Abstract
Background
Late complications of allogeneic hematopoietic stem cell transplantation (HSCT) include a risk of secondary malignancies, including oral cancers. Optimization of best clinical practices for early diagnosis and treatment of oral premalignant or malignant lesions requires an assessment of potential predisposing risk factors as well as treatment outcomes.
Methods
The medical records of patients who developed oral epithelial dysplasia (OED) and oral squamous cell carcinoma (OSCC) following allogeneic HSCT were reviewed. Data on demographics, HSCT course, chronic graft-versus-host disease (cGVHD), smoking and alcohol consumption, oral lesion characteristics, mode of therapy and clinical outcome were recorded; landmark survival was calculated.
Results
Twenty-six patients with OED (n = 8) and OSCC (n = 18) were identified with a median follow-up of 26.5 and 21.5 months, respectively. Premalignant and malignant oral lesions were diagnosed at a median time of 2.5 and 8 years after HSCT, respectively. Chronic GVHD was present in 96% of patients and of these, 96% had oral involvement. Multifocal oral cancer was found in 28% of cases, and localized recurrence was observed in 44% of cases. Five-year overall survival was 75% and 70% for OED and OSCC, respectively.
Conclusions
These results suggest that oral cGVHD may be considered a potential risk factor for the development of OSCC following allogeneic HSCT. The observation that oral cancers were frequently multifocal and recurred locally supports the concept of field cancerization and suggests that these cancers may be more aggressive compared with the non-HSCT population. Vigilant follow-up and coordination of care between hematologists and oral health specialists are critical to minimize morbidity and mortality.
Introduction
With improved outcomes after allogeneic hematopoietic stem cell transplantation (HSCT), increasing attention has been drawn to late complications in long-term survivors. Among these, survivors of allogeneic HSCT are at significantly increased risk for developing second cancers with the incidence of secondary solid tumors 2-6% at 10 years, and 6-13% at 15 years. (1-3)
Squamous cell carcinoma of the skin and mouth are the most common second solid malignancies, accounting for one-third of all secondary solid tumors, with oral squamous cell carcinoma (OSCC) representing 50% of these cases. (1, 2, 4) Curtis et al analyzed over 19,000 patients from the International Bone Marrow Transplant Registry and the Fred Hutchinson Cancer Research Center in the largest study of second cancers following allogeneic HSCT; the relative risk of OSCC was significantly increased in male patients (9.7), patients with chronic graft-versus-host disease (cGVHD; 6.0) and patients who received total body radiation as part of their conditioning regimen (3.0) as well as with time after HSCT (>10 years; relative risk 77.9). (1) Moreover, several case reports and small case series of OSCC following allogeneic HSCT have been reported. (2, 5-13) Possible mechanisms that have been proposed include radiation mutagenesis, cGVHD-related inflammation, prolonged immunosuppression from cGVHD therapy, immunological dysfunction, and carcinogenic and cytotoxic effects of immunosuppressive therapy, or a combination thereof. (8, 14, 15)
An improved understanding of the clinical features and potential factors associated with secondary OSCC, as well as its course and treatment outcomes, may be beneficial in better predicting, identifying and managing this very serious late toxicity of allogeneic HSCT. The objective of this study was to comprehensively review a multi-center cohort of patients who developed oral malignant lesions or oral epithelial dysplasia (OED) lesions after allogeneic HSCT.
Material and Methods
A retrospective review of clinical records was conducted for patients who had undergone allogeneic HSCT and were subsequently diagnosed with oral malignant or premalignant mucosal lesions. Non-epithelial cancers, such as post-transplantation lymphoproliferative disease, or relapsed hematologic malignancy with oral manifestations were excluded. Cases were collected from three transplantation centers: 1) Dana-Farber/Brigham and Women's Cancer Center, Boston, USA; 2) Hadassah University Medical Center, Jerusalem, Israel; and 3) Bone Marrow Transplant Center, State University of Campinas, Campinas, Brazil. This study was approved by each center's institutional review board. All patients were transplanted between May 1980 and November 2007 and diagnosed with OED and OSCC lesions between May 1995 and March 2010.
Clinical data included HSCT course, cGVHD history, and details of OED and OSCC lesions, including presentation, staging (for carcinomas), management, and treatment outcomes. Tobacco and alcohol histories were obtained because these are well established risk factors in the non-HSCT general population. Oral lesions were classified into two categories: 1) OED, including verrucous hyperplasia (VH) and conventional dysplasia; and 2) malignant lesions (invasive carcinoma) including OSCC and verrucous carcinoma (VC, considered a histopathologic variant of squamous cell carcinoma).
Overall survival (OS) was calculated from the date of diagnosis of secondary oral changes or malignant oral disease to the date of death, censored at the date of last contact. Freedom from recurrence (FFR) was defined as the time from the date of diagnosis of malignant oral disease until the date of recurrence for patients with malignant oral disease, censored at the date of last contact. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Twenty-six patients with a median age of 49 (range 14-67) were diagnosed with oral OED or OSCC after allogeneic HSCT (Tables 1 and 2) with a median follow-up of 26.5 and 21.5 months, respectively. Three patients (12%) developed VH, 5 patients (19%) developed dysplasia and 18 patients (69%) developed invasive carcinoma. Twenty-four patients (96%) had cGVHD, and of these patients, 96% (23/24) presented with prominent oral features, requiring a variety of systemic and topical immunosuppressive and immunomodulatory therapies (Table 3). The median time from the first allogeneic transplantation to diagnosis of OED was 3 years for VH (range 1-13) and 2 years for dysplasia (range 1-3 years), whereas the median time to diagnosis of OSCC was 8 years (range 1-14 years; Table 4). Smoking and alcohol consumption was common in this cohort: 42% smoked or previously smoked and 35% regularly consumed alcohol (Table 5).
Table 1.
Patient Distribution
| Institute | No. of Patients (N=26) | Verrucous hyperplasia | Dysplasia | Invasive carcinoma |
|---|---|---|---|---|
| Dana-Farber/Brigham and Women's Cancer Center, Boston, USA | 16 (62%) | 3 (19%) | 3 (19%) | 10 (63%) |
| Hadassah University Medical Center, Jerusalem, Israel | 7 (27%) | - | 2 (29%) | 5 (71%) |
| Bone Marrow Transplant Center, State University of Campinas, Campinas, Brazil | 3 (12%) | - | - | 3 (100%) |
Table 2.
Patient Characteristics
| N (%) | |
|---|---|
| N | 26 |
| Age, median (range) | 49 (14, 67) |
| Sex | |
| Female | 7 (27) |
| Male | 19 (73) |
| Primary diagnosis | |
| CML | 9 (35) |
| NHL | 5 (19) |
| AML | 4 (15) |
| AA | 2 (8) |
| CLL | 2 (8) |
| ALL | 1 (4) |
| MDS | 1 (4) |
| MM | 1 (4) |
| NHL/MDS† | 1 (4) |
| Cases with >1 HSCT (Type of 1st Transplant) | 7 (27) |
| Autologous | 6 (23) |
| Allogeneic | 1 (4) |
| Type of conditioning for 1st allogeneic HSCT†† | |
| Myeloablative | 14 (54) |
| Cy/TBI | 13 (50) |
| Flu/TBI | 1 (4) |
| Non-myeloablative | 12 (46) |
| Bu/Cy | 4 (15) |
| Cy | 1 (4) |
| Bu/Flu | 6 (23) |
| Bu | 1 (4) |
| No conditioning††† | 1 (4) |
Abbreviations: CML=chronic myeloid leukemia; NHL=non-Hodgkin lymphoma; AML=acute myeloid leukemia; AA=aplastic anemia; CLL=chronic lymphocytic leukemia; ALL=acute lymphocytic leukemia; MDS=myelodysplastic syndrome; MM=multiple myeloma; Bu= busulfan, Cy= cyclophosphamide, Flu= fludarabine; N/A= not available.
Patient was originally diagnosed with NHL, treated with autologous HSCT, and was subsequently diagnosed with secondary MDS and underwent non-myeloablative allogeneic HSCT.
For patients who underwent multiple allogeneic transplants, only their first conditioning regimen is provided.
After the first myeloablative autologous HSCT, the patient was subsequently treated with a DLI which resulted in marrow aplasia. No further conditioning was given prior to the second allogeneic HSCT.
Table 3.
Summary of cGVHD Summary
| N (%) | |
|---|---|
| Number of patients with history of cGVHD | 24 (96) |
| Sites of cGVHD† | |
| Skin | 23 (96) |
| Oral | 23 (96) |
| Eyes | 11 (46) |
| GI | 10 (42) |
| Hepatic | 9 (38) |
| Pulmonary | 3 (13) |
| Myofascial | 1 (4) |
| Vaginal | 1 (4) |
| Systemic cGVHD treatment† | |
| Corticosteroids | 23 (96) |
| Calcineurin inhibitors | 23 (96) |
| Mycophenolate mofetil | 7 (29) |
| Azathioprine | 6 (25) |
| Phototherapy (ECP/PUVA) | 5 (21) |
| Thalidomide | 2 (8) |
| Rapamycin | 1 (4) |
| Oral cGVHD | 23 (96) |
| Time to onset since allogeneic HSCT in months, median (range)†† | 7 (1, 23) |
| Time from oral cGVHD to diagnosis of dysplasia in years, median (range) | 0.8 (0.6, 2.1) |
| Time from oral cGVHD to diagnosis of VH in years, median (range) | 0.95 (0.1, 1.8) |
| Time from oral cGVHD to diagnosis of malignancy in years, median (range) | 6 (1, 14) |
| Topical oral cGVHD ancillary treatment† | |
| Corticosteroids | 16 (69) |
| Calcineurin Inhibitors | 6 (26) |
| UVB | 2 (9) |
Abbreviations: ECP = extracorporeal photopheresis; PUVA = psoralen-ultraviolet A phototherapy; VH = verrucous hyperplasia; UVB = ultraviolet B phototherapy.
Multiple sites or treatments per patient are possible, so the frequencies do not sum to N=23 and the percentages do not sum to 100%.
Time to oral cGVHD was calculated from the date of the corresponding allogeneic HSCT; only 18 patients had the date of onset available.
Table 4.
Characteristics and Management of Oral Lesions.
| Verrucous hyperplasia | Dysplasia | Invasive carcinoma | |
|---|---|---|---|
| N | 3 | 5 | 18 |
| Time to Development in years, median (range)† | 3 (1, 13) | 2 (1, 3) | 8 (1, 14) |
| Cancer Stage | |||
| Stage I (T1N0M0) | - | - | 7 (39) |
| Stage II | - | - | 6 (33) |
| T2N0M0 | 5 (28) | ||
| T2N1M0 | - | - | 1 (6) |
| Stage III | - | - | 4 (22) |
| T3N0M0 | - | - | 2 (11) |
| T3N0MX | - | - | 2 (11) |
| Stage IVa (T4N0M0) | - | - | 1 (6) |
| Focal versus Multifocal | |||
| Focal | 2 (67) | 5 (100) | 13 (72) |
| Multifocal | 1 (33) | 0 (0) | 5 (28) |
| Location†† | |||
| Tongue | 0 (0) | 1 (20) | 10 (55) |
| Lower Lip††† | 0 (0) | 4 (80) | 3 (17) |
| Buccal mucosa | 1 (33) | 0 (0) | 7 (39) |
| Gingiva | 2 (66) | 0 (0) | 4 (22) |
| Hard Palate | 1 (33) | 0 (0) | 1 (5) |
| Alveolar mucosa | 0 (0) | 0 (0) | 2 (10) |
| Color | |||
| Red | 0 (0) | 0 (0) | 1 (6) |
| Red/White | 0 (0) | 2 (40) | 8 (44) |
| White | 3 (100) | 3 (60) | 9 (50) |
| Clinical appearance†††† | |||
| Plaque | 3 (100) | 2 (40) | 9 (50) |
| Exophytic | 0 (0) | 0 (0) | 7 (39) |
| Ulceration | 0 (0) | 2 (40) | 5 (28) |
| Papillary | 0 (0) | 2 (40) | 2 (11) |
| Crusting | 0 (0) | 2 (40) | 1 (6) |
| Erythema | 0 (0) | 0 (0) | 3 (17) |
| Pain | 0 (0) | 1 (20) | 11 (61) |
| Anesthesia/Paresthesia | 0 (0) | 0 (0) | 2 (11) |
| Management | |||
| Surgery alone | 3 (100) | 4 (80) | 12 (67) |
| Surgery/Radiotherapy | 0 (0) | 0 (0) | 1 (6) |
| Surgery/topical 5FU | 0 (0) | 1 (20) | 0 (0) |
| Surgery/Chemotherapy/Radiotherapy | 0 (0) | 0 (0) | 4 (22) |
| Chemotherapy/Radiotherapy | 0 (0) | 0 (0) | 1 (6) |
Measured from the date of the 1st allogeneic transplant to the date of development of initial SCC. The overall median was 5 years (range 1-14).
There may have been multiple sites involved per patient, so the frequencies do not sum to N=26 and the percentages do not sum to 100%.
All lesions appeared to originate on the lip; 3 out of 7 cases also extended to the labial mucosa intraorally.
There may have been multiple clinical features per patient, so the frequencies do not sum to N=25 and the percentages do not sum to 100%.
Table 5.
Tobacco and Alcohol History
| N (%) | |
|---|---|
| Smoking status | |
| Daily smoker | 4 (15) |
| Former smoker | 7 (27) |
| Non-smoker | 15 (58) |
| Alcohol consumption | |
| Occasion (1-2 drinks/wk) | 8 (31) |
| Daily (6-7 drinks/wk) | 1 (4) |
| None | 17 (65) |
OED was most commonly observed on the lower lip, as a solitary lesion (Table 4). The median lesion dimensions at diagnosis were 2.0 × 1.0 cm and 1.0 × 1.0 cm for VH and dysplasia, respectively. All OED presented as leukoplakia (verrucous or otherwise), erythroleukoplakia, or proliferative verrucuous leukoplakia (Figure 1).(16) One case of dysplasia was associated with pain; all other cases of OED were asymptomatic. All premalignant lesions were treated surgically; one was treated with surgery followed by topical 5-fluorouracil (Figure 2).
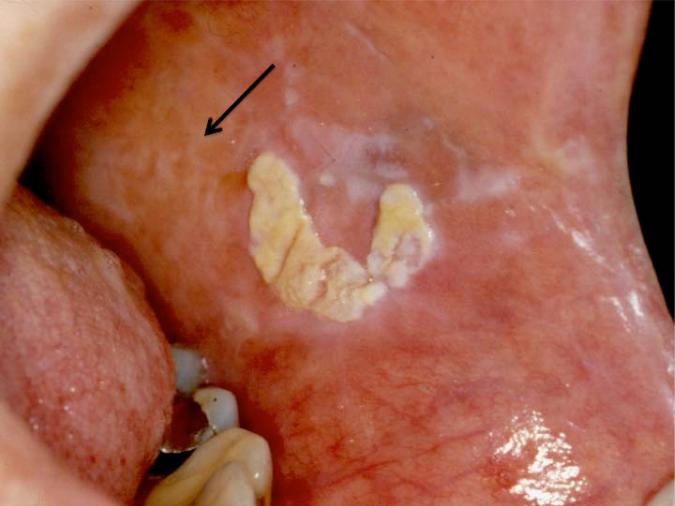
Figure 1.
Exophytic plaque on the left buccal mucosa that demonstrated verrucous hyperplasia histopathologically. Note the lighter white reticulations attributed to long-standing oral cGVHD (arrow).
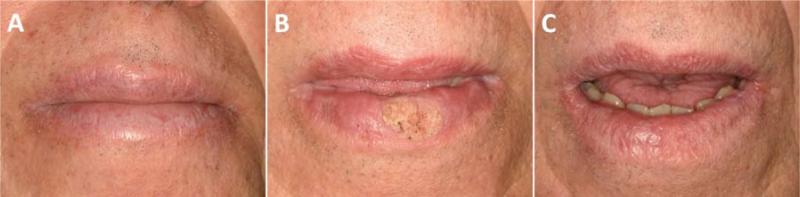
Figure 2.
Exophytic plaque on the lower lip that demonstrated dysplasia histopathologically (Day +390 post allo-HSCT) showing A) white reticular changes involving the uppper and lower lip; B) round dysplastic plaque on the lower lip; and C) complete healing of the lower lip dysplastic lesion after excision and topical treatment with 5-FU (Day +592).
Invasive carcinomas were predominately OSCC (n=15; 88%) with only 2 cases of VC observed (Figure 3 and 4; Table 5). Three cases of OSCC were preceded by a histopathological diagnosis of dysplasia (3/16, 19%; median time from dysplasia to OSCC 29 months, data not shown). The majority of invasive carcinomas (72%) were diagnosed as either Stage I or Stage II. The tongue was the most common site (n=10; 56%), followed by buccal mucosa (n=7; 39%) and lower lip (n=3; 17%); 28% presented with multifocal disease. Half of cases were purely white (50%), followed by red and white (44%) and purely red lesions (6%), and the median lesion size was 2.0 × 1.5 cm. The most frequent clinical features at diagnosis were plaques (50%), exophytic masses (39%) and ulcers (28%). Eleven cases (61%) presented with pain and two (11%) with paresthesia/anesthesia.
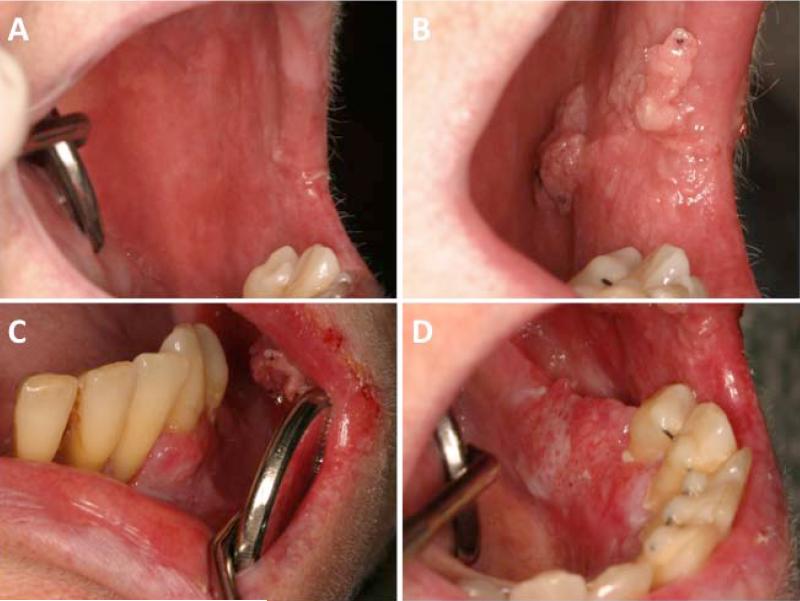
Figure 3.
Invasive squamous cell carcinoma that initially presented as persistent erythema of the left buccal mucosa (Panel A; Day +5080) that then developed into multiple pink exophytic verrucous masses (Panel B; Day +5290) as well as more flat, erythematous and speckled involvement of the right mandibular facial gingiva (Panel C) and left lingual alveolar ridge (Panel D).
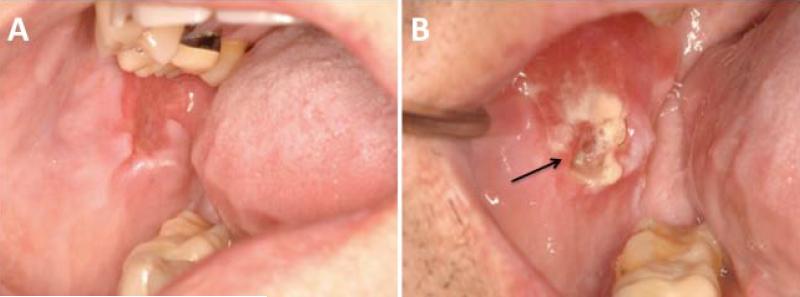
Figure 4.
Invasive squamous cell carcinoma of the right buccal mucosa. A) Lesion prior to biopsy that presented as a distinct area of erythema and atrophy in the context of bilateral oral cGVHD changes; B) painful, exophytic indurated white and red mass with focal ulceration (arrow).
All but one case was managed with surgery with or without adjuvant radiation and/or chemoradiation therapy (Table 4). Eight cases (47%) had neck dissection as a part of the surgical management and only one patient had metastasis which involved the regional lymph nodes. Radiation therapy was not administered in 12 cases, including two Stage III cases (T3N0M0 and T3N0MX) and one Stage IVa (T4N0M0). This was in part due to concerns of further increasing the risk of cancer in the treatment field and in part due to favorable histopathologic features. In addition, in one T3 case radiation therapy was initiated but then discontinued due to cancer progression.
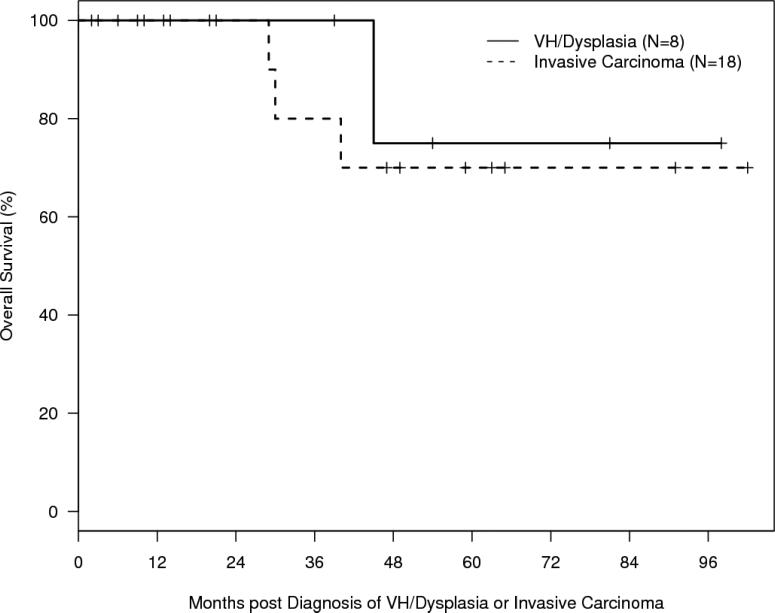
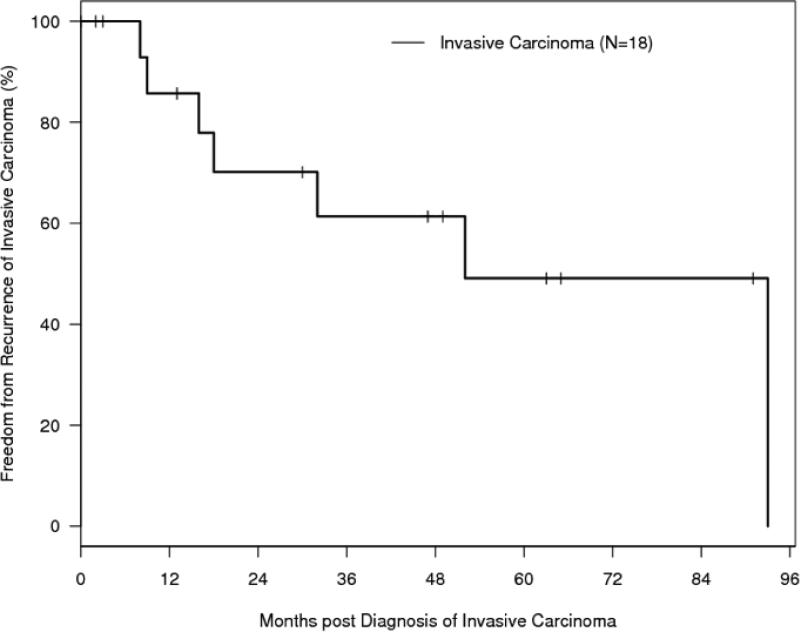
Outcomes are summarized in Table 6 and Figure 5. Local recurrence of invasive carcinoma occurred in 44% of cases with a median time to recurrence of 17 months (range 8-93) from the time of diagnosis. Five-year freedom from recurrence of invasive carcinoma was 46% (SE, 15%). In three cases (12%) a second primary OSCC occurred at a non-contiguous site from the original tumor. Five-year overall survival was 75% (SE, 22%) for patients with premalignant changes and 70% (SE, 14%) for patients with carcinoma. The median follow-up time for the entire cohort of patients was 47 months (95% CI 14-63).
Table 6.
Outcome Summary
| VH/Dysplasia | Invasive Carcinoma | |
|---|---|---|
| No. of Patients | 8 | 18 |
| Recurrence of Invasive Carcinoma | N/A | 8 (44) |
| New site | N/A | 5 (28) |
| Same site | N/A | 3 (12) |
| 5 year FFR ± SE (%)† | N/A | 46 ± 15 |
| 5 year OS ± SE (%)†† | 75 ± 22 | 70 ± 14 |
| Time to Recurrence in months, median (range) | N/A | 17 (8, 93) |
SE: Standard error; FFR = Freedom from recurrence. FFR was calculated from the date of diagnosis of invasive carcinoma to the date of recurrence.
OS = Overall survival; OS was calculated from the date of diagnosis of secondary oral changes to the date of death.
Discussion
There are approximately 10,000 new cases of oral cancer in the US annually, with an incidence of 10.4 cases per 100,000 and overall five-year survival rate of 51.5%. (17, 18) The primary risk factors include tobacco and alcohol, with an emerging role for HPV infection in a subset of patients. (12, 15, 19, 20)
We were interested describing clinical characteristics and outcomes of OED and OSCC complicating long-term survivors of allogeneic HSCT. (8) The overall risk of second malignancies after allogeneic HSCT has been well reported in the literature in retrospective, observational and cohort studies. (1-3, 5, 21-34) However, details of the clinical aspects of oral cancers following HSCT have only been described in case reports or small case series. (4, 14, 35-37) This lack of information about the nature of oral cancer in this high-risk patient population has hindered clinical decision-making and the ability to develop preventive strategies and screening recommendations.
Most of our patients (24 out of 26 patients) developed cGVHD prior to the diagnosis of OED and OSCC, and in all but one of them (23 out of 24 patients) the oral tissues were involved. Eighty per cent of the patients with oral cGVHD received some type of localized therapy (e.g. topical steroids) specifically for their oral cGVHD, suggesting that the disease burden was considerable. The median interval from the diagnosis of oral cGVHD to the diagnosis of oral cancer was six years. These findings support the model that the presence of cGVHD, and specifically in the oral mucosa, plays a significant role in the pathogenesis of oral cancer after allogeneic HSCT. Since GVHD is an alloimmune inflammatory process, long-term immunologically-mediated injury of the mucosa by T-cells may predispose to genomic instability and eventually to malignant transformation, particularly in the context of prior tobacco and alcohol use. (1) A recent study by Khan et al demonstrated genomic instability in oral, but not nasal (which is a rare site of cGVHD) cytological samples in patients with a history of cGVHD, providing both a direct role for cGVHD as well as a potential mechanism of oncogenesis, as genomic instability has been recently associated with epithelial malignancies. (1, 38-40) This may be more specifically mediated by long-term upregulation of cytokines, such as type I interferon, that are highly active in both cGVHD as well as OSCC. (41, 42, 39, 40) Furthermore, there is an emerging body of evidence suggesting that, at least in some cases of secondary solid cancers, donor-derived cells may play a role in carcinogenesis. (43, 44)
The majority of the patients with oral cGVHD were treated with topical and/or intralesional immunomodulators, including corticosteroids and calcineurin inhibitors (tacrolimus and cyclosporine). While there have been case reports of skin cancer developing following local treatment with topical tacrolimus, and two cases of oral cancer following intraoral topical therapy, a causal relationship remains uncertain. (45-50) Nonetheless, topical tacrolimus ointment has a FDA black box warning regarding its potential for increasing the risk of skin cancer. It has been suggested that topically applied tacrolimus may promote carcinogenesis through its immunosuppressive properties (51), by activating mitogen-activated protein kinase pathways (49), which promotes cell division, or by inhibiting keratinocyte DNA repair. (52) Systemic immunosuppressive therapy has also been identified as a potential risk factor for oral cancer; however similar to topical therapies, the exposure and the presence of cGVHD are closely interconnected and it therefore remains difficult to attribute any specific carcinogenic risk. There is no information about malignant transformation associated with the use of other topical immunosuppressive agents. Two patients from our series had previously been managed with localized intraoral ultraviolet B phototherapy for their oral cGVHD; however, unlike PUVA, this modality of therapy has not been associated with an increased incidence of skin cancer when used for the management of psoriasis and other inflammatory skin disorders.(53)
The majority of patients in our study were found to have low stage oral cancer (39% Stage I, 33% Stage II). It is noteworthy that most of these patients were routinely examined by oral medicine specialists, due to the presence of oral cGVHD. These frequent oral evaluations may have contributed to an earlier diagnosis of oral cancer, and perhaps to the favorable 5-year survival rate of 70%, compared with an overall five-year survival rate of 51.5% in the general population. (17, 18, 54)
Eight out of the 18 patients with oral cancer were complicated with a second oral cancer or recurrence of the first tumor. In the non-HSCT population, the incidence of second primary oral cancers is reported to be between 3% and 10%, compared with 45% in our series. (55, 56) Furthermore, in five patients the primary carcinoma was found in several foci. These findings suggest that the nature of oral cancer after allogeneic HSCT may be different and possibly more aggressive than in non-HSCT patients, perhaps due to field changes (e.g. genomic instability) occurring as a result of long-standing oral mucosal inflammation, as well as underlying mutagenic injury from conditioning, alcohol, and tobacco use. (40, 43, 57) This is further supported by the fact that the buccal mucosa, one of the most commonly affected intraoral sites by cGVHD, was also one of the most frequent sites of OSCC in our series; however, buccal mucosa is not considered a high-risk site in non-HSCT patients. (58, 59) Despite these features, only one case had clinical evidence of lymph node metastasis on presentation. Although HPV testing was not performed in this study, none of the cases were located in the oropharynx, tonsils, or base of tongue, the three sites that have been linked epidemiologically to HPV 16 infection.(60)
There are several limitations of this study. First, due to the retrospective study design, data collection was limited to only those patients who had developed OSCC with the depth and accuracy of the available medical records. Second, although this study included a relatively large series of cancer cases, the number of patients with advanced stage (III and IV) tumors was insufficient to evaluate with respect to efficacy of treatment or the effect of treatment modality on the recurrence rate or survival. Considering that radiotherapy is a risk factor for cancer (42), the question remains as to whether radiotherapy should be employed for the treatment of oral cancer in patients with multiple other risk factors, such as conditioning regimen, underlying hematological malignant disease, cGVHD and treatment with immunomodulators. Lastly, while the goal of the study was to include all OED lesions in order to delineate the malignant potential of cGVHD, the actual risks of malignant transformation with dysplasia and VH are unknown. For this reason, cases of frank carcinoma were considered separately from dysplasia and VH.
In summary, this is a descriptive analysis of a large and highly characterized series of patients with OED and OSCC after allogeneic HSCT in which oral cGVHD may be considered a potential risk factor for oral cancer and demonstrates the aggressive nature of this serious late complication of allogeneic HSCT. Carefully coordinated long-term follow-up by a comprehensive cancer team that includes oral medicine expertise is recommended, and patients should be well-informed of cancer risk.(61) Large prospective multicenter studies are necessary to formally identify risk factors that can be used to develop preventive and screening strategies.
Figure 5A. Overall Survival.
Kaplan-Meier curve of overall survival (OS) for patients who developed VH/dysplasia (N = 8) or invasive carcinoma (N = 18) post-HSCT. Curves are calculated from the time of diagnosis of VH/dysplasia or invasive carcinoma.
Figure 5B. Freedom from Recurrence (FFR).
Kaplan-Meier curve of freedom from recurrence (FFR) for patients who developed invasive carcinoma (N = 18) post-HSCT. Curves are calculated from the time of diagnosis of invasive carcinoma.
Acknowledgements
The oral medicine specialists (HM, SE, MEC, SW and NT) thank the Hematopoietic Cell Transplantation and Head and Neck Oncology departments at their respective institutions for their daily collaboration in the management of these patients. We also extend our gratitude to our patients and their families. Supported in part by NIH grant CA142106.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336(13):897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Louie AD, Bhatia R, O'Donnell MR, Fung H, Kashyap A, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19(2):464–71. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 3.Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21(7):1352–8. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 4.Otsubo H, Yokoe H, Miya T, Atsuta F, Miura N, Tanzawa H, et al. Gingival squamous cell carcinoma in a patient with chronic graft-versus-host disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(2):171–4. doi: 10.1016/s1079-2104(97)90065-2. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman E, Socie G, Devergie A, Bourdeau-Esperou H, Traineau R, Cosset JM. Bone marrow transplantation in 107 patients with severe aplastic anemia using cyclophosphamide and thoraco-abdominal irradiation for conditioning: long-term follow-up. Blood. 1991;78(9):2451–5. [PubMed] [Google Scholar]
- 6.Sacchi S, Marcheselli L, Bari A, Marcheselli R, Pozzi S, Luminari S, et al. Secondary malignancies after treatment for indolent non-Hodgkin's lymphoma: a 16-year follow-up study. Haematologica. 2008;93(3):398–404. doi: 10.3324/haematol.12120. [DOI] [PubMed] [Google Scholar]
- 7.Shimada K, Yokozawa T, Atsuta Y, Kohno A, Maruyama F, Yano K, et al. Solid tumors after hematopoietic stem cell transplantation in Japan: incidence, risk factors and prognosis. Bone Marrow Transplant. 2005;36(2):115–21. doi: 10.1038/sj.bmt.1705020. [DOI] [PubMed] [Google Scholar]
- 8.Demarosi F, Lodi G, Carrassi A, Soligo D, Sardella A. Oral malignancies following HSCT: graft versus host disease and other risk factors. Oral Oncol. 2005;41(9):865–77. doi: 10.1016/j.oraloncology.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Witherspoon RP, Deeg HJ, Storb R. Secondary malignancies after marrow transplantation for leukemia or aplastic anemia. Transplant Sci. 1994;4(1):33–41. [PubMed] [Google Scholar]
- 10.Socie G, Scieux C, Gluckman E, Soussi T, Clavel C, Saulnier P, et al. Squamous cell carcinomas after allogeneic bone marrow transplantation for aplastic anemia: further evidence of a multistep process. Transplantation. 1998;66(5):667–70. doi: 10.1097/00007890-199809150-00023. [DOI] [PubMed] [Google Scholar]
- 11.Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129(1):106–12. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 12.Chen MH, Chang PM, Li WY, Hsiao LT, Hong YC, Liu CY, et al. High incidence of oral squamous cell carcinoma independent of HPV infection after allogeneic hematopoietic SCT in Taiwan. Bone Marrow Transplant. doi: 10.1038/bmt.2010.163. [DOI] [PubMed] [Google Scholar]
- 13.Montebugnoli L, Gissi DB, Marchetti C, Foschini MP. Multiple squamous cell carcinomas of the oral cavity in a young patient with graft-versus-host disease following allogenic bone marrow transplantation. Int J Oral Maxillofac Surg. doi: 10.1016/j.ijom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Abdelsayed RA, Sumner T, Allen CM, Treadway A, Ness GM, Penza SL. Oral precancerous and malignant lesions associated with graft-versus-host disease: report of 2 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(1):75–80. doi: 10.1067/moe.2002.119736. [DOI] [PubMed] [Google Scholar]
- 15.Curtis RE, Metayer C, Rizzo JD, Socie G, Sobocinski KA, Flowers ME, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105(10):3802–11. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagan J, Scully C, Jimenez Y, Martorell M. Proliferative verrucous leukoplakia: a concise update. Oral Dis. 2010;16(4):328–32. doi: 10.1111/j.1601-0825.2009.01632.x. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 18.Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT. Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002;24(2):165–80. doi: 10.1002/hed.10004. [DOI] [PubMed] [Google Scholar]
- 19.Ko YC, Huang YL, Lee CH, Chen MJ, Lin LM, Tsai CC. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J Oral Pathol Med. 1995;24(10):450–3. doi: 10.1111/j.1600-0714.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–83. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeg HJ, Sanders J, Martin P, Fefer A, Neiman P, Singer J, et al. Secondary malignancies after marrow transplantation. Exp Hematol. 1984;12(8):660–6. [PubMed] [Google Scholar]
- 22.Witherspoon RP, Fisher LD, Schoch G, Martin P, Sullivan KM, Sanders J, et al. Secondary cancers after bone marrow transplantation for leukemia or aplastic anemia. N Engl J Med. 1989;321(12):784–9. doi: 10.1056/NEJM198909213211203. [DOI] [PubMed] [Google Scholar]
- 23.Socie G, Henry-Amar M, Devergie A, Wibault P, Neiger M, Cosset JM, et al. Poor clinical outcome of patients developing malignant solid tumors after bone marrow transplantation for severe aplastic anemia. Leuk Lymphoma. 1992;7(5-6):419–23. doi: 10.3109/10428199209049797. [DOI] [PubMed] [Google Scholar]
- 24.Witherspoon RP, Storb R, Pepe M, Longton G, Sullivan KM. Cumulative incidence of secondary solid malignant tumors in aplastic anemia patients given marrow grafts after conditioning with chemotherapy alone. Blood. 1992;79(1):289–91. [PubMed] [Google Scholar]
- 25.Deeg HJ, Witherspoon RP. Risk factors for the development of secondary malignancies after marrow transplantation. Hematol Oncol Clin North Am. 1993;7(2):417–29. [PubMed] [Google Scholar]
- 26.Socie G, Henry-Amar M, Bacigalupo A, Hows J, Tichelli A, Ljungman P, et al. Malignant tumors occurring after treatment of aplastic anemia. European Bone Marrow Transplantation-Severe Aplastic Anaemia Working Party. N Engl J Med. 1993;329(16):1152–7. doi: 10.1056/NEJM199310143291603. [DOI] [PubMed] [Google Scholar]
- 27.Lowsky R, Lipton J, Fyles G, Minden M, Meharchand J, Tejpar I, et al. Secondary malignancies after bone marrow transplantation in adults. J Clin Oncol. 1994;12(10):2187–92. doi: 10.1200/JCO.1994.12.10.2187. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia S, Ramsay NK, Steinbuch M, Dusenbery KE, Shapiro RS, Weisdorf DJ, et al. Malignant neoplasms following bone marrow transplantation. Blood. 1996;87(9):3633–9. [PubMed] [Google Scholar]
- 29.Deeg HJ, Socie G, Schoch G, Henry-Amar M, Witherspoon RP, Devergie A, et al. Malignancies after marrow transplantation for aplastic anemia and fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients. Blood. 1996;87(1):386–92. [PubMed] [Google Scholar]
- 30.Andre M, Henry-Amar M, Blaise D, Colombat P, Fleury J, Milpied N, et al. Treatment-related deaths and second cancer risk after autologous stem-cell transplantation for Hodgkin's disease. Blood. 1998;92(6):1933–40. [PubMed] [Google Scholar]
- 31.Kolb HJ, Socie G, Duell T, Van Lint MT, Tichelli A, Apperley JF, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med. 1999;131(10):738–44. doi: 10.7326/0003-4819-131-10-199911160-00004. [DOI] [PubMed] [Google Scholar]
- 32.Socie G, Curtis RE, Deeg HJ, Sobocinski KA, Filipovich AH, Travis LB, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18(2):348–57. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- 33.Lishner M, Patterson B, Kandel R, Fyles G, Curtis JE, Meharchand J, et al. Cutaneous and mucosal neoplasms in bone marrow transplant recipients. Cancer. 1990;65(3):473–6. doi: 10.1002/1097-0142(19900201)65:3<473::aid-cncr2820650316>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 34.Vose JM, Kennedy BC, Bierman PJ, Kessinger A, Armitage JO. Long-term sequelae of autologous bone marrow or peripheral stem cell transplantation for lymphoid malignancies. Cancer. 1992;69(3):784–9. doi: 10.1002/1097-0142(19920201)69:3<784::aid-cncr2820690328>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 35.Bradford CR, Hoffman HT, Wolf GT, Carey TE, Baker SR, McClatchey KD. Squamous carcinoma of the head and neck in organ transplant recipients: possible role of oncogenic viruses. Laryngoscope. 1990;100(2 Pt 1):190–4. doi: 10.1288/00005537-199002000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Millen FJ, Rainey MG, Hows JM, Burton PA, Irvine GH, Swirsky D. Oral squamous cell carcinoma after allogeneic bone marrow transplantation for Fanconi anaemia. Br J Haematol. 1997;99(2):410–4. doi: 10.1046/j.1365-2141.1997.3683184.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Epstein JB, Poh CF, Berean K, Lam WL, Zhang X, et al. Comparison of HPV infection, p53 mutation and allelic losses in post-transplant and non-posttransplant oral squamous cell carcinomas. J Oral Pathol Med. 2002;31(3):134–41. doi: 10.1034/j.1600-0714.2002.310302.x. [DOI] [PubMed] [Google Scholar]
- 38.Khan FM, Sy S, Louie P, Ugarte-Torres A, Berka N, Sinclair G, et al. Genomic instability after allogeneic hematopoietic cell transplantation is frequent in oral mucosa, particularly in patients with history of chronic graft-vs-host disease, and rare in nasal mucosa. Blood. 2010 doi: 10.1182/blood-2009-10-249201. [DOI] [PubMed] [Google Scholar]
- 39.Faber P, Fisch P, Waterhouse M, Schmitt-Graff A, Bertz H, Finke J, et al. Frequent genomic alterations in epithelium measured by microsatellite instability following allogeneic hematopoietic cell transplantation in humans. Blood. 2006;107(8):3389–96. doi: 10.1182/blood-2005-08-3431. [DOI] [PubMed] [Google Scholar]
- 40.Themeli M, Petrikkos L, Waterhouse M, Bertz H, Lagadinou E, Zoumbos N, et al. Alloreactive microenvironment after human hematopoietic cell transplantation induces genomic alterations in epithelium through an ROS-mediated mechanism: in vivo and in vitro study and implications to secondary neoplasia. Leukemia. 2010;24(3):536–43. doi: 10.1038/leu.2009.284. [DOI] [PubMed] [Google Scholar]
- 41.Imanguli MM, Swaim WD, League SC, Gress RE, Pavletic SZ, Hakim FT. Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood. 2009;113(15):3620–30. doi: 10.1182/blood-2008-07-168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chi AC. Squamous cell carcinoma. In: Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Oral and maxillofacial pathology. 3rd edn. St. Louis, Missouri; Saunders Elsevier: 2009. p. 409.p. 413. [Google Scholar]
- 43.Janin A, Murata H, Leboeuf C, Cayuela JM, Gluckman E, Legres L, et al. Donor-derived oral squamous cell carcinoma after allogeneic bone marrow transplantation. Blood. 2009;113(8):1834–40. doi: 10.1182/blood-2008-07-171702. [DOI] [PubMed] [Google Scholar]
- 44.Tomihara K, Dehari H, Yamaguchi A, Abe M, Miyazaki A, Nakamori K, et al. Squamous cell carcinoma of the buccal mucosa in a young adult with history of allogeneic bone marrow transplantation for childhood acute leukemia. Head Neck. 2009;31(4):565–8. doi: 10.1002/hed.20931. [DOI] [PubMed] [Google Scholar]
- 45.Lerche CM, Philipsen PA, Poulsen T, Wulf HC. Topical tacrolimus in combination with simulated solar radiation does not enhance photocarcinogenesis in hairless mice. Exp Dermatol. 2008;17(1):57–62. doi: 10.1111/j.1600-0625.2007.00617.x. [DOI] [PubMed] [Google Scholar]
- 46.Patel TS, Greer SC, Skinner RB., Jr. Cancer concerns with topical immunomodulators in atopic dermatitis: overview of data and recommendations to clinicians. Am J Clin Dermatol. 2007;8(4):189–94. doi: 10.2165/00128071-200708040-00001. [DOI] [PubMed] [Google Scholar]
- 47.Ormerod AD. Topical tacrolimus and pimecrolimus and the risk of cancer: how much cause for concern? Br J Dermatol. 2005;153(4):701–5. doi: 10.1111/j.1365-2133.2005.06899.x. [DOI] [PubMed] [Google Scholar]
- 48.Murphy D. Briefing Information. Food and Drug Administration, Pediatric Advisory Committee; 2005. [Google Scholar]
- 49.Becker JC, Houben R, Vetter CS, Brocker EB. The carcinogenic potential of tacrolimus ointment beyond immune suppression: a hypothesis creating case report. BMC Cancer. 2006;6:7. doi: 10.1186/1471-2407-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattsson U, Magnusson B, Jontell M. Squamous cell carcinoma in a patient with oral lichen planus treated with topical application of tacrolimus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 110(1):e19–25. doi: 10.1016/j.tripleo.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 51.Niwa Y, Terashima T, Sumi H. Topical application of the immunosuppressant tacrolimus accelerates carcinogenesis in mouse skin. Br J Dermatol. 2003;149(5):960–7. doi: 10.1111/j.1365-2133.2003.05735.x. [DOI] [PubMed] [Google Scholar]
- 52.Yarosh DB, Pena AV, Nay SL, Canning MT, Brown DA. Calcineurin inhibitors decrease DNA repair and apoptosis in human keratinocytes following ultraviolet B irradiation. J Invest Dermatol. 2005;125(5):1020–5. doi: 10.1111/j.0022-202X.2005.23858.x. [DOI] [PubMed] [Google Scholar]
- 53.Hearn RM, Kerr AC, Rahim KF, Ferguson J, Dawe RS. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. 2008;159(4):931–5. doi: 10.1111/j.1365-2133.2008.08776.x. [DOI] [PubMed] [Google Scholar]
- 54.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345(26):1890–900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 55.Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, Byers RM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323(12):795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 56.Sturgis EM, Miller RH. Second primary malignancies in the head and neck cancer patient. Ann Otol Rhinol Laryngol. 1995;104(12):946–54. doi: 10.1177/000348949510401206. [DOI] [PubMed] [Google Scholar]
- 57.Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45(4-5):301–8. doi: 10.1016/j.oraloncology.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Treister NS, Cook EF, Jr., Antin J, Lee SJ, Soiffer R, Woo SB. Clinical evaluation of oral chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(1):110–5. doi: 10.1016/j.bbmt.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 59.Epstein JB, Gorsky M, Cabay RJ, Day T, Gonsalves W. Screening for and diagnosis of oral premalignant lesions and oropharyngeal squamous cell carcinoma: role of primary care physicians. Can Fam Physician. 2008;54(6):870–5. [PMC free article] [PubMed] [Google Scholar]
- 60.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 61.Rethman MP, Carpenter W, Cohen EE, Epstein J, Evans CA, Flaitz CM, et al. Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. J Am Dent Assoc. 141(5):509–20. doi: 10.14219/jada.archive.2010.0223. [DOI] [PubMed] [Google Scholar]