Abstract
Objective
To test the hypothesis that women with full and subthreshold bulimia nervosa show abnormal neural activation in response to food intake and anticipated food intake relative to healthy control women.
Method
Females with and without full/subthreshold bulimia nervosa recruited from the community (N = 26) underwent functional magnetic resonance imaging (fMRI) during receipt and anticipated receipt of chocolate milkshake and a tasteless control solution.
Results
Women with bulimia nervosa showed trends for less activation than healthy controls in the right anterior insula in response to anticipated receipt of chocolate milkshake (versus tasteless solution) and in the left middle frontal gyrus, right posterior insula, right precentral gyrus, and right mid dorsal insula in response to consumptions of milkshake (versus tasteless solution).
Discussion
Bulimia nervosa may be related to potential hypo-functioning of the brain reward system, which may lead these individuals to binge eat to compensate for this reward deficit, though the hypo-responsivity might be a result of a history of binge eating highly palatable foods.
Reward Abnormalities Among Women with Full and Subthreshold Bulimia Nervosa: A Functional Magnetic Resonance Imaging Study
Bulimia nervosa is associated with psychiatric distress, functional impairment, medical complications, and increased risk for future obesity, depression, suicide attempts, substance abuse, and health problems (1-2). As well, individuals with subthreshold bulimia nervosa, such as those who report a frequency of binge eating and compensatory behavior below diagnostic thresholds, evidence psychiatric distress, functional impairment, medical complications, and seek treatment (3-5). Thus, numerous studies have investigated biological correlates of bulimic pathology, with the hope of elucidating the etiological processes that give rise to this pernicious disorder.
Theorists have hypothesized that people who show elevated reward from food intake may be at risk for bulimic pathology (6-7). Specifically, it may be that individuals who show a particularly strong activation in mesolimbic reward circuitry in response to food intake are at increased risk for binge eating. In support of the hyper-responsivity theory, women with bulimia nervosa show greater sensitivity to financial reward than healthy controls when measured by behavioral performance in some (8-9), but not all studies (10). Bulimic symptoms also correlate positively with self-reported reward sensitivity (11). Further, women with versus without bulimia nervosa prefer sweeter and higher-fat foods than healthy controls (12-13).
In contrast to the results reviewed above, a few brain imaging studies have produced findings that suggest that individuals with bulimia nervosa may show reduced responsivity of brain reward circuitry. Specifically, positron emission tomography (PET) studies found that women who had recovered from bulimia nervosa showed less activation of the right anterior cingulate cortex and left cuneus in the occipital cortex in response to receipt of glucose versus artificial saliva and lower baseline medial OFC serotonin 2A receptor binding, even after a glucose preload (14-15). Another PET study found less μ-opioid binding in the temporinsular cortex at rest among women with bulimia nervosa compared to controls (16). Because these frontal and mesolimbic brain regions appear to encode reward from food intake (17-18), these results imply that individuals with bulimic pathology might show a blunted responsivity of reward circuitry to food. These results appear to accord with the evidence that obese relative to lean individuals show reduced activation of the dorsal striatum to consumption of a palatable food versus a tasteless solution (19-20).
Thus, self-report and behavioral data seem to imply that individuals with bulimic pathology may show hyper-responsivity of reward circuitry, whereas the brain imaging studies imply that these individuals potentially show hypo-responsivity of reward circuitry. One explanation for this pattern of findings is that brain imaging studies yield different findings because it is more objective than self-report or behavioral data, which are both sensitive to self-presentation biases. In addition, this literature review revealed a key gap in the literature: no brain imaging studies have examined activation of reward circuitry in response to food receipt among individuals with and without current bulimic pathology.
Theorists have also hypothesized that elevated anticipated reward from food intake increases risk for binge eating (21). Incentive salience theory posits that over repeated presentations of a rewarding substance (e.g., food), individuals learn to associate cues with the reward and that consummatory reward decreases while anticipatory reward increases (22). Cues such as sight and smell of food may eventually lead to physiological responses that trigger food craving and increase risk for binge eating (23). Naïve monkeys initially showed firing of mesotelencephalic dopamine neurons only in response to food taste, but this firing began to precede food delivery after conditioning, with maximal firing eventually elicited by the conditioned stimuli that predict subsequent food delivery rather than the actual food receipt (24-25). Blackburn, Phillips, Jakubovic, and Fibiger (26) found that dopaminergic firing was greater in the nucleus accumbens of rats after presentation of a conditioned stimulus that usually signaled food receipt than after delivery of an unexpected meal. Thus, reward activation in response to anticipation of food may be more important than response to consumption of food in predicting whether someone initiates an eating episode.
Some evidence suggests that different neural regions are involved in consummatory and anticipatory food reward. Anticipated receipt of a palatable food, versus anticipated receipt of unpalatable food or a tasteless food, results in greater activation in the OFC, amygdala, cingulate gyrus, striatum (caudate nucleus and putamen), ventral tegmental area, midbrain, parahippocampal gyrus, and fusiform gyrus (17, 27). Anticipation of a pleasant taste, versus actual taste, resulted in greater activation in the dopaminergic midbrain, nucleus accumbens, and the posterior right amygdala (17). Small and associates (28) found that anticipation of a pleasant drink resulted in greater activation in the amygdala and mediodorsal thalamus, whereas the receipt of the drink resulted in greater activation in the left insula/operculum. These results suggest that the amygdala, midbrain, nucleus accumbens, and mediodorsal thalamus are more responsive to anticipated consumption versus actual consumption of food, whereas the frontal operculum/insula is more responsive to consumption versus anticipated consumption of food.
Individuals with bulimia nervosa or recurrent binge eating rate pictures of food as more interesting and arousing and report a greater desire to eat than healthy controls (29-30). Food craving is cited by 70% of patients with bulimia nervosa as a reason for binge eating (31). These data suggest that individuals with bulimia nervosa may experience greater anticipatory reward from eating than healthy controls, which is congruent with findings that they report greater urges to binge and less confidence in their ability to control their food intake after exposure to the sight, smell, and taste of food (32-33).
Salivary response, a key component of the cephalic phase response, correlates positively with self-reported hunger and desire to binge eat, suggesting it may serve as a useful proxy for food craving and is related to anticipatory reward (34). However, findings have been mixed; some studies find that women with bulimia nervosa show more (34-35), less (29, 32), or similar (33) salivary response to food cues compared to healthy controls. These studies suggest that there are no reliable differences between individuals with and without bulimia nervosa in salivary response to food, although it could be that the measures themselves are unreliable or that these studies had small sample sizes that led to inconsistent results. It is also possible that individuals with bulimia nervosa have an approach-avoidance response to food cues, evidenced by a drive to consume food coupled with negative feelings toward food due to guilt and shame from prior binge eating or high levels of thin-ideal internalization. These positive and negative responses to food cues could lead to inconsistent physiological responses. Indeed, individuals with bulimia nervosa often report more negative feelings while looking at, smelling, or touching food (30, 32-34, 36).
One brain imaging study found that individuals with bulimia nervosa using whole brain analysis showed greater activation in the medial OFC and anterior cingulate cortex (ACC) in response to presentations of pictured food versus non-food images relative to healthy controls (36). Schienle and colleagues (37) found that individuals with bulimia nervosa showed greater insula and ACC activation than healthy controls and individuals with binge eating disorder in response to pictures of food versus household items. Interestingly, fMRI studies indicate that obese versus lean individuals show greater activation in reward areas, including the insula, frontal operculum, orbitofrontal cortex, amygdala, and striatum in response to pictures of palatable foods (38-40) and anticipated receipt of palatable food (20). These data suggest that individuals with bulimic pathology show greater anticipatory reward than healthy controls.
Method
Participants
A total of 26 females, aged 18-26 (M = 20.3, SD = 1.87) were recruited over one year from introductory psychology courses and through flyers posted around a university campus. This sample included 11 women with subthreshold bulimia nervosa, 2 women with full threshold bulimia nervosa, and 13 healthy controls matched on body mass index (BMI). The sample was 4% Hispanic, 80% Caucasian, 12% Asian, and 4% African American. Participants had a mean body mass index (BMI) of 23.6 (Range = 19.5-28.2, SD = 2.6).
Procedure
Students in introductory psychology courses were screened with the Eating Disorder Diagnostic Scale (41). Those reporting at least 4 binge episodes and 4 compensatory behaviors in the prior month and those reporting no bulimic pathology were invited to participate. Additional participants were recruited via flyers posted on campus and surrounding areas. Participants were excluded if they had any contraindication for MRI scanning procedure (metal in body, braces, claustrophobia, etc.), if they had a food allergy to chocolate milkshake, or if they did not like chocolate milkshake. Participants with any Axis I psychiatric disorder (based on screening questions from the Structured Clinical Interview for DSM-IV) or who were taking psychoactive medications other than selective serotonin-reuptake inhibitors were excluded. These criteria were selected in order to decrease heterogeneity related to comorbid disorders and thus increase power to detect effects related specifically to bulimic pathology. Although we intended to exclude all psychoactive medications, the difficulty in recruiting participants with full threshold bulimia nervosa forced us to allow at least one class of medication: selective serotonin reuptake inhibitors (SSRI). Sertraline and fluoxetine were the only medications used by participants in the study. Post-hoc analyses revealed no change in results when controlling for SSRI use. Participants excluded because of Axis I psychiatric disorder were provided with referrals to local counselors.
Study procedures were described to interested individuals over email or telephone and eligibility questions were administered. Those who remained eligible completed two assessments. On the first appointment, after providing informed consent, participants completed a diagnostic interview. If the diagnostic interview confirmed a threshold or subthreshold diagnosis of bulimia nervosa or no eating disorder symptoms, the participant scheduled a second appointment. Subthreshold bulimia nervosa was defined as engaging in binge eating and compensatory behaviors at least once per week for a 3-month period, rather than the more stringent twice per week for a full threshold diagnosis. It should be noted that the criteria used to define subthreshold bulimia nervosa in this study is equivalent to the proposed criteria for bulimia nervosa in the DSM-V. If the proposed changes are accepted and incorporated into the final version of the DSMV, all 13 participants in the bulimia nervosa group would meet criteria for DSM-V bulimia nervosa. If the participants did not fall into the eating disordered or non-eating disordered group (e.g., reported partial symptoms or symptoms of anorexia nervosa), they were excluded. Exclusionary criteria resulted in 14 excluded participants (3 for metal contraindicators, 5 for psychotropic medications, 2 for Axis I disorders, and 4 due to the presence of partial symptoms (e.g., bingeing without compensatory behaviors)). No potential participants were excluded due to food allergies or not liking chocolate milkshake.
On the second appointment, participants completed a series of self-report measures. They ate a standardized snack, consisting of a Nutri-Grain bar and fruit (e.g., apple, banana, or pear) to control for effects of acute food deprivation. They also rated the tastes used in the study on pleasantness and craving and reported their intake of chocolate milkshake over the prior month. Finally, they completed a functional MRI scan. This second appointment was scheduled during the mid-follicular phase of the menstrual cycle for each participant to control for effects of menstrual phase on reward.
During the paradigm, two pictures were presented: a glass of chocolate milkshake labeled “milkshake” and a glass of water labeled “water.” We used a standard highly palatable and hedonically pleasurable milkshake for the milkshake condition. We used tasteless solution for the comparison condition to control for the effects of receiving and swallowing a liquid. Pictures were presented for 3 seconds followed by a jitter of 1-7 seconds during which time the screen was blank. Following 60% of the picture cues, a 3 second delivery of 0.5cc of the milkshake/tasteless solution was delivered following the jitter; for the remaining 40% of the pictures cues, no milkshake/tasteless solution was delivered (invalid presentations). A second jitter of 1-7 seconds followed milkshake/tasteless solution delivery. In total, there were at least 20 each of the valid and invalid pictures, in which the milkshake or tasteless solution was and was not delivered when cued: which are the key conditions in this paradigm. This was accomplished in 5 runs of 7.5 min duration (plus 13-secs of dummy scanning at the beginning of each scan to allow equilibrium to be reached). This design allowed us to identify the brain regions that were activated in response to expecting to get a taste of chocolate milkshake versus expecting tasteless solution and also in response to actual receipt of the chocolate milkshake versus tasteless solution. This paradigm has proven sensitive to differences in brain activation in response to chocolate milkshake versus control solution among obese and lean participants in prior studies (19-20).
In addition to the neural measure, subjects used a visual analogue scale to rate the perceived pleasantness of the milkshake and the intensity of the overall flavor. This provided us with hedonic and sensory measures of our stimuli. The scales chosen provided ratio-like data equivalent to magnitude estimation with the added ability to compare individual differences in a more meaningful and sensitive way than traditional category scales (42).
The milkshake was made fresh each day with 1 cup of vanilla Häagen Dazs ice cream, 1 cup of 2% milk, and 2 tablespoons of Hershey's chocolate syrup. The tasteless solution was made from USP grade KCL and NaHCO3, to mimic the ionic components of saliva. The mixture was composed of 0.0125M KCl and 0.00125M NaHCO3 M dissolved in water. Stimuli were stored in a refrigerator and brought to room temperature before use. New liquid tasteless solution was made every 5 days.
fMRI Scanner and Data Acquisition
We used a Siemans Allegra 3T scanner to collect functional and anatomical imaging data. Participants practiced the paradigm prior to data collection, including swallowing without moving their heads. Foam padding and a vacuum pillow were used to limit involuntary head movement. Visual stimuli were presented with a digital projector/reverse screen display system. Taste stimuli were delivered with programmable syringe pumps (Braintree Scientific BS-8000). Participants completed scanning in one 60-minute session. Laterality for image processing was confirmed by taping a vitamin E capsule to the right temporal region in every subject. Echo planar imaging measured blood oxygen level dependent (BOLD) signal as an indication of cerebral brain activation. The OFC and amygdala were of particular interest, which are subject to signal distortions in fMRI (43). To improve BOLD signal detection and minimize susceptibility-based distortion effects, we used a protocol that utilized a high readout bandwidth, a shorter echo time, and localized shimming in the region of the OFC and amygdala to reduce the magnetic field distortion. Specifically, a susceptibility weighted single shot echo planar sequence was used to image the regional distribution of the BOLD signal with TR = 2100 ms, TE = 20ms, flip angle = 80°, with an in plane resolution of 3.0 × 3.0 mm2 (64 × 64 matrix; 192 × 192 mm2 field of view). To cover the whole brain, 32 4mm slices (interleaved acquisition, no skip) were acquired along the AC-PC transverse, oblique plane as determined by the midsagittal section. Slices were acquired in an interleaved mode to reduce the cross-talk of the slice selection pulse. At the beginning of each functional run, the MR signal was allowed to equilibrate over 6 scans for a total of 12.6 sec, which was excluded from analysis. This procedure has consistently been able to measure signal in the amygdala and OFC (44-45). For each subject, a high resolution, T1 weighted 3D volume was acquired in 8 minutes (MP-RAGE with a TR/TE of 2100ms/2.4ms, flip angle of 15°, TI of 1100ms, matrix size of 256×256, FOV of 22cm, slice thickness of 1mm). The orientation of this 3D volume was identical to the functional slices and was used in conjunction with the activation maps to localize the function and determine the anatomic regions for investigation of the time course data. Distortion in EPI images was corrected based on the estimated parameters of the phase map (46).
We monitored head movement in vivo during the scan and re-administered any block in which head movement exceeded 1 mm. Specifically, we used the Prospective Acquisition CorrEction (PACE) program to monitor head movement in real time. If head movement exceeded 1 mm during a scan, the operator was notified so that the scan could be stopped and the block rerun. In addition, for smaller movements, PACE adjusts slice position, orientation and regrids the residual volume-to-volume motion during data acquisition. PACE combines techniques of prospective and retrospective motion correction by estimating motion parameters for subsequent volume acquisition based on detecting motion from reconstructed image data. There is a high level of consistency and accuracy for detected motion parameters in phantom experiments with PACE, (translation<40μm; rotation< 0.05°) and in vivo experiments demonstrate significant reduction of variance in pre- and post-motion volumes (47).
Measures
Screening Measure for Bulimic Pathology
The Eating Disorder Diagnostic Scale (41) was used to screen students for bulimic pathology. The EDDS assesses DSM-IV diagnostic criteria for anorexia nervosa, bulimia nervosa, and binge eating disorder. A frequency count of binge episodes and compensatory behaviors was used to select individuals to participate in the study. The EDDS has shown high agreement (κ = .78 - .83) with eating disorder diagnoses made with the Eating Disorder Examination (EDE; 48), internal consistency (α = .89), 1-week test-retest reliability (r = .87), sensitivity to detecting intervention effects, and predictive validity for future onset of eating pathology and depression (41, 49).
Screening Measure for Axis I Disorders
The screening questions from the Structured Clinical Interview for DSM-IV Disorders was used to screen potential participants for psychiatric disorders. Specifically, the rule-out questions for the most common disorders (e.g., major depression, bipolar disorder, substance abuse, and anxiety disorders) were administered. The SCID shows good inter-rater reliability and test-retest reliability for major depression (r = .80 and .61 respectively), alcohol dependence/abuse (r = 1.00 and .77, respectively), and anxiety disorders (r = .57-.88 and .44-.78, respectively) (50-51).
Handedness
Handedness was assessed with the Edinburgh Handedness Questionnaire (52), and both the laterality quotient and the laterality scale were calculated (53).
Bulimic Symptoms
The diagnostic items from the Eating Disorder Examination, a structured psychiatric interview, were used to assess DSM-IV criteria for bulimia nervosa over the past year (EDE 12th Edition; 48). The shortened version of this interview (Eating Disorder Diagnostic Interview) has been used extensively in studies conducted by our group. The continuous symptom composite has shown internal consistency (α = .92), 1-week test-retest reliability (r = .90), sensitivity to detecting intervention effects, and predictive validity for future onset of depression and the eating disorder diagnoses have show high inter-rater agreement between independent and blinded assessors (κ = .86) and high 1-week test-retest reliability (κ = .96; 54). Moreover, clinical interviewers for this study were trained to produce high inter-rater agreement with supervisors using recorded interviews (κ = .90 or higher) before collecting data.
Body Mass Index (BMI; Kg/M2)
Height was measured to the nearest millimeter using a portable direct reading stadiometer. Participants were measured without shoes and with the body positioned such that the heels and buttocks were against the vertical support of the stadiometer and the head aligned so that the auditory canal and lower rim of the orbit were in a horizontal plane. Weight was assessed to the nearest 0.1 kg using digital scales with participants wearing light clothing without shoes or coats. Two measures of height and weight were obtained and averaged.
Depressive Symptoms
The Beck Depression Inventory (BDI: 55) measures the severity of 21 depressive symptoms. The BDI has shown internal consistency (α = .73 to .95), test-retest reliability (r = .60 to .90), and convergent validity with clinician ratings of depressive symptoms (M r = .75; 55).
Anxiety Symptoms
Anxiety symptoms were measured with the Beck Anxiety Inventory (BAI: 56). The BAI consists of 21 items assessing the severity of anxiety symptoms. This scale has shown internal consistency (α = .92), test-retest reliability over 1 week (r = .75) and discrimintated anxious diagnosotic groups from nonanxious diagnostic groups (57).
Data Analysis
Data were analyzed with Matlab software (MathWorks, Inc., Sherborn, MA) using SPM5 (Wellcome Department of Cognitive Neurology, London, UK). The functional images were time-acquisition corrected to the slice obtained at 50% of the TR. All functional images were then realigned to the scan immediately preceding the anatomical T1 image. The images (anatomical and functional) were normalized to the Montreal Neurological Institute template (MNI-305), which approximates the anatomical space delineated by Talairach and Tournoux (58). Functional images were smoothed with a 7 mm FWHM isotropic Gaussian kernel. For time series analysis on all participants, a high-pass filter was included in the filtering matrix, per SPM5 convention, to remove low frequency noise and slow drifts in the signal, which could bias the estimates of the error.
Condition specific effects at each voxel were computed independently at each voxel for each subject, using the general linear model. The response to events (i.e. indicated by stimulus onsets) were modeled by a canonical hemodynamic response function (HRF) with temporal derivatives, consisting of a mixture of 2 gamma functions that emulate the early peak at 5 seconds and the subsequent undershoot. Our paradigm had 4 events of interest. The anticipatory aspect of the paradigm included the picture of milkshake and the picture of water that signal impending receipt of the solutions. For the consummatory aspect of the paradigm the 2 events included receipt of milkshake and tasteless solution. The temporal derivative of the hemodynamic function was also included as part of the basis set to enable examination of differences in timing between events (59).
Within and between-group comparisons were performed using a random effects model to account for inter-subject variability. SPM assigns significance t-fields from all analyses using the theory of Gaussian Random Fields (60-61). We conducted region of interest analyses using regions based on prior research on brain response to food tastes and/or food reward. These regions (limbic lobe, anterior cingulate, caudate, cingulate gyrus, fusiform gyrus, insula, medial frontal gyrus, middle frontal gyrus, parahippocampal gyrus, precentral gyrus, precuneus, superior frontal gyrus, thalamus) were selected using the WFU Pickatlas (62-63). Significant clusters had voxel continguity of at least 3 voxels.
Results
Descriptive and group differences
Participants who endorsed bulimic pathology reported an average of 10.8 binges per month for the prior 3 months (SD = 11.4, Range = 4-31.7). Overall, participants in the BN group reported engaging in some type of compensatory behavior an average of 10.9 times per month (SD = 8.2, Range = 4-31.7). Eight participants reported vomiting behavior, eight reported fasting, and nine reported excessive exercise. Participants reported vomiting on average 5.6 times per month (SD = 9.8, Range = 0-31.7), engaging in excessive exercise on average 3.4 times per month (SD = 3.7, Range = 0-9), and fasting on average 1.6 times per month (SD = 2.5, Range = 0-5.3). No participants reported laxative or diuretic use. No participants had a history of anorexia nervosa or binge eating disorder. Three participants in the BN group were taking SSRI medication (sertraline and fluoxetine) and controlling for SSRI use did not impact results.
We conducted independent samples t-tests to determine whether women with versus without bulimia nervosa differed on the measures (see Table 1). Women with bulimia nervosa reported higher depressive symptoms. Depression scores were negatively correlated with activation in the right precentral gyrus during anticipation and receipt of milkshake, but were not correlated with any other activation clusters, implying that differences in depressive symptoms were not operating as a confound. There were no group differences in age or ethnicity. In this sample, women with bulimia nervosa tended to be more left-handed than healthy controls, suggesting that laterality effects in brain response should be interpreted cautiously. There were no significant differences between the groups on the number of hours they had eaten prior to their fMRI scan, the amount of time since they last drank a chocolate milkshake, and the frequency with which they had chocolate milkshakes. There were also no significant differences between the pleasantness ratings of the chocolate milkshake and tasteless solution for the two groups. These data suggest that any differences in brain response to the milkshake are not related to acute food restriction, frequency of milkshake consumption, or pleasantness ratings.
Table 1.
Means, standard deviations, and independent sample t-tests on self-report measures
| Bulimia Nervosa (N = 13) M (SD) | Control (N = 13) M (SD) | t(24) | η 2 | |
|---|---|---|---|---|
| Depressive symptoms | 20.04 (10.30) | 6.08 (6.19) | -4.19 | .42* |
| Anxiety symptoms | 1.54 (.44) | 1.33 (.39) | -1.25 | .06 |
| BMI | 23.93 (2.82) | 23.19 (2.42) | -.712 | .02 |
Note:
p<.001
Anticipatory Food Reward Group Differences
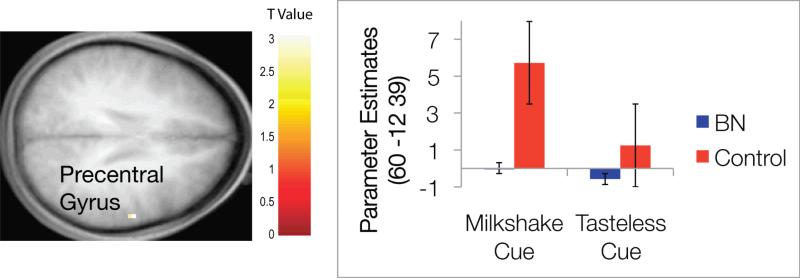
ANOVAs were conducted using SPM5 to determine whether group differences existed between brain activation in regions of interest to anticipating receipt of chocolate milkshake versus anticipating receipt of the tasteless control solution (the anticipatory food reward contrast). This contrast utilized the invalid trials (e.g., when the cue signaled subsequent delivery of chocolate milkshake, but no taste was delivered). This allowed us to separate the effect of anticipation from the effect of taste delivery. There was no main effect of bulimia nervosa diagnosis for this contrast significant at the p < .05 corrected level. There was one effect at the p = .005 uncorrected level; women with bulimia nervosa showed less activation in the right precentral gyrus (see Table 2 for a summary of brain areas and contrasts). Figure 1 shows the activation location and a graph of parameter estimates for each group and each type of cue. Although control participants showed an expected heightened activation of the precentral gyrus in response to the cue signaling chocolate milkshake delivery, and decreased activation in response to the cue signaling tasteless solution delivery, those with bulimia nervosa did not show marked change in this brain region between the two cue types.
Table 2.
Significant brain regions (MNI coordinates of cluster centers), cluster Z-score, and p-value for each contrast
| Contrast | x | y | z | Max Z | p | Brain Region |
|---|---|---|---|---|---|---|
| Anticipatory Reward | ||||||
| 60 | -12 | 39 | -3.22 | .001 | Right Precentral Gyrus | |
| Consummatory Reward | ||||||
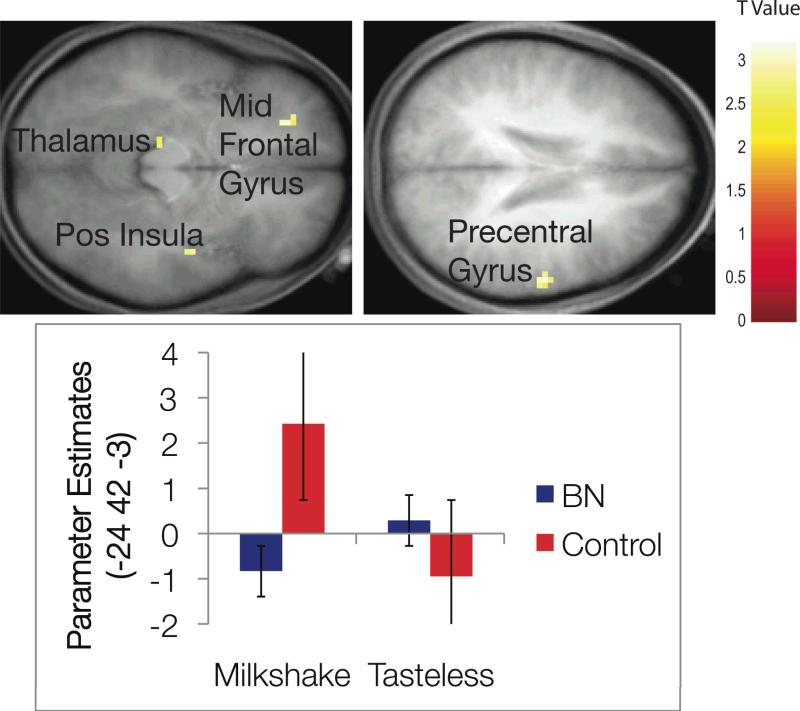
| 45 | -9 | -6 | -3.41 | <.001 | Right Posterior Insula | |
| 60 | -15 | 36 | -3.30 | <.001 | Right Precentral Gyrus | |
| -24 | 42 | -3 | -3.10 | .001 | Left Middle Frontal Gyrus | |
| -12 | -27 | -3 | -2.81 | .003 | Left Thalamus | |
Figure 1.
Results from the ANOVA model of anticipatory food reward. The color bar represents the t values representative for the figure. Axial sections of differential activation in the right precentral gyrus in response to anticipated receipt of chocolate versus tasteless control solution between the two groups. The bar graph represents relative activation in this region [60 -12 39] in response to anticipatory reward.
Consummatory Food Reward Group Differences
We conducted a similar ANOVA investigating group differences in response to milkshake receipt versus tasteless solution receipt (the consummatory food reward contrast). There was no main effect of bulimia nervosa diagnosis for this contrast significant at the p < .05 corrected level. There were four effects at the p < .005 uncorrected level; participants with versus without bulimic pathology showed less activation in the left middle frontal gyrus, right posterior insula, right precentral gyrus, and left thalamus. Echoing the pattern of findings for anticipatory reward, for each effect control participants showed the expected greater activation in these regions in response to milkshake receipt and less activation in response to tasteless solution receipt, whereas, those with bulimia nervosa did not show significant change in activation for these conditions. Figure 2 shows activation locations and a graph of parameter effects for one of the brain regions. Each brain region significant at the p < .005 uncorrected level showed the same pattern of activation for each group and condition.
Figure 2.
Results from the ANOVA model of consummatory food reward. The color bar represents the t values representative for all figures. Axial sections of differential activation between groups in the left middle frontal gyrus, right posterior insula, right precentral gyrus, and left thalamus in response to receipt of chocolate versus tasteless control solution. The bar graph represents relative activation in the left middle frontal gyrus [-24 42 -3] in response to consummatory reward. Results from other regions followed the same overall pattern of activation.
Discussion
As noted, no fMRI brain imaging study has examined activation in reward circuitry in response to actual food intake and anticipated intake during brain scans among individuals with current bulimic pathology versus healthy controls. This is important because brain activation in response to intake and anticipated intake may differ. Prior PET studies with individuals who have recovered from bulimia nervosa showed decreased brain response to taste of glucose (14-15), whereas other imaging studies suggest that individuals with bulimic pathology versus without show increased activation of reward circuitry to food images, which may tap anticipatory food reward. It is unclear whether the mixed findings emerged because some studies focused on recovered versus currently ill participants or because they examined activation in response to glucose administration versus pictures of foods. This preliminary study attempted to address these gaps in the literature by measuring fMRI brain response among women with and without bulimia nervosa while anticipating and receiving a hedonically pleasurable and calorically dense taste. This study also was novel in controlling for the effects of acute food restriction. Although prior studies have implemented a standard fast before scans, this study added a standardized snack consumed 1-hour prior to the scan. Based on prior findings, we hypothesized that women with versus without bulimic pathology will show blunted activation of reward circuitry to food receipt, but greater activation of this circuitry to anticipated receipt of food. Results suggest that women with bulimia nervosa show less response in the right precentral gyrus in both anticipatory and consummatory conditions relative to healthy controls and less response in the left middle frontal gyrus, left thalamus, and right posterior insula in the consummatory condition relative to healthy controls, though none of these effects were significant using the most conservative corrected .05 alpha level.
The thalamus is commonly considered a communication center between sensory areas and frontal regions, often sending information about pleasure and pain (64). Thus, it is implicated in reward processing. Additionally, the insula is considered a primary gustatory sensation region, and has been implicated in response to pleasurable taste (64). The middle frontal gyrus is activated in response to taste (65). The precentral gyrus is primary motor cortex, and activation in response could reflect preparation for swallowing. This area has been related to gustation in prior studies (64, 66).
On the one hand, it is surprising that women with bulimia nervosa would show weaker activation in gustatory and reward regions given the evidence from self-report scales and behavioral data that they show greater reward sensitivity (e.g., 8-9, 11) and the evidence that they show greater activation of reward circuitry in response to pictures of palatable foods (e.g., 36-37). On the other hand, these findings are consistent with evidence that individuals who have recovered from bulimia nervosa show blunted brain activation in response to tastes of a glucose solution (14-15). These results also converge with data indicating that obese relative to lean individuals show reduced activation of the dorsal striatum in response to consumption of a palatable food versus a tasteless solution (19-20). Perhaps repeated binge eating episodes results in decreased activation of gustatotory and reward circuitry and these individuals need to consume a large quantity of highly palatable foods to experience a strong sensation from food. This is a likely explanation due to the finding that women with bulimia nervosa did not appear to differentiate neurally between the milkshake and tasteless stimuli as much as the healthy controls. It may be that individuals with bulimia nervosa have down-regulation of dopamine receptors and change in opioid receptors after recurrent binge eating. This could, in turn, lead to continued binge eating in order to compensate for this reward deficit, as has been proposed by obesity research (e.g., 67). In line with this possible explanation, weight gain over 6-months was associated with reduced activation in the striatum in overweight women (68). Animal studies have found down-regulation of post-synaptic D2 receptors, increased D1 receptor binding, and decreased D2 sensitivity and μ-opioid binding after repeated intake of sweet and fatty foods (69-71). Thus, binge eating among women with bulimia nervosa could impact the brain function, as it does in animals, and serve to maintain the disorder. It is possible that reduced responsivity of gustatory and reward regions of the brain increase risk for both obesity and bulimia nervosa and that other factors, such as thin-ideal internalization, may lead an individual toward one outcome versus the other.
This study was the first imaging study of women with current bulimia nervosa to include a standardized snack prior to the imaging session, although a prior study of recovered participants utilized a glucose preload (15). Theoretically, hunger may result in heightened reward activation in response to pleasurable taste in all populations, so differences between women with and without bulimia nervosa may be more evident after a snack. This was not true in the current study, suggesting that it may be important for future studies to pay close attention to the time of last food intake prior to scan. Schienle and colleagues (37) instructed participants to fast overnight and found no difference in self-reported time of intake, but reported lower levels of blood glucose in women with bulimia nervosa, suggesting a longer fast than controls. Uher and colleagues (36) instructed participants to eat 3 hours prior to the scan, although patients with bulimia nervosa reported a longer duration of fast (M = 4.5 hours compared to M = 3.3 hours for the control group). Because it appears that women with bulimia nervosa may go longer between eating episodes, feeding them 1-hour prior to the brain scan may have attenuated differences in reward activity naturally present during a more deprived state in which binge eating may occur.
Another potential explanation for the abated results in this study compared to prior studies of women with current bulimic pathology is the difference in type of stimuli. It is possible that brain response to images of food, but no impending receipt of food taste is different among women with bulimia nervosa.
Although this study provides preliminary and novel findings about the functioning of gustatory and reward brain regions among women with a current diagnosis of bulimia nervosa in response to actual food intake rather than just food images, it is not without limitations. First, the small sample may have limited our power to detect effects in a number of brain regions. Although we discussed the effects with p < .005 uncorrected, no activations met our more stringent p < .05 corrected requirements. Further, the community-based sample and predominantly subthreshold level of bulimia nervosa in the sample may have limited our ability to detect differences between groups. Although the criteria used in this study were more conservative than those used in other studies of subthreshold bulimia nervosa (binge eating and compensatory behaviors occurring at least once per week for prior 3 months, as opposed to twice per month; e.g., 5), the inclusion of less frequent and severe cases may have diminished effects.
A second limitation is the cross-sectional design of the study. Although it is possible that abnormalities in food reward increase vulnerability to bulimic pathology, it is also possible that this eating disorder contributes to these abnormalities. Cross-sectional studies, like the current one, cannot differentiate between risk factors, causes, or consequences of a disorder. Although it seems logical to first investigate reward differences among women currently suffering from bulimia nervosa to know how to focus future work, prospective studies will be necessary to determine whether blunted responsivity of gustatory and reward circuitry to food intake and anticipated intake increase risk for future escalation in bulimic symptoms. Third, the brain response to a taste of chocolate milkshake or the anticipation of a taste is fundamentally different than the anticipation or experience of a full binge episode. Although women with bulimia nervosa showed blunted reward activation in response to these tastes in the current study, it is possible that they would show greater response to anticipation of an actual binge or to the binge experience.
In sum, results suggested that individuals with bulimic pathology may experience less activation in gustatory and reward regions during anticipation and receipt of palatable foods. This could suggest that women with bulimia nervosa are more similar to women with obesity in response to food reward, which may explain the tendency to engage in binge eating. As posited in the obesity literature, the blunted response could lead to greater amounts of food consumed in order to achieve the same level of satisfaction as healthy controls (67).
Future research should evaluate these findings prospectively in order to determine temporal precedence for decreased gustatory and reward activation and onset of bulimia nervosa. Finally, if these marginally significant findings are supported in larger samples and replications, addressing these abnormalities in treatment and prevention interventions may be important for alleviating this chronic and pernicious disorder.
Acknowledgments
This research was supported in part by a Ruth L. Kirschstein National Research Service Award (Award Number F31MH081588 from the National Institute of Mental Health). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health of the National Institutes of Health.
Footnotes
Financial Disclosure
The authors of this manuscript have no conflicts of interest regarding this publication.
References
- 1.Stice E, Bulik C. Eating disorders. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. Wiley and Sons; Hoboken, NJ: 2008. pp. 643–669. [Google Scholar]
- 2.Wilson GT, Becker CB, Heffernan K. Eating disorders. In: Barkley RA, Mash EJ, editors. Child psychopathology. 2nd ed. The Guilford Press; New York: 2003. pp. 687–715. [Google Scholar]
- 3.LeGrange D, Binford RB, Peterson CB, Crow SJ, Crosby RD, Klein MH, Bardon-Cone AM, Joiner TE, Mitchell JE, Wonderlich SA. DSM-IV threshold versus subthreshold bulimia nervosa. Int J Eat Disord. 2006;39:462–467. doi: 10.1002/eat.20304. [DOI] [PubMed] [Google Scholar]
- 4.Mond J, Hay P, Rodgers B, Owen C, Crosby R, Mitchell J. Use of extreme weight control behaviors with and without binge eating in a community sample: Implications for the classification of bulimic-type eating disorders. Int J Eat Disord. 2006;39:294–302. doi: 10.1002/eat.20265. [DOI] [PubMed] [Google Scholar]
- 5.Spoor ST, Stice E, Burton E, Bohon C. Relations of bulimic symptom frequency and intensity to psychosocial impairment and health care utilization: Results from a community-recruited sample. Int J Eat Disord. 2007;40:505–514. doi: 10.1002/eat.20410. [DOI] [PubMed] [Google Scholar]
- 6.Davis C, Strachan S, Berkson M. Sensitivity to reward: Implications for overeating and obesity. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Farmer RF, Nash HM, Field CE. Disordered eating behaviors and reward sensitivity. J Behav Ther Exper Psychiat. 2001;32:211–219. doi: 10.1016/s0005-7916(01)00036-2. [DOI] [PubMed] [Google Scholar]
- 9.Kane TA, Loxton NJ, Staiger PK, Dawe S. Does the tendency to act impulsively underlie binge eating and alcohol use problems? An empirical investigation. Pers Individ Dif. 2004;36:83–94. [Google Scholar]
- 10.Loxton NJ, Dawe S. How do dysfunctional eating and hazardous drinking women perform on behavioural measures of reward and punishment sensitivity? Pers Individ Dif. 2007;42:1163–1172. [Google Scholar]
- 11.Ahern AL, Field M, Yokum S, Bohon C, Stice E. Cognitive biases and reward sensitivity in dietary restraint. Appetite. Under Review. doi: 10.1016/j.appet.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Drewnowski A, Bellisle F, Aimez P, Remy B. Taste and bulimia. Physiol Behav. 1987;41:621–626. doi: 10.1016/0031-9384(87)90320-9. [DOI] [PubMed] [Google Scholar]
- 13.Sunday SR, Halmi KA. Taste perceptions and hedonics in eating disorders. Physiol Behav. 1990;113:173. doi: 10.1016/0031-9384(90)90196-b. [DOI] [PubMed] [Google Scholar]
- 14.Frank GK, Wagner A, Achenbach S, McConaha C, Skovira K, Aizenstein H, Carter CS, Kaye WH. Altered brain activity in women recovered from bulimic-type eating disorders after a glucose challenge: A pilot study. Int J Eat Disord. 2006;39:76–79. doi: 10.1002/eat.20210. [DOI] [PubMed] [Google Scholar]
- 15.Kaye WH, Frank GK, Meltzer CC, Price JC, McConaha CW, Crossan PJ, Klump KL, Rhodes L. Altered serotonin 2A receptor activity in women who have recovered from bulimia nervosa. Am J Psychiatry. 2001;158:1152–1155. doi: 10.1176/appi.ajp.158.7.1152. [DOI] [PubMed] [Google Scholar]
- 16.Bencherif B, Guarda AS, Colantuoni C, Ravert HT, Dannals RF, Frost JJ. Regional mu-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. J Nucl Med. 2005;46:1349–1351. [PubMed] [Google Scholar]
- 17.O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 18.Small DM, Jones-Gotman M, Dagher A. Feeding induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 19.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by the TAqIA1 gene. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stice E, Spoor S, Bohon C, Veldhuizen M, Small D. Relation of reward from food intake and anticipated intake to obesity: A functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roefs A, Herman CP, MacLeod CM, Smulders FT, Jansen A. At first sight: How do restrained eaters evaluate high-fat palatable foods? Appetite. 2005;44:103–114. doi: 10.1016/j.appet.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 23.Jansen A. A learning model of binge eating: Cue reactivity and cue exposure. Behav Res Ther. 1998;36:257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 24.Kiyatkin EA, Gratton A. Electrochemical monitoring of extracellular dopamine in nucleus accumbens of rats lever-pressing for food. Brain Res. 1994;652:225–234. doi: 10.1016/0006-8993(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 25.Schulz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC. Dopamine and preparatory behavior: II A neurochemical analysis. Behav Neurosci. 1989;103:15–23. doi: 10.1037//0735-7044.103.1.15. [DOI] [PubMed] [Google Scholar]
- 27.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: Food-craving activation during fMRI. NeuroImage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Small D, Veldhuizen M, Felsted J, Mak Y, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;57:786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karhunen LJ, Lappalainen RI, Vanninen EJ, Kuikka JT, Uusitupa MI. Regional cerebral blood flow during food exposure in obese and normal-weight women. Brain. 1997;120:1675–1684. doi: 10.1093/brain/120.9.1675. [DOI] [PubMed] [Google Scholar]
- 30.Mauler BI, Hamm AO, Weike AI, Tuschen-Caffier B. Affect regulation and food intake in bulimia nervosa: Emotional responding to food cues after deprivation and subsequent eating. J Abnorm Psychol. 2006;115:567–579. doi: 10.1037/0021-843X.115.3.567. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell JE, Hatsukami D, Pyle RL, Eckert ED. Characteristics of 275 patients with bulimia. Am J Psychiatry. 1985;142:482–485. doi: 10.1176/ajp.142.4.482. [DOI] [PubMed] [Google Scholar]
- 32.Bulik CM, Lawson RH, Carter FA. Salivary reactivity in restrained and unrestrained eaters and women with bulimia nervosa. Appetite. 1996;27:15–24. doi: 10.1006/appe.1996.0030. [DOI] [PubMed] [Google Scholar]
- 33.Staiger P, Dawe S, McCarthy R. Responsivity to food cues in bulimic women and controls. Appetite. 2000;35:27–33. doi: 10.1006/appe.2000.0327. [DOI] [PubMed] [Google Scholar]
- 34.Legenbauer T, Vogele C, Ruddel H. Anticipatory effects of food exposure in women diagnosed with bulimia nervosa. Appetite. 2004;42:33–40. doi: 10.1016/S0195-6663(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 35.LeGoff D, Leichner P, Spigelman M. Salivary responses to olfactory food stimuli in anorexics and bulimics. Appetite. 1988;11:15–25. doi: 10.1016/s0195-6663(88)80018-7. [DOI] [PubMed] [Google Scholar]
- 36.Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW, Andrew CM, Williams SCR, Campbell IC, Treasure J. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. 2004;161:1238–1246. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- 37.Scheinle A, Schäfer A, Herman A, Vaïtl D. Binge-eating disorder: Reward sensitivity and brain activation to images to food. Biol Psychiatry. 2009;65:654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 38.Rothemund Y, Preuschhof C, Bohner G, Bauknecht H, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: Moderating effects of DRD2 and DRD4. NeuroImage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JF. Widespread reward-system activation in obese women in response to pictures of high-caolorie foods. NeuroImage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Stice E, Telch CF, Rizvi SL. Development and validation of the eating disorder diagnostic scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol Assess. 2000;12:123–131. doi: 10.1037//1040-3590.12.2.123. [DOI] [PubMed] [Google Scholar]
- 42.Bartoshuck LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: Problems, solutions, and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2006;361:1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parrish TB, Gitelman DR, LaBar KS, Mesulam MM. Impact of signal-to-noise on functional MRI. Magn Reson Med. 2000;44:925–932. doi: 10.1002/1522-2594(200012)44:6<925::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 44.Small DM, Gregory MD, Mak YE, Gitelman DR, Mesulam MM, Parrish TB. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;19:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- 45.Veldhuizen MG, Bender G, Constable RT, Small DM. Tasting in the absence of taste: Modulation of early gustatory cortex by attention to taste. Chem Senses. 2007;32:569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- 46.Jezzard P, Balaban RS. Correction for geometric distortion in echoplanar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- 47.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 48.Fairburn CG, Cooper Z. The eating disorder examination. In: Fairburn CG, Wilson GT, editors. Binge eating: nature, assessment, and treatment. 12th ed The Guilford Press; New York: 1993. pp. 317–331. [Google Scholar]
- 49.Stice E, Fisher M, Martinez E. Eating disorder diagnostic scale: Additional evidence of reliability and validity. Psychol Assess. 2004;16:60–71. doi: 10.1037/1040-3590.16.1.60. [DOI] [PubMed] [Google Scholar]
- 50.Zanarini MC, Frankenburg FR. Attainment and maintenance of reliability of axis I and axis II disorders over the course of a longitudinal study. Compr Psychol. 2001;42:369–374. doi: 10.1053/comp.2001.24556. [DOI] [PubMed] [Google Scholar]
- 51.Zanarini MC, Skodol AE, Bender D, Dolan R, Sanislow C, Schaefer E, Morey LC, Grilo CM, Shea MT, McGlashan TH, Gunderson JG. The collaborative longitudinal personality disorders study: Reliability of axis I and II diagnoses. J Pers Disord. 2000;14:291–299. doi: 10.1521/pedi.2000.14.4.291. [DOI] [PubMed] [Google Scholar]
- 52.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 53.Schacter SC. Studies of handedness and anomalous dominance. In: Galaburda AM, editor. Dyslexia and development. Harvard University Press; Cambridge, MA: 1993. pp. 269–296. [Google Scholar]
- 54.Stice E, Marti N, Shaw H, Jaconis M. An 8-year longitudinal study of the natural history of threshold, subthreshold, and partial eating disorders from a community sample of adolescents. J Abnorm Psychol. 2009;118:587–597. doi: 10.1037/a0016481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beck AT, Steer RA, Garbin M. Psychometric properties of the beck depression inventory: 25 years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 56.Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. The Psychological Corporation; San Antonio, TX: [Google Scholar]
- 57.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric propoerties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 58.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- 59.Henson RNA, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: Application to words versus nonwords and initial versus repeated face presentations. NeuroImage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- 60.Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- 61.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisted – again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 62.Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 63.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the talairach atlas. NeuroImage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 64.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating choclate. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 65.Kringelbach ML, de Araujo IET, Rolls ET. Taste-related activity in the human dorsolateral prefrontal cortex. NeuroImage. 2004;21:781–788. doi: 10.1016/j.neuroimage.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 66.Barry MA, Gatenby JC, Zeiger JD, Gore JC. Hemispheric dominance of cortical activity evoked by focal elecrogustatory stimuli. Chem Senses. 2001;26:471–482. doi: 10.1093/chemse/26.5.471. [DOI] [PubMed] [Google Scholar]
- 67.Wang G, Volkow N, Logan J, Pappas N, Wong C, Zhu W, Netusil N, Fowler J. Brain dopamine and obesity. Lancet. 2001;257:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 68.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.2105-10.2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport. 2002;13:1557–1578. doi: 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 71.Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18:2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]