across encoding trials. ROI = region of interest; L = leftl; R = right; Comp = comparison; Yng = young; Old = old; En1 = encoding trial 1; En2= encoding trial 2; En3 = encoding trial 3; DF = degrees of freedom; Err = error.
Acknowledgments
We are indebted to the volunteers who participated in this study. We would like to thank Janice Fairhurst, George Chiuo, Seung-Schik Yoo, and Istvan Akos Morocz for their help with scan acquisition at the Center for Advanced Imaging at Brigham and Women’s Hospital, and Meghan Frey for collecting the neuropsychological data. This work was supported by Karolinska Institutet foundations (Fobi0794) [P.V], the Swedish Research Council [P.V]; the Royal Swedish Academy of Sciences: FOAO7L501 [P.V]; and Marie Curie Fellowship: FP7-PEOPLE-2007-4-1-IOF from the European Union [P.V]. National Institutes of Health: K24 AG035007 [R.S.], R01 AG027435-S1 [R.S and K.J], P01AG036694 [R.S. and K.J], P50AG00513421 [R.S and K.J], and the Alzheimer’s Association: IIRG-06-27374 [R.S] and IIRG-08-90934 [D.R].
Footnotes
Disclosure statement
[K.J.] has consulted for GE Healthcare, who holds the commercial distribution rights for PiB PET imaging. The rest of the authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent Amyloid Deposition Without Significant Cognitive Impairment Among the Elderly. Arch Neurol. 2008;65(11):1509–17. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, Wahlin Å, Larsson M. Cognitive functioning in very old age. In: Craik FIM, Salthouse TA, editors. Handbook of cognitive aging. NJ: Erlbaum, Hillsdale; 1999. pp. 499–558. [Google Scholar]
- Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends in neurosciences. 1994;17(1):13–8. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Buckner R, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. The Year in Cognitive Neuroscience 2008. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical Hubs Revealed by Intrinsic Functional Connectivity: Mapping, Assessment of Stability, and Relation to Alzheimer’s Disease. J Neurosci. 2009;29(6):1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk W, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s Disease: Evidence for a relationship between default activity, amyloid, and memory. J Cogn Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold K-H, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of Hyperactive Neurons Near Amyloid Plaques in a Mouse Model of Alzheimer’s Disease. Science. 2008;321(5896):1686–9. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Celone K, Calhoun VD, Dickerson BD, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks i Mild Cognitive Impairment and Alzheimer’s disease: An independent component analysis. J Neurosci. 2006;26(4):10222–31. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus. 2007;17:1071–80. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. MIT press; Cambridge (MA): 1993. [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of Healthy Aging on Hippocampal and Rhinal Memory Functions: An Event-Related fMRI Study. Cereb Cortex. 2006;16(12):1771–82. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23(3):921–7. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer’s neuropatologic alterations in aged cognitively normal subjects. Journal of neuropathology and experimental neurology. 1999;58:376–88. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- Dee L, Benton AL. A cross-modal investigation of spatial performance in patients with unilateral cerebral disease. Cortex. 1970;6:261–72. doi: 10.1016/s0010-9452(70)80015-6. [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb Cortex. 2009;19(3):733–44. doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison PM, Archer HAM, Hinz RP, Hammers AP, Pavese NM, Tai YFM, Hotton GM, Cutler DB, Fox NP, Kennedy AM, Rossor MMDD, Brooks DJMDD. Amyloid, hypometabolism, and cognition in Alzheimer disease: An [11C]PIB and [18F]FDG PET study. Neurology. 2007;68(7):501–8. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- Engler H, Forsberg A, Almkvist O, Blomquist G, Larsson E, Savitcheva I, Wall A, Ringheim A, Langstrom B, Nordberg A. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129(11):2856–66. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically Parcellating the Human Cerebral Cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Sherzai A, Taylor C, Langbaum JBS, Chen K, Buxton RB. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuroimage. 2009;47(4):1678–90. doi: 10.1016/j.neuroimage.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHough PR. “Mini-Mental State”: a practical method for grading cognit9ive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forsberg A, Engler H, Almkvist O, Blomquist G, Hagman G, Wall A, Ringheim A, Långström B, Nordberg A. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiology of aging. 2008;29(10):1456–65. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Freir DB, Fedriani R, Scully D, Smith IM, Selkoe DJ, Walsh DM, Regan CM. A[beta] oligomers inhibit synapse remodelling necessary for memory consolidation. Neurobiology of aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.001. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golby A, Silverberg G, Race E, Gabrieli S, O’Shea J, Knierim K, Stebbins G, Gabrieli J. Memory encoding in Alzheimer’s disease: an fMRI study of explicit and implicit memory. Brain. 2005;128(4):773–87. doi: 10.1093/brain/awh400. [DOI] [PubMed] [Google Scholar]
- Gomperts SNMDP, Rentz DMP, Moran EB, Becker JAP, Locascio JJP, Klunk WEMDP, Mathis CAP, Elmaleh DRP, Shoup TP, Fischman AJM, Hyman BTMDP, Growdon JHM, Johnson KAM. Imaging amyloid deposition in Lewy body diseases SYMBOL. Neurology. 2008;71(12):903–10. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory Strength and Repetition Suppression: Multimodal Imaging of Medial Temporal Cortical Contributions to Recognition. Neuron. 2005;47(5):751–61. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16(9):1484–92. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101(13):4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsych. 1991;13:933–49. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Grön G, Bittner D, Schmitz B, Wunderlich AP, Tomczak R, Riepe MW. Variability in memory performance in aged healthy individuals: an fMRI study. Neurobiology of Aging. 2003;24(3):453–62. doi: 10.1016/s0197-4580(02)00128-8. [DOI] [PubMed] [Google Scholar]
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary Tangles, Amyloid, and Memory in Aging and Mild Cognitive Impairment. Arch Neurol. 2003;60(5):729–36. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Hay J, Jacoby LL. Separating habit and recollection in young and older adults: effects of elaborative processing and distinctiveness. Psychol Aging. 1999;14:122–34. doi: 10.1037//0882-7974.14.1.122. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KRA, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of Functional Connectivity in Clinically Normal Older Adults Harboring Amyloid Burden. J Neurosci. 2009;29(40):12686–94. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R, Rugg M. Neural response suppression, haemodynamic repetition effects, and behavioral priming. Neuropsychologia. 2003;41:263–70. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131(3):665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL. Ironic effects of repetition: measuring age-related differences in memory. J Exp Psychol Learn Mem Cogn. 1999;25:3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Smith EE, Rosand J, Rentz DM, Klunk WE, Mathis CA, Price JC, DeKosky ST, Fischman AJ, Greenberg SM. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Annals of Neurology. 2007;62(3):229–34. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Susskind-Wilder L, Conngor DJ, Sabbagh MN, Caselli RJ. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42:980–9. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Asthana S, Gluck MA, Myers C. Associative learning over trials activates the hippocampus in healthy elderly but not mild cognitive impairment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15(2):129–45. doi: 10.1080/13825580601139444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Kemppainen NMMDP, Aalto SM, Wilson IAP, Nagren KP, Helin SM, Bruck AMDP, Oikonen VM, Kailajarvi MMDP, Scheinin MMDP, Viitanen MMDP, Parkkola RMDP, Rinne JOMDP. Voxel-based analysis of PET amyloid ligand [11C]PIB uptake in Alzheimer disease. Neurology. 2006;67(9):1575–80. doi: 10.1212/01.wnl.0000240117.55680.0a. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang G-F, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Annals of Neurology. 2004;55(3):306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Knopman DSM, Parisi JEM, Salviati AM, Floriach-Robert MM, Boeve BFM, Ivnik RJP, Smith GEP, Dickson DWM, Johnson KAB, Petersen LEB, McDonald WCM, Braak HM, Petersen RCPM. Neuropathology of Cognitively Normal Elderly. Journal of Neuropathology & Experimental Neurology. 2003;62(11):1087–95. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39(2):184–99. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100:14504–9. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis CA, Bacskai BJ, Kajdasz ST, McLellan ME, Frosch MP, Hyman BT, Holt DP, Wang Y, Huang G-F, Debnath ML, Klunk WE. A Lipophilic Thioflavin-T Derivative for Positron Emission Tomography (PET) Imaging of Amyloid in Brain. Bioorganic & Medicinal Chemistry Letters. 2002;12:295–8. doi: 10.1016/s0960-894x(01)00734-x. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–98. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008;105(6):2181–6. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, LaRossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ the Alzheimer’s Disease Neuroimaging Initiative. Episodic memory loss is related to hippocampal-mediated {beta}-amyloid deposition in elderly subjects. Brain. 2009;132(5):1310–23. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and Death of Neurons in the Aging Brain. Science. 1997;278(5337):412–9. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Morrow D, Leirer VO, Carver LM, Tanke ED, McNally AD. Repetition improves older and younger adult memory for automated appointment messages. Hum Factors. 1999;41:194–204. doi: 10.1518/001872099779591268. [DOI] [PubMed] [Google Scholar]
- Orfanidou E, Marslen-Wilson WD, Davis MH. Neural response suppression predicts repetition priming of spoken words, and pseudo words. J Cogn Neurosci. 2006;18:1237–52. doi: 10.1162/jocn.2006.18.8.1237. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001;11(19):1528–30. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Synaptic Depression and Aberrant Excitatory Network Activity in Alzheimer’s Disease: Two Faces of the Same Coin? NeuroMolecular Medicine. 2010;12(1):48–55. doi: 10.1007/s12017-009-8097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank A, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgement tasks. Frontiers in human neuroscience. 2010;3:1–12. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Kalpouzos G, Nilsson LG, Ryberg M, Nyberg L. Preserved hippocampus activation in normal aging as revealed by fMRI. Hippocampus. 2010 doi: 10.1002/hipo.20794. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Sleegers K, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Altered deactivation in indivdiuals with genetic risk for Alzheimer’s disease. Neuropsychologia. 2008;46:1679–87. doi: 10.1016/j.neuropsychologia.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–32. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Prince SE, Wang L, Hellegers C, Doraiswamy PM. Prognostic value of posteromedial cortex deactivation in mild cognitive impairment. PLoS ONE. 2007;2(10):e1104. doi: 10.1371/journal.pone.0001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamäki M, DePeau K, Blacker D, Sperling RA. Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. AM J Geriatric Psychiatry. 2008;16(4):283–91. doi: 10.1097/JGP.0b013e318162a0a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamäki M, O’Keefe K, Bertram L, Tanzi RE, Dickerson BD, Blacker D, Albert MS, Sperling RA. Evidence of altered posteromedial cortical fMRI activity in subjects at risk for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;24:28–36. doi: 10.1097/WAD.0b013e3181a785c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. {beta}-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(11):2837–44. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb blood flow metab. 2005;25(11):1528–47. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and preclinical Alzheimer’s disease. Annals of Neurology. 1999;45(3):358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA. Hippocampal and neocortical activation during repetitive encoding in older adults. Neurobiol aging. 2006;27:173–82. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Sperling RA, Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Annals of Neurology. 2010;67(3):353–64. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, Dannals RF, Mathis CA, Klunk WE, Ferrucci L, Kraut MA, Wong DF. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010 doi: 10.1212/WNL.0b013e3181d3e3e9. WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of aging. 2010 doi: 10.1016/j.neurobiolaging.2010.04.007. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Rowe CCM, Ng SM, Ackermann UP, Gong SJP, Pike KB, Savage GP, Cowie TFBMBA, Dickinson KLB, Maruff PP, Darby DP, Smith CM, Woodward MF, Merory JF, Tochon-Danguy HP, O’Keefe GP, Klunk WEM, Mathis CAP, Price JCP, Masters CLM, Villemagne VLM. Imaging [beta]-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–25. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid Plaques Disrupt Resting State Default Mode Network Connectivity in Cognitively Normal Elderly. Biological psychiatry. 2010;67(6):584–7. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. decreases in cerebral cortex. J Cogn Neurosci. 1997;9(5):648–63. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL. Neural mechanisms of visual object priming: evidence for perceptual and semantic distinctions in fusiform cortex. Neuroimage. 2003;19(3):613–26. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Small SA. Age-related memory decline. Archives of neurology. 2001;58:360–4. doi: 10.1001/archneur.58.3.360. [DOI] [PubMed] [Google Scholar]
- Soldan A, Gazes Y, Hilton HJ, Stern Y. Aging Does Not Affect Brain Patterns of Repetition Effects Associated with Perceptual Priming of Novel Objects. Journal of Cognitive Neuroscience. 2008;20(10):1762–76. doi: 10.1162/jocn.2008.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: Successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20(2):1400–10. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74(1):44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS. Encoding novel face-name associations: a functional MRI study. Hum Brain Mapp. 2001;14:129–39. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BD, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional alterations in memory networks in early Alzheimer’s disease. NeuroMol Med. 2010 doi: 10.1007/s12017-009-8109-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–88. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L, Ojemann J, Miezin F, Petersen S, Videen T, Raichle M. Activation of the hippocampus in normal humans: A functional anatomical study of memory. Proc Natl Acad Sci USA. 1992;89(5):1837–41. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2006;20:112–7. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive Decline and Brain Volume Loss as Signatures of Cerebral Amyloid-{beta} Peptide Deposition Identified With Pittsburgh Compound B: Cognitive Decline Associated With A{beta} Deposition. Arch Neurol. 2009;66(12):1476–81. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers; Stuttgart (DE): 1988. [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42(10):1394–413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Vannini P, O’Brien J, O’Keefe K, Pihlajamaki M, LaViolette PS, Sperling RA. What goes down must come up: Role of the posteromedial cortices in encoding and retrieval. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. Journal of Neurochemistry. 2007;101(5):1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the Hippocampus During Encoding and Retrieval of Face-Name Pairs. Science. 2003;299(5606):577–80. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Gilewski MJ. Assessment of memory complaints by rating scales and questionnaires. Psychopharmacology Bulletin. 1988;24:523–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical differences in bold.
Mean and standard error (S.E.) of the beta-weight across encoding trials. ROI = region of interest; L = leftl; R = right; Comp = comparison; Yng = young; Old = old; En1 = encoding trial 1; En2= encoding trial 2; En3 = encoding trial 3; DF = degrees of freedom; Err = error.