Abstract
Systemic inflammation (SI) is associated with impairment of cardiac autonomic modulation (CAM), which is associated with cardiac disease. However, there is limited data about SI on CAM circadian pattern, which this study is aimed to investigate in a middle-aged sample. C-reactive protein (CRP) was used as a SI marker. We performed HRV analysis on each 5-minute segment RRs from of a 24-hour 12-lead ECG to obtain time and frequency domain HRV indices as measures of CAM. The circadian pattern of CAM was analyzed by a two-stage modeling. Stage one, for each individual we fit a cosine periodic model based on the 288 segments of 5-minute HRV data to produce three individual-level cosine parameters that quantity the circadian pattern: mean (M), amplitude (Â), and acrophase time (θ), measure the overall average, the amplitude of the oscillation, and the timing of the highest oscillation, respectively. Stage two, we used random-effects-meta-analysis to summarize the effects of CRP on the three circadian parameters obtained in stage one. CRP was adversely associated with lower M of log-HF, log-LF, SDNN, and RMSSD [β (SE): −0.22 (0.07) ms2, −0.20 (0.06) ms2, −3.62 (0.99) ms, and −2.32 (0.73) ms, respectively, with all p-values<0.01]. More importantly, CRP was also adversely associated with lower  of SDNN and RMSSD [β (SE): −0.84 (0.44) ms and −0.86 (0.38) ms, respectively, both p-values<0.05]. SI is adversely associated with circadian pattern of CAM, suggesting that the cardiac risk associated with SI may be partially mediated via inflammation-related changes in CAM.
Keywords: C-reactive Protein, Inflammatory Marker, Heart Rate Variability, Periodic Regression, Random-effects Model, Meta-analysis, Community Population
1. Introduction
Cytokine production by the immune system plays an important role in pathophysiological processes of chronic inflammatory diseases, including atherosclerosis (Libby, 2002). Excessive expression of pro-inflammatory cytokines is a reliable marker of atherosclerosis risk. Higher levels of C-reactive protein (CRP) and white blood cell (WBC) count are major markers of higher burden of systemic inflammation, and have been associated with increased risk of cardiovascular disease morbidity and mortality in population-based studies (Blake et al., 2003; Kucharska-Newton et al., 2009; Nelson et al., 2000; Phillips et al., 1992; Ridker et al., 1997).
Heart rate variability (HRV) is regulated by the balance of sympathetic and parasympathetic modulations, and it is a commonly used noninvasive measurement of cardiac autonomic modulation (CAM) (Task force of the European society of cardiology and the North American society of pacing and electrophysiology, 1996). Many previous prospective studies in patients and in population-based samples have demonstrated that lower HRV calculated from short-term normal RR intervals, ranging from several minutes to several hours, was associated with significantly higher risk of mortality (Bigger et al., 1992; Dekker et al., 2000), sudden cardiac death (La Rovere et al., 2003), and incidence of coronary heart disease (CHD) (Liao et al., 1997; Tsuji et al., 1996).
Several recent studies have reported an inverse association between various HRV indices, calculated from short-time or 24-hour ECG, and the burden of systemic inflammation in patients with stable or unstable CHD (Lanza et al., 2006; Madsen et al., 2007) and in subjects without CHD (Araujo et al., 2006; Jensen-Urstad et al., 1998; Sajadieh et al., 2004; Sloan et al., 2007; Thayer, and Fischer, 2009). Others have reported a lack of such association in patients with suspected CHD (Yue et al., 2007). However, all of these studies were based on the overall mean levels of HRV. Several studies have described a circadian pattern of CAM (Lombardi et al., 1992; Malpas, and Purdie, 1990; Massin et al., 2000; Nakagawa et al., 1998), which can be quantified with a cosine periodic regression model consisting with three cosine function parameters: mean (M), amplitude (Â), and acrophase (θ). The cosine function parameter M measures the overall average of a HRV index, the  measures the amplitude of the oscillation of a HRV index, and the θ measures the clock time when the highest oscillation (amplitude) is reached. The disturbances of the circadian pattern of CAM can be at the levels of Mean, amplitude, and/or acrophase. However, to our knowledge, no study has examined the effects on inflammation on the three circadian parameters that quantify the circadian pattern of CAM. Therefore, the objective of this study is to examine the effects of inflammation markers on the circadian pattern of CAM, reflected as the overall mean, the amplitude of oscillation, and the time of acrophase, in a community-dwelling sample.
2. Methods
2.1. Study population
For this report, we used the data collected for the Air Pollution and Cardiac Risk and its Time Course (APACR) study, which we designed to investigate the mechanisms and the time course of the adverse effects of fine particulate matter (PM2.5) on cardiac electrophysiology, blood coagulation, and systemic inflammation. Recruitment methods for the APACR study have been published elsewhere (He et al., 2010; Liao et al., 2010). All study participants were recruited from communities in central Pennsylvania, primarily from the Harrisburg metropolitan area. The inclusion criteria for the study included nonsmoking adults, ≥ 45 years old, who had not been diagnosed with severe cardiac problems (defined as diagnosed valvular heart disease, congenital heart disease, acute myocardial infarction or stroke within 6 months, or congestive heart failure). Approximately 75% of the individuals who were contacted and who met our inclusion criteria were enrolled in the APACR study. We enrolled and examined 106 individuals for the APACR study between November, 2007 and June, 2009. The study protocol was approved by Penn State University College of Medicine IRB. All study participants provided written informed consent.
Study participants were examined in the Penn State College of Medicine General Clinical Research Center (GCRC) in the morning between 8 and 10 AM of the first visit (Day 1). All participants fasted for at least 8 hours before the clinical examination. After completing a health history questionnaire, a trained research nurse measured seated blood pressure three times, height, and weight, and drew 50 ml of blood. A trained investigator connected the Holter ECG recorders between 9 and 10 AM. Participants were then released to go on with their regular daily routines in the period of ongoing Holter recording. The next morning (Day 2), the participants came back to the GCRC to disconnect the Holter monitors, and had another 50 ml of blood drawn.
2.2. Biomarkers of systemic inflammation
The average of Day 1 and Day 2 markers of inflammation (high sensitivity CRP and WBC count), measured at the Penn State GCRC Central Laboratory, were used in this report.
2.3. Holter ECG recording and HRV variables
A high fidelity (sampling frequency 1000 Hz) 12-lead H-Scribe Holter System (Mortara Instrument, Inc.) was used for 24-hour beat-to-beat ECG data collection. The standardized operating procedures for the APACR study developed by the study investigators were followed rigorously in the data collection, retrieval, offline processing and HRV analysis. The main objective of the offline processing was to verify the Holter-identified ECG waves, and to identify and label additional electronic artifacts and arrhythmic beats in the ECG recording.
After removing artifacts and ectopic beats, any RR interval <400 ms, >2000 ms, or where the ratio from two adjacent RR intervals was <0.80 or >1.20 were also excluded from the HRV analysis. The remaining normal RR intervals were then divided into 288 5-minute segments. The time- and frequency-domain HRV analysis were performed on each of the 5-minute segments, if the total length of such normal RR intervals was greater than 4 minutes (80% of original data), using the Fast Fourier Transformation (FFT) method. Briefly, the adjacent RR interval data were interpolated using a piecewise cubic spline interpolation approach, with a 2 Hz sampling rate. The FFT was performed on the equidistantly interpolated RR time series. We used a second order polynomial model to remove the slow non-stationary trends of the HRV signal. The following HRV indices were calculated as measures of CAM: standard deviation of all RR intervals (SDNN, ms), square root of the mean of the sum of the squares of differences between adjacent RR intervals (RMSSD, ms), power in the low frequency range (0.04–0.15 Hz, LF), power in the high frequency range (0.15–0.40 Hz, HF), and the ratio of LF to HF (LF/HF). Following current recommendations (Task force of the european society of cardiology and the north american society of pacing and electrophysiology, 1996), we performed logarithmic transformations on HF and LF prior to statistical analysis.
2.4. Statistical Analysis
From the 106 individuals, we excluded 5 subjects from this report because of the following reason(s): failure to draw blood (n=1), technical problems with the Holter recording (n=1), and insufficient normal RR intervals for HRV analysis (less than 20 hours of 24 recording) (n=3). As a result, this report uses the data from the remaining 101 individuals. Each individual contributed up to 288 segments of 5-minute RR interval data within 24 hours, resulting in up to 29,088 data segments. We analyzed 28334 segments of HRV data after excluding segments with less than 4 minutes of normal RR interval data.
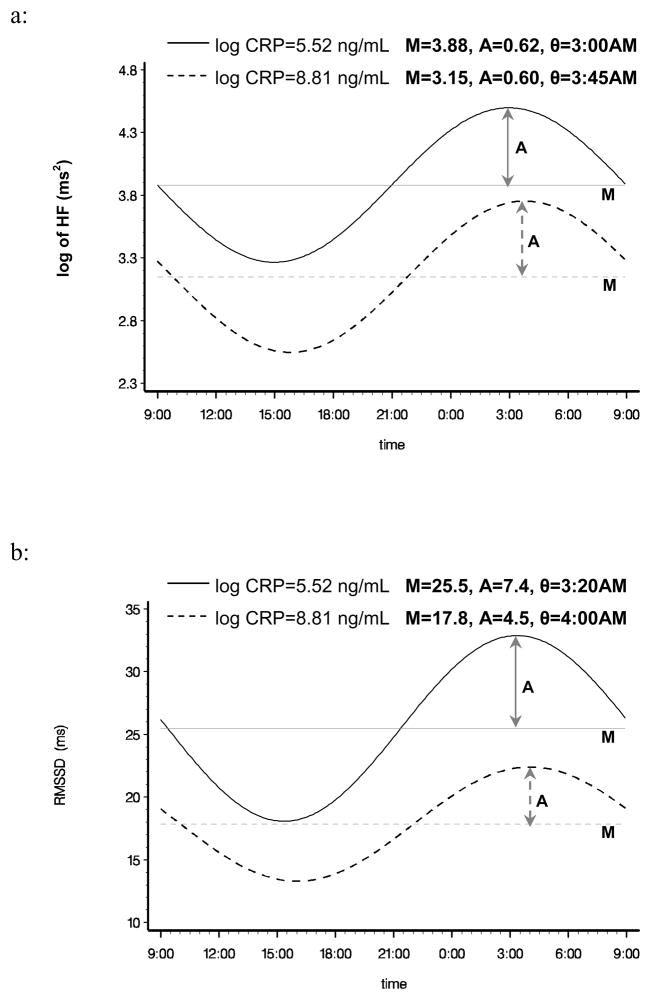
A two-stage analysis was performed to assess the relationship between inflammation markers and the circadian pattern of HRV. In the first stage, for each individual we fit the HRV data based on all available 5-minute segments to a cosine periodic regression model (D’Negri et al., 2005): HRVi(t)= Mi + Ai·cos [2π ·(t−θi)/T] + εi, i=1, …, 101, where Mi is the daily average of HRV of the ith subject, Ai is the amplitude of HRV of the ith subject around Mi, t is the time-specific segment order number, T is the total number of 5-minute segments in 24 hours, θi is the acrophase (the lag from the reference time point (9 AM) to the time of the zenith of the cosine curve fit to the data of the ith subject), and εi is the error term for the ith subject. One unit of t corresponds to 5 minutes, with 1 indicating 9:00 AM to 9:05 AM, 2 indicating 9:05 AM to 9:10 AM, etc. Thus, from the above described cosine model, we obtained the estimated individual-level cosine periodic regression parameters, namely the M, Â, and θ to quantify the periodicity of the HRV variables. In the second stage, we used random-effects meta-analyses to obtain overall estimates of M, Â and θ, and their 95% confident intervals (CIs) to assess the associations between inflammation markers and the three components of the circadian pattern of HRV (DerSimonian, and Laird, 1986). To visualize the associations between circadian patterns of HRV with inflammatory biomarkers, we plotted in Figure 1 the cosine periodic model estimates for log HF (as an example of frequency domain HRV) and RMSSD (as an example of time domain HRV) over the clock time at lower and higher levels of CRP (corresponding to approximately 10th and 90th percentile of CRP). To confirm the associations between the biomarkers of systemic inflammation and the means from the random-effects meta-analysis models, we also applied linear mixed-effects models, specifying a first-order autoregressive covariance structure, to assess the associations between HRV variables and the biomarkers of systemic inflammation (Laird, and Ware, 1982) using the entire 24-hour data, as well as analyzing the daytime (9 AM to 9 PM) and nighttime (9 PM to 9 AM next day) data separately, in order to examine whether inflammation affect daytime and nighttime CAM differently. In this approach we ignored the assumption of the specific cosine form for the data and treated the HRV variables from each 5-minute segment as repeated measures. Residual diagnostics were used to assess the appropriateness of modeling assumptions, and no sizeable departures were detected. Meier-Ewert HK et al. (Meier-Ewert et al., 2001) reported that CRP concentrations remained stable over 24 hours. Thus, for all of our analyses, we averaged the inflammatory markers from the first and the second visits (24 hours apart), and the inflammatory markers were used as continuous variables. We logarithmically transformed CRP prior to analysis. All analyses were performed using SAS version 9.1 software (SAS Institute Inc., Cary, NC, USA).
Figure 1.
Multivariable adjusted periodic regression curves of log HF and RMSSD at different values of CRP concentration (approximately the 10th and 90th percentiles).
Curves: The estimated diurnal circadian periodic curves over the time of HRV collection.
Horizontal lines: Mean of periodic curve.
Lines with arrows: Amplitude of periodic curve.
3. Results
The demographic characteristics, cardiovascular disease risk factors, and the HRV indices of the study sample are shown in Table 1. The mean age of the participants was 56.5 years (SD= 7.7, with 47%, 40%, 9%, and 4% in 45–54, 55–64, 65–69, and ≥ 70 years group, respectively), with 74% non-Hispanic white, 26% minorities (including Black, Hispanic, and Chinese), 61% female, and 50% having chronic cardiovascular related disease (mostly hypertension, and 8 and 7 individuals had diabetes and a history of coronary heart disease, respectively). In general, persons with chronic cardiovascular related diseases were older, had higher levels of systemic inflammation, and lower HF, LF, SDNN, and RMSSD than persons without chronic cardiovascular related diseases. Therefore, we adjusted for age, sex, ethnicity, and chronic cardiovascular related disease in the subsequent analyses.
Table 1.
The study population characteristics and summaries of HRV indices*
| CVD, Hypertension or Diabetes |
All (n=101) | ||
|---|---|---|---|
| Yes (n=42) | No (n=59) | ||
| Age (years) | 57.5 ± 6.8 | 55.7 ± 8.2 | 56.5 ± 7.7 |
| Male (%) | 38.1 | 39.0 | 38.6 |
| Non-Hispanic White (%) | 76.2 | 72.9 | 74.3 |
| Obesity (%) | 33.3 | 15.3 | 22.8 |
| Hypertension (%) | 83.3 | - | 34.7 |
| Diabetes (%) | 19.0 | - | 7.9 |
| CVD (%) | 16.7 | - | 6.9 |
| Body Mass Index (kg/m2) | 30.0 ± 7.1 | 26.0 ± 4.2 | 27.7 ± 5.9 |
| Systolic Blood Pressure (mmHg) | 129.6 ± 16.7 | 116.8 ± 11.8 | 122.1 ± 15.4 |
| Diastolic Blood Pressure (mmHg) | 77.9 ± 9.7 | 72.9 ± 8.3 | 75.0 ± 9.2 |
| Fasting Glucose (mg/dl) | 94.6 ± 37.0 | 84.6 ± 10.1 | 88.8 ± 25.5 |
| Log of CRP (ng/mL) | 7.4 ± 1.3 | 6.9 ± 1.1 | 7.1 ± 1.2 |
| WBC count (×103) | 6.2 ± 1.2 | 5.3 ± 1.2 | 5.7 ± 1.3 |
| HRV variables†: | |||
| Log of HF (ms2) | 3.3 ± 0.8 | 3.7 ± 0.9 | 3.5 ± 0.9 |
| Log of LF (ms2) | 4.4 ± 0.7 | 4.9 ± 0.8 | 4.7 ± 0.8 |
| LF/HF Ratio | 4.7 ± 2.6 | 4.6 ± 2.1 | 4.6 ± 2.3 |
| SDNN (ms) | 37.7 ± 11.4 | 44.4 ± 13.5 | 41.6 ± 13.0 |
| RMSSD (ms) | 20.0 ± 8.5 | 23.2 ± 9.5 | 21.8 ± 9.2 |
| Heart Rate (/min) | 77 ± 10 | 76 ± 10 | 76 ± 10 |
Continuous variables reported as mean ± SD; binary variables reported as %.
Calculated by first computing the individual-level means from all available 5-minute segments of data, and then averaging over the individual-level means from the entire study sample.
Abbreviations: CRP, C-reactive protein; HF, high frequency powers; HRV, heart rate variability; LF, low frequency powers; MI, myocardial infarction; RMSSD, the square root of the mean of the sum of the squared differences of the adjacent RR intervals; SDNN, standard deviation of RR intervals; WBC, white blood cell count.
The associations between the markers of systemic inflammation and each of the cosine parameters (M, A, and θ) estimated from the entire sample using random-effects meta-analysis regression models are presented in Table 2, as both unadjusted (Model 1) and age, sex, ethnicity, and CVD-related diseases adjusted (Model 2) models. In general, higher CRP was significantly associated with lower mean levels of HF, LF, SDNN, and RMSSD in both models (p<0.01), indicative of an inverse association between the burden of inflammation and lower cardiac autonomic modulation. More importantly, higher CRP was also inversely associated with lower amplitude of SDNN and RMSSD, indicating an inverse association between the burden of inflammation and the lower circadian amplitude of the oscillation of CAM. CRP was not associated with the circadian parameter θ, indicating no adverse effects of inflammation on the timing of the circadian pattern of CAM. The associations between higher levels of WBC and the three cosine periodic regression parameters are similar to that from CRP, but less pronounced. The circadian variations of HF and RMSSD over the clock time at lower and higher levels of CRP (corresponding to approximately the 10th and 90th percentiles of CRP) are graphically presented in Figure 1. These plots showed the overall reduction of the means of HF (as a frequency domain HRV index) and RMSSD (as a time domain HRV index) at higher levels of CRP. More importantly, these plots also showed a clear decrease in the amplitude of RMSSD oscillation at higher levels of CRP.
Table 2.
The regression coefficients (β) estimates and standard error (SE) of inflammatory markers on cosine parameters of the circadian patterns of HRV from random-effects meta-analysis.
| log of HF (ms 2) |
log of LF (ms2) |
SDNN (ms) |
RMSSD (ms) |
LF/HF Ratio |
Heart Rate (/min) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β† | SE | β | SE | β | SE | β | SE | β | SE | β | SE | |||
| Log CRP (ng/mL) | Model 1‡ | M | −0.22 | 0.07** | −0.22 | 0.06** | −3.92 | 1.01** | −2.35 | 0.73** | 0.06 | 0.19 | 1.25 | 0.81 |
| A | −0.01 | 0.03 | −0.02 | 0.02 | −0.98 | 0.44* | −0.96 | 0.38* | 0.03 | 0.09 | −0.59 | 0.37 | ||
| θ§ | 3.10 | 3.77 | −2.75 | 5.41 | −2.66 | 3.94 | 3.28 | 4.01 | 0.74 | 4.98 | 1.21 | 1.51 | ||
| Model 2‡ | M | −0.22 | 0.07** | −0.20 | 0.06** | −3.62 | 0.99** | −2.32 | 0.73** | 0.16 | 0.17 | 1.23 | 0.81 | |
| A | −0.004 | 0.028 | −0.02 | 0.02 | −0.84 | 0.44# | −0.86 | 0.38* | 0.07 | 0.08 | −0.57 | 0.38 | ||
| θ | 2.94 | 3.89 | −2.63 | 5.33 | −3.48 | 3.85 | 2.43 | 4.09 | 1.53 | 4.84 | 1.52 | 1.53 | ||
| WBC (×103) | Model 1 | M | −0.12 | 0.07# | −0.11 | 0.06# | −2.01 | 1.01* | −1.05 | 0.72# | 0.05 | 0.18 | 2.40 | 0.74** |
| A | 0.001 | 0.027 | −0.01 | 0.02 | −0.22 | 0.42 | −0.06 | 0.37 | −0.03 | 0.08 | −0.53 | 0.36 | ||
| θ | 7.13 | 3.53* | 2.27 | 5.20 | −0.97 | 3.77 | 5.35 | 3.81** | 5.53 | 4.75 | 1.27 | 1.45 | ||
| Model 2 | M | −0.11 | 0.06# | −0.12 | 0.06* | −2.11 | 0.97* | −1.06 | 0.71 | 0.01 | 0.16 | 2.44 | 0.73** | |
| A | −0.001 | 0.027 | −0.01 | 0.02 | −0.27 | 0.42 | −0.09 | 0.37 | −0.05 | 0.08 | −0.54 | 0.36 | ||
| θ | 7.26 | 3.59* | 2.47 | 5.06 | −0.63 | 3.64 | 5.60 | 3.82** | 5.18 | 4.57 | 1.14 | 1.45 | ||
p<0.10;
p<0.05;
p<0.01.
The estimates of regression coefficients correspond to the changes of cosine periodic function parameters of HRV for every one-unit increase in inflammatory markers.
Model 1: Unadjusted; Model 2: Adjusted for age, sex, ethnicity, and CVD-related conditions.
Difference of acrophase of cosine function, where one unit of θ corresponds to 5 minutes.
Abbreviations: CRP, C-reactive protein; HF, high frequency powers; HRV, heart rate variability; LF, low frequency powers; RMSSD, the square root of the mean of the sum of the squared differences of the adjacent RR intervals; SDNN, standard deviation of RR intervals; WBC, white blood cell count.
Ignoring the specific cosine form for the data and treating HRV variables from each 5-minute segment as repeated measures, the regression coefficients, SE, and P value relating CRP and WBC count and HRV indices according to daytime (9 AM to 9 PM) and nighttime (9 PM to 9 AM next day) are presented in Table 3. These analyses are based on linear mixed-effects models, which allow us to examine the time of the day specific associations between inflammatory markers and CAM. In general, higher levels of inflammation marker are significantly and consistently associated with lower levels of HRV indices and higher heart rate, in models based on the entire 24-hour data and on time of the day (daytime/nighttime) data. Moreover, the larger effect sizes of the regression coefficients from nighttime HRV data indicate a stronger relationship between inflammation and nighttime CAM.
Table 3.
The regression coefficients (β) estimates and standard error (SE) of inflammatory markers on HRV indices using 24-hour data, daytime (9 AM to 9 PM) and nighttime (9 PM to 9 AM next day) data separately from linear mixed-effects models analysis.
| log of HF (ms2) |
log of LF (ms2) |
SDNN (ms) |
RMSSD (ms) |
LF/HF Ratio |
Heart Rate (/min) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β† | SE | β | SE | β | SE | β | SE | β | SE | β | SE | |||
| Log CRP (ng/mL) | Model 1‡ | 24-h | −0.23 | 0.02** | −0.23 | 0.01** | −4.01 | 0.21** | −2.41 | 0.23** | 0.09 | 0.06* | 1.30 | 0.29** |
| day | −0.23 | 0.02** | −0.22 | 0.02** | −3.59 | 0.25** | −1.94 | 0.24** | 0.17 | 0.06** | 1.01 | 0.41* | ||
| night | −0.23 | 0.03** | −0.24 | 0.02** | −4.47 | 0.33** | −2.88 | 0.38** | −0.02 | 0.06 | 1.70 | 0.38** | ||
| Model 2‡ | 24-h | −0.23 | 0.02** | −0.21 | 0.01** | −3.71 | 0.20** | −2.38 | 0.22** | 0.18 | 0.04** | 1.29 | 0.29** | |
| day | −0.23 | 0.02** | −0.20 | 0.02** | −3.39 | 0.24** | −1.97 | 0.23** | 0.30 | 0.05** | 1.03 | 0.42* | ||
| night | −0.23 | 0.03** | −0.22 | 0.02** | −4.05 | 0.32** | −2.79 | 0.37** | 0.05 | 0.06 | 1.66 | 0.38** | ||
| WBC (×103) | Model 1 | 24-h | −0.12 | 0.02** | −0.11 | 0.01** | −2.05 | 0.20** | −1.06 | 0.22** | 0.06 | 0.04 | 2.37 | 0.27** |
| day | −0.13 | 0.02** | −0.13 | 0.02** | −2.19 | 0.25** | −1.11 | 0.23** | 0.04 | 0.05 | 2.18 | 0.38** | ||
| night | −0.11 | 0.03** | −0.10 | 0.02** | −1.93 | 0.32** | −1.00 | 0.38** | 0.09 | 0.06 | 2.64 | 0.35** | ||
| Model 2 | 24-h | −0.12 | 0.02** | −0.12 | 0.01** | −2.18 | 0.20** | −1.08 | 0.22** | 0.03 | 0.04 | 2.42 | 0.26** | |
| day | −0.12 | 0.02** | −0.13 | 0.02** | −2.25 | 0.23** | −1.09 | 0.22** | −0.01 | 0.05 | 2.21 | 0.38** | ||
| night | −0.11 | 0.03** | −0.11 | 0.02** | −2.11 | 0.31** | −1.04 | 0.36** | 0.06 | 0.06 | 2.69 | 0.34** | ||
p<0.05;
p<0.01;
p<0.10.
The estimates of regression coefficients correspond to the changes in HRV for every one-unit increase in inflammatory markers.
Model 1: Unadjusted; Model 2: Adjusted for age, sex, ethnicity, and CVD-related conditions.
Abbreviations: CRP, C-reactive protein; HF, high frequency powers; HRV, heart rate variability; LF, low frequency powers; RMSSD, the square root of the mean of the sum of the squared differences of the adjacent RR intervals; SDNN, standard deviation of RR intervals; WBC, white blood cell count.
4. Discussion
Several published studies have investigated the overall associations between HRV and inflammatory markers, including time and frequency domain HRV measures, and utilizing patient-based (Lanza et al., 2006; Madsen et al., 2007) or population-based samples (Araujo et al., 2006; Jensen-Urstad et al., 1998; Sajadieh et al., 2004; Sloan et al., 2007; Thayer, and Fischer, 2009). In general, these previous studies have demonstrated significant associations between the elevated burdens of systemic inflammations and reduced mean levels of HRV. However, none of previous studies have examined the inflammation and HRV association in all three demotions of the circadian pattern of HRV, a well established characteristic of CAM in human. Therefore, the main objective of this study is to quantify the relationship between the burden of systemic inflammation and the circadian pattern of cardiac autonomic modulation in all three cosine parameters. To achieve this aim, we used a two-stage approach, with the first stage using cosine periodic regression to fit the individual-level 24-hour HRV data on a 5-minute per segment basis to estimate the three cosine parameters, and the second stage summarizing these three parameters from the entire study sample using the random-effects meta-analysis approach. This two-stage approach enabled us to investigate the effects of systemic inflammation on the mean levels, the amplitude and the acrophrase of HRV indices, thus providing insight into the circadian impact of inflammation on CAM. As summarized in Table 2 and graphically shown in Figure 1, our data suggest an overall adverse relationship between systemic inflammation and lower mean levels of CAM, which are consistent with previous findings (Araujo et al., 2006; Jensen-Urstad et al., 1998; Lanza et al., 2006; Madsen et al., 2007; Sajadieh et al., 2004; Sloan et al., 2007; Thayer, and Fischer, 2009). Most importantly, our data also clearly indicate an adverse relationship between systemic inflammation and lower circadian amplitude of CAM, which we have not been able to find similar report from the published literature. For instance, one unit increment of log CRP in these data is associated with 2.3 ms decrease in the mean of RMSSD and a 0.8 ms lower amplitude of RMSSD. To our knowledge, this is the first report of the impact of inflammation on the circadian variation of CAM in terms of the amplitude. In this community-dwelling sample of nonsmokers, inflammation was not associated with the timing of circadian oscillation of CAM
When we stratified the HRV data according to the time of the day, our findings of a consistent relationship between the levels of inflammation and the mean levels of HRV are also in agreement with previous reports of an adverse relationship between inflammation and CAM (Araujo et al., 2006; Jensen-Urstad et al., 1998; Lanza et al., 2006; Madsen et al., 2007; Sajadieh et al., 2004; Sloan et al., 2007; Thayer, and Fischer, 2009). Moreover, our data on the stronger association for the nighttime CAM are suggestive of a larger effect of inflammation on nighttime CAM than on the daytime CAM. For example, the regression coefficient (effect size) of RMSSD from CRP is 42% larger from the nighttime than from the daytime based on the data presented in Table 3. This daytime and nighttime differences in the effect size are also supportive of the effect of inflammation on the amplitude of CAM, with the highest amplitude occurred at nighttime.
The exact mechanisms linking elevated systemic inflammation and impaired circadian rhythm of cardiac autonomic modulation are not well-understood presently. It is plausible that the underlying causes of elevated systemic inflammation can lead to the overall activation of sympathetic outflow, and thus lead to the imbalance of the sympatovagal modulation of the heart rhythms. This hypothesis is supported by studies that have identified the products and mediators of inflammation that can serve as stimuli on the autonomic modulation system (Banks et al., 1991; Goehler et al., 2000). Alternatively, it is also plausible that activated sympathetic modulation or reduced vagal modulation (reflected as reduced HRV) can enhance the production of cytokines, which in turn can lead to higher levels of blood inflammatory markers. This latter hypothesis is supported by several recently published experimental studies of sepsis in animal models and myocardial ischemia models. Such animal models demonstrated that stimulation of the vagus nerve significantly inhibits tumor-necrosis-factor-a release (Borovikova et al., 2000) and an inhibition of cytokine activity (Saeed et al., 2005). Unfortunately, our cross-sectional data did not allow us to elucidate the temporal relationship between systemic inflammation and CAM. In addition, we only have data from nonsmokers who did not have any cardiac event in the 6 months prior to participating in our study. Thus, the results may not be generalizable to smokers or individuals with recent acute cardiac events. We did not have detailed information on the use of anti-inflammation medications, or medications that affect the balance of sympathetic and parasympathetic modulation, such as beta-blockers. As a result, we cannot elucidate the potential effect modification of the inflammation and CAM relationship by these medications, which are known to impact the levels of inflammatory markers and/or CAM (Niemela et al., 1994).
In summary, in this middle-aged sample of community-dwelling individuals, higher burden of systemic inflammation is related to lower overall CAM and reduced amplitude of the circadian oscillation of CAM, suggesting that increased cardiovascular risk associated with inflammation may be partially mediated via inflammation related changes in CAM. More studies are needed to establish the temporality of, and to elucidate the potential mechanisms underlying, this association.
Acknowledgments
This study was funded by NIH grant number 1 R01 ES014010. The authors wish to thank the participants of the APACR study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araujo F, Antelmi I, Pereira AC, Latorre MdoR, Grupi CJ, Krieger JE, Mansur AJ. Lower heart rate variability is associated with higher serum high-sensitivity c-reactive protein concentration in healthy individuals aged 46 years or more. Int J Cardiol. 2006;107:333–337. doi: 10.1016/j.ijcard.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci. 1991;48:117–121. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- Biggerm JTJ, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Blake GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, c-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108:2993–2999. doi: 10.1161/01.CIR.0000104566.10178.AF. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- D’Negri CE, Marelich L, Vigo D, Acunzo RS, Girotti LA, Cardinali DP, Siri LN. Circadian periodicity of heart rate variability in hospitalized angor patients. Clin Auton Res. 2005;15:223–232. doi: 10.1007/s10286-005-0280-9. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC study. Atherosclerosis Risk in Communities. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- He F, Shaffer M, Li X, Rodriguez-Colon S, Wolbrette D, Williams R, Cascio W, Liao D. Individual-level pm(2.5) exposure and the time course of impaired heart rate variability: the APACR study. J Expo Sci Environ Epidemiol. 2011;21:65–73. doi: 10.1038/jes.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Urstad M, Jensen-Urstad K, Ericson M, Johansson J. Heart rate variability is related to leucocyte count in men and to blood lipoproteins in women in a healthy population of 35-year-old subjects. J Intern Med. 1998;243:33–40. [PubMed] [Google Scholar]
- Kucharska-Newton AM, Couper DJ, Pankow JS, Prineas RJ, Rea TD, Sotoodehnia N, Chakravarti A, Folsom AR, Siscovick DS, Rosamond WD. Hemostasis, inflammation, and fatal and nonfatal coronary heart disease: long-term follow-up of the atherosclerosis risk in communities (aric) cohort. Arterioscler Thromb Vasc Biol. 2009;29:2182–2190. doi: 10.1161/ATVBAHA.109.192740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lanza GA, Sgueglia GA, Cianflone D, Rebuzzi AG, Angeloni G, Sestito A, Infusino F, Crea F, Maseri A. Relation of heart rate variability to serum levels of c-reactive protein in patients with unstable angina pectoris. Am J Cardiol. 2006;97:1702–1706. doi: 10.1016/j.amjcard.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC study. Atherosclerosis Risk in Communities study. Am J Epidemiol. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- Liao D, Shaffer ML, Rodriguez-Colon S, He F, Li X, Wolbrette DL, Yanosky J, Cascio WE. Acute adverse effects of fine particulate air pollution on ventricular repolarization. Environ Health Perspect. 2010;118:1010–1015. doi: 10.1289/ehp.0901648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Lombardi F, Sandrone G, Mortara A, La Rovere MT, Colombo E, Guzzetti S, Malliani A. Circadian variation of spectral indices of heart rate variability after myocardial infarction. Am Heart J. 1992;123:1521–1529. doi: 10.1016/0002-8703(92)90804-5. [DOI] [PubMed] [Google Scholar]
- Madsen T, Christensen JH, Toft E, Schmidt EB. C-reactive protein is associated with heart rate variability. Ann Noninvasive Electrocardiol. 2007;12:216–222. doi: 10.1111/j.1542-474X.2007.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpas SC, Purdie GL. Circadian variation of heart rate variability. Cardiovasc Res. 1990;24:210–213. doi: 10.1093/cvr/24.3.210. [DOI] [PubMed] [Google Scholar]
- Massin MM, Maeyns K, Withofs N, Ravet F, Gerard P. Circadian rhythm of heart rate and heart rate variability. Arch Dis Child. 2000;83:179–82. doi: 10.1136/adc.83.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of c-reactive protein concentrations in healthy human subjects. Clin Chem. 2001;47:426–430. [PubMed] [Google Scholar]
- Nakagawa M, Iwao T, Ishida S, Yonemochi H, Fujino T, Saikawa T, Ito M. Circadian rhythm of the signal averaged electrocardiogram and its relation to heart rate variability in healthy subjects. Heart. 1998;79:493–496. doi: 10.1136/hrt.79.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JJ, Liao D, Sharrett AR, Folsom AR, Chambless LE, Shahar E, Szklo M, Eckfeldt J, Heiss G. Serum albumin level as a predictor of incident coronary heart disease: the atherosclerosis risk in communities (aric) study. Am J Epidemiol. 2000;151:468–477. doi: 10.1093/oxfordjournals.aje.a010232. [DOI] [PubMed] [Google Scholar]
- Niemela MJ, Airaksinen KE, Huikuri HV. Effect of beta-blockade on heart rate variability in patients with coronary artery disease. J Am Coll Cardiol. 1994;23:1370–1377. doi: 10.1016/0735-1097(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Phillips AN, Neaton JD, Cook DG, Grimm RH, Shaper AG. Leukocyte count and risk of major coronary heart disease events. Am J Epidemiol. 1992;136:59–70. doi: 10.1093/oxfordjournals.aje.a116421. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. Rr interval variability is inversely related to inflammatory markers: the cardia study. Mol Med. 2007;13:178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task force of the European society of cardiology and the North American society of pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Fischer JE. Heart rate variability, overnight urinary norepinephrine and c-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J Intern Med. 2009;265:439–447. doi: 10.1111/j.1365-2796.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJJ, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. the framingham heart study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- Yue W, Schneider A, Ruckerl R, Koenig W, Marder V, Wang S, Wichmann H, Peters A, Zareba W. Relationship between electrocardiographic and biochemical variables in coronary artery disease. Int J Cardiol. 2007;119:185–191. doi: 10.1016/j.ijcard.2006.07.129. [DOI] [PubMed] [Google Scholar]