Abstract
The use of microneurography to measure muscle sympathetic nerve activity has provided important insights in human physiology. However, placing microelectrodes into nerves can be challenging, particularly in certain patient populations. In this paper, we describe the use of real-time ultrasound guidance to assist with microneurography, including advantages, disadvantages, and proper training.
Introduction
Microneurography is a technique used in humans to measure post-ganglionic sympathetic neural activity directed towards muscle and skin. It is commonly performed by introducing a microelectrode percutaneously into the nerve to be studied and then adjusting the electrode until a satisfactory signal is recorded that represents the targeted neural output and has adequate signal-to-noise ratio for analysis and quantification. This technique relies on the placement of the electrode primarily based on the sounds heard via an amplifier system (usually 80,000 to 100,000-fold amplification) and requires an extensive amount of training, knowledge of anatomy, recognition of the subtle and characteristic sounds associated with the target signal, and experience. Variable success rates are reported (ranging from ~ 60 – 85%) and the success rate in certain subject populations (e.g. subjects who are obese) may be lower. We report here the first use of real-time ultrasound guided percutaneous microneurography. We also discuss important considerations of the use of ultrasound in microneurography, including advantages and disadvantages as well as suggestions for introducing ultrasound into practice.
History of microneurography
The use of microneurography for the direct measurement of sympathetic neural activity (SNA) in human subjects was first developed in Uppsala, Sweden in the late 1960s (Vallbo et al., 2004). This group of clinical neurophysiologists came across SNA by chance during studies designed to record activity from large muscle spindle afferents. Post-ganglionic sympathetic nerves directed to the skeletal muscle vasculature are noradrenergic vasoconstrictor nerves, which exhibit tonic resting activity that occurs in a so-called “bursting” pattern. This muscle sympathetic neural activity (MSNA) is the most commonly measured using microneurography. Each burst of sympathetic nerve activity represents a collection of action potentials from individual neurons. Because of the strong influence of the arterial baroreflex on efferent MSNA, the bursts exhibit a regular relationship with the cardiac cycle. However, non-baroreflex influences also affect the gating of efferent activity, such that (normally) not every cardiac cycle is associated with a sympathetic burst.
Currently, the most common way to search for the MSNA signal is to start with external (transcutaneous) electrical stimulation of the nerve to identify candidate sites for the nerve search. Subsequently, the microelectrode is introduced across the skin and manipulated with small, slow movements while the investigators listen for signals from an amplifier system. Usually, the placement of the electrode into the nerve bundle results in a “discharge” of activity. Further micro-adjustment of the electrode is necessary to locate, isolate and optimize the MSNA signal. In addition to the relationship with the cardiac cycle, other characteristics helpful in identifying a good MSNA signal include an afferent response when the muscle spindle is stretched but not when the skin is stroked (which would indicate a skin SNA signal), an increase in activity during voluntary endexpiratory apnea, no change in activity in response to a sudden startle stimulus (such as a loud noise), and an increase in activity during decreases in blood pressure or orthostasis (Vallbo et al., 2004).
In some populations, including individuals who are overweight or obese, longer microelectrodes may be necessary to be able to reach the nerve. Both the increased distance from the skin to the nerve and the difficulty controlling longer microelectrodes adds to the difficulty of microneurography in these populations.
We have been using ultrasound for several years for invasive procedures such as the placement of arterial and central venous catheters and nerve blocks for pain and anesthesia. The advantages of the use of ultrasound for this purpose are now widely accepted and the use of live ultrasound guidance is policy in our institution for most central venous catheterizations. These experiences have resulted in a high level of familiarity with the use of ultrasound and we reasoned that it could be used to guide the placement of microelectrodes for measurement of MSNA. Based on our subsequent experience, we believe individuals who are trained in the use of ultrasound can utilize it successfully to locate peripheral nerves and to guide the placement of microelectrodes into the nerve. It may be especially beneficial in those populations where locating nerves using conventional non-image guided microneurography is more difficult (e.g. subjects with peripheral obesity).
Advances in ultrasound
In the last two decades, relatively inexpensive, user-friendly, portable ultrasound machines capable of generating high-resolution images in real-time have been developed. In particular, higher frequency ultrasound probes (transceivers) and better post-processing has made the identification of small anatomical objects such as peripheral nerves easier. Consequently ultrasound is now widely used in research and clinical applications, including guiding needle-based procedures under live visualization. These advances also make ultrasound attractive for use in microneurography. However, it is important that the advantages and limitations of ultrasonography are understood before it is used for any diagnostic or interventional procedure, including microneurography. Additionally, development of expertise in both microneurography and interventional ultrasound procedures is essential before the use of ultrasound in microneurography is considered.
Technique
We have used ultrasound to image the peroneal nerve along the later aspect of the lower leg at the fibular head as well as at the popliteal fossa posteriorly. We have also imaged the three major nerves of the upper extremity (radial, ulnar, and median) at various locations along the arm. In all cases, the nerves were clearly distinguishable from surrounding tissues. We have performed ultrasound-guided microneurography of the radial nerve but the majority of our studies involve measurement of MSNA at the peroneal nerve near the fibular head. As this is also the most commonly used site in the literature, we will focus on this site in our description of the technique.
Microneurography is an invasive procedure and therefore aseptic technique is used. The skin and ultrasound probe are cleaned with a chlorhexidine and/or alcohol solution and sterile microelectrodes and ultrasound gel are used. A linear, high frequency (12–15 MHz) probe is used to image the nerve and microelectrode during placement. The depth of the image required is dependent on the nerve to be imaged and the body habitus of the subject, but the machine display is typically set at 2–3 cm. The probe is placed posterior to the tibial tuberosity at the lateral aspect of the leg with minimal pressure using adequate ultrasound gel to ensure probe contact (Figure 1). The peroneal nerve can be identified in a cross-sectional view by its oval or round shape and its bright, speckled appearance due to the reflective nature of the nerve fascicles and the connective tissue (Figure 2).
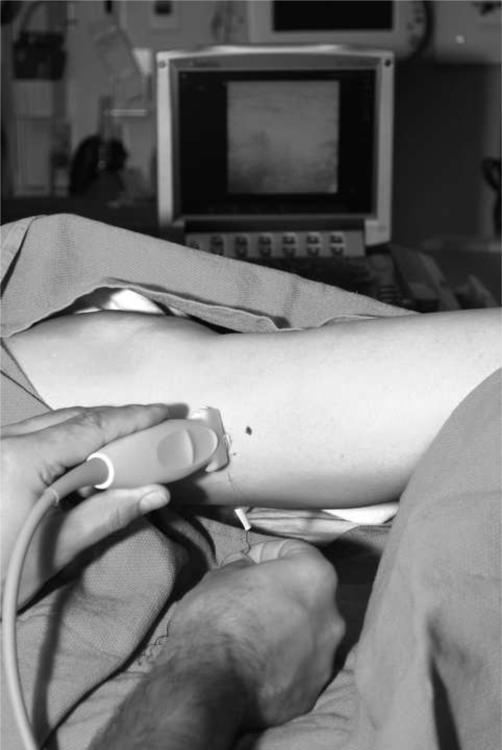
Figure 1.
Photo showing the placement of probe at the lateral aspect of the lower leg to image the peroneal nerve in cross-section at the fibular head (black dot on skin represents caudal border of the fibular head). It is important that attention be paid to ergonomics, including placement of the ultrasound screen, and that an assistant be available to help with adjustments of the ultrasound machine.
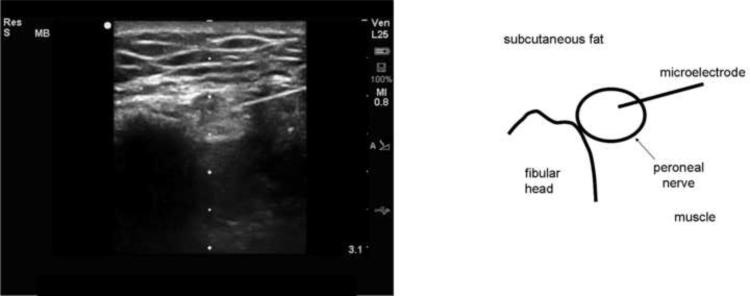
Figure 2.
Ultrasound image and drawing of structures of interest of a microelectrode being introduced into the peroneal nerve at the lateral aspect of the leg at the fibular head. The image is rotated 90° counterclockwise so that the lateral aspect of the leg is represented by the top of the screen. A high-frequency (12 MHz) linear probe was used to image the nerve in cross-section and the microelectrode inserted in an in-plane approach. The dots at the center of the image are 0.5 cm marks and the maximum depth is 3.1 cm. The peroneal nerve is a round, echogenic object posterolateral to the fibular head. The microelectrode can be seen entering the nerve from the right side of the image. The insertion point of the microelectrode across the skin cannot be seen in the image.
The microelectrode is inserted in an in-plane approach percutaneously 1–2 cm posterior to the probe. This allows the microelectrode to stay as close to 90° to the ultrasound beam for best wave reflection and image production. This cross-sectional, in-plane technique requires that the lower leg of the subject be elevated to allow space for the microelectrode and guiding hand. This may require that a longer microelectrode (40–50 mm) be utilized due to the increased distance this approach creates from the microelectrode insertion site to the nerve. The microelectrode is visualized as it is advanced anteriorly and medially to the external border of the nerve and then into the nerve. Ultrasound-guided microneurography at other sites, such as the peroneal nerve at the popliteal fossa (Figure 3) and nerves of the upper extremity (radial and median nerves) (Figures 4 and 5) can be performed using similar techniques. In-plane imaging of the needle being advanced into a cross-sectional image of the nerve allows for the most accurate placement of microelectrode. Out of plane techniques can be used, but it is difficult to know if the very tip of the microelectrode is in the field of view of the ultrasound beam and the therefore the operator must rely on movement of the surrounding tissues to determine the approximate location of the microelectrode. We have occasionally placed microelectrodes while imaging the nerve longitudinally as well, but it is more difficult to maintain a proper longitudinal image of the nerve while keeping the full length of the microelectrode in view.
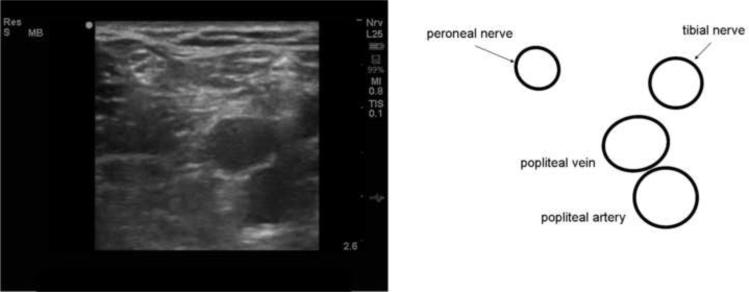
Figure 3.
Ultrasound image and drawing of structures of interest of the peroneal nerve at the posterior aspect of the leg at the popliteal fossa. The image is rotated 180° so that the posterior of the popliteal fossa is represented by the top of the screen. The maximum depth is 2.6 cm. A high-frequency (12 MHz) linear probe was used to image the nerve in cross-section. The peroneal nerve is a round, echogenic object lateral to the tibial nerve and popliteal artery and vein. As the nerve is imaged in a more cephalad location, it joins with the tibial nerve to become the sciatic nerve; caudally it is found more laterally.
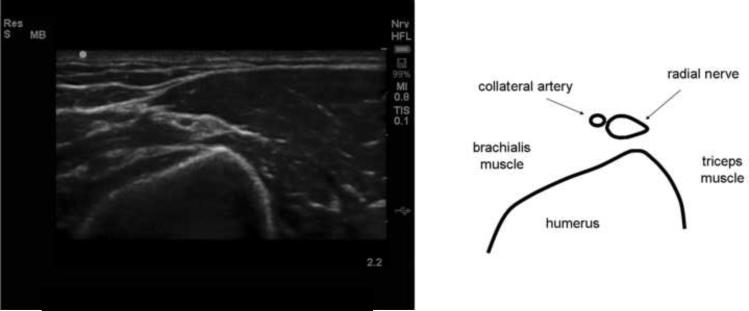
Figure 4.
Ultrasound image and drawing of structures of interest of the radial nerve at the posterior aspect of the distal humerus proximal to the radial head. The image is rotated approximately 180° so that the posterior aspect of the arm is represented by the top of the screen. The maximum depth is 2.2 cm. A high-frequency (12 MHz) linear probe was used to image the nerve in cross-section. The radial nerve is a small teardrop-shaped, echogenic object adjacent to a collateral artery.
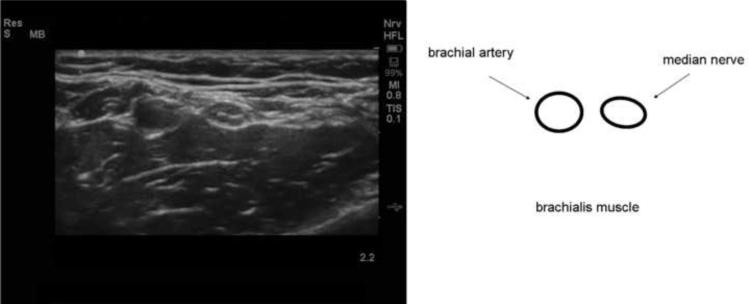
Figure 5.
Ultrasound image and drawing of structures of interest of the median nerve at anterior aspect of the arm at the antecubital fossa. The anterior aspect of the arm is represented by the top of the screen. The maximum depth is 2.2 cm. A high-frequency (12 MHz) linear probe was used to image the nerve in cross-section. The median nerve is a round, echogenic object medial to the brachial artery.
We have found that with ultrasound the time required to place the microelectrode into the nerve can be reduced to just a few minutes. In many cases, the characteristic discharge of nerves is heard immediately and the subject experiences sensations that are associated with intraneuronal placement. This is reproducible enough that we will alert subjects just prior to entry of the microelectrode into the nerve that they may start to feel transient paresthesias or cramping sensations. In some cases, immediate bursts of MSNA can be observed, but usually further small adjustments of the electrode are needed in order to be able to obtain a satisfactory signal using the criteria described above. If an adequate signal cannot be obtained, the use of ultrasound facilitates rapid movement of the electrode to a new site. Due to the highly amplified signals, electrical interference is a concern in any microneurography practice. The vibration of the piezoelectric crystal of the ultrasound probe can cause significant electrical interference and it may be necessary to experiment with different preamplifier, reference electrode, and ultrasound probe cord positions to minimize this interference and to keep contact between the skin and the hand holding the transducer. We turn off the ultrasound machine during recording of the microneurography signal to minimize noise.
The ideal method of introducing ultrasound into a microneurography practice is not clear, but the ability to use ultrasound to find the peroneal nerve does not replace the need for basic training in the technique of microneurography. Without a proper understanding of microneurography theory, technique, and interpretation and analysis of the signal with, the researcher takes the risk of obtaining uninterpretable data and causing unnecessary discomfort to the subject. It is also important that prior to attempting to introduce ultrasound into a laboratory microneurography program experience with ultrasound and knowledge of basic principles of image generation and device operations, including the physics of ultrasound, machine operation and use, optimization of images, selection of appropriate transducer, depth, focus, time gain compensation, overall gain settings, and the effects of transducer pressure and movements. Guidelines for training in ultrasound-guided regional anesthesia have been developed and may serve as a model (Sites et al., 2010). Ultrasound guidance should be first practiced using simulators, cadavers, or models such as gel blocks before applying to human subjects. Pitfalls are common with ultrasound guided procedures and again, the regional anesthesia literature can serve as a guide in understanding and avoiding these errors (Sites et al., 2007).
Advantages of ultrasound guidance
In place of transcutaneous stimulation, ultrasound can be used to locate the anatomical location of the nerve and its depth to guide placement of the microelectrode, allowing for greater accuracy. It also allows identification of surrounding anatomical structures (such as vascular structures and bones) which may affect the placement of a microelectrode. However, the greatest advantage of ultrasound is the ability to visualize the electrode and the nerve simultaneously and place the microelectrode into the nerve under “live” ultrasound guidance. Even though tungsten microelectrodes are extremely small in diameter, they are very echogenic and, if coincident with the ultrasound beam, are quite visible in the generated image as a bright white line (Figure 1). We report here our experience with these advantages of ultrasound-guided microneurography; however, we have not conducted prospective statistical analyses comparing success rates with and without use of ultrasound. Therefore this report must be considered a preliminary description of the technique. Clearly, prospective randomized trials would be needed to evaluate whether the use of ultrasound results a meaningful improvement in success rates for microneurography, although they would be difficult to perform.
Disadvantages of ultrasound guidance
The use of ultrasound in microneurography is not without important limitations. While in the number of manufacturers and machines has grown and machines and probes have become more affordable, even used units can cost thousands of dollars and maintenance is expensive. We have found that locating the nerve with transcutaneous stimulation is not necessary with the use of ultrasound but it takes additional time to set up the ultrasound unit and obtain satisfactory images, especially when learning the technique. In some cases, nerves are difficult to identify or are misidentified due to factors such as anatomical variation or ultrasound artifacts and this can lead to searching for a nerve signal in the wrong structure. Without a significant amount of training and practice in ultrasound guided invasive procedures, it is common for the microelectrode to not be fully visualized or for the electrode to be advanced without proper visualization of both electrode and nerve. This can lead to the operator not realizing the tip of the microelectrode is not in the nerve or for structures other than the nerve (e.g. periosteum) being inadvertently contacted, which can be uncomfortable for the subject.
Most importantly, using ultrasound does not guarantee that an adequate signal can be obtained. For example, we have observed the microelectrode entering or passing through nerves without hearing the characteristic discharge. Additionally, the types of nerve fibers that the tip of the microelectrode is in contact with can not be determined with ultrasound and even after placing the microelectrode into a nerve under ultrasound, additional adjustments of the electrode are usually needed to obtain an appropriate signal. Specifically, ultrasound is not of help in avoiding skin SNA or “mixed” skin and muscle signals, a frequently encountered problem, although it does facilitate rapid repositioning of the microelectrode. Again, training in recognizing and obtaining a proper signal is essential before ultrasound guided microneurography can be of use.
Summary
We believe ultrasound-guided microneurography is a powerful approach to this important technique for direct measurement of sympathetic neural activity in intact humans. While our laboratory has used non-image guided microneurography for many years, ultrasound guided microneurography is being utilized increasingly as its utility becomes apparent. However, further experience and ultimately randomized studies of the effect of ultrasound on the success of microneurography are needed to determine if ultrasound increases success, reduces the time spent locating an appropriate signal, and increases patient comfort during the procedure. Finally, the developers of microneurography have stated “… it cannot be too strongly emphasized that it is essential to have a proper education in a laboratory with adequate experience of the technique before microneurography is adopted in a new laboratory.” (Vallbo et al., 2004) We extend this caution to adopting the use of ultrasound for microneurography.
Supplementary Material
Real-time video showing placement of microelectrode into peroneal nerve. The image is rotated 90° counterclockwise so that the lateral aspect of the leg is represented by the top of the screen. A high-frequency (12 MHz) linear probe was used to image the nerve in cross-section and the microelectrode was inserted in an in-plane approach. The dots at the center of the image are 0.5 cm marks and the maximum depth is 2.2 cm. The peroneal nerve is a round, echogenic object posterolateral to the fibular head. The microelectrode can be seen entering the nerve from the right side of the image. Note the amount of movement of the nerve as it is contacted by the microelectrode and the movement of the fascicles in the nerve as the microelectrode is adjusted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- de Kool BS, Blok JH, Walbeehm ET, van Neck JW, Hovius SE, Visser GH. Ultrasound-guided near-nerve neurography for early evaluation of nerve regeneration. J Neurosci Methods. 2008;174:265–271. doi: 10.1016/j.jneumeth.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Howry DH, Bliss WR. Ultrasonic visualization of soft tissue structures of the body. J Lab Clin Med. 1952;40:579–592. [PubMed] [Google Scholar]
- Kapral S, Krafft P, Eibenberger K, Fitzgerald R, Gosch M, Weinstabl C. Ultrasound-guided supraclavicular approach for regional anesthesia of the brachial plexus. Anesth Analg. 1994;78:507–513. doi: 10.1213/00000539-199403000-00016. [DOI] [PubMed] [Google Scholar]
- Kristensen JK, Holm HH, Rasmussen SN, Barlebo H. Ultrasonically guided percutaneous puncture of renal masses. Scandinavian journal of urology and nephrology. 1972;6(Suppl 15):49–56. doi: 10.3109/00365597209133645. [DOI] [PubMed] [Google Scholar]
- Sites BD, Brull R, Chan VW, Spence BC, Gallagher J, Beach ML, Sites VR, Hartman GS. Artifacts and pitfall errors associated with ultrasound-guided regional anesthesia. Part I: understanding the basic principles of ultrasound physics and machine operations. Reg Anesth Pain Med. 2007;32:412–418. doi: 10.1016/j.rapm.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Sites BD, Chan VW, Neal JM, Weller R, Grau T, Koscielniak-Nielsen ZJ, Ivani G. The American Society of Regional Anesthesia and Pain Medicine and the European Society of Regional Anaesthesia and Pain Therapy joint committee recommendations for education and training in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2010;35:S74–80. doi: 10.1097/AAP.0b013e3181d34ff5. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol. 2004;96:1262–1269. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Real-time video showing placement of microelectrode into peroneal nerve. The image is rotated 90° counterclockwise so that the lateral aspect of the leg is represented by the top of the screen. A high-frequency (12 MHz) linear probe was used to image the nerve in cross-section and the microelectrode was inserted in an in-plane approach. The dots at the center of the image are 0.5 cm marks and the maximum depth is 2.2 cm. The peroneal nerve is a round, echogenic object posterolateral to the fibular head. The microelectrode can be seen entering the nerve from the right side of the image. Note the amount of movement of the nerve as it is contacted by the microelectrode and the movement of the fascicles in the nerve as the microelectrode is adjusted.