Summary
Hydroxycarbamide (hydroxyurea) provides laboratory and clinical benefits for adults and children with sickle cell anaemia (SCA). Given its mechanism of action and prior reports of genotoxicity, concern exists regarding long-term toxicities and possible carcinogenicity. We performed cross-sectional analyses of chromosome stability using peripheral blood mononuclear cells (PBMC) from 51 children with SCA and 3-12 years of hydroxycarbamide exposure (mean age 13.2±4.1 years), compared to 28 children before treatment (9.4±4.7 years). Chromosome damage was less for children receiving hydroxycarbamide than untreated patients (0.8±1.2 versus 1.9±1.5 breaks per 100 cells, p=0.004). There were no differences in repairing chromosome breaks after in vitro radiation; PBMC from children taking hydroxycarbamide had equivalent 2Gy-induced chromosome breaks compared to untreated patients (30.8±16.1 versus 31.7±8.9 per 100 cells, p=not significant). Radiation plus hydroxycarbamide resulted in similar numbers of unrepaired breaks in cells from children on hydroxycarbamide compared to untreated patients (95.8±44.2 versus 76.1±23.1 per 100 cells, p=0.08), but no differences were noted with longer exposure (97.9±42.8 breaks per 100 cells for 3-6 years of hydroxycarbamide exposure versus 91.2±48.4 for 9-12 years of exposure). These observations provide important safety data regarding long-term risks of hydroxycarbamide exposure for children with SCA, and suggest low in vivo mutagenicity and carcinogenicity.
Keywords: sickle cell anaemia, hydroxycarbamide, DNA damage, chromosome breakage
Introduction
Hydroxycarbamide (hydroxyurea) is a potent antimetabolite and antineoplastic agent that has been used for almost 50 years to treat a variety of human diseases, including myeloproliferative neoplasms (Finazzi, et al 2000, Harrison, et al 2005, Lofvenberg and Wahlin 1988, Nand, et al 1996, Sterkers, et al 1998), leukaemia (Kennedy & Yarbro 1966), melanoma (Cassileth & Hyman 1967), brain tumours (Levin, et al 1984), ovarian cancer (Ariel 1970), psoriasis (Leavell & Yarbro 1970), and human immunodeficiency virus (Lori, et al 1994). Nearly 30 years ago, animal studies demonstrated that anaemic monkeys treated with hydroxycarbamide showed an increase in fetal haemoglobin (HbF) (Letvin, et al 1984), which led to the first clinical application of hydroxycarbamide for sickle cell anaemia (SCA). Platt et al (1984) treated two adults with SCA with hydroxycarbamide, and both had a rapid increase in HbF-containing reticulocytes with no significant short-term toxicities. These initial experiments led to a critical multicentre phase I/II trial for adults with SCA, demonstrating the short-term safety, dose-dependent response, and consistent increase in HbF resulting from hydroxycarbamide therapy at the maximum tolerated dose (MTD) (Charache, et al 1992).
Based on these results, the definitive Multicenter Study of Hydroxycarbamide in Sickle Cell Anemia (MSH) was developed. This National Heart Lung and Blood Institute (NHLBI)-funded phase III double-blinded, placebo-controlled randomized trial enrolled 299 adult patients with severe symptoms and demonstrated the dramatic clinical efficacy of hydroxycarbamide at MTD; the trial was halted early due to highly significant reductions in the time to first painful vaso-occlusive event (Charache, et al 1995). Additional clinical improvements were noted including significant reductions in the frequency of painful events, acute chest syndrome, transfusions, and hospitalizations in the hydroxycarbamide-treated subjects. The subsequent NHLBI-funded multicentre paediatric trial, HUG-KIDS, was a phase I/II study of 84 children with severe SCA, which demonstrated similar laboratory and clinical efficacy as observed for adults, as well as minimal short-term toxicities (mostly mild transient and reversible cytopenias) of hydroxycarbamide therapy at MTD (Kinney, et al 1999).
Investigations of the risks and benefits of hydroxycarbamide are still ongoing, but to date no significant, identifiable long-term toxicities have been identified for patients with SCA (de Montalembert, et al 2006, Gulbis, et al 2005, Hankins, et al 2005, Heeney and Ware 2010, Kinney, et al 1999, Steinberg, et al 2003, Wang, et al 2001, Zimmerman, et al 2004). However, given its primary mechanism of action as a potent inhibitor of ribonucleotide reductase (Lewis & Wright 1974) and previous reports identifying hydroxycarbamide as a clastogen (Friedrisch, et al 2008, Oppenheim and Fishbein 1965), mutagen (Ziegler-Skylakakis, et al 1985), teratogen (Chaube and Murphy 1966, Murphy and Chaube 1964) and potential carcinogen (Sakano, et al 2001), there is still ongoing concern about the adverse long-term side effects of hydroxycarbamide therapy in SCA, particularly when treatment is initiated in the early years of life and spans a prolonged period of time.
We prospectively investigated the long-term effects of hydroxycarbamide exposure on DNA integrity and repair capacity in a large cohort of children with SCA receiving continuous hydroxycarbamide therapy. We report cross-sectional analyses of patient samples, quantifying chromosome breaks in long-lived peripheral blood mononuclear cells (PBMC). We have also tested the ability of PBMC to repair induced DNA breaks, thereby providing an in vitro correlate for in vivo mutagenicity and carcinogenicity. Taken together, these studies provide important long-term data regarding the safety and genotoxicity concerns of hydroxycarbamide exposure at MTD for children with SCA.
Patients and Methods
Patient samples
Blood samples were obtained from children with SCA receiving care at St. Jude Children’s Research Hospital who were enrolled on the Hydroxycarbamide Study of Long-term Effects (HUSTLE, ClinicalTrials.gov NCT00305175). The HUSTLE protocol was reviewed and approved by the St. Jude Institutional Review Board. The hospital is located in the mid-South region of the United States, with no major toxic industrial exposure and no nuclear energy plants within 100 miles. Patients were recruited for the HUSTLE study in two cohorts, based on previous hydroxycarbamide exposure. New cohort subjects had no prior hydroxycarbamide exposure; they were enrolled in this prospective study after hydroxycarbamide was recommended for clinical severity, most commonly as a preventive therapy for frequent acute vaso-occlusive events. Samples were collected at baseline before treatment exposure. Old cohort subjects were already receiving hydroxycarbamide treatment at the time of study enrollment, and samples were collected at three-year treatment anniversaries. For both cohorts, hydroxycarbamide dosing was escalated to MTD as previously described (Heeney and Ware 2010, Kinney, et al 1999, Zimmerman, et al 2004), with serial monitoring to document laboratory effects and toxicities. There were 79 subjects included in the cross-sectional analyses of chromosome breakage, ranging from 0 to 12 years of hydroxycarbamide exposure. Normal adult healthy control samples (n=11) were also included in the analyses (range 29-60 years, mean 44 years). Healthy children were not used as controls due to ethical concerns of phlebotomy in healthy children and the lack of availability of truly healthy children at our institution that specializes in paediatric haematology and oncology.
In vitro analyses
PBMC were isolated and purified from venous blood samples using Ficoll-Hypaque density centrifugation as previously described (Denning, et al 1987). In three separate flasks (A, B, C) 5 × 106 cells were diluted in Dulbecco’s Modified Eagle’s Medium (DMEM) and 10% Human A Serum. Cells were stimulated with phytohemagglutinin (PHA 2 μg/ml, Remel, Inc. Lanexa, KS) and incubated for 48 h at 37°C in a humidified incubator. After PHA stimulation, the cells were then handled differently: Flask A received neither irradiation nor hydroxycarbamide; Flask B was irradiated with 2 Gray (Gy) external beam radiation (XRT) from a Cesium Cell Irradiator (Gammacell 40 Exactor, MDS Norton, Ottawa, ON); and in Flask C, 100μM hydroxycarbamide (Sigma-Aldrich, Inc., St. Louis, MO) was added just prior to 2 Gy irradiation. All flasks were then incubated at 37°C for an additional 24 h. 2 Gy was chosen because pilot experiments demonstrated numerous chromosome breaks without cell death (data not shown).
Chromosome Analyses
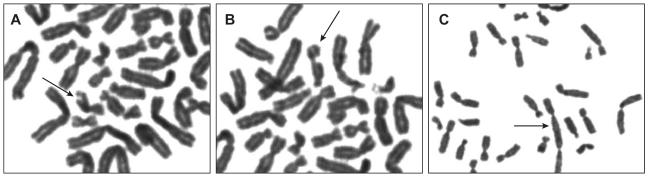
After 72 h of total incubation, 0.5 μg/ml of colcemid (stock 10 μg/ml), a mitotic spindle inhibitor used to arrest cells in metaphase, was added to each flask, followed by an additional incubation for 4 h at 37°C. Cells were then pelleted by centrifugation (1500 rpm × 10 min), resuspended in a hypotonic solution (0.075% KCl) and incubated for 15 min at 37°C. The cells were then pelleted again, resuspended in Carnoy’s fixative (1:3; glacial acetic acid: methanol) and incubated for 15 min. This fixation procedure was repeated two additional times. Chromosome spreads were prepared by dropping cells on wet slides, air-dried and stained using the Giemsa-banding technique (Gustashaw 1991). After harvesting and staining, 100 metaphase cells from each flask were analysed and scored for total numbers of chromosome breaks (including chromosome fragments), chromatid breaks, and chromosome fusions (examples in Figure 1).
Figure 1.
Chromosome Abnormalities
Peripheral blood mononuclear cells were isolated from venous blood samples as described in Methods. After phytohaemagglutinin stimulation, DNA damage was induced by 2Gy radiation therapy and DNA repair was inhibited by incubation with 100μM hydroxycarbamide. After lymphocytes were harvested and stained, 100 metaphase cells were examined for evidence of DNA damage, including chromatid breaks (A), chromosome breaks (B), and chromosome fusion events (C).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 4 graphical and statistical software (GraphPad Software, Inc., LaJolla, CA). The Mann-Whitney U test was used to determine differences among groups. Tests with p value <0.05 were considered statistically significant.
Results
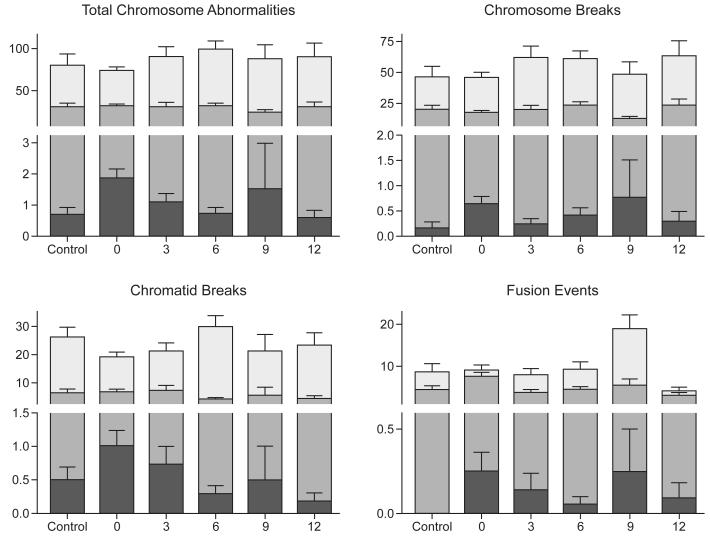
A total of 79 subjects were included in the cross-sectional analyses, and the results were summarized by 3-year increments of hydroxycarbamide exposure, ranging from 0-12 years (Figure 2). There was no positive correlation between hydroxycarbamide exposure and baseline (uninduced) DNA damage. As a group, the 51 children with SCA and 3-12 years of hydroxycarbamide exposure actually had significantly fewer total chromosome abnormalities compared to the 28 children with SCA and no prior hydroxycarbamide exposure (0.8 ± 1.2 breaks per 100 cells versus 1.9 ± 1.5 breaks, p=0.004, Table I) and equivalent to normal adult controls (0.6 ± 0.7 breaks, p=0.83). Similar results were observed when specific types of chromosomal breakage abnormalities (e.g., chromatid breaks, fusion events) were analysed (Table I). Prolonged hydroxycarbamide exposure was not associated with a different number of chromosome abnormalities; children with 9 or 12 years of hydroxycarbamide treatment had similar numbers of uninduced abnormalities (0.8± 1.6 breaks per 100 cells) as those with 3 or 6 years of exposure (0.9 ± 1.0 breaks, p=0.33).
Figure 2.
Effect of hydroxycarbamide exposure on chromosomal integrity
For each vertical data group, the black bar represents “Flask A,” reflecting uninduced chromosome abnormalities; the grey bar represents “Flask B,” reflecting induced damage with 2Gy irradiation; the white bar represents “Flask C” reflecting induced damage and direct repair inhibition after treatment with 100μM hydroxycarbamide and 2Gy irradiation. Standard error of the mean is represented above each data point. y-axis = number of events per 100 cells; x-axis = hydroxycarbamide exposure in years. Control, n= 11 subjects; no prior hydroxycarbamide exposure, n=28; 3 years hydroxycarbamide exposure, n=15; 6 years hydroxycarbamide exposure, n=21; 9 years hydroxycarbamide exposure, n=4; 12 years hydroxycarbamide exposure, n=11.
Table I.
Chromosome abnormalities (e.g., double-stranded chromosome breaks, chromatid breaks, fusion events) in controls and subjects with or without prior hydroxycarbamide exposure. Results represent mean number of events per 100 cells examined in metaphase chromosome spreads ± standard deviation. Final column represents p value obtained comparing subjects with and without prior hydroxycarbamide exposure using the Mann-Whitney U test. HC, hydroxycarbamide; XRT, external beam radiation
| Control | No HC Exposure |
HC Exposure | p-value | |
|---|---|---|---|---|
| Patients (n) | 11 | 28 | 51 | |
| Age at Sample (years) | 44.3 ± 11.0 | 9.4 ± 4.7 | 13.2 ± 4.3 | |
| 0 Gy XRT/0 HC | ||||
| Chromosome Breaks | 0.1 ± 0.4 | 0.6 ± 0.8 | 0.3 ± 0.7 | 0.18 |
| Chromatid Breaks | 0.5 ± 0.5 | 1.0 ± 1.2 | 0.4 ± 0.8 | 0.04 |
| Fusion Events | 0.0 ± 0.0 | 0.3 ± 0.6 | 0.1 ± 0.3 | 0.52 |
| Total Abnormalities | 0.6 ± 0.7 | 1.9 ± 1.5 | 0.8 ± 1.2 | <0.01 |
| 2Gy XRT/0 HC | ||||
| Chromosome Breaks | 19.7 ± 11.4 | 17.1 ± 7.0 | 21.4 ± 13.1 | 0.32 |
| Chromatid Breaks | 6.4 ± 4.7 | 6.8 ± 5.0 | 5.1 ± 4.2 | 0.07 |
| Fusion Events | 4.6 ± 2.9 | 7.8 ± 5.0 | 4.3 ± 2.9 | <0.01 |
| Total Abnormalities | 30.7 ± 14.9 | 31.7 ± 8.9 | 30.8 ± 16.1 | 0.20 |
| 2 Gy XRT/100μM HC | ||||
| Chromosome Breaks | 46.3 ± 29.3 | 46.4 ± 22.7 | 61.1 ± 32.6 | 0.052 |
| Chromatid Breaks | 26.1 ± 11.8 | 20.2 ± 8.9 | 25.9 ± 15.1 | 0.16 |
| Fusion Events | 8.7 ± 6.7 | 9.5 ± 6.1 | 8.8 ± 7.2 | 0.36 |
| Total Abnormalities | 81.1 ± 45.1 | 76.1 ± 23.1 | 95.8 ± 44.2 | 0.08 |
Sublethal XRT-induced DNA damage was manifest as a significantly increased number of chromosome abnormalities, particularly chromosome breaks and chromatid breaks (Figure 2). Chronic in vivo hydroxycarbamide exposure did not affect the amount of induced DNA damage, as subjects with 3-12 years of hydroxycarbamide exposure had similar numbers of XRT-induced abnormalities as subjects with no exposure (30.8 ± 16.1 breaks per 100 cells versus 31.7 ± 8.9 breaks, p=0.20, Table I), and were also comparable to normal adult controls (30.7 ± 14.9 breaks, p=0.83). Like the findings for uninduced DNA damage, prolonged in vivo hydroxycarbamide exposure was not associated with an increased number of XRT-induced chromosome abnormalities; children with 9 or 12 years of hydroxycarbamide exposure had similar numbers of XRT-induced chromosome abnormalities (29.1 ± 16.0 per 100 cells) as children with 3 or 6 years of exposure (31.6 ± 16.4 abnormalities per 100 cells, p=0.56).
Finally, we analysed the ability of hydroxycarbamide to inhibit XRT-induced DNA damage repair in vitro, as one means to mimic the potential of in vivo DNA damage to accumulate in patients with continuous hydroxycarbamide exposure. Unrepaired DNA damage was manifest as substantially increased numbers of chromosome abnormalities, particularly chromosome breaks and chromatid breaks (Table I). Subjects with hydroxycarbamide exposure had a similar total number of chromosome abnormalities compared to those without hydroxycarbamide exposure (95.8 ± 44.2 breaks per 100 cells versus 76.1 ± 23.1, p=0.08, Table I) and a similar number of abnormalities compared to healthy controls (81.1 ± 45.1, p=0.30). These differences did not increase with prolonged hydroxycarbamide exposure; children with 9 or 12 years of in vivo hydroxycarbamide exposure had a similar number of chromosome abnormalities (91.2 ± 48.4 abnormalities per 100 cells) as children with 3 or 6 years of exposure (97.9 ± 42.8 abnormalities per 100 cells, p=0.43). Further analyses of specific types of chromosome damage showed similar findings for chromosome breaks among children with hydroxycarbamide exposure (61.1 ± 32.6 breaks per 100 cells, Table I) compared to those without prior exposure (46.4 ± 22.7 breaks per 100 cells, p=0.052, Table I), and no difference in chromatid breaks (25.9 ± 15.1 chromatid breaks per 100 cells in hydroxycarbamide-treated subjects versus 20.2 ± 8.9 chromatid breaks per 100 cells for subjects without hydroxycarbamide exposure, Table I, p=0.16). These slight increases in chromosome breaks also were not significantly different when compared to healthy controls (46.3 ± 29.3 chromosome breaks per 100 cells, p=0.14) and the number of chromosome breaks was not cumulative with prolonged hydroxycarbamide exposure (59.8 ± 36.5 chromosome breaks per 100 cells for subjects with 9 or 12 years of hydroxycarbamide exposure versus 61.7 ± 31.3 for subjects with 3 or 6 years of exposure, p=0.57).
Discussion
The clinical and laboratory efficacy of hydroxycarbamide in the treatment of SCA has been convincingly demonstrated over the past 25 years (Ware 2010). These consistent results leave little question as to the clinical benefits of hydroxycarbamide therapy for both children and adults with SCA. The demonstrated decrease in morbidity and mortality with hydroxycarbamide use, coupled with the modest short-term toxicity profile and ease of once daily oral administration, make hydroxycarbamide an ideal treatment option for this patient population (Ware 2010). However, the long-term efficacy and safety of hydroxycarbamide use for patients with SCA remains one of the most critical unanswered questions (Brawley, et al 2008). This is especially important for the paediatric population, since the starting age for hydroxycarbamide is steadily becoming younger and the potential duration of exposure could span several decades (Heeney and Ware 2010).
Hydroxycarbamide is a potent inhibitor of ribonucleotide reductase, the enzyme responsible for the formation of deoxyribonucleotides, which are the substrates required for both DNA synthesis and repair. Hydroxycarbamide has been identified as an S-phase specific agent that interrupts the cell cycle at the G1 and S phases (de Lima, et al 2003, Yarbro 1992). Several in vitro studies suggest hydroxycarbamide is a clastogen and mutagen; cells subjected to prolonged incubations with high concentrations of hydroxycarbamide developed a progressive increase in the number of chromosome strand breaks and hypoxanthine –guanine phosphoribosyltransferase gene (HPRT1) mutations, and also had a reduction in their ability to repair damaged DNA (Francis, et al 1979, Li and Kaminskas 1987, Oppenheim and Fishbein 1965, Ziegler-Skylakakis, et al 1985). However, the concentration and length of hydroxycarbamide exposure used in these in vitro experiments were supraphysiological (prolonged incubations using hydroxycarbamide concentrations of 1-10mM) in comparison to the pharmacokinetic profile in children with SCA, who demonstrate typical maximum serum concentrations of only 200-500 μM (Rogers, et al 2005, Ware, et al 2008). These previous in vitro reports documented the potential genotoxic effects of hydroxycarbamide and support the careful clinical monitoring of hydroxycarbamide use in patients, but probably did not measure or predict the true mutagenic potential of daily oral hydroxycarbamide use in human patients.
In vivo studies of the genotoxic effects of hydroxycarbamide in patients with SCA have produced mixed results. We previously measured the effect of hydroxycarbamide on acquired DNA mutations using the HPRT1 and IGHV@/IGHD@/IGHJ@ (VDJ) recombination assays in 68 patients with SCA (32 with short-term hydroxycarbamide exposure and 36 without hydroxycarbamide exposure) and 59 controls, including 27 patients with myeloproliferative neoplasms (MPN) and hydroxycarbamide exposure up to 18 years (Hanft, et al 2000). Adults with MPN and prolonged hydroxycarbamide exposure surprisingly had a significantly lower number of illegitimate IGHV@/IGHD@/IGHJ@ mutations than those with low exposure and controls. Children with SCA and short-term hydroxycarbamide exposure had increased numbers of IGHV@/IGHD@/IGHJ@ mutations (but still within the normal range) when compared to children with no hydroxycarbamide exposure, and the number of HPRT1 mutations was equivalent (Hanft, et al 2000). Subsequently, Khayat et al (2006) reported no significant differences in the mitotic index or the frequency of chromosome aberrations in peripheral blood lymphocytes of 8 patients with SCA treated with hydroxycarbamide over the course of one year. More recently, an investigation of DNA damage in peripheral blood leucocytes of SCA patients treated with hydroxycarbamide using the comet assay demonstrated a significantly higher DNA damage index in the hydroxycarbamide group compared to the control group (Friedrisch, et al 2008). However, there was a negative correlation between the duration of hydroxycarbamide exposure and damage index. Finally, we reported that children with SCA exhibited a significantly higher number of circulating micronuclei-containing erythrocytes after hydroxycarbamide exposure, which was observed within the first 3 months of therapy but did not accumulate over time (Flanagan, et al 2010). Taken together, these previous studies provide evidence for measurable genotoxicity from hydroxycarbamide exposure in patients with SCA, but little evidence to support cumulative mutagenicity or carcinogenic potential.
In an effort to clarify the genotoxic effects of prolonged hydroxycarbamide exposure, we devised experiments to quantify baseline chromosomal damage and the ability to repair experimentally induced DNA damage, reasoning that these would be relevant clinical endpoints for children with SCA and hydroxycarbamide exposure. Our patients had no known exposure to additional environmental genotoxins that could have potentially confounded the laboratory measurements. Our results provide the first direct analysis of chromosome integrity and DNA repair capacity in lymphocytes of patients with SCA and up to 12 years of in vivo hydroxycarbamide exposure. These results are encouraging, as children receiving hydroxycarbamide therapy demonstrated neither increased baseline chromosome damage nor inhibition in the ability to repair damaged DNA, in comparison to healthy controls and to young SCA patients with no prior hydroxycarbamide exposure. Children treated with hydroxycarbamide for 3-12 years actually had a significantly lower amount of uninduced chromosome damage compared to untreated patients, possibly reflecting lower lymphocyte counts (Table I). It is also possible that the oxidative stress and generalized inflammation of severe untreated sickle cell anaemia produces a mild genotoxic effect, and hydroxycarbamide therapy may help to ameliorate this process. The slightly increased number of chromosome aberrations in treated patients with the combination of 2 Gy XRT exposure and additional hydroxycarbamide incubation was not statically significant, and reflects radiation exposure that is not clinically relevant. Patients are unlikely to ever receive such a genotoxic dose of radiation, even with generous diagnostic imaging; for example, routine posterior–anterior/lateral chest X-ray provides 0.00016 Gy exposure and abdominal computerized tomography scan provides only 0.01 Gy exposure (Brenner & Hall, 2007). These results suggest that the true genotoxicity, and thus perhaps the carcinogenic potential, of hydroxycarbamide treatment is low for patients with SCA.
Hydroxycarbamide can provide substantial life-enhancing and life-prolonging benefits for individuals with SCA, a condition for which there previously has been no effective therapy. However, despite continued proven efficacy, hydroxycarbamide remains underutilized, in part due to long-term safety concerns and with particular concern for its possible malignant potential. The recently completed BABY-HUG study (NCT00006400) will provide important information about the safety of hydroxycarbamide therapy in very young children. It will remain critical to closely monitor children with prolonged hydroxycarbamide exposure and to further investigate and confirm the safety profile and low genotoxic potential that has been documented to date, particularly in children initiating hydroxycarbamide therapy at a very young age. The long-term safety and efficacy of hydroxycarbamide in children with SCA have not yet been fully defined, but this study provides important and reassuring long-term data for children with SCA receiving hydroxycarbamide therapy and documents a low genotoxic effect.
Acknowledgments
This work was supported in part by R01-HL-090941 and U54-HL070590-07 (REW) from the National Heart, Lung, and Blood Institute, by the National Cancer Institute Cancer Center Support Grants 2 P30 CA021765 (JML) and T32-CA070089 (PTM) and by the American Lebanese Syrian Associated Charities (ALSAC). The authors would especially like to thank Marc Valentine and Susan Ragsdale, CLSp(CG), in the Comprehensive Cancer Center Cytogenetic Share Resource for performing the extensive cytogenetic analyses and Nicole A. Mortier, MHS PA-C, Amy C. Kimble, FNP and the St. Jude Children’s Research Hospital clinical staff for providing outstanding patient care including hydroxycarbamide management, and to all patients and families for study participation.
References
- Ariel IM. Therapeutic effects of hydroxyurea. Experience with 118 patients with inoperable solid tumors. Cancer. 1970;25:705–714. doi: 10.1002/1097-0142(197003)25:3<705::aid-cncr2820250331>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Brawley OW, Cornelius LJ, Edwards LR, Gamble VN, Green BL, Inturrisi C, James AH, Laraque D, Mendez M, Montoya CJ, Pollock BH, Robinson L, Scholnik AP, Schori M. National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med. 2008;148:932–938. doi: 10.7326/0003-4819-148-12-200806170-00220. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Hall EJ. Computed Tomography – An Increasing Source of Radiation Exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- Cassileth PA, Hyman GA. Treatment of malignant melanoma with hydroxyurea. Cancer Res. 1967;27:1843–1845. [PubMed] [Google Scholar]
- Charache S, Dover GJ, Moore RD, Eckert S, Ballas SK, Koshy M, Milner PF, Orringer EP, Phillips G, Jr., Platt OS. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992;79:2555–2565. [PubMed] [Google Scholar]
- Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- Chaube S, Murphy ML. The effects of hydroxyurea and related compounds on the rat fetus. Cancer Res. 1966;26:1448–1457. [PubMed] [Google Scholar]
- de Lima PD, Cardoso PC, Khayat AS, Mde O. Bahia, Burbano RR. Evaluation of the mutagenic activity of hydroxyurea on the G1-S-G2 phases of the cell cycle: an in vitro study. Genet Mol Res. 2003;2:328–333. [PubMed] [Google Scholar]
- de Montalembert M, Brousse V, Elie C, Bernaudin F, Shi J, Landais P. Long-term hydroxyurea treatment in children with sickle cell disease: tolerance and clinical outcomes. Haematologica. 2006;91:125–128. [PubMed] [Google Scholar]
- Denning SM, Tuck DT, Singer KH, Haynes BF. Human thymic epithelial cells function as accessory cells for autologous mature thymocyte activation. J Immunol. 1987;138:680–686. [PubMed] [Google Scholar]
- Finazzi G, Ruggeri M, Rodeghiero F, Barbui T. Second malignancies in patients with essential thrombocythaemia treated with busulphan and hydroxyurea: long-term follow-up of a randomized clinical trial. Br J Haematol. 2000;110:577–583. doi: 10.1046/j.1365-2141.2000.02188.x. [DOI] [PubMed] [Google Scholar]
- Flanagan JM, Howard TA, Mortier N, Avlasevich SL, Smeltzer MP, Wu S, Dertinger SD, Ware RE. Assessment of genotoxicity associated with hydroxyurea therapy in children with sickle cell anemia. Mutat Res. 2010;698:38–42. doi: 10.1016/j.mrgentox.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis AA, Blevins RD, Carrier WL, Smith DP, Regan JD. Inhibition of DNA repair in ultraviolet-irradiated human cells by hydroxyurea. Biochim Biophys Acta. 1979;563:385–392. doi: 10.1016/0005-2787(79)90057-1. [DOI] [PubMed] [Google Scholar]
- Friedrisch JR, Pra D, Maluf SW, Bittar CM, Mergener M, Pollo T, Kayser M, da Silva MA, Henriques JA, da Rocha Silla LM. DNA damage in blood leukocytes of individuals with sickle cell disease treated with hydroxyurea. Mutat Res. 2008;649:213–220. doi: 10.1016/j.mrgentox.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Gulbis B, Haberman D, Dufour D, Christophe C, Vermylen C, Kagambega F, Corazza F, Devalck C, Dresse MF, Hunninck K, Klein A, Le PQ, Loop M, Maes P, Philippet P, Sariban E, Van Geet C, Ferster A. Hydroxyurea for sickle cell disease in children and for prevention of cerebrovascular events: the Belgian experience. Blood. 2005;105:2685–2690. doi: 10.1182/blood-2004-07-2704. [DOI] [PubMed] [Google Scholar]
- Gustashaw K. In: The ACT Cytogenetics Laboratory Manual. Second Edition MJ B, editor. Raven Press, Ltd.; New York: 1991. [Google Scholar]
- Hanft VN, Fruchtman SR, Pickens CV, Rosse WF, Howard TA, Ware RE. Acquired DNA mutations associated with in vivo hydroxyurea exposure. Blood. 2000;95:3589–3593. [PubMed] [Google Scholar]
- Hankins JS, Ware RE, Rogers ZR, Wynn LW, Lane PA, Scott JP, Wang WC. Long-term hydroxyurea therapy for infants with sickle cell anemia: the HUSOFT extension study. Blood. 2005;106:2269–2275. doi: 10.1182/blood-2004-12-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, Wilkins BS, van der Walt JD, Reilly JT, Grigg AP, Revell P, Woodcock BE, Green AR. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353:33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Hematol Oncol Clin North Am. 2010;24:199–214. doi: 10.1016/j.hoc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BJ, Yarbro JW. Metabolic and therapeutic effects of hydroxyurea in chronic myeloid leukemia. JAMA. 1966;195:1038–1043. [PubMed] [Google Scholar]
- Khayat AS, Antunes LM, Guimaraes AC, Bahia MO, Lemos JA, Cabral IR, Lima PD, Amorim MI, Cardoso PC, Smith MA, Santos RA, Burbano RR. Cytotoxic and genotoxic monitoring of sickle cell anaemia patients treated with hydroxyurea. Clin Exp Med. 2006;6:33–37. doi: 10.1007/s10238-006-0091-x. [DOI] [PubMed] [Google Scholar]
- Kinney TR, Helms RW, O’Branski EE, Ohene-Frempong K, Wang W, Daeschner C, Vichinsky E, Redding-Lallinger R, Gee B, Platt OS, Ware RE. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood. 1999;94:1550–1554. [PubMed] [Google Scholar]
- Leavell UW, Jr., Yarbro JW. Hydroxyurea. A new treatment for psoriasis. Arch Dermatol. 1970;102:144–150. doi: 10.1001/archderm.102.2.144. [DOI] [PubMed] [Google Scholar]
- Letvin NL, Linch DC, Beardsley GP, McIntyre KW, Nathan DG. Augmentation of fetal-hemoglobin production in anemic monkeys by hydroxyurea. N Engl J Med. 1984;310:869–873. doi: 10.1056/NEJM198404053101401. [DOI] [PubMed] [Google Scholar]
- Levin VA, Edwards MS, Wara WM, Allen J, Ortega J, Vestnys P. 5-Fluorouracil and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) followed by hydroxyurea, misonidazole, and irradiation for brain stem gliomas: a pilot study of the Brain Tumor Research Center and the Childrens Cancer Group. Neurosurgery. 1984;14:679–681. doi: 10.1227/00006123-198406000-00006. [DOI] [PubMed] [Google Scholar]
- Lewis WH, Wright JA. Altered ribonucleotide reductase activity in mammalian tissue culture cells resistant to hydroxyurea. Biochem Biophys Res Commun. 1974;60:926–933. doi: 10.1016/0006-291x(74)90403-3. [DOI] [PubMed] [Google Scholar]
- Li JC, Kaminskas E. Progressive formation of DNA lesions in cultured Ehrlich ascites tumor cells treated with hydroxyurea. Cancer Res. 1987;47:2755–2758. [PubMed] [Google Scholar]
- Lofvenberg E, Wahlin A. Management of polycythaemia vera, essential thrombocythaemia and myelofibrosis with hydroxyurea. Eur J Haematol. 1988;41:375–381. doi: 10.1111/j.1600-0609.1988.tb00212.x. [DOI] [PubMed] [Google Scholar]
- Lori F, Malykh A, Cara A, Sun D, Weinstein JN, Lisziewicz J, Gallo RC. Hydroxyurea as an inhibitor of human immunodeficiency virus-type 1 replication. Science. 1994;266:801–805. doi: 10.1126/science.7973634. [DOI] [PubMed] [Google Scholar]
- Murphy ML, Chaube S. Preliminary Survey of Hydroxyurea (Nsc-32065) as a Teratogen. Cancer Chemother Rep. 1964;40:1–7. [PubMed] [Google Scholar]
- Nand S, Stock W, Godwin J, Fisher SG. Leukemogenic risk of hydroxyurea therapy in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Am J Hematol. 1996;52:42–46. doi: 10.1002/(SICI)1096-8652(199605)52:1<42::AID-AJH7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, Fishbein WN. Induction of chromosome breaks in cultured normal human leukocytes by potassium arsenite, hydroxyurea and related compounds. Cancer Res. 1965;25:980–985. [PubMed] [Google Scholar]
- Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, Nathan DG. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest. 1984;74:652–656. doi: 10.1172/JCI111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers Z, Thompson B, Ware R, Wang W, Iver R, Miller S, Minniti C, Rana S, Barredo J, Toledano S, Zimmerman S, Casella J, Files B, Waclawiw M, Gerber A, Bonds D. Pharmacokinetics of hydroxyurea in young children with sickle cell anemia: a report from the BABY HUG trial. Blood (ASH Annual Meeting Abstracts) 2005;106:3184. [Google Scholar]
- Sakano K, Oikawa S, Hasegawa K, Kawanishi S. Hydroxyurea induces site-specific DNA damage via formation of hydrogen peroxide and nitric oxide. Jpn J Cancer Res. 2001;92:1166–1174. doi: 10.1111/j.1349-7006.2001.tb02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, Varma M, Ramirez G, Adler B, Smith W, Carlos T, Ataga K, DeCastro L, Bigelow C, Saunthararajah Y, Telfer M, Vichinsky E, Claster S, Shurin S, Bridges K, Waclawiw M, Bonds D, Terrin M. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- Sterkers Y, Preudhomme C, Lai JL, Demory JL, Caulier MT, Wattel E, Bordessoule D, Bauters F, Fenaux P. Acute myeloid leukemia and myelodysplastic syndromes following essential thrombocythemia treated with hydroxyurea: high proportion of cases with 17p deletion. Blood. 1998;91:616–622. [PubMed] [Google Scholar]
- Wang WC, Wynn LW, Rogers ZR, Scott JP, Lane PA, Ware RE. A two-year pilot trial of hydroxyurea in very young children with sickle-cell anemia. J Pediatr. 2001;139:790–796. doi: 10.1067/mpd.2001.119590. [DOI] [PubMed] [Google Scholar]
- Ware R, He J, NA M, Cheng C, Flanagan J, Sparreboom A. Distinct phenotypes of hydroxyurea absorption among children with sickle cell anemia. Blood (ASH Annual Meeting Abstracts) 2008;112:709. [Google Scholar]
- Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbro JW. Mechanism of action of hydroxyurea. Semin Oncol. 1992;19:1–10. [PubMed] [Google Scholar]
- Ziegler-Skylakakis K, Schwarz LR, Andrae U. Microsome- and hepatocyte-mediated mutagenicity of hydroxyurea and related aliphatic hydroxamic acids in V79 Chinese hamster cells. Mutat Res. 1985;152:225–231. doi: 10.1016/0027-5107(85)90065-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman SA, Schultz WH, Davis JS, Pickens CV, Mortier NA, Howard TA, Ware RE. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103:2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]