Abstract
Background
Telomeres shorten with each cell division and are essential for chromosomal stability. Short telomeres in surrogate tissues (e.g., blood cells) are associated with increased cancer risk in several case-control studies, but findings are inconsistent in prospective studies.
Methods
We systematically reviewed studies published prior to August 30, 2010 on the association between telomere length (TL) in surrogate tissues and cancer. There were 27 reports on 13 cancers and/or incident cancer investigating this association. The majority, 16, were retrospective case-control studies, 11 were prospective studies. Meta-analyses were conducted to determine odds ratios (ORs) and 95% confidence intervals (CIs) for these studies.
Results
Studies on bladder, esophageal, gastric, head and neck, ovarian, renal, and overall incident cancer found associations between short telomeres and these cancers. Non-Hodgkin lymphoma, breast, lung and colorectal cancer reports were inconsistent. Single studies on endometrial, prostate, and skin cancers were null. In a random effects meta-analysis, short TL was significantly associated with cancer in retrospective studies (pooled OR for the shortest TL quartile compared with the longest: 2.9, 95%CI 1.73 – 4.8, P<0.0001). The pooled OR for prospective studies was 1.16 (95%CI 0.87 – 1.54, P=0.32). All studies combined yielded a pooled OR of 1.96 (95%CI 1.37 – 2.81, P=0.0001) for the association of short TL and cancer.
Conclusion and Impact
There is suggestive evidence that short surrogate tissue TL is associated with cancer; the strongest evidence exists for bladder, esophageal, gastric, and renal cancers. Additional prospective studies with consistent methodology are needed to confirm this hypothesis.
Keywords: telomere length, cancer, meta-analysis, biomarker, cancer risk, telomeres
INTRODUCTION
Chromosome ends are capped by telomeres which consist of tandem (TTAGGG)n nucleotide repeats and an associated protein complex called shelterin (1). Telomeric DNA terminates in a G-rich single-strand overhang of 50 to 300 nucleotides and folds back upon itself to form the telomeric “T-loop”. Absolute telomere length (TL) depends on an individual’s age, cellular replicative history, and tissue type (2). The telomerase complex, consisting of telomerase (gene name TERT), an RNA component (TERC), and other regulatory proteins elongate telomeres. Telomeres shorten with each cell division, partly due to incomplete replication of the 3′end of chromosomes.
Telomerase is repressed in human somatic cells during extra-uterine life and telomere attrition is part of normal aging of replicating somatic cells (2-4). When telomeres reach a critical length, loss of telomere protection leads to replicative senescence and apoptosis (5, 6). If apoptosis does not occur and cells continue to divide, the resultant genomic instability causes chromosomal abnormalities. Somatic cancer cells, which lack normal DNA damage response mechanisms, continue to divide despite critically short telomeres by upregulating of telomerase or utilizing the alternative lengthening of telomeres mechanism (7, 8).
Very short telomeres and germline mutations in telomere biology genes occur in patients with dyskeratosis congenita (DC), an inherited bone marrow failure and cancer predisposition syndrome. Patients with DC have an 11-fold increased overall cancer risk and 1000-fold increased risk of squamous cell tongue cancer (9). These patients start life with telomeres much shorter than the 1st percentile for their age (10) and have very early age of cancer onset (9). While DC represents the extreme of the short telomere phenotype, it is conceivable that smaller, less pronounced differences in TL in the general population may also contribute to cancer risk.
The number of epidemiologic studies of associations between TL and a variety of diseases has grown rapidly in recent years. Herein, we review published reports and present meta-analyses on the association between TL in surrogate tissues and cancer risk.
METHODS
We aimed to identify studies of the association of TL and risk of cancer in surrogate tissues (e.g., blood and buccal cells).We conducted a literature review using PubMed to search Medline (US Library of Medicine, Bethesda, MD). The search terms were “cancer or risk of cancer”, “telomere”, “telomere length”, and “epidemiology”, for studies published on or before August 30, 2010. We included only full reports (abstracts only were excluded) in English. A total of 40 publications were identified, and their titles and abstracts reviewed for relevance. Twenty-three reports were excluded because they either focused on diseases other than cancer or analyzed TL in tumor tissues. Seventeen reports fit our selection criteria. A subsequent, more refined search including the terms “blood” or “buccal cells”, “odds ratio”, “case control”, and “short telomeres”, yielded an additional 20 publications of which 10 fit our selection criteria. Thus, we included a total of 27 reports on 13 different cancer types and one report on overall incident cancer in this review.
Laboratory methods utilized in published studies
Four different laboratory methods of TL determination were used in the reviewed reports (Table 1). Wu et al. used TRF analysis by Southern blot to determine TL (11). This method requires larger amounts (hundreds of nanograms) of unfragmented, high-quality DNA, is labor intensive and thus not high-throughput, which limits its utility for larger studies.
Table 1.
Association studies of Telomere Length and Cancer.
| Tumor Site | Study Design | (#Cases/#Controls) | DNA Source | Method of TL Measurement |
Main findings | Reference |
|---|---|---|---|---|---|---|
| Bladder | Case-Control | 135/135 | PBLs¶ | Q-FISH | Short telomeres increase bladder cancer risk | (11)* |
| Case-Control | 63/93 | Buccal cells | Q-PCR+ (triplicates) | Short telomeres increase bladder cancer risk | (12)* | |
| Nested Case- Control |
61/67 (females), 123/125 (males) |
PBLs | Q-PCR (triplicates) | Short telomeres increase bladder cancer risk | (22)* | |
| Breast | Population-based Case-Control |
1067/1110 | WBCs† (NOS)‡ | Q-PCR (duplicates) | Short telomeres increase breast cancer risk in premenopausal women. |
(29)* |
| Nested Case- Control |
1122/1147 | PBLs | Q-PCR (triplicates) | TL is not associated with breast cancer risk in postmenopausal women. |
(13)* | |
| Case-Control | 265/446 | Buffy coats and granulocyte preparations§ |
Q-PCR (triplicates) | Blood cell telomeres are longer in breast cancer patients than controls |
(30)* | |
| Case-Control | 287/350 | WBCs (NOS) | Q-PCR (triplicates) | Mean TL is shorter in cases than in controls. More pronounced in premenopausal women. |
(28)* | |
| Case-Control | 152/176 | PBLs | Q-PCR (triplicates) | TL is not associated with risk of breast cancer | (37)* | |
| Case-Control | 153/159 | cultured lymphocytes |
Q-FISH | TL is not associated with risk of breast cancer. | (37)* | |
| Case-Control | 153/159 | cultured lymphocytes |
Q-FISH | Short telomeres on chromosome 9p increase breast cancer risk. |
(38) | |
| Case-Control | 102/50 | PBLs | Q-PCR (triplicates) | Longer TL increased risk of breast cancer. | (14)* | |
| Case-Control | Prospective EPIC: 199/420 |
PBLs | Q-PCR (duplicate and triplicates) |
No association between risk of breast cancer and short TL. |
(25)* | |
| Case-Control | Retrospective SEARCH: 2243/2181 |
PBLS | Q-PCR (duplicates or triplicates) |
Short TL increased risk of breast cancer in retrospective study. |
(25)* | |
| Colorectal | Nested Case- Control |
191/306 | PBLs | Q-PCR (duplicates) | No association of leukocyte mean TL and risk of incident CRC¶¶ in men. |
(33)* |
| Nested Case- Control |
134/357 | PBLs | Q-PCR (duplicates) | No association of leukocyte mean TL and risk of incident CRC in women. |
(20)* | |
| Case-Control | Prospective EPIC:185/406 |
PBLs | Q-PCR | No association of increased risk of CRC and short telomeres. |
(25)* | |
| Case-Control | Retrospective SEARCH: 2249/2161 |
PBLs | Q-PCR | Short TL increased risk of CRC in retrospective study. | (25)* | |
| Endometrial | Nested Case- Control |
279/791 | PBLs | Q-PCR (triplicates) | No association between relative TL and risk of endometrial cancer. |
(26)* |
| Esophagus | Cohort | 300 (38 cancers) | Buffy coats | Q-PCR (triplicates) | Short TL increased risk of esophageal adenocarcinoma in patients with Barrett's esophagus. |
(27)* |
| Case-Control | 94/94 | PBLs | Q-PCR (triplicates), STELA++ |
Short TL significantly increases risk of esophageal cancer. Short TL on 17p and 12q show increased risk. |
(32)* | |
| Gastric | Case-Control | 396/378 | PBLs | Q-PCR (duplicates) | Short TL increases risk of gastric cancer. | (21)* |
| Population-based Case-Control |
300/416 | PBLs | Q-PCR (duplicates) | Increased risk of gastric cancer among study participants with shortest TL. |
(17)* | |
|
Head and
Neck |
Case-Control | 92/92 | PBLs | Southern Blot | Short telomeres increase risk of head and neck cancer. | (11)* |
|
Incident
Cancer |
Population-based Case-Control |
92/787 | PBLs | Q-PCR (quadruplicate) |
Short TL increased risk of incident cancer (any cancer type). |
(31)* |
| Lung | Population-based Case-Control |
111/99 | First morning sputum |
Q-PCR (duplicates) | Short TL is not associated with risk of lung cancer. | (16) |
| Case-Control | 243/243 | PBLs | Q-PCR (duplicates) | Short telomeres increase risk of lung cancer. | (18* | |
| Case-Control | 54/54 | PBLs | Q-FISH | Short telomeres increase risk of lung cancer. | (11)* | |
|
Non-
Hodgkin Lymphoma |
Case-Control | 40/40 | PBLs | Flow-FISH | Telomeres in WBC subsets are significantly shorter in patients with NHL††. |
(40)* |
| Case-Control | 107/107 | WBCs | Q-PCR | Risk of NHL is increased with longer TL. | (19)* | |
| Ovarian | Population-based Case-Control |
99/100 | Buffy coats | Q-PCR (triplicates) | TL is significantly shorter in patients with serous ovarian cancer. |
(24)* |
| Prostate | Nested Case- Control |
612/1049 | Buffy coats | Q-PCR (triplicates) | TL is not associated with risk of prostate cancer. | (23)* |
| Renal | Case-Control | 32/32 | PBLs | Q-FISH | Short telomeres increase risk of renal cancer. | (11)* |
| Case-Control | 65/65 | PBLs | Q-FISH | Short telomeres increase risk of renal cancer. | (36)* | |
| Skin | Nested Case- Control |
218 melanoma cases, 285 SCC‡‡, 300 BCC§§/870 |
PBLs | Q-PCR (triplicates) | TL is not associated with skin cancer risk. | (15)* |
indicates the studies included in the meta-analysis.
peripheral blood lymphocytes;
WBCs, white blood cells; ‡not otherwise specified;
DNA was derived from granulocytes for 146 controls. DNA from the rest of the controls and all of the cases was from buffy coats.
colorectal cancer;
single telomere length analysis;
non-Hodgkin lymphoma;
squamous cell carcinoma;
basal cell carcinoma; Nested case-control studies were conducted within prospective cohort studies. One study combined analyses of two independent case-control series of breast and colorectal cancer in their report (retrospective SEARCH and prospective EPIC case-control study) (25). One study combined two independent case-control studies of breast cancer in their report (37).
The majority of the reviewed reports used quantitative PCR (Q-PCR) TL measurement (12-33). This method was recently updated, and can be high-throughput (34, 35). The telomere repeat content is expressed as a ratio of telomere-specific amplification to a single copy gene. Q-PCR is fast, sensitive, and requires smaller amounts (few nanograms) of DNA. Published coefficients of variation (CVs) for this method ranged from 0.37% to 28%. This wide range suggests substantial variability between laboratories. Four studies used quantitative fluorescence in situ hybridization (Q-FISH) analysis (11, 36-38). One publication which used Q-FISH reported a CV of 12.4% (38). However, Q-FISH is limited to cells that proliferate well in culture which could result in a different population of cells than a study that combined all cell-types. Fresh cells are also required for flow-FISH, which combines FISH and flow cytometry (39), and determines TL in subpopulations of hematopoietic cells. This method is useful for studying telomeres of the immune system and its diseases [used by (40)]. One study of esophageal cancer (32) used the single telomere length assay (STELA) assay which measures chromosome-specific TL at high resolution (41).
Statistical Methods
Meta-analyses were performed with Stata version 11.0 (StataCorp, College Station, TX) using the “metan” command. Twenty-five reports (using blood and buccal cells as DNA source) were included in the main meta-analysis. One publication reported an association between chromosome-specific TL and breast cancer risk (38), but no overall TL and therefore was excluded from the meta-analysis. One lung cancer study measured TL in cells of morning sputum which could have included tumor cells; therefore, we excluded this report. Begg’s statistical test was used to assess publication bias (42). Log-odds ratios, 95% confidence intervals (95%CIs), and standard errors (SEs) of the referent quartile compared to the quartile with the shortest telomeres were used to compute the summary log-OR. Hazard ratios reported in Risques et al. (27) were converted into unadjusted odds ratios (ORs). Studies reporting mean TL among controls (20, 33) were converted to quartile categories. We obtained data from two studies that reported tertiles and converted these to quartiles (24, 31). Sufficient data was given in Xing et al. (32) to convert to quartiles. We used the reported means and standard errors in cases and controls, and assumed that the TL arose from a normal distribution and computed the 25th, 50th, and 75th quartiles, Q1, Q2 and Q3, respectively, in the controls using that Q1 = mean-0.67*stderror, Q2 = mean, and Q3 = mean + 0.67*stderror. We then computed the probabilities among cases to fall into Q1, Q2, and Q3 and multiplied these probabilities by the total number of cases. Using that 25% of controls fall into each of the four categories, we created a two by four table to compute the OR for the highest compared to the lowest TL quartile and its standard error.
We first computed a pooled summary estimate assuming a fixed effects model (43). Because there was significant heterogeneity between estimates across studies, we then used the random effects model (44) that we present in the results section. Heterogeneity was described using the I2-statistic, the approximate proportion of total variability in the point estimates that can be attributed to heterogeneity (45).
RESULTS
The published data on association between TL and risk of cancer are shown in Table 1 and Figure 1. For consistency, we present the association findings (adjusted ORs) for TL categorized into quartiles or tertiles, and comparisons between the shortest and longest (referent) quartile or tertile, unless otherwise noted. All studies adjusted for age in their analyses, but adjustment for other exposures or conditions (e.g., smoking or body mass index) varied. We refer the reader to the specific publications for details.
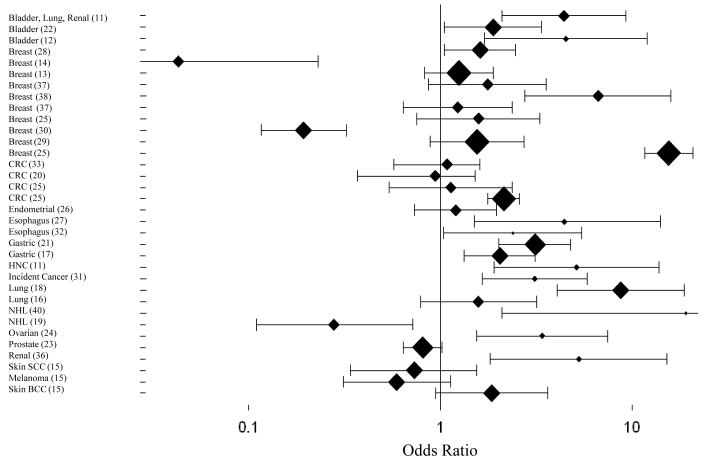
Figure 1. Association between Telomere Length and Risk of Cancer in Surrogate Tissues.
Association findings for TL are presented categorized into quartiles or tertiles and comparisons between the shortest and longest (referent) group. The graph was created using study-specific, adjusted odds ratios and 95% confidence intervals with SigmaPlot Version 11.0. (37) include two independent case-control series.
(11) included bladder, lung, and renal cell carcinoma cases; (14) and (19) reported a significant association of longer TL and risk of breast cancer and NHL, respectively; here we plot the inverse log odds ratios.
(37) Case-control study at Lombardi Comprehensive Cancer Center; (38) Chromosome 9-specific OR; (37) Case-control study at Roswell Park Cancer Institute; (25) Prospective EPIC study; (25) Retrospective SEARCH study; (25) Prospective EPIC study; (25) Retrospective SEARCH study;
Abbreviations: CRC, colorectal cancer; HNC, head and neck cancer; NHL, non-Hodgkin lymphoma; SCC, squamous cell carcinoma; BCC, basal cell carcinoma.
Head and Neck Cancer
Imbedded in a comparison of four different cancer types (head and neck, lung, renal, and bladder), Wu et al. (11), evaluated the association between TL and risk of head and neck cancer. Using 92 patients with head and neck cancer TL was measured with Southern Blot analysis of DNA from lymphocytes. A dose-response relationship between risk for head and neck cancer and TL was seen in quartile analyses. The OR for the shortest TL quartile compared to the longest was 5.11 (95%CI 1.90 – 13.77, Ptrend≤0.001). Follow-up studies of TL in head and neck cancer have not yet been published.
Breast Cancer
Eight studies on TL and breast cancer were identified in our review. Two studies found no association between breast cancer risk and TL (13, 37), three studies found that short telomeres were significantly associated with increased breast cancer risk (28, 29, 38), and two case-control studies found that telomeres were significantly longer in breast cancer cases than controls (14, 46).
One retrospective case-control study (28) in 268 sister sets found no association between TL and breast cancer, but suggested an association of shorter telomeres and premenopausal breast cancer (OR=2.1, 95%CI 0.80 – 5.5, Ptrend=0.17). The CVs were 28% and 19% for interbatch and intrabatch variability, respectively. The same group replicated this finding in a larger, population-based case-control study with 1,067 breast cancer cases and 1,110 controls; an increased risk of premenopausal breast cancer was noted for shortest telomeres (OR=1.61, 95%CI 1.05 – 2.45, Ptrend=0.01) (29).
A second study with 1,122 cases and 1,147 controls found no association between TL and breast cancer risk in postmenopausal women (13) after adjustment for known breast cancer risk factors, (OR=1.25, 95%CI 0.83 – 1.88, Ptrend=0.20). This study was prospective and DNA was obtained prior to cancer diagnosis, which could explain the inconsistency between this and other findings of TL and breast cancer.
One breast cancer study which found that longer TL increased risk of breast cancer, used 265 cases and 446 matched controls from two population-based groups (OR= 5.17, 95%CI 3.09 – 8.64, Ptrend≤0.01) (46). DNA was extracted from granulocyte preparations for 146 controls. While granulocytes typically have shorter telomeres than lymphocytes the authors found no difference in TL between the two control groups. A second small study replicated these findings in 102 patients and 50 controls (14) and found increased risk of breast cancer with each longer quartile of TL; the OR for the longest TL quartile compared to the shortest was 23.3 (95%CI 4.40 – 122.3, P=0.0003, CV=5%).
Zheng et al.,(37) investigated TL and breast cancer risk in two independent case-control settings. Neither study nor both combined found associations of TL and breast cancer (OR for both studies combined=1.23, 95%CI 0.89 – 1.71, Ptrend=0.35). The CV was 8.1%. However, a subsequent study by the same group, found that short TL specifically on chromosome 9p was significantly associated with increased risk of breast cancer (OR=6.62, 95%CI 2.75 – 15.94, Ptrend≤0.01 ) (38). The CV for the Q-FISH method was 12.4%.
Pooley et al. (25) compared TL and cancer risk in retrospective and prospective studies of breast and colon cancer. Breast cancer cases in the retrospective study had significantly shorter telomeres than age-matched controls (OR=15.5, 95%CI 11.6 – 20.8, Ptrend=2.1×10−80). This association was weaker and not significant in the prospective component of the study when samples were collected ≥ 6 months prior to cancer diagnosis (OR=1.58, 95%CI 0.75 – 3.31, Ptrend=0.18).
Lung Cancer
TL in peripheral blood leukocytes (PBLs) from 54 lung cancer cases was significantly shorter compared to 54 controls (P<0.001) (11). This study also found that shorter telomeres were associated with an increased risk of lung cancer (combined OR for lung, renal, and bladder cancer=4.41, 95%CI 2.1 – 9.28, Ptrend=0.001).
A second retrospective study of TL and lung cancer showed a very strong association in PBLs of individuals with short telomeres and increased risk of lung cancer compared to individuals with longer telomeres (OR=8.73, 95%CI 4.08 – 18.71, Ptrend≤0.0001) (18). The interbatch and intrabatch CVs were 7.5% and 1.7%, respectively. The effect of short telomeres was most pronounced in small cell lung cancer. Another population-based case-control study (16) showed no association between short telomeres and risk of lung cancer (OR=1.58, 95%CI 0.79 – 3.18) based on TL in DNA derived from morning sputum (111 patients, 99 controls). The possible presence of tumor cells in sputum complicates the interpretation of these findings. The CV was 23% and the ICC was 0.87 for the Q-PCR assay.
Esophageal Carcinoma
Two studies found significant associations between short TL and increased esophageal cancer risk. The first study measured TL by Q-PCR in a cohort of 300 individuals with Barrett’s esophagus of who 38 developed esophageal adenocarcinoma. The OR was 4.66 (95%CI 1.28 – 16.93, P=0.02) for those with shortest telomeres (27). The CVs were 6% and 7% for the intra-assay and inter-assay variability, respectively. The second study evaluated both average and chromosome-specific (17p, 12q, 2p, and 11q) TL in 94 cases with esophageal carcinoma and 94 matched controls (32). Individuals with short overall TL and with short 17p and 12q TL had a significant increased risk of esophageal cancer (OR for overall TL=2.52, 95%CI 1.29 – 4.94, P=0.03).
Gastric Cancer
Two case control studies showed increased an association between short TL and gastric cancer. A Polish population-based study included 300 patients and 416 matched controls. The OR for gastric cancer for subjects with the shortest compared to the longest TL quartile was 2.04 (95%CI 2.01 – 4.79, Ptrend<0.001) (17). Another gastric cancer case-control study (396 cases, 378 controls) presented a significantly increased risk (OR=3.12, 95%CI 2.01 – 4.79, Ptrend<0.001) in individuals with shortest TL (21). The interbatch CV was 8.1%.
Colorectal Cancer
Two case-control studies of the association between colorectal cancer and TL showed no association. Both studies were restricted to Caucasian males (OR=1.25, 95%CI 0.86 – 1.81, P=0.24) (191) and females (OR=0.94, 95%CI 0.65 – 1.38, P=0.76) (134) from large cohorts (Women’s Health Study and Physician’s Health Study) and assessed mean leukocyte TL (20, 33) by Q-PCR using blood samples obtained at study randomization. Pooley et al. (25) also investigated TL and cancer risk in retrospective and prospective studies on colon cancer. Colon cancer cases in the retrospective study had significantly shorter telomeres than controls (OR=2.14, 95%CI 1.77 – 2.59, Ptrend=1.8×10−13). The prospective component found no association (OR=1.13, 95%CI 0.54 – 2.36, Ptrend=0.82).
Ovarian Cancer
A pilot case-control study of buffy coat TL evaluated 99 ovarian cancer cases and 100 controls from the population-based Polish Ovarian Cancer Study (24). The risk of ovarian cancer was highest in the shortest TL tertile (OR=3.39, 95%CI 1.54 – 7.46, Ptrend=0.002). Stratified analyses showed an increased association with short telomeres only in those with poorly differentiated tumors (TL dichotomized: OR=4.89, 95%CI 1.93 – 12.34, P<0.001, versus OR=0.82, 95%CI 0.29 – 2.28, P=0.07). A significant interaction between TL and tumor grade was observed (P=0.02). The CV for repeats was 6.4%.
Endometrial Cancer
A nested case-control study (279 cases, 791 controls) found no relationship between leukocyte TL and endometrial cancer (26). Women with the shortest telomeres and endometrial cancer had a multivariate adjusted OR=1.2 (95%CI 0.73 – 1.96, Ptrend=0.37). Among postmenopausal women, the OR was 1.04 (95% CI 0.61 – 1.79). In this study telomere and single-gene assay coefficients of variation for triplicates were 0.87% and 1.09%.
Prostate Cancer
A prospective study with 612 individuals with prostate cancer and 1,049 controls from the PLCO cohort (Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial) showed that TL was not associated with prostate cancer (OR=0.81, 95%CI 0.64 – 1.02, Ptrend=0.34) (23). The CVs within triplicates of the telomere and single-gene assay were 1.11% and 0.77%, respectively, and the inter-assay CVs were 5.6% and 2.6%, respectively.
Renal Cell Cancer
Wu et al. (11) also included 32 patients and 32 controls with renal cancer. Individuals with short telomeres had a significantly increased risk of all smoking related cancers (bladder, lung, and renal cell cancer) (OR=4.41, 95%CI 2.1 – 9.28, Ptrend=0.001). A second, small case-control study (65 cases, 65 controls) found TL to be associated with renal cancer cases (OR=5.26, 95%CI 1.82 – 15.2, Ptrend=0.001) (36).
Bladder Cancer
Consistently strong associations between surrogate tissue TL and bladder cancer have been observed. A study of 135 bladder cancer patients and 135 controls measured TL by Q-FISH on PBLs (11). The OR for shortest TL compared to longest was 4.41 (95%CI 2.1 – 9.28, Ptrend=0.001). A second case-control study with 63 bladder cancer patients and 93 controls (12) measured TL in buccal cells with Q-PCR and found an OR=4.5 (95%CI 1.7 – 12) for the shortest quartile compared to the longest.
The third study including 61 female and 123 male bladder cancer cases and 67 and 125 controls, respectively, (22) yielded an OR=1.88 (95%CI 1.05 – 3.36, Ptrend=0.006) in cases with the shortest TL. The CVs of the telomere and single-gene assay were between 2.22% and 2.46% (cases were ascertained from two different cohorts), and the intra-assay CVs were between 0.37% and 0.55%. These findings suggest that surrogate tissue TL could be a marker of bladder cancer risk. However, all three studies used samples obtained around the time of cancer diagnosis, so reverse causation bias could have contributed to these findings.
Non-Hodgkin Lymphoma
One small study (40 cases, 40 controls) of TL in non-Hodgkin lymphoma (NHL) found an OR=19.0 (95%CI 2.1 – 170.4) for short TL (40). TL was measured in B and T cells in cases without peripheral blood involvement, and in granulocytes in controls, possibly confounding the results because of shorter telomeres in granulocytes. A second, prospective study with 107 cases showed a dose-response relationship between quartiles of increasing TL and risk of NHL (OR=3.6, 95%CI 1.4 – 8.9, Ptrend=0.003) (19). This association was similar across the most common subtypes. Tumors cells circulating in peripheral blood of undiagnosed cases in this study could have caused to bias the effect towards null. The authors suggested that individuals with longer telomeres have an elevated NHL risk.
Skin Cancer
One prospective case-control study on skin cancer nested in the Nurses’ Health Study included 803 women with incident skin cancer, including melanoma, squamous cell carcinoma (SCC), basal cell carcinoma (BCC) (15). Shorter telomeres were associated with a non-significant decreased risk of melanoma (OR=0.59, 95%CI 0.31 – 1.13, Ptrend=0.09) and SCC (OR=0.73, 95%CI 0.34 – 1.55, Ptrend=0.3). In contrast, shorter TL was correlated with non-significant higher risk of BCC (OR=1.85, 95% CI 0.94 – 3.62, Ptrend=0.09).
Overall Incident Cancer
The association of TL and incident cancers was conducted in a prospective, population-based study of 787 participants who were cancer-free at enrollment (31). After 10 years of follow-up, 92 individuals developed a cancer. The hazard ratio for incident cancer was highest in those in the shortest TL tertile compared to the longest (OR=3.11, 95%CI 1.65 – 5.84, P<0.001). Higher cancer mortality was also noted among individuals with shorter TL. However, this study was based on a small numbers of incident cancers.
Meta-Analysis of the Association between Telomere Length and Cancer
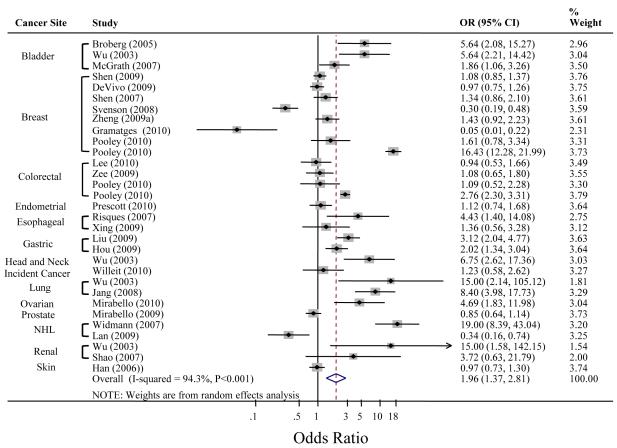
A total of 25 studies on 13 different cancers (bladder, breast, colorectal, esophageal, endometrial, gastric, head and neck, lung, NHL, ovarian, prostate, renal, and skin cancers) and overall incident cancer were included in the meta-analyses based on the following criteria: DNA derived from blood or buccal cells, and analysis by quartiles of TL among controls (studies included are noted in Table 1). A study which measured TL on chromosome 9p only (38) and one on lung cancer (16) which used cells from sputum were excluded from the analysis. Publication dates ranged from August 2003 (11) to August 2010 (31). The largest report (25) involved 2,249 patients with colorectal cancer; the smallest included 32 patients with renal cancer (11).
We first computed a fixed effects meta-analytic estimate, and found appreciable heterogeneity among the studies (I2-statistic=94.3% [τ2=0.8879, P<0.001]). Therefore, we present the results of the random effects meta-analyses. The random effect meta-analytic pooled OR for all cancer types combined was 1.96 (95%CI 1.37 – 2.81, P<0.0001) for the quartile with the shortest telomeres compared with the referent quartile of TL with longest telomeres (Figure 2). Based on Begg’s test, there was no evidence of publication bias (P=0.072).
Figure 2. Meta-Analysis of the Association Between Telomere Length and Cancer.
Studies included in the meta-analysis were selected based on the following criteria: DNA derived from blood and buccal cells, and analysis by quartiles of TL among controls. Unadjusted odds ratios were used to conduct analysis (exception: Pooley et al. [25]: ORs are adjusted for batch to avoid confounding). Significant study heterogeneity was present (I2=94.3%, P<0.001), so the random effects meta-analysis was used. The weight percents are from the random effects analysis. The dashed line indicates the OR of the metaanalysis. (14), (19), and (30) reported a significant association of longer TL and risk of breast cancer and NHL, respectively; we used the inverse log odds ratios to compute the summary OR.
The forest plot was created using Stata version 11.0 (StataCorp, College Station, TX). Abbreviations: OR, odds ratio; CI, confidence interval; NHL, non-Hodgkin Lymphoma.
Sensitivity analyses
Han et al. (15) reported on each skin cancer entity (melanoma, squamous cell, and basal cell carcinoma) separately. We thus computed and used a summary OR for all skin cancers in the main analysis to avoid under-representing heterogeneity. We repeated the analysis using the separated estimates of each skin cancer. Since the controls were matched individually, the estimates were independent. By splitting a single study into three sub-studies, we underestimate the inter-study variability, but better account for the differing etiology of telomere attrition in those cancers. The random effects pooled OR over all studies (using the three separate skin cancer estimates) was 1.95 (95%CI 1.43 – 2.66, P<0.001). The I2-statistic showed comparably high heterogeneity of 94.2% (τ2 = 0.6738, P<0.001).
We also separately estimated a pooled OR for the seven breast cancer studies included in the meta-analysis (13, 14, 25, 28, 29, 37, 46). The studies enrolled between 152 (37) and 1,122 (13) breast cancer cases. The random effects pooled OR was 1.06 (95%CI 0.41 – 2.75, P=0.9) indicating no association of breast cancer with TL. Lacking sufficient data, we could not further stratify by age or menopausal status.
We repeated the analyses using only studies (for any cancer) which used Q-PCR for TL measurement to lessen possible confounding due to differing laboratory methodology. The random effect pooled OR was 1.59 (95%CI 1.07 – 2.36, P<0.0001).
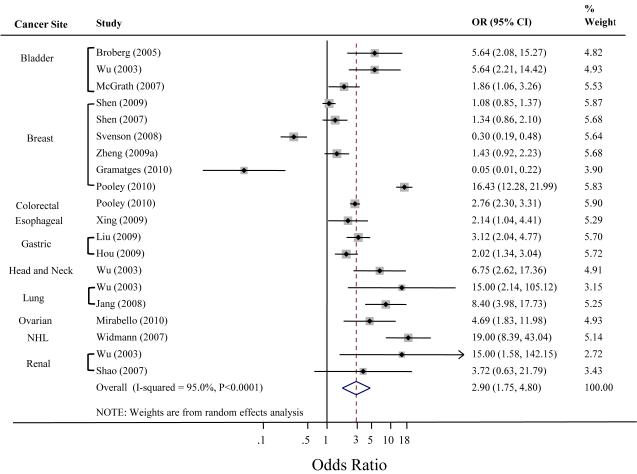
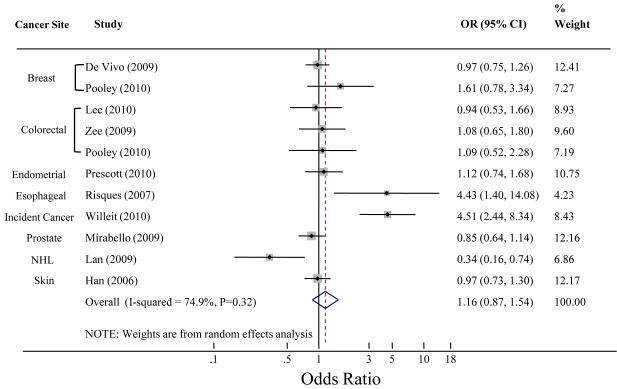
Lastly, we analyzed retrospective and prospective studies separately to better account for reverse causation bias. The pooled estimate for the retrospective studies was 2.9 (95%CI 1.76 – 4.8, P<0.0001) (Figure 3). The prospective studies combined yielded a pooled OR=1.16, 95%CI 0.87 – 1.54, P=0.32) (Figure 4). This finding is agreement with Pooley et al. (25) who published significant findings in retrospective breast and colon cancer and null results in the prospective cohorts.
Figure 3. Meta-Analysis of Telomere Length and Cancer in Retrospective studies.
The plot was created using Stata version 11.0 (StataCorp, College Station, TX). Svenson et al. and Gramatges et al. found an association with longer telomeres; here inverse log odds ratios were used to compute the summary OR. The dashed line indicates the pooled OR of the analysis. Because there was significant heterogeneity described by the I2-statistic between estimates across studies, we present results from the random effects model.
Abbreviations: OR, odds ratio; NHL, non-Hodgkin lymphoma.
Figure 4. Meta-Analysis of Telomere Length and Cancer in Prospective studies.
The plot was created using Stata version 11.0 (StataCorp, College Station, TX). Lan et al. found an association with longer telomeres and NHL; here inverse log odd ratios were used to compute the summary OR. The weight percents are from the random effects analysis. Heterogeneity was described using the I2-statistic, the approximate proportion of total variability in the point estimates that can be attributed to heterogeneity. The dashed line indicates the pooled estimate of the analysis. Han et al. reported on each skin cancer entity (melanoma, squamous cell, and basal cell carcinoma) separately. We computed and used a summary OR for all skin cancers in this analysis to avoid under-representing heterogeneity.
Abbreviations: OR, odds ratio; NHL, non-Hodgkin lymphoma.
DISCUSSION
Telomere shortening results in chromosomal instability which, in the absence of normal cellular senescence processes, can lead to cancer development. Patients with germline defects in telomere biology, such as those with DC, have a very high risk of cancer (9). Most somatic cancer cells have significant aberrations in telomere biology. Therefore, it is biologically plausible that individuals with short telomeres, even if they are not as short as in DC, might be at increased risk of cancer compared to individuals with longer telomeres. This hypothesis formed the basis for the association studies of cancer and TL described in our report.
Studies on bladder, esophageal, gastric, head and neck, ovarian, renal, and overall incident cancer found that shorter telomeres were significantly associated with these cancers. Findings from studies on non-Hodgkin lymphoma, breast, lung and colorectal cancer were inconsistent. Single studies on endometrial, prostate, and skin cancers found no associations. Our meta-analysis showed a statistically significant positive OR and therefore suggests an association between short TL and cancer in the studies evaluated. This estimate, however, is heavily weighted by the larger studies and cancer types on which most studies were conducted. Results from very small studies and in cancer types, for which only one or two reports (e.g., head and neck, ovarian cancer) have been conducted so far, need to be interpreted with caution. Thus, these results may not be representative of all cancer types.
This meta-analysis accounted for the variability in TL measurement methods between studies. Most studies used Q-PCR, a high-throughput method which is suitable for large epidemiologic studies. While the CVs have improved significantly since its development, the CVs in the reviewed reports are highly variable. In addition, only one report published an ICC which is more suitable to assess repeatability of an assay. The reproducibility between laboratories, the effects of DNA extraction, and DNA storage need to be addressed in future studies. Methodologic studies of TL measured by terminal restriction fragment (TRF) analysis by Southern blot, Q-PCR, Q-FISH, and correlations with single TL measurement are also needed to assess the evidence from different studies. These details should be reported in future TL association studies.
Our findings are intriguing, but several questions remain to be answered: 1.) What is the role of TL in surrogate tissues in the etiology of specific cancers? 2.) Could reverse causation bias explain the discrepant findings in studies with variable findings in the same cancer type? and 3.) How is telomere shortening related to or influenced by common cancer risk factors (e.g., smoking or inflammation)?
Surrogate tissue TL may be a marker of genetic risk and/or environmental exposures that are related to cancer etiology. A limited number of intra-individual TL studies suggest that TL differs between tissues, but that it is correlated within an individual (36, 40, 47-49). Thus measuring TL within a surrogate tissue, such as blood or buccal cells may aid in understanding TL in other tissues. However, it is possible that variability in surrogate tissue TL, and the potential direct exposure of that tissue to carcinogens (e.g., buccal cell exposure to cigarette smoke) could explain discrepant results in some of the studies described. For example, it is not known if there are differential effects of smoking on TL in oral or lung tissues, which are directly exposed to cigarette smoke, compared to tissues without direct contact, such as blood cells.
The majority of the published studies reviewed were case-control studies which obtained DNA from the cases after cancer diagnosis. This could result in reverse causation bias, where changes in surrogate tissue TL could be a consequence of the presence of malignant disease rather than an etiologic marker. Studies of incident prostate, skin, colon, and breast cancers (13, 15, 20, 23, 33), in which samples were collected months or years prior to cancer diagnosis, did not find significant evidence for associations between TL and cancer risk.
The majority of the reviewed studies do not give information on administration of chemotherapy or radiation therapy prior to DNA collection. There is theoretical consideration and empirical data that both therapeutic modalities shorten telomeres (50-52). Only two small studies of the reviewed reports [ovarian cancer, (24), and breast cancer, (14)] commented on treatment. They, however, did not find a difference between cases who received chemotherapy prior to sample collection and those who did not. Comparison of prospectively collected samples to samples collected at the time of enrollment after breast or colon cancer diagnosis suggested that telomere shortening occurred primarily after diagnosis (25). Additional prospective cohort studies, with collection of serial samples, and inclusion of treatment data are required to better understand the differences in these findings.
Studies which assess potential interactions between known cancer risk factors on TL and cancer risk are important to understanding these associations. Data suggesting that other risk factors, such as obesity, other hormones, even stress or lifestyle, influence TL and risk of cancer have been discussed elsewhere (53, 54). Cancer types with no or inconsistent associations with TL include breast and prostate cancers. These cancer types are predominantly influenced by hormones. The role of cigarette smoke or inflammation in the development of these cancers is either minimal or ill-defined (55). Notably, estrogen has anti-inflammatory and antioxidant activities which may have protective properties against the development of cancer. In addition, estrogen can stimulate telomerase (56). This might explain some discrepancy in the breast cancer reports in pre- and postmenopausal patients.
All cancers found to date to be associated with short TL have either an inflammatory component (e.g., bladder and gastric cancers) and/or are strongly associated with a known carcinogen, such as smoking (e.g., bladder and lung cancers). Telomere shortening can occur as a result of oxidative stress (57). The reactive oxygen species present in cigarette smoke may explain, in part, the studies suggesting an association between the cumulative lifetime exposure to cigarette smoke and more rapid telomere shortening (58, 59). Studies with detailed smoking exposure data, telomere length, and cancer are needed to better understand this potential interaction.
Chronic inflammation is a known risk factor for some cancers (e.g., esophageal, bladder, and gastric cancer) and is also associated with high granulocyte turnover (60). Since telomeres shorten with each cell division, this may result in shorter granulocyte telomeres causing a reduction of the reported TL of DNA from total leukocytes. Several cytokines may activate telomerase which could, in part, compensate for telomeric loss (61, 62). Ulcerative colitis is another example of the complex interplay between inflammation, cancer, and telomere attrition. In this chronic inflammatory condition, individuals are at increased risk of colon cancer; patients with higher rates of chromosomal instability and shorter telomeres are at greater risk of progression to colon cancer (63). It is possible that individuals who are most susceptible to smoking and/or inflammation-related cancers may also be susceptible to more rapid telomere attrition due to a combination of genetics and environmental exposures.
In summary, our meta-analysis showed suggestive evidence for an association between short TL and overall cancer, but this effect may be driven by stronger effects in specific cancers, the presence of reverse causation bias, and/or the potential effect of prior cancer therapy in case-control studies. The ORs for retrospective studies were much higher than for prospective studies (2.9 versus 1.16) which is consistent with the presence of reverse causation bias and possible contribution of cancer therapy prior to sample collection. Notably, the sub-analysis on breast cancers showed no significant association with TL. Heterogeneity between all studies was substantial. Some studies were small and the number of studies for each cancer type is still limited. Conclusions for cancer types in which only or very few studies have been conducted have to be interpreted with caution. For many cancers, the association between TL and risk of cancer has not yet been investigated. It is also biologically conceivable that analyzing average TL over all chromosomes combined might blur effects since the shortest telomere in a cell may be the most critical. Future studies of chromosome specific TL will be required to better understand this aspect. Large, prospective studies of specific cancer types which evaluate TL before and after cancer diagnosis with detailed information on treatment modalities will also be required to better understand the role of surrogate tissue TL and cancer risk.
ACKNOWLEDGEMENTS
We thank Drs. Mark H. Greene and Shahinaz Gadalla, National Cancer Institute, for insightful discussions, Drs. Jonine Figueroa and Gwen Murphy, National Cancer Institute, for their support with analyses, and Linh Duong, National Cancer Institute and Center for Disease Control and Prevention, for help with the initial literature review. We also thank Drs. Karen Pooley and Peter Willeit, Department of Public Health, University of Cambridge, UK, and Dr. Ulrika Svenson, Department of Medical Biosciences, Umea, Sweden, for generously providing additional data. This study was funded by the in intramural research program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
This work was supported by the intramural research program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
No conflicts of interest.
Reference List
- (1).Palm W, de LT. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–34. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- (2).Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–79. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- (3).Hornsby PJ. Short telomeres: cause or consequence of aging? Aging Cell. 2006;5(6):577–8. doi: 10.1111/j.1474-9726.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- (4).Shawi M, Autexier C. Telomerase, senescence and ageing. Mech Ageing Dev. 2008;129(1-2):3–10. doi: 10.1016/j.mad.2007.11.007. [DOI] [PubMed] [Google Scholar]
- (5).Baird DM. Mechanisms of telomeric instability. Cytogenet Genome Res. 2008;122(3-4):308–14. doi: 10.1159/000167817. [DOI] [PubMed] [Google Scholar]
- (6).Campisi J, Kim SH, Lim CS, Rubio M. Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol. 2001;36(10):1619–37. doi: 10.1016/s0531-5565(01)00160-7. [DOI] [PubMed] [Google Scholar]
- (7).Hackett JA, Greider CW. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene. 2002;21(4):619–26. doi: 10.1038/sj.onc.1205061. [DOI] [PubMed] [Google Scholar]
- (8).Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001;10(7):677–85. doi: 10.1093/hmg/10.7.677. [DOI] [PubMed] [Google Scholar]
- (9).Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113(26):6549–57. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Alter BP, Baerlocher GM, Savage SA, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110(5):1439–47. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wu X, Amos CI, Zhu Y, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003;95(16):1211–8. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- (12).Broberg K, Bjork J, Paulsson K, Hoglund M, Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26(7):1263–71. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]
- (13).De V I, Prescott J, Wong JY, et al. A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1152–6. doi: 10.1158/1055-9965.EPI-08-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Gramatges MM, Telli ML, Balise R, Ford JM. Longer relative telomere length in blood from women with sporadic and familial breast cancer compared with healthy controls. Cancer Epidemiol Biomarkers Prev. 2010;19(2):605–13. doi: 10.1158/1055-9965.EPI-09-0896. [DOI] [PubMed] [Google Scholar]
- (15).Han J, Qureshi AA, Prescott J, et al. A prospective study of telomere length and the risk of skin cancer. J Invest Dermatol. 2009;129(2):415–21. doi: 10.1038/jid.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hosgood HD, III, Cawthon R, He X, Chanock S, Lan Q. Genetic variation in telomere maintenance genes, telomere length, and lung cancer susceptibility. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hou L, Savage SA, Blaser MJ, et al. Telomere length in peripheral leukocyte DNA and gastric cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(11):3103–9. doi: 10.1158/1055-9965.EPI-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Jang JS, Choi YY, Lee WK, et al. Telomere length and the risk of lung cancer. Cancer Sci. 2008;99(7):1385–9. doi: 10.1111/j.1349-7006.2008.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lan Q, Cawthon R, Shen M, et al. A prospective study of telomere length measured by monochrome multiplex quantitative PCR and risk of non-Hodgkin lymphoma. Clin Cancer Res. 2009;15(23):7429–33. doi: 10.1158/1078-0432.CCR-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Lee IM, Lin J, Castonguay AJ, et al. Mean leukocyte telomere length and risk of incident colorectal carcinoma in women: a prospective, nested case-control study. Clin Chem Lab Med. 2010;48(2):259–62. doi: 10.1515/CCLM.2010.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Liu X, Bao G, Huo T, et al. Constitutive telomere length and gastric cancer risk: case-control analysis in Chinese Han population. Cancer Sci. 2009;100(7):1300–5. doi: 10.1111/j.1349-7006.2009.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).McGrath M, Wong JY, Michaud D, Hunter DJ, De V I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16(4):815–9. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- (23).Mirabello L, Huang WY, Wong JY, et al. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009 doi: 10.1111/j.1474-9726.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Mirabello L, Garcia-Closas M, Cawthon R, et al. Leukocyte telomere length in a population-based case-control study of ovarian cancer: a pilot study. Cancer Causes Control. 2010;21(1):77–82. doi: 10.1007/s10552-009-9436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Pooley KA, Sandhu MS, Tyrer J, et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res. 2010;70(8):3170–6. doi: 10.1158/0008-5472.CAN-09-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Prescott J, McGrath M, Lee IM, Buring JE, De V I. Telomere length and genetic analyses in population-based studies of endometrial cancer risk. Cancer. 2010 doi: 10.1002/cncr.25328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Risques RA, Vaughan TL, Li X, et al. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2649–55. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- (28).Shen J, Terry MB, Gurvich I, et al. Short telomere length and breast cancer risk: a study in sister sets. Cancer Res. 2007;67(11):5538–44. doi: 10.1158/0008-5472.CAN-06-3490. [DOI] [PubMed] [Google Scholar]
- (29).Shen J, Gammon MD, Terry MB, et al. Telomere length, oxidative damage, antioxidants and breast cancer risk. Int J Cancer. 2009;124(7):1637–43. doi: 10.1002/ijc.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Svenson U, Roos G. Telomere length as a biological marker in malignancy. Biochim Biophys Acta. 2009;1792(4):317–23. doi: 10.1016/j.bbadis.2009.01.017. [DOI] [PubMed] [Google Scholar]
- (31).Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- (32).Xing J, Ajani JA, Chen M, et al. Constitutive short telomere length of chromosome 17p and 12q but not 11q and 2p is associated with an increased risk for esophageal cancer. Cancer Prev Res (Phila Pa) 2009;2(5):459–65. doi: 10.1158/1940-6207.CAPR-08-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Zee RY, Castonguay AJ, Barton NS, Buring JE. Mean telomere length and risk of incident colorectal carcinoma: a prospective, nested case-control approach. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2280–2. doi: 10.1158/1055-9965.EPI-09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Shao L, Wood CG, Zhang D, et al. Telomere dysfunction in peripheral lymphocytes as a potential predisposition factor for renal cancer. J Urol. 2007;178(4 Pt 1):1492–6. doi: 10.1016/j.juro.2007.05.112. [DOI] [PubMed] [Google Scholar]
- (37).Zheng YL, Ambrosone C, Byrne C, et al. Telomere length in blood cells and breast cancer risk: investigations in two case-control studies. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Zheng YL, Loffredo CA, Shields PG, Selim SM. Chromosome 9 arm-specific telomere length and breast cancer risk. Carcinogenesis. 2009;30(8):1380–6. doi: 10.1093/carcin/bgp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Baerlocher GM, Vulto I, de JG, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat Protoc. 2006;1(5):2365–76. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- (40).Widmann TA, Herrmann M, Taha N, Konig J, Pfreundschuh M. Short telomeres in aggressive non-Hodgkin’s lymphoma as a risk factor in lymphomagenesis. Exp Hematol. 2007;35(6):939–46. doi: 10.1016/j.exphem.2007.03.009. [DOI] [PubMed] [Google Scholar]
- (41).Baird DM. New developments in telomere length analysis. Exp Gerontol. 2005;40(5):363–8. doi: 10.1016/j.exger.2005.02.008. [DOI] [PubMed] [Google Scholar]
- (42).Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- (43).MANTEL N, HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48. [PubMed] [Google Scholar]
- (44).DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- (45).Higgins JP. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–60. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- (46).Svenson U, Nordfjall K, Stegmayr B, et al. Breast cancer survival is associated with telomere length in peripheral blood cells. Cancer Res. 2008;68(10):3618–23. doi: 10.1158/0008-5472.CAN-07-6497. [DOI] [PubMed] [Google Scholar]
- (47).Fordyce CA, Heaphy CM, Joste NE, et al. Association between cancer-free survival and telomere DNA content in prostate tumors. J Urol. 2005;173(2):610–4. doi: 10.1097/01.ju.0000143195.49685.ce. [DOI] [PubMed] [Google Scholar]
- (48).Gadalla SM, Cawthon R, Giri N, Alter BP, Savage SA. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging (Albany NY) 2010 doi: 10.18632/aging.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Heaphy CM, Bisoffi M, Fordyce CA, et al. Telomere DNA content and allelic imbalance demonstrate field cancerization in histologically normal tissue adjacent to breast tumors. Int J Cancer. 2006;119(1):108–16. doi: 10.1002/ijc.21815. [DOI] [PubMed] [Google Scholar]
- (50).Engelhard M, Sack H. [Radiotherapy alone in early stages of low malignancy non-Hodgkin lymphomas] Praxis (Bern 1994 ) 1998;87(23):801–5. [PubMed] [Google Scholar]
- (51).Schroder CP, Wisman GB, de JS, et al. Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplantation. Br J Cancer. 2001;84(10):1348–53. doi: 10.1054/bjoc.2001.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Lee JJ, Nam CE, Cho SH, et al. Telomere length shortening in non-Hodgkin’s lymphoma patients undergoing chemotherapy. Ann Hematol. 2003;82(8):492–5. doi: 10.1007/s00277-003-0691-4. [DOI] [PubMed] [Google Scholar]
- (53).Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens ) 2009;8(1):7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- (54).Kim S, Parks CG, DeRoo LA, et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009;18(3):816–20. doi: 10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Radpour R, Barekati Z, Haghighi MM, et al. Correlation of telomere length shortening with promoter methylation profile of p16/Rb and p53/p21 pathways in breast cancer. Mod Pathol. 2010;23(5):763–72. doi: 10.1038/modpathol.2009.195. [DOI] [PubMed] [Google Scholar]
- (56).Kyo S, Takakura M, Kanaya T, et al. Estrogen activates telomerase. Cancer Res. 1999;59(23):5917–21. [PubMed] [Google Scholar]
- (57).Stindl R. Tying it all together: telomeres, sexual size dimorphism and the gender gap in life expectancy. Med Hypotheses. 2004;62(1):151–4. doi: 10.1016/s0306-9877(03)00316-5. [DOI] [PubMed] [Google Scholar]
- (58).von ZT, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220(1):186–93. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- (59).Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- (60).Rufer N, Brummendorf TH, Kolvraa S, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190(2):157–67. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Friedrich U, Griese E, Schwab M, et al. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000;119(3):89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]
- (62).Mondello C, Petropoulou C, Monti D, et al. Telomere length in fibroblasts and blood cells from healthy centenarians. Exp Cell Res. 1999;248(1):234–42. doi: 10.1006/excr.1999.4398. [DOI] [PubMed] [Google Scholar]
- (63).Fern L, Pallis M, Ian CG, et al. Clonal haemopoiesis may occur after conventional chemotherapy and is associated with accelerated telomere shortening and defects in the NQO1 pathway; possible mechanisms leading to an increased risk of t-AML/MDS. Br J Haematol. 2004;126(1):63–71. doi: 10.1111/j.1365-2141.2004.05006.x. [DOI] [PubMed] [Google Scholar]