Abstract
Androgens regulate body composition in youth and declining testosterone that occurs with aging is associated with muscle wasting, increased fat mass and osteopenia. Transgenic mice with targeted androgen receptor (AR) overexpression in mesenchymal stem cells (MSC) were generated to explore the role of androgen signaling in the regulation of body composition. Transgenic males, but not females, were shorter and have reduced body weight and visceral fat accumulation. Dual energy x-ray absorptiometry (DXA) revealed significant reductions in fat mass with a reciprocal increase in lean mass, yet no difference in food consumption or locomotor activity was observed. Adipose tissue weight was normal in brown fat but reduced in both gonadal and perirenal depots, and reduced hyperplasia was observed with smaller adipocyte size in visceral and subcutaneous white adipose tissue. Although serum leptin, adiponectin, triglyceride, and insulin levels were no different between the genotypes, intraperitoneal glucose tolerance testing showed improved glucose clearance in transgenic males. High levels of the AR transgene are detected in MSCs but not in mature fat tissue. Reduced fibroblast colony forming units indicate fewer progenitor cells resident in the marrow in vivo. Precocious expression of GLUT4, PPARγ and C/EBPα was observed in proliferating precursor cultures from transgenic mice compared to controls. In more mature cultures, there was little difference between the genotypes. We propose a mechanism where enhanced androgen sensitivity can alter lineage commitment in vivo to reduce progenitor number and fat development, while increasing the expression of key factors to promote smaller adipocytes with improved glucose clearance.
Keywords: body composition, MSC, lean mass, adipogenesis, testosterone, androgen receptor
Introduction
It is generally recognized that there is no body compartment that is not directly or indirectly affected by androgens [Bhasin, 2003]. With aging and declining testosterone levels, changes in body composition, bone quality, physical and cognitive function occur that are of clinical importance [Matsumoto, 2002]. Androgen treatment [Wang et al., 2004] and anabolic steroid use/abuse [Hartgens and Kuipers, 2004] can alter and/or ameliorate these changes. However, while there is now general agreement that testosterone administration increases muscle mass [Storer et al., 2003], the effects of androgen administration on fat mass are not as well characterized.
Clinically, increased total body and visceral fat are associated with low testosterone concentrations [Seidell et al., 1990]. Declining testosterone concentrations have also been associated with an increase in the risk for type 2 diabetes and metabolic syndrome [Bassil et al., 2009]. In addition, androgen deficiency induced by gonadotropin-releasing hormone agonist administration in healthy men results in increased adipose tissue mass [Mauras et al., 1998]. In contrast, testosterone replacement in eugonadal men results in a reduction in adipose tissue mass and alteration of regional distribution [Woodhouse et al., 2004]. Thus, clinical evidence suggests that androgens also regulate body fat accumulation in humans.
In the development of obesity, the increase in adipose tissue mass is due to an increase in both the size and number of adipocytes [Jo et al., 2009]. The increase in cell number can result of recruitment of preadipocytes from a population of multipotent stem cells in the bone marrow [Crossno et al., 2006] or from sub-populations of cells resident in mature white adipose tissue (WAT) [Rodeheffer et al., 2008]. Bone marrow mesenchymal stem cells (MSCs) can differentiate into a variety of cell types including fat, muscle, cartilage, and bone [Bianco et al., 2006]. With aging, marrow adipogenesis accelerates in vivo, while the ability of MSCs to form bone decreases. It has been suggested that MSC precursors differentiate into adipose rather than bone with a reciprocal relationship [Pei and Tontonoz, 2004], and thus may contribute to age-related body composition changes. In addition, in vitro data has demonstrated an inhibition of adipogenesis with androgen treatment in committed preadipocyte cell lines [Singh et al., 2006], in the more multipotential C3H 10T1/2 fibroblasts [Singh et al., 2003], and in human MSC cultures [Gupta et al., 2008].
Genetic evidence also implicates androgen signaling in the modulation of obesity, with the AR locus on the X chromosome repeatedly associated with measures of obesity including fat mass, percentage of body fat, sum of skinfolds etc [Snyder et al., 2004]. In addition, Cre-loxP global AR knockout male mice show differences in the number and size of adipocytes [Yeh et al., 2002] and develop late-onset obesity, with an increase in WAT but not brown fat [Sato et al., 2003]. Combined inactivation of AR and estrogen receptor α (ERα) in mice indicates that bone and body composition are differentially regulated by sex steroids; while AR is required to maintain trabecular bone mass, both AR and ERα are needed for muscle and cortical bone integrity [Callewaert et al., 2009]. Since ERα inactivation, with or without combined AR knockdown, increased fat mass, the effects of androgen on fat may be mediated through the ER after aromatization to 17-ß estradiol. However, increased lean mass and decreased fat was observed when the AR was overexpressed in myocytes of male rats, likely through increases in oxidative metabolism [Fernando et al., 2010]. Combined, in vitro and in vivo studies suggest that androgens are key effectors of MSC lineage determination and function, and support the clinical evidence of an effect of androgen on lean mass and fat accumulation.
Nevertheless, a mechanistic understanding of androgen regulation of body composition is currently lacking and the cell types involved in mediating responses to androgen treatment in vivo have not been established, limiting progress on novel therapeutic approaches. In addition, studying the in vivo action of androgen (e.g., testosterone) is complex because it can be metabolized to estrogen via aromatase activity. As a consequence, some testosterone action results from activation of ER after conversion to 17-ß estradiol. Transgenic rodents with targeted AR overexpression or AR knockdown have proven to be excellent model systems to dissect out the contribution of androgens to the regulation of bone mineral homeostasis and body composition. We have engineered transgenic mice with tissue-specific AR overexpression driven by a 3.6 kb fragment from the type I α1 collagen gene promoter (AR3.6-transgenic mice). Fragments of the type I collagen promoter provide for a high level of gene expression in vivo that is well characterized [Kalajzic et al., 2002]. AR3.6-transgenic mice exhibit overexpression of AR in mesenchymal precursor cells, cells of the osteoblastic lineage including osteoblasts and osteocytes, as well as cells in the periosteum [Wiren et al., 2004]. An important advantage of this model is increased sensitivity to androgen in a tissue-specific manner, without changes in circulating hormones. The purpose of the current study was to analyze the consequences of enhanced androgen sensitivity in AR3.6-transgenic mice as a model to better understand the role of androgens to influence fat mass and body composition.
Materials and Methods
Animal procedures
The generation of AR transgenic mice employing the 3.6 kb α11 collagen promoter fragment to drive expression has been described previously [Wiren et al., 2004]. AR3.6-transgenic animals (hereafter referred to as AR3.6-tg mice) were bred to wild-type B6D2F1 mice (Jackson Labs, Bar Harbor, ME) employing both genders. The mice were maintained under a 12 h light-dark cycle, had free access to tap water and were fed ad libitum a standard rodent chow containing 4.5% fat and 23% protein (LabDiet 5001, PMI Nutrition Int., St. Louis, MO). All animal studies were performed according to institutional, local, state, federal and NIH guidelines for the use of animals in research under an Institutional Animal Use and Care Committee (IACUC)-approved protocol.
Body composition analysis
All animals were weighed weekly, and body length (nose to rump) was determined at monthly intervals over six months (n = 6). Body composition was measured by dual-energy X-ray absorptiometry (DXA) using a mouse densitometer (PIXImus2, software version 2.1, GE-Lunar, Madison WI, USA). Total tissue mass, lean mass, and fat mass as a percentage of total tissue were determined at 6 months of age (n = 10).
Food intake and locomotor activity measurements
Food consumption was determined for male mice of both genotypes (n = 8-9) after 48 h of acclimation to being singly-housed. Food pellets were weighed morning and evening over a five day period and the average daily food intake for each period was expressed as kcal/day based on the total metabolizable energy per gram of chow. For spontaneous activity measurements, mice (n = 12) were singly housed and allowed to acclimate for 24 h. Baseline locomotor activity was assessed under low light conditions in automated, open-field activity monitors (Accuscan Instruments Inc., Columbus, OH). The total time spent moving and the total time resting was calculated in 5 min bins for 60 min. In addition, horizontal distance traveled, margin time, i.e., the amount of time spent near the periphery when placed in an open field (thigmotaxis), and vertical activity (rearing) were determined.
Tissue collection and histological analysis
For marrow analysis, femurs were placed in 70% ethanol for storage. Following embedding in methylmethacrylate and sectioning, slides were stained with toluidine blue. Adipocytes are evidenced by large empty spaces after fixation. Adipose tissue depots, including visceral WAT (both gonadal and perirenal) and interscapular brown adipose tissue (BAT), were dissected and immediately weighed. The tissues were then snap frozen and stored at −80°C until RNA extraction. For histological analysis, subcutaneous (inguinal) and visceral (gonadal) WAT were dissected and fixed in Bouin's solution (Sigma-Aldrich, St. Louis, MO) for 48 h and then stored in 70% ethanol. Tissues were dehydrated through graded alcohols, cleared with xylenes and infiltrated with paraffin by standard procedures as previously described [Wiren et al., 2004]. Tissues were embedded in a random orientation and the blocks were sectioned at 5 μm and stained with hematoxylin and eosin. For adipocyte size determinations, non-serial sections from gonadal and inguinal fat pads were photographed. The number of adipocytes in the captured images and their mean areas were determined using computer-assisted image analysis software (Leica Application Suite v3.3.0, Leica Microsystems Inc., Bannockburn, IL). For each genotype, at least 300 adipocytes were analyzed in 3 or more sections from 2-3 different animals. A histogram showing adipocyte size distribution patterns for each genotype was generated using Prism software v5.02 (GraphPad software Inc., San Diego, CA). For analysis of bone marrow adiposity, femurs were dissected from wild-type and AR3.6-tg mice and fixed for histological analysis. Slides were stained with alcian blue and the extent of adiposity was qualitatively assessed.
Serum analysis and intra-peritoneal glucose tolerance testing (IPGTT)
Following an overnight fast, mice are heavily anaesthetized using a cocktail of ketamine, xylazine and acepromazine and blood was collected by cardiac puncture. Serum was aliquotted and stored at −80 C. Serum triglycerides were determined by enzymatic hydrolysis using the glycerol phosphate oxidase (GPO) assay kit (Sigma Chemical Co., St. Louis, MO). Serum insulin levels were determined using an ultra-sensitive enzyme-linked immunosorbent assay (Crystal Chem. Inc., Downers Grove, IL). Serum leptin and adiponectin concentrations were determined by ELISA according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). For IPGTT, mice were fasted overnight for 18 h and tail vein blood glucose was measured at baseline and at the indicated time points following an intra-peritoneal injection of D-glucose administered at 1 g/kg of body weight using a glucometer (One Touch Ultra II, Lifescan, Milpitas, CA).
MSC Colony Formation and Lipid Accumulation Assays
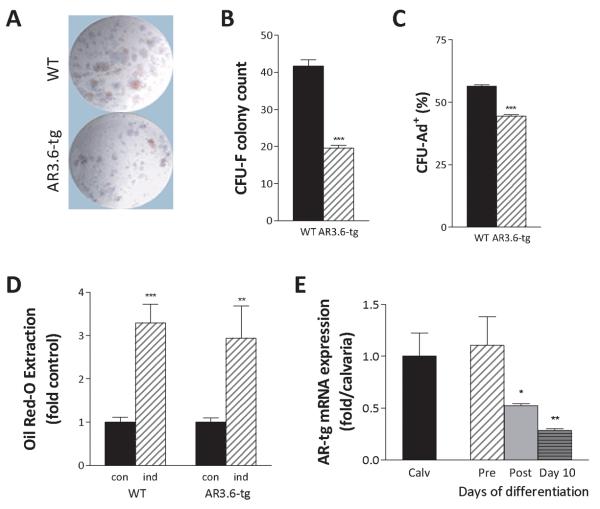
Bone marrow was flushed from long bones (femurs and tibiae) with α-MEM containing 15% lot-selected fetal bovine serum (Hyclone, Logan, UT) and antibiotics. A single cell suspension was generated by gently passing through a 22 g needle and cell number was determined following red blood cell lysis and trypan blue exclusion. For CFU fibroblastic colony formation (CFU-F), cells harvested directly from marrow were plated at 1.5 × 106 cells/well in 6-well plates and cultured at 37° C in a humidified chamber containing 5% CO2 essentially as described [Moerman et al., 2004]. On day 6, one half of the media was replaced and growth was continued for 6 more days. For quantification of CFU-F numbers, cells were fixed with 4% paraformaldehyde, rinsed with methanol and stained with Giemsa. Colonies of 50 or more cells were counted manually using a dissecting scope. For CFU adipocyte (CFU-Ad+) differentiation, cells were switched to media containing 1μm rosiglitazone and growth was continued for 4 days with a media change after day two. To determine colony number, cells were fixed, rinsed with 60% isopropano, stained with 0.5% Oil red-O in isopropanol, rinsed clear and then counter stained with 0.5% methyl green in 0.1M sodium acetate, pH 4.2. Total CFUs and colonies containing at least 10% Oil red-O positive cells were counted as described [Moerman et al., 2004]. A representative image is shown in Fig. 7A.
Figure 7. Reduced CFU-F numbers in marrow but nearly normal adipogenic differentiation in AR transgenic cells.
Bone marrow MSCs were isolated from male wild-type and AR3.6-tg mice and plated for CFU-F assays or adipogenic colony formation (CFU-Ad+). A. Representative image of colony assays. B. Quantitation of CFU-F numbers between wild-type (WT) and AR3.6-tg male mice shows a significant reduction in CFU-F in marrow populations from AR3.6-tg mice. Result shown is the mean ± SEM from 3 independent experiments. ***, p < 0.001 compared to WT. C. Analysis of CFU-Ad+ colony percentage between WT and AR3.6-tg male mice, with a modest reduction in colonies in marrow from AR3.6-tg mice. ***, p < 0.001. D. Lipid accumulation in differentiated adipocytes after plating at equal numbers of progenitors shown by Oil red-O extraction and quantiation. Undifferentiated (con) and cells induced (ind) with 1 μM rosiglitazone show a modest but non-significant reduction in Oil red-O accumulation between genotypes. Result shown is the mean ± SEM from 12-16 individual wells from 2 independent experiments. **, p < 0.01; ***, p < 0.001 all vs. controls. E. Temporal expression profile for AR transgene expression at different stages of adipogenic differentiation from progenitor populations. Expression was compared to the high level observed in calvaria (calv) in precursor (pre) proliferating cultures, after confluence (post) and after differentiation at day 10. *, p < 0.05; **, p < 0.01 all compared to precursor cells.
Since bone marrow MSC can contribute to adipose tissue in the adult [Crossno et al., 2006], we also assessed adipocyte differentiation and lipid accumulation in ex vivo cultures. For adipocyte differentiation, cells were isolated as above and expanded in culture for 7-10 days with one half of the media changed on day 6. Cultures were washed with PBS, detached and cell number was determined. Cells from both genotypes were then plated at a uniform density of 30,000 cells/cm2 in 12-well dishes. At confluence, the media was supplemented with 1 μM rosiglitazone and growth was continued for 4 days. To determine the extent of adipogenesis, cells were stained with Oil red-O as above. Levels were quantitated after extraction with 4% IGEPAL (Sigma, St. Louis, MO) in 100 % isopropanol and the absorbance of each well was determined at 490 nm.
Gene expression analysis by quantitative real time RT-PCR (qPCR)
To assess gene expression changes in mature adipose tissue, epididymal fat pads were dissected, immediately snap frozen and stored at −80°C until processing. RNA was extracted from fat tissue using the RNeasy lipid tissue mini-kit (Qiagen, Carlsbad, CA) according to the manufacturer's instructions. For adipogenic differentiation analysis, MSCs cultures were derived as described above and plated at equal densities. At confluence, adipogenic differentiation was induced by treating with 1 μM rosiglitazone. RNA was isolated using RNA-Stat 60 (TelTest, Inc., Friendswood, TX) in proliferating progenitor cultures, at confluence and after adipogenic induction at 10 days of differentiation. To remove potential DNA contamination, RNA samples were treated with DNAse I and purified with Zymo-spin columns (Zymo Research, Orange, CA). RNA concentration was determined spectrophotometrically and integrity was confirmed by agarose gel electrophoresis and ethidium bromide staining. Real time qPCR was performed using primers purchased from Qiagen; the kruppel-like factor Klf-5, glucose transporter 4 (GLUT4), CCAAT enhancer-binding protein family (C/EBP) C/EBPα, C/EBPβ, C/EBPδ, peroxisome proliferator-activated receptor gamma (PPARγ) PPARγ1/2, hormone sensitive lipase (HSL), lipoprotein lipase (LPL) and uncoupling protein 1 (UCP-1). The AR3.6 transgene was amplified using specific primers designed not to detect endogenous AR by including the sequence for the bovine growth hormone poly-adenylation signal on the antisense primer; forward, 5′-AAGTGCCCAAGATCCTT-3′ and reverse, 5′-ACAACAGATGGCTGGCAACT-3′. qPCR analysis was performed using the iCycler IQ Real Time RT-PCR detection system (Bio-Rad Laboratories Inc., Hercules, CA) in a one-step reaction using the Quantitect SybrGreen real time RT-PCR reagent (Qiagen, Carlsbad, CA). Expression differences were determined using the ΔΔCt method [Winer et al., 1999], normalized to Ribogreen [Hashimoto et al., 2004].
Statistical analysis
All data were analyzed using Prism v5.02 software (GraphPad Software Inc., San Diego, CA). Significant differences between wild-type and AR3.6-tg mice were assessed by an unpaired two-tailed t-test. Two-way ANOVA for the effects of time and genotype was used for analysis of body weights, lengths and for IPGTT, with Bonferroni post-tests when appropriate. All data are expressed as mean ± standard error of the mean (SEM).
Results
Altered body composition in male AR transgenic mice
We have previously described the production and skeletal characterization of transgenic mice with AR overexpression driven by the 3.6 kb type I α1 collagen promoter. Phenotypic analysis has revealed that AR3.6-tg animals develop a complex bone phenotype in males characterized as low turnover, with a reduction in bone resorption and bone formation with the only anabolic action in bone at the periosteal surface [Wiren et al., 2004]. In females, with lower endogenous androgen levels, little or no effect of the transgene was noted. This low turnover phenotype is analgous to that observed in a second transgenic mouse line employing a 2.3 kb collagen promoter fragment (AR2.3-tg mice) that does not exhibit transgene expression in precursor cells [Wiren et al., 2008]. Notably, in addition to the bone phenotype reported, male AR3.6-tg mice also have reduced body weight compared to wild-type littermates.
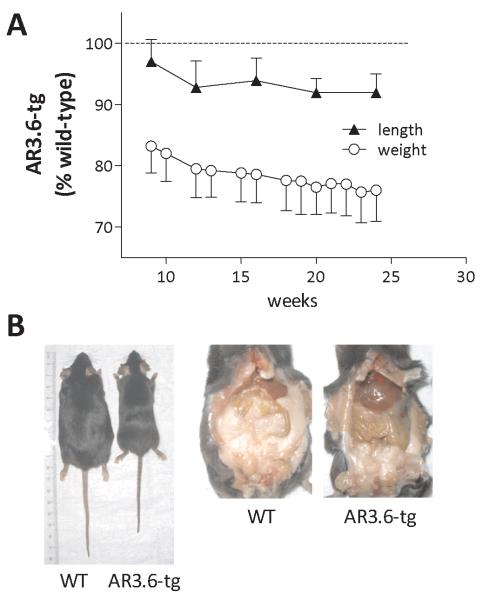
To assess the kinetics of the body weight reduction and to determine at which stage of development changes in body composition begin to manifest, we followed growth from puberty out to 6 months of age (Fig. 1A). At birth the genotypes were indistinguishable. However, as the mice developed, male AR3.6-tg mice weighed significantly less at each time point measured. They were also shorter than wild-type littermates. Analysis for the effects of time and genotype by repeated measures two-way ANOVA revealed an extremely significant effect of genotype (F = 54.57; p < 0.0001) and time (F = 36.51; p < 0.0001) with no interaction. The reduced body weight phenotype was apparent as early as 5 weeks of age in male AR3.6-tg mice where it began at ~ 17% below that of wild type mice and by 24 weeks had reached 24%. The reduction in nose-rump length is consistent beginning at 12 weeks of age with AR3.6-tg mice ~ 8% shorter, a difference that remained fairly constant out to 6 months of age. Thus, percent change in body weight in the transgenic cohort relative to control continues to decline in adult mice after 12 weeks (linear regression analysis with slope = −0.3088 ± 0.027, p < 0.0001 from zero), while nose-to-rump length percent changes are relatively unaffected (slope = −0.1175 ± 0.100, not significantly different from zero). Representative images of external and intra-abdominal appearance of male transgenic versus wild-type mice (Fig. 1B) clearly show the reduced body weight and length, as well as a major reduction in visceral fat content.
Figure 1. Reduced body weight and visceral fat accumulation in AR transgenic male mice.
Growth curves of wild-type and AR3.6-tg male mice. A. Body weight and nose-to-rump length determinations were carried out weekly or monthly, respectively, over a 6 month period. Data shown are expressed as fold change from wild-type, mean ± SEM (n = 6). B. Whole body external and intra-abdominal appearance of representative 6.5-month-old male wild-type and AR3.6-tg mice.
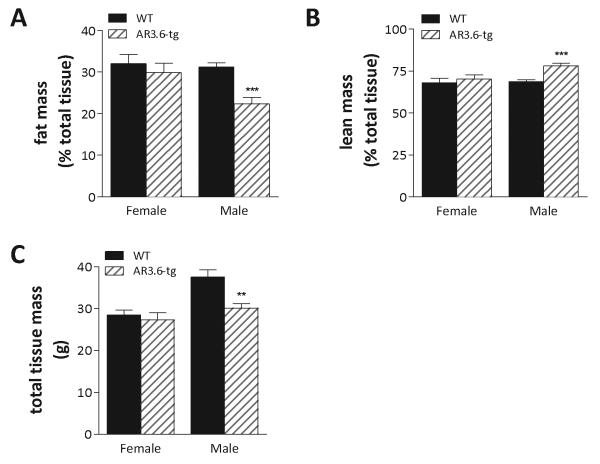
To better characterize the reduced body weight phenotype in male AR3.6-tg mice and evaluate the potential underlying mechanisms, body composition was analyzed in 6-month-old mice by dual-energy x-ray absorptiometry (DXA). DXA revealed highly significant reductions in both total fat (56% of wild-type) and fat as a percentage of body mass (72% of wild-type; Fig. 2A). Adipose tissue accounted for 31% of the total tissue in wild-type mice compared to only 22% in AR3.6-tg males. In addition, in AR3.6-tg male mice, lean mass was also significantly increased (Fig. 2B), accounting for 78% of total tissue compared to only 69% in wild-type littermates. Statistical analysis by student's t-test confirmed that the increase in lean mass and decrease in fat were both highly significant (p < 0.001). Total tissue mass (TTM) determined by DXA also demonstrates that the body size of AR3.6-tg male mice is 20% less than wild-type (Fig. 2C, p < 0.01). In contrast, female AR3.6-tg mice did not demonstrate significant alterations in body composition as a result of AR overexpression (Fig. 2A-C), likely due to the lower endogenous testosterone concentrations in females compared with males. Males only were thus evaluated in all further studies.
Figure 2. AR transgenic male but not female mice exhibit increased lean mass and reduced fat mass compared to wild-type littermates.
Six-month-old adult male and female AR3.6-tg and wild-type mice were analyzed by DXA. A. Fat mass as a percent of total body weight. B. Lean mass as a percent of total body weight. C. Total tissue mass. Data presented as mean ± SEM; n = 10. **, p < 0.01 and ***, p < 0.001.
Normal food intake and locomotor activity in AR transgenic mice
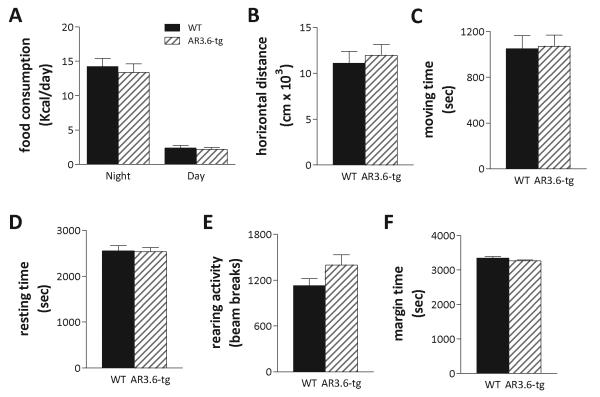
To identify potential mechanisms underlying the observed differences in transgenic male body composition, potential changes in food intake and/or activity was evaluated. Food consumption was determined morning and night for 5 consecutive days, to evaluate diurnal patterns of activity and feeding behavior. The amount of food consumed was converted to kcal/day based on the total metabolizable energy in the diet (Fig. 3A). During dark hours, wild-type mice consumed an average of 14.24 ± 1.2 kcal of rodent chow per day over 5 days compared to 13.36 ± 1.25 kcal for AR3.6-tg mice during the same period. As expected, less food was consumed during the daytime, with wild-type mice consuming 2.38 ± 0.345 kcal of chow versus 2.17 ± 0.3 kcal for AR3.6-tg mice. Food intake was not significantly different between genotypes during either period. In addition, possible changes in locomotor activity were assessed in automated activity monitors (Fig. 3B-F). After an acclimation period, wild-type and AR3.6-tg male mice were placed in monitors for 60 min and 5 different parameters were assessed. Results shown in indicate no significant difference in any parameter measured between wild-type and AR3.6-tg male mice, including no difference in horizontal distance, total time moving or total time resting, or time spent at the periphery (an indirect assessment of potential changes in stress hormones). Thus, increased lean mass and reduced fat is not due to differences in food consumption nor in behavioral changes that would be manifest as alterations in spontaneous locomotor activity.
Figure 3. No differences in food intake or baseline locomotor activity in AR transgenic mice.
A. Food consumption for wild-type and AR3.6-tg male mice (n = 8-9) was determined overnight (~16 h) and during each day (~ 8 h) for 5 consecutive days. Data are shown in kcals/day as mean ± SEM. No significant differences between the genotypes were noted. Spontaneous locomotor activity shown as B. horizontal distance, C. total time moving, D. total time resting, E. rearing activity and F. margin time (thigmotaxis). Data presented as mean ± SEM; n = 12 per genotype. No significant differences were observed between the genotypes.
AR transgenic male mice display depot-specific fat reduction
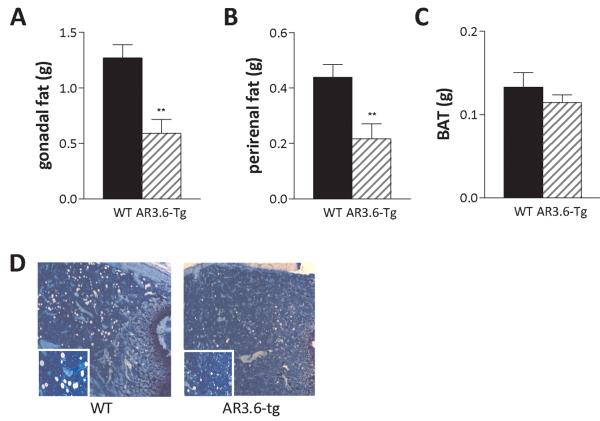
To assess the regional specificity of fat reduction observed in male AR3.6-tg mice, individual adipose tissue depots were dissected and weighed. Specific tissue included interscapular BAT and both perirenal and gonadal visceral WAT depots (Fig. 4A-C). There were significant reductions in both visceral fat depots in AR3.6-tg mice compared to wild-type. The mean wet tissue weight of gonadal fat depots from wild-type mice was 1.27 ± 0.12 grams compared to 0.59 ± 0.13 grams for AR3.6-tg mice. This amounts to a ~ 53% reduction. Similarly, perirenal fat reduction was 50% (0.44 ± 0.046 grams versus 0.22 ± 0.055 grams). These reductions were both statistically significant (p < 0.01). There was no significant alteration in BAT, with a mean wet tissue weight of 0.13 ± 0.017 grams for wild-type and 0.11 ± 0.009 grams for AR3.6-tg mice. In addition, UCP-1, HSL and LPL transcript levels as assessed by qPCR were similar in both genotypes (data not shown). Even though it has been suggested that brown and white fat derive from the same lineage, these results are consistent with data indicating that androgen action in adipose-tissue is depot specific [Rodriguez-Cuenca et al., 2005]. Thus, body weight and fat, percent fat mass and WAT depot weights were all significantly reduced in transgenic males. However, percent lean mass was significantly increased. Because mesenchymal stem cells (MSC) are the common precursors to both fat and muscle tissue, we hypothesized that AR transactivation by androgen in MSC has a direct role in reducing body weight and fat accumulation in transgenic mice. To evaluate this potential mechanism, AR3.6 transgene expression in MSCs was confirmed (see Fig. 7E). Transgene expression is high in proliferating cultures and was not different between male and female cells (data not shown). Since transgene expression is low to undetectable in mature fat, muscle and liver tissue in AR3.6-tg mice [Wiren et al., 2004], enhanced androgen sensitivity in MSCs in transgenic males may influence the differentiation of progenitor cells in vivo. Consistent with this possibility, histological analysis of femurs from wild-type and AR3.6-tg mice demonstrates reduced bone marrow adipogenesis from MSC, shown by the voids left behind from lipid filled cells after processing of transgenic bones (Fig. 4D).
Figure 4. Decreased visceral adipose tissue mass and reduced marrow adipogenesis in AR transgenic male mice.
Individual fat depots were dissected from 6-month-old AR3.6-tg and wild-type male mice and wet tissue weights were determined. A. Gonadal (epididymal) fat, B. Perirenal fat, and C. Interscapular BAT. Data presented as mean ± SEM; n = 15-25, **, p < 0.01. D. Histological analysis after toluidine blue staining of the bone marrow compartment from the metaphyseal region of the femur in wild-type and AR3.6-tg males. Adipocytes are represented by the large empty spaces in the marrow after processing and staining. Total magnification at 50 X; inset image at 100 X total magnification.
Reduced adipocyte area in fat pads from male AR transgenic mice
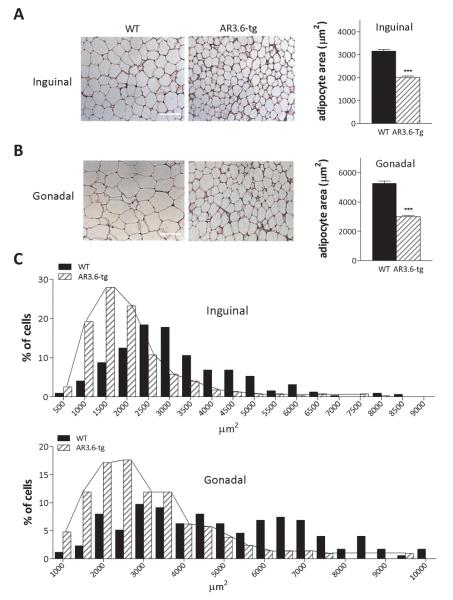
We next performed histological analysis on hematoxylin/eosin stained sections from inguinal and gonadal WAT depots (Fig. 5A, B). The mean area of individual adipocytes in μm2 was then determined. In inguinal fat, adipocyte area was reduced ~36% from AR3.6-tg mice compared to wild-type (2012 ± 56 versus 3155 ± 79; p < 0.001). In gonadal depots, adipocyte size was also significantly smaller in AR3.6-tg mice compared to wild-type fat (~ 3000 ± 84 vs. 5272 ± 167; p < 0.001). Histograms of adipocyte area (Fig. 5C) reveal a typical Gaussian distribution in wild-type cells, ranging from 1000 μm2 up to 10,000 μm2 with no individual group comprising more than 10% of the total cells measured. In contrast, results show that ~75% of the cells measured 3500 μm2 or less in gonadal depots from transgenic mice. Similarly, frequency distribution plots of cell size from inguinal depots show that more than 83% of the cells were 2500 μm2 or smaller while only 45% of cells from wild-type fat were in that size range.
Figure 5. Adipocytes in fat pads from AR transgenic male mice are smaller with reduced area compared to wild-type littermates.
Histological analysis of A. subcutaneous (inguinal) and B. visceral (gonadal) fat pads from wild-type and AR3.6-tg male mice (scale bars = 100 μm). Quantification is shown next to each histological image and demonstrates significantly smaller mean areas for adipocytes from both subcutaneous and visceral depots in AR3.6-tg males, *** p < 0.001. C. Histogram showing the frequency distribution of adipocyte areas for each depot (inguinal and gonadal) from WT and AR3.6-tg mice. Connecting lines highlight the leftward shift to smaller adipocytes in both depots from AR3.6-tg mice.
AR transgenic mice show reduced glucose levels and improved clearance
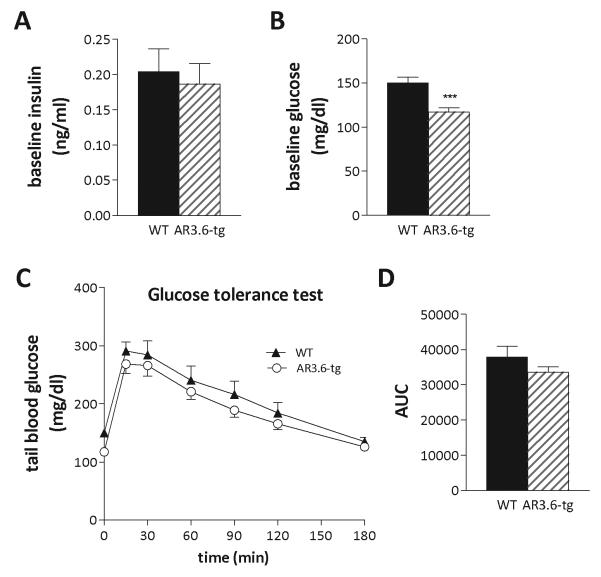
Since fat tissue also regulates glucose homeostasis, we next evaluated levels of fasting insulin and glucose from wild-type and AR3.6-tg mice. Serum insulin levels were measured following an overnight fast (Fig. 6A). Insulin was not significantly different between the genotypes (0.20 ± 0.033 ng/ml vs. 0.18 ± 0.029 ng/ml for wild-type vs. AR3.6-tg males, respectively). There were also no significant differences in serum leptin (3.62 ± 0.840 ng/ml vs. 3.33 ± 0.588 ng/ml), adiponectin (3.7 ± 0.268 μg/ml vs. 3.8 ± 0.275 μg/ml) or serum triglycerides concentrations (0.74 ± 0.069 mmol/L vs. 0.68 ± 0.073 mmol/L). However, baseline glucose levels (Fig. 6B) were significantly reduced in transgenic males compared to wild-type mice (114 ± 4.6 mg/dl vs. 150 ± 6.7 mg/dl; p < 0.001). Intra-peritoneal glucose tolerance tests (IPGTT) were next performed, again on fasted mice, by measuring blood glucose at timed intervals after a 1g/kg bolus of D-glucose. The ability to clear the glucose load was similar between genotypes, but AR3.6-tg mice did maintain slightly lower blood glucose levels over the 2 hour challenge and neither genotype fully returned to baseline until 180 min after the glucose injection. Analysis of the IPGTT time course curve (Fig. 6C) by repeated measure 2-way ANOVA using unweighted means shows increased glucose clearance in AR3.6-tg mice, with a significant effect of genotype [F(1, 175) = 6.788; p = 0.01] and time [F(6, 175) = 32.65; p < 0.001], with no interaction. Areas under the curve from individual animals were combined (Fig. 6D) and show a trend for increased glucose clearance in AR3.6-tg mice.
Figure 6. Serum insulin and blood glucose determinations during an intra-peritoneal glucose tolerance test (IPGTT) in wild-type and AR transgenic male mice.
A. Serum insulin was determined using an ultra-sensitive EIA kit after an overnight fast (n = 8). B. Tail blood glucose was determined after an 18h fast using a glucometer (n = 12-15); *** p < 0.001. C. IPGTT of wild-type and AR3.6-tg mice after an 18 h fast. Blood glucose was measured at baseline and at the indicated time points following an ip injection if D-glucose administered at 1g/kg (n = 12-15). A significant effect of genotype [F(1, 175) = 6.788; p = 0.01] and time [F(6, 175) = 32.65; p < 0.001 is observed. D. Area under the curve determinations were assembled from individual animal GTT curves and the mean ± SEM was calculated.
Reduced colony formation in progenitors derived from AR transgenic mice
To characterize stem cell lineage commitment in AR3.6 transgenic mice, colony-forming unit assays were performed (representative image shown in Fig. 7A). The fibroblastic colony forming unit (CFU-F) is used as a measure of the pluripotent progenitor cells residing in the marrow. Bone marrow MSCs were isolated from wild-type and AR3.6-tg male mice and CFU-F frequency was determined. Bone marrow from wild-type mice contained an average of ~42 CFU-F per 1.5 × 106 cells while AR3.6-tg marrow produced only 20 CFU-F (Fig. 7B). Thus, transgenic marrow contains ~52% fewer pluripotent progenitor cells compared to wild-type mice (p < 0.001). The ability of marrow cells to differentiate was also assessed by counting adipogenic colonies (CFU-Ad+) in these cultures (Fig. 7C). Transgenic cultures demonstrated a much more modest ~20% reduction in CFU-Ad+ numbers (p < 0.001). To better characterize the temporal sequence of adipogenic differentiation, MSC cultures were first expanded ex vivo and then seeded at equal densities (30,000 cells/cm2) to account for the reduced number of progenitors in AR3.6-tg derived marrow populations. Cultures from both genotypes reached confluence at similar times. At confluence, cultures were switched to media containing the PPARγ agonist rosiglitazone. After 4 days, cultures were evaluated by Oil red-O staining and quantitation. Qualitatively, adipocyte differentiation was clearly evident in both genotypes compared to uninduced cultures. Interestingly, similar to adipocytes in fat pads, evaluation of cell size in adherent cells also showed visually smaller adipocyte size in transgenic cultures (data not shown). Following Oil red-O extraction and quantitation, results indicated that there were only modest differences in lipid accumulation between wild-type and AR3.6-tg MSC (3.29 vs. 2.93-fold vehicle control for wild-type and AR3.6-tg, respectively, Fig. 7D). Transgene expression was also evaluated in proliferating, confluent and day 10 cultures. The level of transgene expression is high in proliferating precursor populations (Fig. 7E), equivalent to the high level observed in calvaria [Wiren et al., 2004]. As the cultures reach confluence and begin differentiation, transgene level is significantly reduced and by day 10, expression of the transgene is only ~20% the level observed in precursor cells. This result is consistent with the undetectable level of expression observed in mature fat depots in vivo [Wiren et al., 2004]. Thus, reduced CFU-F formation is consistent with reduced fat accumulation in vivo as a consequence of fewer adipogenic progenitors; this reduction occurs while lean mass is increased.
Gene expression changes observed in proliferating progenitor cells from AR transgenic mice but not in mature fat or during adipogenic differentiation of MSCs
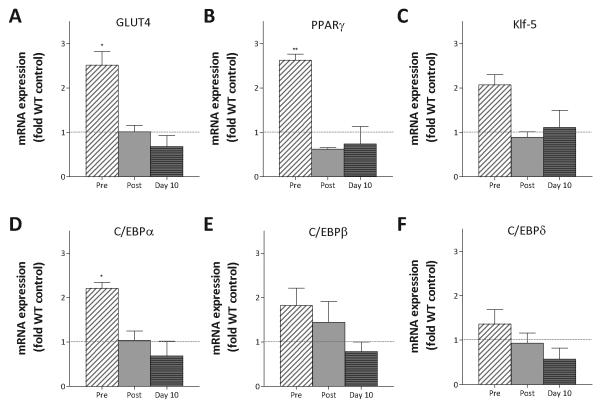
Targeted AR overexpression may regulate fat accumulation by directing the commitment of MSCs away from the adipocyte lineage at early stages of differentiation, or by modulating the differentiation process during adipogenesis. To assess gene expression changes in adipose tissue and during the temporal sequence of adipogenic differentiation, bone marrow MSC were expanded, plated at equal densities and RNA was isolated from rapidly proliferating cells at 50% confluence, in just confluent cultures and in differentiating cultures at 10 days after treatment with rosiglitazone. Expression differences were determined by qPCR analysis. There were no significant differences in mature fat depots in levels of HSL, LPL and UCP-1 (data not shown). However, during the course of adipogenic differentiation , expression differences were observed (Fig. 8). Since GLUT4 is the primary insulin-stimulated glucose transporter in muscle and adipose tissue and because glucose clearance was improved in transgenic males, we characterized GLUT4 expression during differentiation. Notably, GLUT4 mRNA expression was 2.5-fold higher in proliferating progenitor transgenic cultures compared to wild-type (p < 0.05). Expression levels of key adipogenic transcription factors [Lefterova and Lazar, 2009] including PPARγ, C/EBPα, C/EBPβ, C/EBPδ, and Kruppel-like factors including Klf-5 were also determined. In proliferating cultures, PPARγ expression was elevated over 2.5-fold (p < 0.01) while C/EBPα expression was ~ 2-fold above wild-type (p < 0.05). Thus, two transcription factors essential for adipocyte differentiation were both up-regulated in proliferating progenitor cells from AR3.6-tg mice compared to control cells, before the initiation of differentiation. During the course of differentiation after confluence and after induction of differentiation at day 10, there were no significant differences in expression between the gentoypes.
Figure 8. Increased expression of adipogenic transcription factors during proliferation in pluripotent marrow progenitors from AR transgenic male mice.
MSCs from bone marrow of wild-type and AR3.6-tg male mice were expanded ex vivo and induced to differentiate with rosiglitazone. Total RNA was isolated from uninduced proliferating MSC cultures at ~50% confluency, in confluent cultures and after adipocyte induction at 10 days of differentiation. Gene expression differences in proliferating cultures was assessed by qPCR for A.GLUT4, B. PPARγ, C. Klf-5, D. C/EBPα, E. C/EBPβ, and F. C/EBPδ. Data for AR3.6-tg cultures is shown normalized to WT at each time point. Data is presented as mean ± SEM; n = 3 replicates in three independent experiments for both genotypes. *, p < 0.05; **, p < 0.01 all compared to WT control levels.
Discussion
A substantial body of evidence demonstrates that androgens influence body composition in vivo, yet the mechanisms are incompletely characterized. In the studies reported here, transgenic mice with targeted AR overexpression driven by the type I collagen promoter were characterized. To our knowledge, this analysis represents the first characterization of the consequences of AR overexpression in stem cell populations in vivo. A body composition phenotype was observed with increased lean mass but reduced fat mass in males. Adipose tissue weight from transgenic males was normal in brown fat but reduced in both gonadal and perirenal WAT depots. In addition, adipocyte size was smaller in both visceral and subcutaneous WAT and glucose tolerance testing showed improved glucose clearance in transgenic males. The reduction in fat accumulation may result from inhibition of fat development since fewer progenitor cells were observed in MSC derived from transgenic males, with reduced numbers of CFU-F and a modest reduction CFU-Ad+ colony number. Elevated expression of PPARγ and C/EBPα in pluripotent progenitor cultures during the proliferation stage from transgenic mice suggests that premature adipogenic differentiation of progenitors occurs, and is associated with elevated expression of GLUT4 compared to controls. Combined, these results are consistent with the hypothesis that enhanced androgen sensitivity in stem cells alters lineage commitment early at the progenitor stage in vivo to reduce fat development, while increasing the expression of key transcription factors and GLUT4 during proliferation to promote smaller, more sensitive adipocytes but with little effect on mature fat.
Androgen administration has been shown to influence body composition, reducing fat mass but increasing muscle mass. MSCs have been characterized as pluripotent stem cells with the ability to differentiate into multiple lineages under appropriate conditions, including both fat and muscle [Bianco et al., 2006]. Our results demonstrate that progenitor CFU-F colony formation is dramatically inhibited in marrow from transgenic mice. Previous work has shown that treatment of adipocytes differentiated from MSCs using the nonaromatizable androgen dihydrotestosterone (DHT) resulted in adipocytes that were smaller [Gupta et al., 2008]. This ex vivo result is consistent with the response in vivo noted here. Transcription factors are essential mediators of adipogenesis, and C/EBPα and PPARγ are considered the two primary transcription factors that mediate the development of fat cells [Lefterova and Lazar, 2009]. Expression of key transcription factors was altered in proliferating progenitor cells from transgenic males, with elevated expression of GLUT4, PPARγ and C/EBPα observed prior to the induction of differentiation. Notably, GLUT4 expression or activity has been inversely correlated with adipocyte size [Eckertova et al., 2011; Ruschke et al., 2010] and activation of PPARγ in vivo has also been shown to result in smaller adipocytes [Minoura et al., 2007]; these expression differences may play a role in the smaller adipocytes seen in mature fat depots in male mice. Elevated expression of GLUT4 in precursors may also underlie improved glucose clearance observed in male transgenic mice. The differences seen in proliferating progenitors was transient as no significant expression differences were observed in confluent preadipocytes nor in mature differentiated cultures at day 10. Although elevated PPARγ expression was observed during proliferation of precursor cells, there was no corresponding increase in target gene expression in more mature cultures. Interestingly, androgen has been shown to inhibit PPARγ transcriptional activity [Du et al., 2009], so that elevations in expression may result in increased signaling only transiently and may not influence target gene expression profiles downstream in more mature cells as AR transgene levels decline in this model. Because transgene expression is low in mature cells, this model does not address the effects of androgen on mature fat function. Consistent with the lack of expression difference in differentiating adipocytes, a modest non-significant reduction in the ultimate differentiation of the cultures was observed as assessed by lipid accumulation between the genotypes. The absence of changes in mRNA expression in both mature adipocyte cultures and in mature fat tissue observed in transgenic mice suggests a model of directed adipogenesis in which the AR is critical for very early steps in adipocyte lineage allocation in progenitor populations, mediated by AR overexpression in stem cells in this model. Combined, these results suggest that the cellular target of androgen in vivo may be predominantly directed at pluripotent progenitor cells and the very immature adipocyte lineage, consistent with higher AR levels in vivo in preadipocytes than in mature adipocytes [Dieudonne et al., 1998]. Notably, the progenitor population also appears to be the target in androgenetic alopecia (male pattern baldness), with normal stem cell numbers but a defect in conversion of hair follicle stem cells to early progenitor cells driven by testosterone action [Garza et al., 2011].
The association between adipocyte size and insulin sensitivity remains controversial. In vivo, adipocyte diameter, more than BMI and fat mass, is inversely associated with glucose metabolism. In clinical studies in obese children, adipocyte size was shown to be associated with insulin sensitivity independent of fat mass [Maffeis et al., 2007]. Nevertheless, this association is not always observed [Mundi et al., 2010]. Inflammatory markers, which are thought to be the primary mediators of the pathological consequences of obesity, are also strongly correlated with adipocyte diameter in subcutaneous adipose tissue [Maffeis et al., 2007]. In middle-aged women, adipocyte size was reported to be a predictor of type 2 diabetes, independent from total body fat and distribution [Lonn et al., 2010]. It is also interesting to note that de novo lipogenesis is down-regulated in large adipocytes [Roberts et al., 2009], and in this cross-sectional study, the relationship between small adipocytes and insulin sensitivity was also reported to be independent of body mass index (BMI). Adipocyte size was also higher in subjects with diabetes in a population pre-disposed to obesity, again inversely correlating adipocyte size with insulin sensitivity [Weyer et al., 2000]. Thus, hypertrophic adipocytes are proposed to be functionally impaired and less metabolically active. In AR3.6-tg mice, smaller adipocytes are associated with significantly increased glucose clearance and reduced fasting blood glucose levels, consistent with most of the clinical literature.
The comparison of phenotypic differences between AR3.6-tg mice with different patterns of overexpression has also allowed us to differentiate between the site/envelope specific direct effects of androgens in the bone compartment [Wiren et al., 2008; Wiren et al., 2004]. In AR3.6-tg mice, AR is expressed in immature cells and throughout the osteoblast lineage, in the periosteum, and in marrow stromal cell progenitors, while AR2.3-tg expression is restricted to mature osteoblasts and osteocytes. Characterization of the phenotypes of these mice revealed overall similar effects on bone homeostasis [Wiren et al., 2008; Wiren et al., 2004]. In contrast to the conventional view, we have shown in both transgenic lines that androgen is not anabolic in mature bone and in fact, enhanced androgen sensitivity in osteoblasts/osteocytes is associated with a low turnover phenotype and is detrimental to overall bone strength and quality, an effect that may be mediated by increased osteoblast apoptosis [Wiren et al., 2006] and changes in gene expression to modify matrix quality [Wiren et al., 2010]. As expected, AR3.6-tg but not AR2.3-tg males showed increased bone formation at the periosteal surface, an effect likely mediated by the distinct expression patterns of the AR-transgene. The most important distinction between transgenic lines observed is the alterations in body composition seen in AR3.6-tg males. Since no changes in fat mass or lean body mass were observed in AR2.3-tg mice, the body composition phenotype is likely not mediated by changes in the bone compartment. Both the bone and body composition phenotype were sex-specific and observed in males but not females, likely due to the 10-fold lower concentration of circulating testosterone in females compared to males.
A novel aspect of the model is the ability to evaluate the consequences of changes in body composition in vivo, in particular a reduction in visceral fat, not mediated by hormone administration, calorie restriction or by increased activity levels, as these measures were not different between AR3.6-tg and wild-type mice. As noted, we did observe a modest increase in glucose clearance associated with smaller adipocytes. Decreased adipose tissue mass and reduced adipocyte size may also be associated with altered lipid metabolism, either through increased lipolysis or reduced lipogenesis. Circulating leptin concentrations are known to be proportional to fat mass [Considine et al., 1996], and since AR3.6-tg males are smaller, leptin levels were comparable to wild-type mice when normalized to body weight. HSL is an androgen-responsive lipolytic enzyme involved in triglyceride hydrolysis to glycerol and free fatty acids, and testosterone has also been shown to decrease HSL expression and catecholamine-stimulated lipolysis in mature adipocytes derived from subcutaneous depots in human subjects [Dicker et al., 2004]. We observed no difference in HSL expression in mature gonadal fat, but did not assess expression in preadipocytes or in MSCs. LPL is a primary lipogenic enzyme catalyzing the rate-limiting step in the hydrolysis of triacylglycerol from circulating lipoproteins. In AR3.6-tg mice, a small but nonsignificant increase in LPL expression was seen. There was also no difference between the genotypes in UCP-1 expression. Combined with the absence of alterations in serum adiponectin or triglycerides, these data suggest that changes in fat mass of the magnitude observed here do not dramatically influence serum hormone and adipokine expression, and again suggest that the phenotypic differences observed in the transgenic model are due to enhanced androgen sensitivity in MSCs to influence progenitors and not through modulation of mature fat function in vivo. It is interesting to speculate that with a diet-induced obesity model in AR3.6-tg mice, changes in adipokine levels may be observed.
A wealth of information regarding androgen effects on bone and body composition have come from both global and tissue-specific AR knockout mice. In general, results from these studies are opposite to the phenotype observed with AR overexpression reported here. As stated previously, global ARKO mice develop late-onset obesity [Fan et al., 2005] and show decreased muscle mass in males [MacLean et al., 2008]. In contrast, transgenic mice display a phenotype with reduced fat mass and reduced adipocyte size but increased lean (fat free) mass. In addition, the generation of mice with myocyte-specific AR ablation has highlighted the importance of androgen for the maintenance of muscle mass and fiber type [Ophoff et al., 2009]. While targeted AR3.6-tg mice showed no changes spontaneous activity, ARKO mice exhibited reduced activity patterns during development and consumed 30% less oxygen compared to wild-type mice as assessed by indirect calorimetry [Fan et al., 2005]. Although global ARKO mice showed no alterations in serum lipid markers [Sato et al., 2003], there have been conflicting reports with regard to adipocytokine secretion and insulin sensitivity in other global ARKO models. Fan et al. reported normal insulin sensitivity and high adiponectin secretion [Fan et al., 2005], while Lin et al. showed insulin resistance and hyperleptinemia, but reduced adiponectin [Lin et al., 2005]. Interestingly, mature adipose-specific AR deletion mice do not develop obesity, yet have high serum leptin concentrations [Yu et al., 2008]. These discrepancies may be explained by differences in the experimental paradigm or genetic backgrounds of the founder lines. The data presented here has focused on the effects of androgen on adipogenesis ex vivo in bone marrow stromal cells that are developmentally derived from a mesodermal origin. It is also likely that there is a neural crest contribution to adipose development [Billon et al., 2008]. Combined, these reports are consistent with an important role for AR signaling in vivo in early adipogenesis but not necessarily mature fat function.
In summary, AR overexpression in MSCs results in body compositional changes exemplified by significant reductions in total fat and fat as percent body weight, but with an increase in lean mass. Reduced adipocyte size and increased glucose clearance but no difference in serum adipokines were noted. The reduction in white fat depot weight may reflect both smaller adipocyte size and reduced fat development. Notably, the number of progenitors resident in the marrow is significantly reduced AR3.6-tg mice, but only modest inhibition of terminal differentiation and accumulation of lipid is observed in mature cells ex vivo. Although proliferating progenitor cultures demonstrate precious expression of the transcription factors PPARγ and C/EBPα with elevated GLUT4 levels in transgenic compared to wild-type controls, expression differences are not observed in mature adipocyte cultures nor in mature fat depots. Thus, the effects of androgen signaling on lineage allocation are complex and stage-dependent to target early progenitor populations in vivo, with the result of reduced fat mass but increased muscle mass. Combined, results suggest that the AR-transgenic model provides the first in vivo confirmation of ex vivo data demonstrating androgen inhibition of fat development specifically in pluripotent cells, and thus proof of principle in vivo for the hypothesis proposed by Bhasin et al, that “the primary site of androgen action is the pluripotent stem cell” [Bhasin et al., 2003]. This hypothesis provides a unifying explanation for observed reciprocal effects of androgen treatment on fat and muscle mass in vivo and for the phenotype observed in AR3.6-tg males. Thus, a growing body of evidence including epidemiological data, clinical studies with androgen administration in hypogonadal populations, in vitro data in pluripotent stem cells treated with DHT and our current observations in AR transgenic mice with reduced adipogenesis, all suggest that androgen can influence lineage allocation in pluripotent cells to affect fat accumulation, fat cell size and glucose clearance. Our results have important implications for the treatment of obesity, diabetes and the detrimental consequences of hypogonadism and aging on body composition.
Acknowledgments
The authors would like to thank Joel Hashimoto for technical assistance. We also thank Dr. Robert Klein (OHSU) for the use of equipment for DXA analysis, Drs. Tamara Phillips and Cheryl Reed (OHSU) for assistance with characterization of locomotor activity, and Drs. Mitch Schaffler (City College of NY) and Karl Jepsen Schaffler (Mt. Sinai School of Medicine) for images of bone marrow adiposity. All work was performed in facilities provided by the Department of Veterans Affairs.
Grant sponsor: National Institute of Diabetes, Digestive & Kidney Disease; Grant number: R01 DK067541 (KMW). Grant sponsor: Department of Defense; Grant number: United States Army Research Acquisition Activity Award No. W81XWH-05-1-0086 (KMW).
References
- Bassil N, Alkaade S, Morley J. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009;5:427–448. doi: 10.2147/tcrm.s3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S. Regulation of body composition by androgens. J Endocrinol Invest. 2003;26:814–822. doi: 10.1007/BF03345230. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Taylor W, Singh R, Artaza J, Sinha-Hikim I, Jasuja R, Choi H, Gonzalez-Cadavid N. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A Biol Sci Med Sci. 2003;58:M1103–1110. doi: 10.1093/gerona/58.12.m1103. [DOI] [PubMed] [Google Scholar]
- Bianco P, Kuznetsov SA, Riminucci M, Gehron Robey P, Klimanskaya Irina, Robert L. Postnatal skeletal stem cells: “Methods in Enzymology. Academic Press; 2006. pp. 117–148. [DOI] [PubMed] [Google Scholar]
- Billon N, Monteiro M, Dani C. Developmental origin of adipocytes: new insights into a pending question. Biol Cell. 2008;100:563–575. doi: 10.1042/BC20080011. [DOI] [PubMed] [Google Scholar]
- Callewaert F, Venken K, Ophoff J, De Gendt K, Torcasio A, van Lenthe GH, Van Oosterwyck H, Boonen S, Bouillon R, Verhoeven G, Vanderschueren D. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-{alpha} FASEB J. 2009;23:232–240. doi: 10.1096/fj.08-113456. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Crossno JT, Jr., Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker A, Rydén M, Näslund E, Muehlen IE, Wirén M, Lafontan M, Arner P. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47:420–428. doi: 10.1007/s00125-003-1324-0. [DOI] [PubMed] [Google Scholar]
- Dieudonne M, Pecquery R, Boumediene A, Leneveu M, Giudicelli Y. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am J Physiol. 1998;274(6 Pt 1):C1645–1652. doi: 10.1152/ajpcell.1998.274.6.C1645. [DOI] [PubMed] [Google Scholar]
- Du J, Zhang L, Wang Z. Testosterone inhibits the activity of peroxisome proliferator-activated receptor gamma in a transcriptional transaction assay. Pharmazie. 2009;64:692–693. [PubMed] [Google Scholar]
- Eckertova M, Ondrejcakova M, Krskova K, Zorad S, Jezova D. Subchronic treatment of rats with oxytocin results in improved adipocyte differentiation and increased gene expression of factors involved in adipogenesis. Br J Pharmacol. 2011;162:452–463. doi: 10.1111/j.1476-5381.2010.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H. Androgen Receptor Null Male Mice Develop Late-Onset Obesity Caused by Decreased Energy Expenditure and Lipolytic Activity but Show Normal Insulin Sensitivity With High Adiponectin Secretion. Diabetes. 2005;54:1000–1008. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]
- Fernando SM, Rao P, Niel L, Chatterjee D, Stagljar M, Monks DA. Myocyte androgen receptors increase metabolic rate and improve body composition by reducing fat mass. Endocrinology. 2010;151:3125–3132. doi: 10.1210/en.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza L, Yang C, Zhao T, Blatt H, Lee M, He H, Stanton D, Carrasco L, Spiegel J, Tobias J, Cotsarelis G. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011;121:613–622. doi: 10.1172/JCI44478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Bhasin S, Guo W, Singh R, Miki R, Chauhan P, Choong K, Tchkonia T, Lebrasseur NK, Flanagan JN, Hamilton JA, Viereck JC, Narula NS, Kirkland JL, Jasuja R. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Molecular and Cellular Endocrinology. 2008;296:32–40. doi: 10.1016/j.mce.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34:513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- Hashimoto J, Beadles-Bohling A, Wiren K. Comparison of RiboGreen and 18S rRNA quantitation for normalizing real-time RT-PCR expression analysis. Biotechniques. 2004;36:54–60. doi: 10.2144/04361BM06. [DOI] [PubMed] [Google Scholar]
- Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalajzic Z, Liu P, Kalajzic I, Du Z, Braut A, Mina M, Canalis E, Rowe D. Directing the expression of a green fluorescent protein transgene in differentiated osteoblasts: comparison between rat type I collagen and rat osteocalcin promoters. Bone. 2002;31:654–660. doi: 10.1016/s8756-3282(02)00912-2. [DOI] [PubMed] [Google Scholar]
- Lefterova M, Lazar M. New developments in adipogenesis. Trends in Endocrinology & Metabolism. 2009;20:107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Lin H-Y, Xu Q, Yeh S, Wang R-S, Sparks JD, Chang C. Insulin and Leptin Resistance With Hyperleptinemia in Mice Lacking Androgen Receptor. Diabetes. 2005;54:1717–1725. doi: 10.2337/diabetes.54.6.1717. [DOI] [PubMed] [Google Scholar]
- Lonn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010;24:326–331. doi: 10.1096/fj.09-133058. [DOI] [PubMed] [Google Scholar]
- MacLean HE, Chiu WSM, Notini AJ, Axell A-M, Davey RA, McManus JF, Ma C, Plant DR, Lynch GS, Zajac JD. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008;22:2676–2689. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- Maffeis C, Silvagni D, Bonadonna R, Grezzani A, Banzato C, Tato L. Fat cell size, insulin sensitivity, and inflammation in obese children. J Pediatr. 2007;151:647–52. doi: 10.1016/j.jpeds.2007.04.053. [DOI] [PubMed] [Google Scholar]
- Matsumoto A. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57:M76–99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, Veldhuis J, Urban R. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83:1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- Minoura H, Takeshita S, Kimura C, Hirosumi J, Takakura S, Kawamura I, Seki J, Manda T, Mutoh S. Mechanism by which a novel non-thiazolidinedione peroxisome proliferator-activated receptor gamma agonist, FK614, ameliorates insulin resistance in Zucker fatty rats. Diabetes Obes Metab. 2007;9:369–378. doi: 10.1111/j.1463-1326.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundi MS, Karpyak MV, Koutsari C, Votruba SB, O'Brien PC, Jensen MD. Body fat distribution, adipocyte size, and metabolic characteristics of nondiabetic adults. J Clin Endocrinol Metab. 2010;95:67–73. doi: 10.1210/jc.2009-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophoff J, Van Proeyen K, Callewaert F, De Gendt K, De Bock K, Vanden Bosch A, Verhoeven G, Hespel P, Vanderschueren D. Androgen Signaling in Myocytes Contributes to the Maintenance of Muscle Mass and Fiber Type Regulation But Not to Muscle Strength or Fatigue. Endocrinology. 2009;150:3558–3566. doi: 10.1210/en.2008-1509. [DOI] [PubMed] [Google Scholar]
- Pei L, Tontonoz P. Fat's loss is bone's gain. J Clin Invest. 2004;113:805–806. doi: 10.1172/JCI21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, Hodson L, Dennis A, Neville M, Humphreys S, Harnden K, Micklem K, Frayn K. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52:882–890. doi: 10.1007/s00125-009-1300-4. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy Kv, Friedman JM. Identification of White Adipocyte Progenitor Cells In Vivo. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, Monjo M, P AM, Roca P. Depot differences in steroid receptor expression in adipose tissue: possible role of the local steroid milieu. Am J Physiol Endocrinol Metab. 2005;288:E200–207. doi: 10.1152/ajpendo.00270.2004. [DOI] [PubMed] [Google Scholar]
- Ruschke K, Illes M, Kern M, Kloting I, Fasshauer M, Schon M, Kosacka J, Fitzl G, Kovacs P, Stumvoll M, Bluher M, Kloting N. Repin1 maybe involved in the regulation of cell size and glucose transport in adipocytes. Biochem Biophys Res Commun. 2010;400:246–251. doi: 10.1016/j.bbrc.2010.08.049. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H, Kato S. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem Biophys Res Commun. 2003;300:167–171. doi: 10.1016/s0006-291x(02)02774-2. [DOI] [PubMed] [Google Scholar]
- Seidell J, Bjorntorp P, Sjostrom L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- Singh R, Artaza J, Taylor W, Gonzalez-Cadavid N, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, Bhasin S. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with {beta}-catenin and T-Cell Factor 4 may bypass canonical wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder E, Walts B, Perusse L, Chagnon Y, Weisnagel S, Rankinen T, Bouchard C. The human obesity gene map: the 2003 update. Obes Res. 2004;12:369–439. doi: 10.1038/oby.2004.47. [DOI] [PubMed] [Google Scholar]
- Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, Casaburi R, Bhasin S. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478–1485. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto A, Snyder P, Weber T, Berman N, Hull L, Swerdloff R. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–2098. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung C, Shackel I, Williams P. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Wiren KM, Semirale AA, Hashimoto JG, Zhang XW. Signaling pathways implicated in androgen regulation of endocortical bone. Bone. 2010;46:710–723. doi: 10.1016/j.bone.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren KM, Semirale AA, Zhang XW, Woo A, Tommasini SM, Price C, Schaffler MB, Jepsen KJ. Targeting of androgen receptor in bone reveals a lack of androgen anabolic action and inhibition of osteogenesis A model for compartment-specific androgen action in the skeleton. Bone. 2008;43:440–451. doi: 10.1016/j.bone.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren KM, Toombs AR, Semirale AA, Zhang X. Osteoblast and osteocyte apoptosis associated with androgen action in bone: requirement of increased Bax/Bcl-2 ratio. Bone. 2006;38:637–651. doi: 10.1016/j.bone.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Wiren KM, Zhang XW, Toombs AR, Kasparcova V, Gentile MA, Harada S, Jepsen KJ. Targeted overexpression of androgen receptor in osteoblasts: unexpected complex bone phenotype in growing animals. Endocrinology. 2004;145:3507–3522. doi: 10.1210/en.2003-1016. [DOI] [PubMed] [Google Scholar]
- Woodhouse LJ, Gupta N, Bhasin M, Singh AB, Ross R, Phillips J, Bhasin S. Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J Clin Endocrinol Metab. 2004;89:718–726. doi: 10.1210/jc.2003-031492. [DOI] [PubMed] [Google Scholar]
- Yeh S, Tsai M, Xu Q, Mu X, Lardy H, Huang K, Lin H, Yeh S, Altuwaijri S, Zhou X, Xing L, Boyce B, Hung M, Zhang S, Gan L, Chang C, Hung M. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci USA. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IC, Lin H-Y, Liu N-C, Wang R-S, Sparks JD, Yeh S, Chang C. Hyperleptinemia without obesity in male mice lacking androgen receptor in adipose tissue. Endocrinology. 2008;149:2361–2368. doi: 10.1210/en.2007-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]