Abstract
The relationship between physical activity and central nervous system mechanisms of pain in fibromyalgia (FM) is unknown. This study determined whether physical activity was predictive of brain responses to experimental pain in FM using functional magnetic resonance imaging (fMRI). Thirty-four participants (n=16 FM; n=18 Control) completed self-report and accelerometer measures of physical activity and underwent fMRI of painful heat stimuli. In FM patients, positive relationships (p<0.005) between physical activity and brain responses to pain were observed in the dorsolateral prefrontal cortex, posterior cingulate cortex, and the posterior insula, regions implicated in pain regulation. Negative relationships (p<0.005) were found for the primary sensory and superior parietal cortices, regions implicated in the sensory aspects of pain. Greater physical activity was significantly (p<0.05) associated with decreased pain ratings to repeated heat stimuli for FM patients. A similar non-significant trend was observed in controls. In addition, brain responses to pain were significantly (p<0.005) different between FM patients categorized as `low' active and those categorized as `high' active. In controls, positive relationships (p<0.005) were observed in the lateral prefrontal, anterior cingulate and superior temporal cortices and the posterior insula. Our results suggest an association between measures of physical activity and central nervous system processing of pain.
PERSPECTIVE
Our data suggest that brain responses to pain represent a dynamic process where perception and modulation co-occur and that physical activity plays a role in balancing these processes. Physically active FM patients appear to maintain their ability to modulate pain while those who are less active do not.
Keywords: chronic pain, functional magnetic resonance imaging, accelerometry, thermal pain stimulus, exercise, pain regulation
INTRODUCTION
Fibromyalgia (FM) is a chronic pain disorder estimatedto affect ~5 percent of the general population in both the United States and Europe.5,26 The American College of Rheumatology criteria for a diagnosis of FM require pain lasting for at least 3 months in all four quadrants of the body and along the axial skeleton, as well as the presence of at least 11 of 18 specific tender points.57 Beyond the cardinal symptom of pain, FM is associated with sleep disturbances, fatigue, cognitive complaints, and exacerbation of symptoms with exertion. Overall health tends to be poor and patients report low health-related quality of life across several psychological and physical domains.17
Though the causes of FM remain poorly understood, research has demonstrated that altered central nervous system (CNS) processing of nociceptive signals is a reliable characteristic of FM and may be responsible for maintaining widespread pain.9,42 Compared to healthy controls, FM patients have altered spinal cord levels of biochemicals with known roles in nociception such as substance P and serotonin.37,39 Several studies have shown augmented windup responses to repetitive pain stimuli36,43 and an absence of diffuse noxious inhibitory controls,24,25 phenomena associated with wide-dynamic range neurons in the spinal cord21 and the dorsal reticular nucleus of the medulla51. Finally, compelling evidence that the CNS is involved in FM pain comes from recent neuroimaging studies demonstrating augmented brain responses to both painful and non-painful stimuli in FM patients.6,10,19
Identifying effective treatments for FM has been a challenge for the medical and scientific communities. One consistently efficacious treatment for FM is exercise training.7 Several studies have demonstrated significant improvements in mood, general well-being and symptomsfollowing exercise training compared to various control conditions.22,56 Results from these studies suggest that being sedentary is associated with greater symptom burden and that maintaining or increasing physical activity levels can improve several symptoms associated with FM. Interestingly, this same relationship, albeit to a lesser extent, has been observed in otherwise healthy individuals.16
Despite the positive effect of exercise training on symptoms, FM patients are sedentary and many are hesitant to engage in physical activity for fear of symptom exacerbation.48 This is supported by data demonstrating that clinical pain is negatively related to physical activity in FM23 and that intense exercise increases central pain processing.50 Integration of the evidence suggests a cyclic relationship between physical activity and FM, wherein decreased activity increases the risk of developing symptoms and these symptoms further depress physical activity.
The neural mechanisms through which FM pain is related to physical activity have not been characterized. Since CNS mechanisms appear to play an important role in maintaining FM pain and evidence suggests that pain limits physical activity, this study explored the relationship between physical activity and brain responses to painful stimulation in FM patients and healthy controls using functional magnetic resonance imaging (fMRI). We hypothesized that both self-reported physical activity and activity measured objectively by accelerometry would be negatively related to brain activity in areas involved in sensory and affective dimensions of pain, and positively related to areas implicated in pain modulation.
MATERIALS AND METHODS
The institutional review board at the University of Wisconsin-Madison approved all experimental procedures, and written informed consent was obtained from each participant. Because FM predominately affects women, only females were recruited for this study. FM patients and healthy controls were recruited by newspaper advertisements, fliers in rheumatology clinics and by mass e-mail to female faculty, staff and students at the University of Wisconsin-Madison. All participants were recruited as part of a larger study investigating brain responses to pain and were paid $200 for completion of the study. Participants for the current study were also included in a study examining physical activity behaviors in FM patient and controls.30 A physician confirmed diagnosis of FM according to ACR criteria57 was required for inclusion in the patient group. Confirmation was obtained from each patient's care provider via a letter indicating that the patient met the widespread pain and tender point criteria for a diagnosis of FM.57 Healthycontrols were required to be free of chronic pain complaints to take part in the study. Participants in both groups were screened for the presence of ferrous metal in their bodies, pregnancy and claustrophobia. Any FM patient with a comorbid painful disorder (i.e. arthritis) and participants in either group who were taking analgesic, cardiovascular, or high-dose anti-depressant medications were also excluded from the study. For practical purposes of study recruitment and a realistic consideration of the FM patient, continuation of low-dose antidepressants was permitted. This criterion was also applied to the healthy control group. Both groups were asked to maintain their current level of medication use and not change it during the course of the study. Five FM patients and two healthy controls reported using low-dose antidepressants. In addition to our entry criteria, participants were instructed to abide by the following criteria prior to testing: 1) no structured exercise for at least 48 hours, 2) abstain from alcohol for at least 24 hours, 3) not to consume drinks or foods containing caffeine for at least 4 hours, and 4) abstain from smoking for at least 2 hours. Participants confirmed compliance with these restrictions prior to brain imaging data collection. We did not exclude for chronic fatigue syndrome (CFS) in the larger study, however none of the FM patients in the current investigation had comorbid CFS. Finally, a trained interviewer screened all participants for exclusionary diagnoses of major depression, substance abuse, and other major Axis I psychiatric disorders using the Structured Clinical Interview for DSM-IV Disorders (SCID).14 Eighteen FM patients and 19 healthy controls met criteria for inclusion in the study.
Experimental design and procedures
Participants visited the laboratory on two days separated by a 7-day physical activity monitoring period. On the initial visit, participants self-reported their physical activity behaviors over the past week using the long form of the International Physical Activity Questionnaire (IPAQ). The total score for the IPAQ is calculated from subscales based on activity type (i.e. work-related, housework, transportation, and recreation) and intensity levels (walking, moderate, and vigorous) and represents activities participated in during the week prior to filling out the form. Data are reported in MET-minutes per week for activity type and minutes per day for intensity level. The questionnaire is widely used and has been demonstrated to have acceptable reliability and validity for physical activity measurement.13
Participants then completed a simulated MRI session to help familiarize participants to the scanning environment, determine sensitivity to a range of heat pain stimuli and to determine the temperature of the heat stimuli to be used during the actual scan day. All thermal stimuli were delivered using the Medoc TSA-II NeuroSensory Analyzer with a 900mm2 Peltier thermode (Medoc Advanced Medical Systems, Israel) to the thenar eminence of the left hand.
Suprathreshold pain sensitivity was assessed while participants were in the mock scanner. Each participant received seven temperatures (whole degree increments between 43 and 49 °C) twice, in random order, and rated the intensity and unpleasantness of each stimulus using separate vertical 0–20 category-ratio scalesanchored by written desriptors.18 Heat stimuli were delivered for 8 seconds each, with 1-minute inter-stimulus intervals. For each participant, linear regression was performed on the intensity ratings to determine the temperature corresponding to “moderate” pain (i.e., between 11 and 13) on the 0–20 scale). We chose a relative pain stimulus designed to be perceived as moderate by our participants because in our previous study10 we reported that FM patients differed from controls when everyone received a 47-°C stimulus, but that these differences were largely diminished when a perceptually relative stimulus that participants rated as a `5' (strong pain) out of `10' was used. Our results were consistent with those of Gracely et al.19 who demonstrated similar neural responses between FM patients and healthy controls using perceptually equivalent pressure stimuli; and provided stronger evidence of sensory augmentation in FM by ensuring that sensitivity to peripheral pain stimuli was controlled for. Use of perceptually relative pain stimuli allows for a more specific test of the relationship between physical activity and brain responses to pain. This temperature was used for each painful stimulus delivered during the fMRI data collection day.
After the MRI simulation, participants were asked to wear an ActiGraph GT1M accelerometer (ActiGraph, LLC, Pensacola, FL) to measure physical activity for the 7-day period preceding their fMRI scan. Participants were provided standard instructions and asked to wear the monitor throughout the day and to remove it only if they were planning to sleep or engage in activities which might expose the monitor to water (e.g. showering or swimming). Briefly, participants were instructed that the monitor should be worn at hip level between their side and navel (verbal and visual demonstration) in the upright position. They were further instructed they could wear it on either the right or left side, but to stick with the chosen side. A research assistant demonstrated proper placement of the device and had the participant place the device on themself. If necessary, adjustments were made to ensure a tight fit. Data were recorded continuously using 1-minute epochs. One week after the simulation day participants returned for fMRI scanning.
Conventional and functional image acquisition
All MR images were acquired on a 3-tesla GE Signa scanner (Waukesha, WI). High-resolution T1 weighted anatomical images were collected in the sagittal plane using an inversion-recovery sequence (Inversion time=600 milliseconds, TR=9 milliseconds, TE=1.7 milliseconds, flip angle=10 °, field of view=240 mm, 128 slices, 1.2 mm slice thickness). T2*-weighted echo-planar blood oxygen level dependent (EPI-BOLD) functional images were collected with a whole-head transmit-receive coil using the following parameters: repetition time=2000 milliseconds; echo time=30 milliseconds; flip angle=90 °; field of view=240 mm; and acquisition matrix of 64 × 64 voxels, yielding whole brain coverage with 30 4-mm slices with a 1-mm gap.
Due to saturation effects, the first threesets of functional images were not used in statistical analyses of functional brain data. Functional scans during heat pain were acquired for all subjects, as part of a larger series of functional scans that included heat pain and cognitive tasks. To control for the potential influence of the tasks involved in the larger series of functional scans on brain responses to heat pain, participants were randomly assigned to four separate functional scanning run orders. Good balance was achieved for both the FM (Order 1: n=3; Order 2: n=6; Order 3: n=5; Order 4: n=4) and healthy control (Order 1: n=5; Order 2: n=4; Order 3: n=6; Order 4: n=5) groups. For the heat pain run, following a 24-second baseline period, each participant received five 20-second heat stimuli followed by a 12-second rating period and an 8-second recovery period in a block design. Both intensity and unpleasantness ratings were collected immediately following each stimulus, using the same 0–20 scales as the simulation day.
Image processing and analysis
All analyses were done with Analysis of Functional Neuroimages (AFNI) software.12 Individual subject data were slice-time, motion and fieldmap corrected. Data were then analyzed using a general linear model with separate regressors for the pain stimulus and rating periods. Regressors were created by convolving the stimulus timings with an ideal hemodynamic response function. The single-subject statistical maps created were then smoothed with an 8-mm full-width at half maximum Gaussian kernel, converted to percent signal change, and normalized to the Montreal Neurological Institute 152 (MNI-152) template included in AFNI for group analysis.
Physical activity data processing
Responses to the IPAQ questionnaire were scored based on the accompanying instructions (available at www.ipaq.ki.se/ipaq.htm). Accelerometer data were processed using ActiLife software to determine the number of minutes spent in sedentary (<100 counts/min), moderate (1952–5724 counts/min) and vigorous (>5724 counts/min) activities. The moderate and vigorous cut points were chosen based on data indicating that they correspond to energy expenditures of 3 to 6 and greater than 6 metabolic equivalents (METs), respectively.15 In addition, we calculated an activities of daily living (ADL) cutpoint (760–1952 counts/min) to test whether low levels of physical activity would be related to brain processing of pain.28 The activity data were then further processed by in-house software to exclude days with less than 9 hours of data and any participants who had less than 3 weekdays and 1 weekend day of usable data. Average minutes spent in each activity level and mean total counts were then calculated for weekdays and weekend days separately and a weighted average (i.e. multiplying the weekday average by 5 and the weekend average by 2) was calculated for each subject's entire monitoring period. Finally, average counts per minute were derived by dividing total activity counts by total wear time. For a more detailed description of our methods and physical activity data for the larger group of FM patients and healthy controls please refer to the study by McLoughlin et al.30.
Statistical analyses
In order to examine the relationship between brain responses to experimental pain and physical activity, regression analyses of whole brain data were performed using AFNI's 3dRegAna program.12 Regions of interest (ROI) were chosen based on the extant literature documenting fMRI brain responses to pain in both healthy individuals and patients with FM. Each pain ROI was defined using the Harvard-Oxford cortical and subcortical structural atlas based on data collected at the Centre for Morphometric Analysis at Harvard University, Boston, MA. This probabilistic atlas is based on manual segmentation of 48 cortical and 21 subcortical brain regions based on 37 T1-weighted brain images registered to the standard MNI-152 brain template. The probability threshold for each brain region was set at 0.25. The regions included the pre- and postcentral gyri, anterior and posterior cingulate gyri, anterior and posterior insular cortices, superior, middle and inferior frontal cortices, left and right thalamus, superior parietal cortex and the brain stem. In addition, the occipital polewas chosen as a control region to further test the specificity of the relationships between brain responses to painful heat stimuli and physical activity. The occipital pole was chosen for this purpose because significant increases in brain activity were expected for this region, due to the change in the image participants were viewing during pain testing; yet we did not expect this activity to be related to physical activity levels.
Separate regression analyses were computed for each of the following physical activity metrics: 1) total self-reported activity from the IPAQ, 2) average accelerometer counts per minute, 3) average daily minutes spent doing ADL, and 4) average daily minutes spent in moderate or vigorous activity. Each of these measures was evaluated independently as it is currently unknown whether perception, level or type of activity is most relevant for understanding FM pain. Due to the limited sample size, the a priori designation of pain relevant brain regions, and the exploratory nature of the study, a voxel-wise threshold for significance was set at p=0.005 with a cluster threshold set at 200 mm3.
For the purpose of a subgroup comparisonbased on self-reported physical activity, FM patients were categorized into `high' and `low' active groups (median split; n=8 for each). Activity subgroups were compared using an independent-samples t-test and the pain ROI. For this comparison, the voxel-wise threshold for significance was set at p=0.01 with a cluster threshold at 200 mm3. Accelerometer data were not analyzed with a similar comparison due to the limited sample size.
Because of the central role of the periaqueductal gray (PAG) in descending modulation of pain and the small volume of the region, a region of interest regression was performed between physical activity and the PAG response to pain. The structure was anatomically defined using a detailed brainstem atlas for reference32 and fMRI activity in response to pain was averaged across the region.
Regressions were also planned to assess relationships between our physical activity measures and pain ratings during fMRI data collection. Because we hypothesized that physical activity would be associated with greater brain activity in regions implicated in pain modulation, we computed a change score between the first and last heat stimulus of the fMRI run as a marker of modulation across the scan. We hypothesized that physical activity would be negatively associated with changes in pain ratings across the scan. Thus, the relationship between physical activity and the change score was evaluated by a Pearson correlation with a one-tailed significance of α=0.05.
RESULTS
Of the original sample recruited for participation, two FM patients and one control participant were excluded because of excessive head motion during the fMRI scan (i.e. greater than 2mm movement). Sixteen FM patients and 18 control participants were included in the analyses. Physical characteristics of the participants included in the final analysis are presented in Table 1. There was no significant difference in age or weight between the patient and control groups (p>0.05), but patients were significantly taller than controls (p=0.035).
Table 1.
Participant characteristics
| FM (n=16) | CO (n=18) | p-value | |
|---|---|---|---|
| Age | 42.88 ± 11.60 | 40.83 ± 9.96 | 0.585 |
| Height (cm) | 168.12 ± 6.91 | 163.70 ± 4.63 | 0.035* |
| Weight (kg) | 75.59 ± 17.82 | 66.17 ± 8.32 | 0.067 |
| Years of chronic pain | 14.13 ± 8.03 |
Significant at p = 0.05
Self-reported and accelerometer physical activity data are presented in Tables 2 and 3. For accelerometer data both FM patients and healthy controls averaged roughly 6 days of wearing the accelerometer (FM: 6.2±0.72 days; Controls: 6.6±0.63 days) and greater than 10 hours per day (FM: 901±168 minutes; Controls: 902±92 minutes) of accelerometer wear time. Physical activity data from this subgroup of FM patients and controls was similar to the data from our larger physical activity study30 and indicated that both groups would be considered sedentary based on current physical activity recommendations.
Table 2.
Self-reported physical activity in FMpatients and healthy controls (CO).
| FM (n=16) | CO (n=18) | |
|---|---|---|
| Job-related(MET-min/wk.) | 534 (1450, 5706) | 1104 (2398, 10020) |
| Transportation(MET-min/wk.) | 189 (225, 660) | 694 (809, 2628) |
| Housework(MET-min/wk.) | 1543 (2059, 7850) | 931 (893, 3735) |
| Recreation(MET-min/wk.) | 581 (1064, 4095) | 1181 (1369, 4692) |
| Total(MET-min/wk.) | 2758 (2227, 7776) | 3779 (2701, 10083) |
| Sitting time(min/day) | 437 (232, 720) | 391 (152, 626) |
| Walking(min/day) | 21 (26, 103) | 62 (63, 174) |
| Moderate(min/day) | 74 (61, 171) | 60 (49, 171) |
| Vigorous(min/day) | 4 (11, 43) | 13 (17, 51) |
All measures are expressed as mean (± standard deviation and range). Participants completed the IPAQ on the first day of testing, one week prior to functional brain scanning. Instructions were to provide
Table 3.
Objectively measured total physical activity in FM patientsand healthy controls (CO).
| FM (n=13) | CO (n=14) | |
|---|---|---|
| Daily counts (×103) | 186 (51, 162) | 264 (44, 191) |
| Counts/min | 208 (50, 144) | 295 (52, 180) |
| Minutes sedentary | 1061 (260, 874) | 1130 (55, 167) |
| Minutes ADL | 60 (25, 80) | 75 (19, 63) |
| Minutes moderate | 15 (7, 22) | 28 (10, 30) |
| Minutes vigorous | 0.3 (0.6, 2) | 1.2 (2, 8) |
Measures are mean (± standard deviation and range) expressed as counts and min per day Sedentary = <100 counts/min, ADL = 760–1952 counts/min, moderate = 1952–5724 counts/min, vigorous = >5724 counts/min.
Self-reported physical activity (IPAQ total) was weaklyand not significantly related to any of the accelerometer measures for either FM patients (range of rvalues: −0.19 to 0.14) or healthy controls (range of rvalues: −0.24 to 0.24). For FM patients, accelerometer average counts per minute were positively and significantly (r=0.745, p=0.003) related to ADL (760–1952) counts. Average accelerometer counts per minute were not significantly related (r=0.44) to moderate and vigorous (>1952) counts, nor were ADL counts significantly related (r=0.24) to moderate and vigorous counts. For healthy controls, accelerometer average counts per minute were positively and significantly (r=0.72, p=0.003) related to moderate and vigorous counts. Average accelerometer counts per minute were not significantly related (r=0.15) to ADL counts, and ADL counts were not significantly related (r=0.26) to moderate and vigorous counts.
Functional MRI analyses
Self-reported physical activity
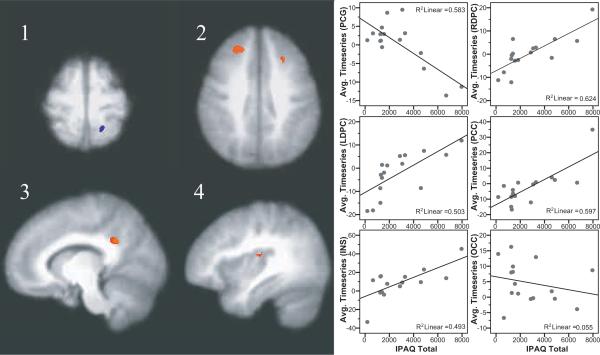
Brain regions showing correlations between self-reported physical activity and the fMRI response to painful heat stimulation are reported in Table 4. In the FM group, responses to painful heat stimuli were positively correlated with self-reported physical activity in the right dorsolateral prefrontal cortex, left dorsolateral prefrontal cortex, left posterior cingulate cortex, and right posterior insula (Figure 1). A negative correlation between self-reported activity and brain responses was found in a region of the postcentral gyrus extending from the left sensory cortex to the left superior parietal cortex. No relationships between brain activity and self-reported physical activity in control participants met our criteria.
Table 4.
Clusters showing relationships between self-reported physical activity and brain responses to pain
| Direction | Peak x, y, z | Volume (mm3) | Peak r2 | F-statistic | |
|---|---|---|---|---|---|
| FM (n=16) | |||||
| R dorsolateral prefrontal | + | −27.5, −35.5, 39.5 | 965 | 0.67 | 27.8 |
| L dorsolateral prefrontal | + | 21.5, −25.5, 39.5 | 371 | 0.63 | 24.1 |
| L superior parietal/postcentral gyrus | − | 16.5, 49.5, 69.5 | 338 | 0.67 | 28.6 |
| L posterior cingulate | + | 12.5, 46.5, 32.5 | 343 | 0.55 | 17.1 |
| R posterior insula | + | −40.5, 17.5, 14.5 | 243 | 0.49 | 13.5 |
| CO (n=18) | |||||
| No significant relationships | |||||
All values significant at voxel-wise threshold of p=0.005 and cluster size threshold at 200 mm3
Figure 1.
Brain regions showing significant associations between self-reported physical activity and responses to heat pain in FM patients. A negative correlation was found in the post-central gyrus, extending into the superior parietal cortex (image 1). Positive correlations were found in the left and right dorsolateral prefrontal cortex (image 2), posterior cingulate cortex (image 3), and the mid to posterior insula (image 4). Images shown are with voxel-wise threshold set a p=0.005 and cluster size thresholding at 200 mm3. Functional timeseries data (average cluster values) for each individual were extracted and are shown plotted against physical activity values with the corresponding r-squared values.
Physical activity measured via accelerometer
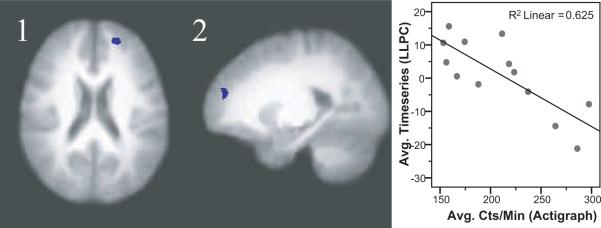
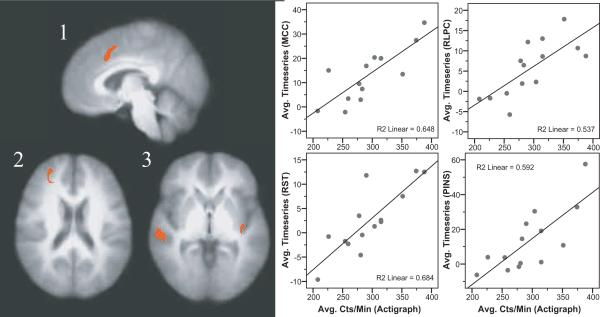
Thirteen FM patients and 14 healthy controls had both accelerometer and fMRI data that met criteria for inclusion. No significant differences in symptoms or physical characteristics were detected between these subsamples and the larger study cohort. Table 5 presents brain areas where pain-evoked activity significantly correlated with objectively measured physical activity. For FM patients, there was a negative relationship between average accelerometer counts and brain responses in the left lateral prefrontal cortex (Figure 2). For healthy controls, brain activity in the right lateral prefrontal cortex, right superior temporal cortex, posterior insular cortex and the anterior portion of the mid-cingulate cortex was positively related to average accelerometer counts per minute of wear time (Figure 3). Regression of the number of minutes spent in moderate or vigorous activity to brain data yielded no significant relationships in either the FM or control groups. For the FM group the results of a regression on the number of minutes spent in ADL (760–1952 counts/min) mirrored those reported for total counts per minute; a significant negative relationship between the amount of time spent in ADL and brain responses to pain in the left lateral prefrontal cortex. For controls, no significant relationship between minutes spent in ADL and brain responses to pain were noted (Table 5).
Table 5.
Relationships between objectively monitored physical activity and brain responses to pain
| Direction | Peak x, y, z | Cluster size (mm3) | Peak r2 | F-statistic | |
|---|---|---|---|---|---|
| FM (n=13) | |||||
| Average accelerometer counts/minute | |||||
| L lateralPFC | − | 19.5, −51.5, 22.5 | 456 | 0.80 | 36.0 |
| Time spent in ADL | − | 22.0, −52.0, 20.0 | 208 | 0.63 | 18.6 |
| L lateralPFC | − | 19.5, −51.5, 22.5 | 456 | 0.80 | 36.0 |
| Time spent in moderate and vigorous activity | |||||
| No significant relationships | |||||
| CO (n=14) | |||||
| Average accelerometer counts/minute | |||||
| R lateralPFC | + | −29.5, −53.5, 22.5 | 493 | 0.66 | 22.1 |
| Anterior MCC | + | −3.5, −20.5, 38.5 | 740 | 0.67 | 24.1 |
| L posterior insula | + | 40.5, 15.5, −1.5 | 217 | 0.60 | 17.9 |
| R superior temporal | + | −62.5, 25.5, −0.5 | 790 | 0.72 | 30.9 |
| Time spent in ADL | |||||
| No significant relationships | |||||
| Time spent in moderate and vigorous activity | |||||
| No significant relationships | |||||
All regions significant at voxel-wise threshold of p=0.005 and cluster size threshold at 200 mm3
Abbreviations: PFC, prefrontal cortex, MCC, mid-cingulate cortex, ADL, activities of daily living
Figure 2.
A significant negative correlation between accelerometer counts per minute and responses to heat pain in FM patients was found in the superior frontal cortex/frontal pole (images 1 and 2). Images shown are with voxel-wise threshold set a p=0.005 and cluster size thresholding at 200 mm3. Functional timeseries data (average cluster values) for each individual were extracted and are shown plotted against physical activity values with the corresponding r-squared values.
Figure 3.
Brain regions showing significant associations between average accelerometer counts per minute of wear time and responses to heat pain in healthy controls.Positive correlations were found in the mid anterior and posterior cingulate cortices (image 1), a region in the lateral prefrontal cortex (image 2), the superior temporal cortex (image 3) and the posterior insular coretx (image 3). Images shown are with voxel-wise threshold set a p=0.005 and cluster size thresholding at 200 mm3. Functional timeseries data (average cluster values) for each individual were extracted and are shown plotted against physical activity values with the corresponding r-squared values.
Control Region
No significant relationships were observed between measures of physical activity and brain responses in the occipital pole for either FM or controls. This occipital ROI was active during our functional pain scan, yet it is typically not considered a pain-relevant brain region. Thus, we considered this region to be a reasonable control to test the specificity of our physical activity relationships.
Group analysis of pain responses between `high' and `low' active FM patients
Significant differences in brain responses to painful stimuli were observed between the high (n=8)and low (n=8)active subgroups of FM patients (p<0.01). FM patients designated as `high' active exhibited greater activity in the left dorsolateral prefrontal cortex and posterior insula extending into the parietal operculum and less activity in the left postcentral gyrus than FM patients designated as `low' active. Total self-reported physical activity for the `low' active subgroup was 1085.4 MET-minutes per week. Total self-reported physical activity for the `high' active subgroup was 4316.5 MET-minutes per week. The `low' active subgroup reported 28 minutes of moderate activity per day while the `high' active subgroup reported 115 minutes of moderate activity per day. The average temperature did not significantly differ between high and low active groups (FM `low': 47.12±0.93, FM `high': 47.00±0.42, p=0.781; Control `low': 48.03±1.00, Control `high': 47.83±1.10, p=0.730).
Periaqueductal gray
For the PAG region of interest, neither self-reported nor objectively monitored physical activity was related to PAG responses in either group (all p>0.05). Analysis of PAG activity in response to the pain stimulus indicated that the PAG was not significantly active above baseline during delivery of the moderate pain stimulus for either FM patients or healthy controls (p>0.05).
Behavioral analyses
For the FM patients, both self-reported physical activity and the time recorded in moderate and vigorous activities by the accelerometer were significantly and negatively related to changes in pain intensity and unpleasantness ratings across the scan (p<0.05). The results are presented in Table 6. Dividing the FM patients into high and low activity groups with the median self-reported physical activity as a cutpoint revealed that high active patients had a significant decrease in their pain ratings from the first to the fifth stimulus (intensity: 10.75±4.33 vs. 7.88±5.69, p=0.038; unpleasantness: 8.5±3.8 vs. 6.25±4.5, p=0.013), whereas the low activity group did not significantly change (intensity: 12.00±4.3 vs. 13.25±4.4, p=0.435; unpleasantness: 12.00±3.9 vs. 12.50±4.4, p=0.725). No significant relationships were found between physical activity and changes in pain ratings across the scan in the control participants. Median split of controls into high and low active groups showed no significant changes in pain ratings for either group, however the direction of change was consistent with that observed in FM with high active controls showing a decrease in their pain ratings from the first to the fifth stimulus (intensity: 9.44±2.2 vs. 7.44±3.2, p=0.189; unpleasantness: 7.00±3.0 vs. 5.78±2.7, p=0.295) and low active controls showing an increase (intensity: 8.89±5.2 vs. 10.78±4.9, p=0.090; unpleasantness: 7.0±4.8 vs. 7.67±5.4, p=0.428). Changes in pain sensitivity were not significantly related to the absolute temperature delivered and the average temperature did not significantly differ between high and low active groups (FM `low': 47.26±0.96, FM `high': 47.21±0.59, p=0.902; Control `low': 48.15±0.84, Control `high': 47.80±1.14, p=0.452).
Table 6.
Correlations between physical activity and change in pain ratings across fMRI scan
| Intensity difference score | Unpleasantness difference score | |
|---|---|---|
| FM | ||
| Total activity counts/minute | −.259 (p =.221) | −.376 (p =.127) |
| Activities of daily living | .028 (p =.936) | −.039 (p =.909) |
| Moderate and vigorous activity | −.532* (p =.046) | −.568* (p =.034) |
| Self reported physical activity | −.519* (p =.020) | −.456* (p =.038) |
| CO | ||
| Total activity counts/minute | −.179 (p =.271) | −.385 (p =.087) |
| Activities of daily living | −.255 (p =.378) | −.017 (p =.953) |
| Moderate and vigorous activity | −.230 (p =.215) | −.393 (p =.082) |
| Self reported physical activity | −.170 (p =.250) | −.148 (p =.279) |
All results are Pearson's r
Significant at p =.05 by one-tailed test
DISCUSSION
Our findings indicate that the responses of several pain-relevant brain regions are related to physical activity in both FM patients and healthy controls. For FM patients, self-reported physical activity had more robust relationships to brain responses to pain, while objectively measured physical activity showed stronger relationships for controls. In general, brain regions previously implicated in pain regulation were positively associated with physical activity, while brain regions implicated in the sensory/discriminative aspects of pain processing were negatively related to physical activity. Categorizing FM patients into `high' and `low' physical activity subgroups revealed greater responses in pain regulatory regions and lesser responses in sensory regions for those with higher self-reported physical activity. Moreover, higher active FM patients demonstrated decreased pain ratings across repeated pain stimuli while lower active did not. Controls demonstrated a similar patter of change that did not reach statistical significance. This may have been due to the initial low pain ratings exhibited by controls. Although our protocol was designed to elicit pain ratings between 11 and 13 on the pain intensity scale, for ethical and patient safety considerations delivery of temperatures above 49 °C was prohibited. This presented a ceiling-effect problem for a number of our healthy control participants. Overall, our results suggest greater pain regulation capacity in FM patients who are more physically active.
Brain regions and direction of relationships
Lateral regions of the prefrontal cortex are frequently studied in relation to pain processing and are considered as critical structures for endogenous pain control.4 Research has demonstrated that the prefrontal cortices are involved in the top-down modulation of pain,27 placebo analgesia53 and the analgesic effects of stimulus controllability.54 Further, patients with low back pain exhibit gray matter loss in this region.2 In our study, positive relationships between self-reported physical activity and the dorsolateral prefrontal cortex were found for FM patients, while average accelerometer counts per minute and minutes spent in ADL were negatively related to brain activity in the left frontal cortex; a region laterally opposite and ventral to that observed for self-reported physical activity. For controls, positive relationships between accelerometer counts per minute and the lateral prefrontal cortex were found. Greater pain-related activity in the lateral prefrontal cortex may indicate that physically active FM patients and healthy controls have a greater capacity to engage endogenous pain modulatory networks. The decreased pain ratings that occurred across the five heat pain stimuli for the FM patients with `high' self-reported physical activity are consistent with this interpretation.
The anterior portion of the midcingulate cortex was significantly related to objectively measured physical activity in healthy controls. With respect to pain, regions of the cingulate cortices have been associated with pain regulation, affective integration and avoidance behaviors.52 Specifically, rostral portions of the anterior cingulate have been associated with placebo analgesia,3,53 while anterior portions of the midcingulate cortex, such as that observed in the present study, have been associated with hypnotic analgesia.52 Because we did not observe significant relationships between changes in pain ratings and physical activity in healthy controls, it is not possible to determine whether the relationship observed in the present study was specific to pain regulation.
Positive relationships between posterior cingulate responses to heat pain and physical activity were observed in both FM patients and healthy controls. Posterior cingulate activity was positively related to self-reported physical activity in FM patients and objectively measured physical activity in healthy controls(part of the MCC cluster listed in Table 5). The posterior cingulate cortex has been characterized as serving evaluative functions such as sensory monitoring and internal body awareness.52 Activity within the posterior cingulate has also been shown to correlate with the magnitude of analgesia achieved in a placebo design.53 The positive relationship between physical activity and brain activity in the posterior cingulate suggests that physically active persons may evaluate and regulate pain to a greater degree than those who are less active.
The insula is commonly observed in studies involving experimental pain stimuli.35 In our study, both FM patients' self-reported physical activity and controls' accelerometer counts were positively related to the brain response to pain in the posterior insula. The involvement of insular regions in pain processing is multifaceted and evidence suggests roles in both the encoding and modulation of pain.34,35 Prior research suggests that the posterior insula has a degree of somatotopic representation of pain29 and is related to the sensory aspects of pain.1,45 More recent neuroimaging studies have reported that gray mater density within the posterior insula activity was associated with the magnitude of placebo analgesia,40 the cognitive modulation of experimental pain4,38 and mu-opioid mediated neurotransmission.58 Tran et al.47 recently reported that posterior insula activity increased during a period when psychophysical pain ratings had plateaued, suggesting that activity within the posterior insula served to gate or regulate pain perception. These results are consistent with data demonstrating that patients with posterior insula lesions appear to have compromised pain control.41 It is likely that the insular cortices play a role in both pro- and antinocicpetive processes. Further research will be necessary to understand its role in mediating a relationship between physical activity and pain.
Our results also indicated a negative relationship between self-reported physical activity and brain responses in the postcentral gyrus for FM patients. Responses to pain stimuli in this sensory cortical region have been associated with the intensity and discriminative aspects of pain.1,35 This is consistent with our hypothesis that physical activity levels would be negatively associated with sensory aspects of pain. It is also consistent with our data showing greater activity in the sensory cortex in low active compared to high active FM patients.
The implication of the negative relationship found between brain activity in the superior parietal cortex and self-reported physical activity in FM patients is less clear. A role for the superior parietal cortex in pain processing has not been clearly elucidated, but it has been suggested to play a role in sustaining attention to pain.8 High active FM patients also exhibited less activity in the superior parietal cortex compared to low active FM patients. This suggests that painful stimuli did not demand as much attention for FM patients who reported greater physical activity. Alternatively, the changes in pain ratings for more active FM patients might indicate that parietal cortex responses decreased in response to pain regulatory processes occurring in other brain regions (i.e. prefrontal, cingulate & insular cortices).
Our data did not support a role for the PAG in the relationship between physical activity and pain in either FM or controls. The PAG has been demonstrated to have powerful pain regulation effects and recent neuroimaging work has supported the PAG as being active during both sensory and modulatory aspects of pain perception.3,35,46 It may be that the moderate pain stimulus in our study was not intense enough to evoke a strong PAG response. Because of the PAG's small size, another possibility is that the relatively large size of our functional voxels reduced our ability to detect PAG activity. Further research, using brainstem-specific imaging methods and stronger pain stimuli may help determine whether physical activity is related to PAG responses during pain modulation.
Limitations& Considerations
Several limitations should be considered when interpreting these results. First, only women were included in our study. The relationship between physical activity and brain responses to pain may be different for men. Substantial evidence has accumulated indicating that women have lower pain thresholds, lower pain tolerances, and a higher incidence of chronic pain states than men.55 Researchers have also begun using neuroimaging tools to investigate sex differences in neural responses to pain and have reported significant differences.31,33,44 Thus, appropriate caution should be taken in generalizing the current results to men.
Our FM patients did not have comorbidCFS; the most common comorbid condition among FM patients. Thus, our results may not apply to FM patients with a concurrent diagnosis of CFS. We have found that cardiorespiratory and perceptual responses to exercise differ between CFS patients with comorbid FM and those with CFS only11, suggesting that CFS and FM do differ in their responses to physical activity. Although using a more homogenous sample of FM patients reduces our ability to generalize to the larger FM population, the results are more likely to be explained by FM rather than another comorbid condition. Five FM patients and two healthy controls that were included in the analyses reported taking low-dose antidepressants. To determine the influence of these participants on our primary outcomes, we reanalyzed our data with and without each individual who reported low-dose antidepressant use (i.e. comparing the full sample to the full sample minus one). None of the individuals substantially altered the pattern of the observed relationships.
Physical activity was not manipulated in our study, thus limiting the conclusions that can be drawn concerning how physical activity might alter brain function. Future research combining exercise training, pain psychophysics and fMRI would be an important next step. Our FM patients and controls would be considered sedentary by currentphysical activity recommendations for 30 minutes of moderate activity on 5 or more days per week.49 According to our accelerometer data, FM patients averaged7.2 minutes of moderate activity per day. Controls averaged 16.0 minutes per day of moderate activity.20 Given the limited range of physical activity data, we consider this a conservative test of our hypothesis. Finally, our results may be limited by the size of the sample tested and the amount of physical activity reported and measured among our groups. This is particularly true considering the relationships observed for the accelerometer data and for the median split data. A larger sample size and the inclusion of broader range of physical activity with more active participants would likely strengthen and clarify the observed relationships between physical activity and brain responses to pain.
Conclusions
In sum, the present data provide initial support for the hypothesis that central nervous system mechanisms of pain processing are related to physical activity. Our findings suggest that physical activity is positively related to brain responses in pain regulatory regions and negatively related to responses sensory/discriminative regions. FM patients who report being more physically active demonstrate greater responses in pain regulatory brain regions while receiving painful stimuli and this is accompanied by decreases in pain ratings. Healthy controls who are more physically active also demonstrate this pattern of responding. These results suggest that how physically active a patient perceives themselves to be can influence how they perceive and modulate pain.At present,it is not clear what dose of physical activity is sufficient to affect pain modulatory mechanisms. Research aimed at manipulating physical activity and examining brain responses to pain would help clarify whether increases in physical activity improve pain regulation in FM.
Acknowledgements
The authors would like to acknowledge the significant assistance of Laura Ellingsonin collecting and processing data for these studies, of Isaac Schwabacher in developing software for analysis of accelerometer data, and of John Ollinger, Ph.D. and Nate Vack in analyzing neuroimaging data.This research was supported by National Institutes of Health grant ROI 5R01AR050969, the National Institute of Arthritis and Musculoskeletal and Skin Disorders (NIAMS).
This study was conducted at the University of Wisconsin-Madison
Supported by NIH ROI 5R01AR050969 (NIAMS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: RACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Bingel U, Schoell E, Büchel C. Imaging pain modulation in health and disease. Curr Opin Neurol. 2007;20:424–431. doi: 10.1097/WCO.0b013e328259c34d. [DOI] [PubMed] [Google Scholar]
- 5.Branco JC, Bannwarth B, Failde I, Abello Carbonell J, Blotman F, Spaeth M, Saraiva F, Nacci F, Thomas E, Caubère JP, Le Lay K, Taieb C, Matucci-Cerinic M. Prevalence of fibromyalgia: A survey in five European countries. Semin Arthritis Rheum. 2010;39:448–453. doi: 10.1016/j.semarthrit.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Burgmer M, Pogatzki-Zahn E, Gaubitz M, Wessoleck E, Heuft G, Pfleiderer B. Altered brain activity during pain processing in fibromyalgia. Neuro Image. 2009;44:502–508. doi: 10.1016/j.neuroimage.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Busch AJ, Schachter CL, Overend TJ, Peloso PM, Barber KA. Exercise for fibromyalgia: A systematic review. J Rheumatol. 2008;35:1130–1144. [PubMed] [Google Scholar]
- 8.Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuro Image. 2006;32:1804–1814. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Clauw DJ. Fibromyalgia: An overview. Am J Med. 2009;122(Suppl 12):S3–S13. doi: 10.1016/j.amjmed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31:364–378. [PubMed] [Google Scholar]
- 11.Cook DB, Nagelkirk PR, Poluri A, Mores J, Natelson BH. The influence of aerobic fitness and fibromyalgia on cardiorespiratory and perceptual responses to exercise in patients with chronic fatigue syndrome. Arthritis Rheum. 2006;54:3351–3362. doi: 10.1002/art.22124. [DOI] [PubMed] [Google Scholar]
- 12.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 13.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSMIV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- 15.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Glass JM, Lyden AK, Petzke F, Stein P, Whalen G, Ambrose K, Chrousos G, Clauw DJ. The effect of brief exercise cessation on pain, fatigue, and mood symptom development in healthy, fit individuals. J Psychosom Res. 2004;57:391–398. doi: 10.1016/j.jpsychores.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Gormsen L, Rosenberg R, Bach FW, Jensen TS. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur J Pain. 2010;14:127.e1–8. doi: 10.1016/j.ejpain.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Gracely RH, McGrath F, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- 19.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 20.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 21.Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: Much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 22.Jones KD, Burckhardt CS, Clark SR, Bennett RM, Potempa KM. A randomized controlled trial of muscle strengthening versus flexibility training in fibromyalgia. J Rheumatol. 2002;29:1041–1048. [PubMed] [Google Scholar]
- 23.Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, Williams DA, Clauw DJ. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 24.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 25.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 28.Matthews CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11 Suppl):S512–22. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
- 29.Mazzola L, Isnard J, Peyron R, Guenot M, Mauguiere F. Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain. 2009;146:99–104. doi: 10.1016/j.pain.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 30.McLoughlin MJ, Colbert LH, Stegner AJ, Cook DB. Are women with fibromyalgia less physically active than healthy women? Medicine and Science in Sport and Exercise. 2010 doi: 10.1249/MSS.0b013e3181fca1ea. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R257–R267. doi: 10.1152/ajpregu.00084.2006. [DOI] [PubMed] [Google Scholar]
- 32.Naidich TP, Duvernoy HM, Delman BN, Sorensen AG, Kollias SS, Haacke EM. Duvernoy's atlas of the human brain stem and cerebellum: High field MRI, surface anatomy, internal structure, vascularization, and 3-D sectional anatomy. Springer; New York: 2008. [Google Scholar]
- 33.Paulson PE, Minoshima S, Morrow TJ, Casey KL. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain. 1998;76:223–229. doi: 10.1016/s0304-3959(98)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 35.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Clin Neurophysiol. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 36.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 37.Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550–556. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- 38.Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;20:7438–7445. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarz MJ, Spath M, Muller-Bardorff H, Pongratz DE, Bondy B, Ackenheil M. Relationship of substance P, 5-hydroxyindole acetic acid and tryptophan in serum of fibromyalgia patients. Neurosci Let. 1999;259:196–198. doi: 10.1016/s0304-3940(98)00937-9. [DOI] [PubMed] [Google Scholar]
- 40.Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J Neurosci. 2009;29:4882–4887. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. Roles of the insular cortex in the modulation of pain: Insights from brain lesions. J Neurosci. 2009;29:2684–2694. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staud R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr Rheumatol Rep. 2002;4:299–305. doi: 10.1007/s11926-002-0038-5. [DOI] [PubMed] [Google Scholar]
- 43.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 44.Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum Brain Mapp. 2009;30:689–698. doi: 10.1002/hbm.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tracey I. Imaging pain. Brit J Anaesthesia. 2008;101:32–39. doi: 10.1093/bja/aen102. [DOI] [PubMed] [Google Scholar]
- 46.Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran TD, Wang H, Tandon A, Hernandez-Garcia L, Casey KL. Temporal summation of heat pain in humans: Evidence supporting thalamocortical modulation. Pain. 2010;150:93–102. doi: 10.1016/j.pain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turk DC, Robinson JP, Burwinkle T. Prevalence of fear of pain and activity in patients with fibromyalgia syndrome. J Pain. 2004;5:483–490. doi: 10.1016/j.jpain.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 49.US Department of Health and Human Services (USDHHS) Physical Activity Guidelines Advisory Committee Report, 2008. USDHHS; Washington, DC: 2008. http://www.health.gov/paguidelines. Available at: http://www.health.gov/paguidelines/report/pdf/CommitteeReport.pdf. [Google Scholar]
- 50.Vierck CJ, Jr, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 51.Villanueva L, Bouhassira D, Le Bars D. The medullary subnucleus reticularis dorsalis (SRD) as a key link in both the transmission and modulation of pain signals. Pain. 1996;67:231–240. doi: 10.1016/0304-3959(96)03121-1. [DOI] [PubMed] [Google Scholar]
- 52.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005:6533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA, Nadeau V, Jones AK. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145:24–30. doi: 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ. Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci. 2006;26:11501–11509. doi: 10.1523/JNEUROSCI.2568-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gender Med. 2005;2:137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 56.Wigers SH, Stiles TC, Vogel PA. Effects of aerobic exercise versus stress management treatment in fibromyalgia. A 4.5 year prospective study. Scand J Rheumatol. 1996;25:77–86. doi: 10.3109/03009749609069212. [DOI] [PubMed] [Google Scholar]
- 57.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 58.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurisci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]