Abstract
The detection of low-abundance DNA variants or mutations is of particular interest to medical diagnostics, individualized patient treatment and cancer prognosis; however, detection sensitivity for low-abundance variants is a pronounced limitation of most currently available molecular assays. We have recently developed coamplification at lower denaturation temperature-PCR (COLD-PCR) to resolve this limitation. This novel form of PCR selectively amplifies low-abundance DNA variants from mixtures of wild-type and mutant-containing (or variant-containing) sequences, irrespective of the mutation type or position on the amplicon, by using a critical denaturation temperature. The use of a lower denaturation temperature in COLD-PCR results in selective denaturation of amplicons with mutation-containing molecules within wild-type mutant heteroduplexes or with a lower melting temperature. COLD-PCR can be used in lieu of conventional PCR in several molecular applications, thus enriching the mutant fraction and improving the sensitivity of downstream mutation detection by up to 100-fold.
Keywords: cancer, coamplification at lower denaturation temperature, COLD-PCR, denaturation temperature, low-abundance mutations, variant and mutation enrichment
In cancer, molecular profiling and disease staging for prognosis, determining personalized treatment and therapy, the assessment of residual disease post-treatment, and the monitoring of therapy outcome and remission/relapse all require accurate identification of DNA mutations and DNA variants. However, clinical and diagnostic applications are often limited by accuracy and sensitivity when clinically significant mutations and minority alleles are present at a low abundance relative to the wild-type component of a clinical specimen. A particular variant or mutant may confer a selective advantage (a driver mutation) or may be carried through in a neutral manner by linkage processes (passenger mutation) throughout disease progression, tumor evolution and metastasis [1–6]. Many of these mutations may easily be detected at a late stage as a clonal mutation (present in frequencies as low as 100−1 in wild-type DNA); however, these variants are much more difficult to identify at an early disease stage when present subclonally (100−1–100−3 as a ratio to wild-type), randomly (100−3–100−6) and spontaneously (100−6–100−8), and especially when the mutation type and position are unknown.
Heterogeneity and mosaicism of mutations within tumors and certain syndromes have been well documented [7–9]. The ability to accurately examine this heterogeneity remains difficult. Furthermore, the difficulty in discerning low-abundance unknown mutations is especially pronounced in heterogeneous specimens from precancerous or cancerous tissue biopsies, sputum, urine, stool and circulating extracellular DNA released in the blood. Similarly, as cancer cells are typically heterogeneous in infiltrating and multifocal cancer types, they will be present at low abundances among an excess of normal cells [10–12]. Tumors with high stromal contamination, such as those found in pancreatic, lung or prostate cancer, often contain mutations that are obscured by the relatively large abundance of wild-type alleles [4,10,13–15], and thus require laborious microdissection to isolate the tumor cells prior to performing molecular analyses. However, microdissection approaches are often expensive, time-consuming and yield small amounts of DNA for downstream evaluation. As such, the detection and identification of minority alleles present at low abundance in heterogeneous specimens remains challenging, and the resulting accuracy for the detection of cancer-related somatic mutations is largely dependent upon the sensitivity and selectivity of the molecular techniques and methods employed.
The significance of identifying these low-abundance mutations is critical in several fields of medicine, including cancer [16,17], genetic and infectious diseases [9], and prenatal diagnosis [18]. For example, in non-small-cell lung cancer, low-abundance EGFR mutations (T790M) may confer drug resistance to tyrosine kinase inhibitors [13,19]. If these mutations exist at a low-abundance, they may be overlooked, thus detrimentally compromising the patient’s therapy. Similarly, mutations in the plasma of colorectal cancer patients often cannot be identified via sequence analysis using conventional methods; however, biomarkers useful for monitoring tumor evolution and treatment response may be present [20,21].
The polymerase chain reaction is often utilized as the basis of most molecular applications that investigate DNA sequence variation. Unless specifically modified, PCR will amplify all alleles with approximately equal efficiency, comparable to their initial concentrations. As such, when analyzing these specimens within which the variant DNA can exist at low abundance in the presence of a large majority of wild-type alleles, the ability to identify the mutation is dependent upon the sensitivity of downstream assays, such as Sanger sequencing, among many others. Sanger sequencing is typically reliable for screening germline or prevalent (clonal) somatic mutations, and is widely available; however, the sensitivity of mutation detection is typically limited to detecting approximately 10–20% mutant fractions [22]. PCR-blocking approaches (using standard oligonucleotides or those containing peptide nucleic acid or locked nucleic acid) or sequencing-by-synthesis (pyrosequencing) approaches have the ability to increase sensitivity for mutational detection, however the region available for analysis is often quite limited (10–30 bp). Screening for specific DNA biomarkers of interest notably alleviates some of the impediments associated with sensitivity and selectivity; however, it remains quite difficult to screen across a mutation spectrum that may contain many novel or unknown mutations [23].
COLD-PCR
To alleviate the limitations discussed above, we developed a novel PCR-based application – coamplification at lower denaturation temperature (COLD)-PCR [24], that preferentially enriches minority alleles from mixtures of wild-type and mutation-containing sequences, irrespective of mutation type and location within the amplicon. COLD-PCR amplification yields amplicons containing enriched proportions of mutant (or variant) alleles, thus permitting the discrete detection and identification of minority alleles by downstream applications.
As PCR is typically an integral step in most genetic analyses, COLD-PCR can be used in place of PCR as a fundamental platform to improve the sensitivity of downstream or combinatorial technologies, including techniques such as Sanger sequencing, pyrosequencing, next-generation sequencing, mutation scanning and mutation genotyping. COLD-PCR is one of relatively few methods that are capable of simultaneously enriching both known and unknown mutations [23]; as such, COLD-PCR is highly advantageous owing to its ability to enrich nearly all low-abundance mutations, regardless of whether they are known or unknown mutations [24,25].
The unique attribute of COLD-PCR is that the selective enrichment of low-abundance mutations (or variants) within a target amplicon is achieved by exploiting a small, but critical and reproducible, difference in amplicon melting temperature (Tm). A single nucleotide variation or mismatch at any position along a double-stranded DNA sequence changes the amplicon Tm. The Tm for amplicons up to 200 bp in length may vary by approximately 0.2–1.5°C and is dependent on the sequence composition [26]. Just below the Tm there is a critical denaturation temperature (Tc) wherein PCR efficiency drops abruptly as a result of the limited number of denatured amplicons. This difference in PCR efficiency, at specifically defined denaturation temperatures, can be used to selectively enrich minority (or low-abundance mutant) alleles throughout the course of PCR.
COLD-PCR platforms
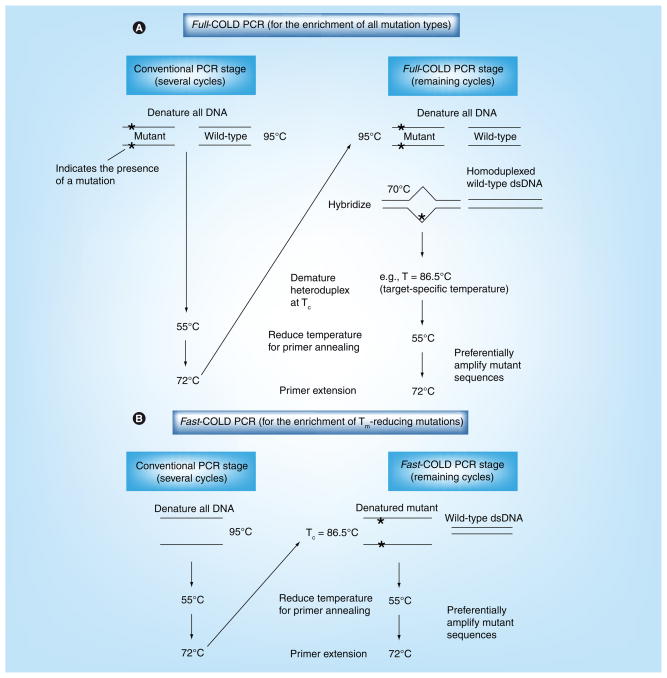
Coamplification at lower denaturation temperature-PCR was initially developed using two approaches, full-COLD-PCR and fast-COLD-PCR [24]; the protocol schematic for each of these two COLD-PCR platforms is illustrated in Figure 1. In full-COLD-PCR, a five-step PCR protocol is performed, which includes: a standard denaturation step; a hybridization step; a critical denaturation step at the defined Tc ; a primer annealing step; and an extension step. The intermediate hybridization step (70°C) is used during PCR cycling to allow hybridization of mutant and wild-type alleles (Figure 1A). Heteroduplexes, which melt at lower temperatures than homoduplexes in almost all cases [27], can be selectively denatured using an amplicon-specific Tc and preferentially amplified throughout the course of PCR; conversely, the denaturation efficiency is reduced for homoduplex molecules, and as such, the majority of the molecules will remain in a double-stranded homoduplex state throughout the course of thermocycling. The efficiency of amplification of the major alleles (typically wild-type) is therefore appreciably reduced. However, by decreasing the denaturation temperature to the Tc, mutations at any position along the sequence are preferentially enriched during COLD-PCR amplification.
Figure 1. Coamplification at lower denaturation temperature-PCR protocol.
Two original forms of COLD-PCR were developed as full-COLD-PCR (A) and fast-COLD-PCR (B). (A) Full-COLD-PCR has the potential to enrich all possible mutations. Several preliminary rounds of conventional PCR enable an initial increase of the target amplicon(s). After denaturation at approximately 95.0°C (or as defined by the polymerase system), the PCR amplicon(s) are incubated (e.g., 70.0°C for 2–8 min) for re-annealing and hybridization. Hybridization of mutant and wild-type alleles forms heteroduplexed molecules (mismatch-containing) that possess a lower Tm than homoduplexed molecules. The PCR temperature is subsequently increased to the Tc (e.g., Tc = 86.5°C) to preferentially denature the heteroduplexed amplicons. The temperature is reduced for primer annealing (e.g., 55.0°C), and then increased to 72.0°C for primer extension, thus preferentially amplifying the mutation-containing alleles. (B) Fast-COLD-PCR can be performed to enrich mutations with melting temperatures lower than the wild-type amplicon. Denaturation at the Tc (rather than the standard 95.0°C) preferentially denatures the strands containing the lower Tm allele; this generates single-stranded DNA for primer annealing and extension.
COLD: Coamplification at lower denaturation temperature; dsDNA: Double-stranded DNA; Tc: Critical denaturation temperature; Tm: Melting temperature.
In fast-COLD-PCR the intermediate hybridization temperature step is not necessary and a three-step thermocycling protocol is performed (denaturation, primer annealing and polymerase extension). However, denaturing the amplicons at the Tc will amplify molecules containing Tm-reducing variants (such as G:C>A:T or G:C>T:A mutations) (Figure 1B); in such cases, the Tm of the mutant-containing homoduplexes is lower than that of the wild-type sequence. Full-COLD-PCR requires more time for completion than conventional PCR owing to the inclusion of the hybridization step. Both the full- and fast-COLD-PCR platforms require typical reagent concentrations and the conditions used in conventional PCR. The decision to perform full- or fast-COLD-PCR depends on the type of mutations to be studied and the aim of the project.
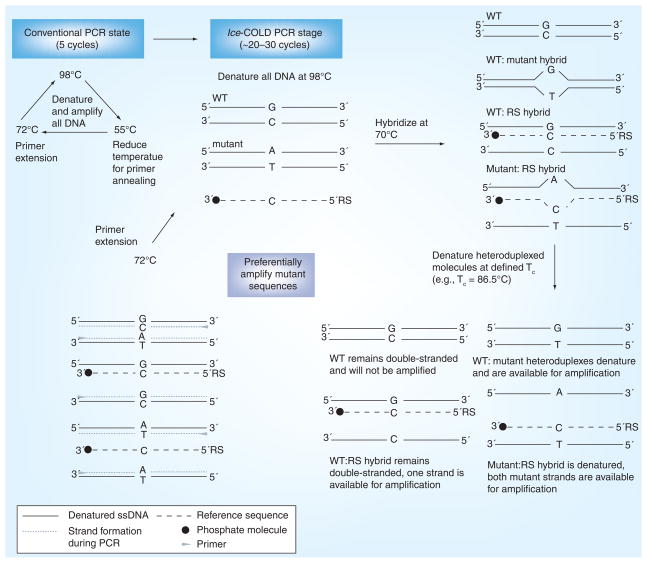
While full-COLD-PCR has the advantageous ability to enrich all mutation types, the enrichment may be modest and the original amplification protocol is time-intensive owing to the hybridization step. In comparison, fast-COLD-PCR can be performed rapidly and with robust enrichment; however, the disadvantage of this platform is that only Tm-reducing mutations can be enriched throughout the course of amplification. To combine the attributes of both original platforms of COLD-PCR, a new platform, ice-COLD-PCR, was recently designed. This platform has been coined ice-COLD-PCR as it provides improved and complete enrichment of all mutation types [28]. In ice-COLD-PCR, we have shortened the hybridization times, improved the enrichment potential and optimized the approach, such that all mutation types can be robustly enriched. To accomplish this, we have incorporated a synthetic, single-stranded, wild-type-specific oligonucleotide reference sequence (RS), which binds to the wild-type template and inhibits its amplification. The oligonucleotide RS contains a 3′-phosphate modification to prevent polymerase extension. The RS is slightly shorter than the length of the PCR amplicon so that it obstructs primer binding (by limiting primer overlap to up to five nucleotides) and prevents amplification of the wild-type alleles. We recommend the use of a high-fidelity polymerase (such as Phusion® [Finnzymes Inc.]) that lacks 5′- to 3′-exonuclease activity to simultaneously inhibit PCR errors and prevent potential problems by hydrolysis of the RS. A schematic for the ice-COLD-PCR platform is presented in Figure 2. Using a five-step thermocycling program similar to that of full-COLD-PCR, the RS inhibits the amplification of the wild-type alleles, while the use of a lower denaturation temperature preferentially enriches low-abundance mutant and variant alleles. In addition, through the use of the Phusion polymerase system, we found that the hybridization time could be considerably shortened (to 30 s) from the original 5–8 min hybridization time performed in full-COLD-PCR. We attribute this shortened time requirement to the high salt concentration present in the manufacturer-supplied high-fidelity buffer, which promotes the hybridization rate at the intermediate step of COLD-PCR. However, owing to the proprietary status of the buffer, the chemical composition remains unknown and we are unable to validate this conclusion.
Figure 2. Ice-coamplification at lower denaturation temperature-PCR protocol schematic.
Ice-COLD-PCR has the potential to enrich all possible mutations and also provides a higher enrichment than full-COLD-PCR. Ice-COLD-PCR is performed in a nested format here (i.e., using a larger PCR amplicon as a template). An initial few rounds of nested conventional PCR are first applied to enable build-up of the target amplicon(s). After denaturation at approximately 98.0°C (or as defined by the polymerase system), the PCR amplicon(s) and the oligonucleotide reference sequence are hybridized at 70.0°C. Mutant-containing heteroduplexed molecules (mismatch-containing) will be denatured at the Tc, and preferentially enriched throughout the course of ice-COLD-PCR.
COLD: Coamplification at lower denaturation temperature; RS: Reference sequence; Tc: Critical denuration temperature; WT: Wild-type. Data from [28].
As with all mutational assays, it is preferable to use high-quality DNA in each assay. As COLD-PCR is designed to enrich minor-variants throughout the course of PCR, any artifacts or contamination within the specimen may either interfere with or be enriched by the reaction. For example, the preservation of tumor specimens through formalin-fixed paraffin-embedding may result in DNA damage that, in principle, can lead to false-positives. Nevertheless, COLD-PCR works best with small-size amplicons, which is compatible with DNA encountered in degraded or formalin-fixed, paraffin-embedded (FFPE) specimens. Recent comparison of frozen and FFPE specimens amplified using COLD-PCR revealed concordance among snap-frozen and FFPE specimens [29].
Anticipated mutation enrichment
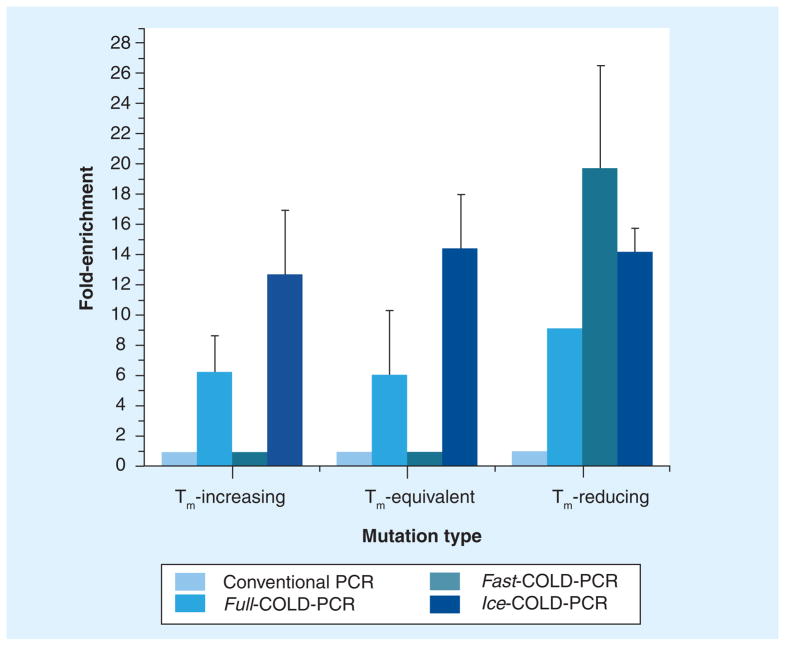
Each of the COLD-PCR platforms (fast-, full- and ice-COLD-PCR) have been evaluated in a variety of scenarios. A recent analysis compared the enrichment potential for each of the different PCR platforms using a 3% mutant mixture for an 87 bp PCR amplicon [28]; our findings for a Tm-equivalent mutation (G>C) are presented in Figure 3. As exhibited in Figure 3, there is no inherent potential for preferential enrichment in conventional PCR. Enrichment by full-COLD-PCR is possible for each mutation type; however, enrichment may be modest relative to the other COLD-PCR platforms, typically exhibiting approximately 5–7-fold enrichment for mutations in this amplicon. Fast-COLD-PCR demonstrates the highest enrichment potential for Tm-reducing mutations (~20-fold); however, it remains unable to enrich both Tm-equivalent and Tm-increasing mutations. Propitiously, ice-COLD-PCR demonstrates, such as full-COLD-PCR, that it can enrich all mutation types, however, its enrichment potential is greater (~13–15-fold). Another tactic that has been successful at improving enrichment is to perform two consecutive rounds of COLD-PCR reactions, wherein products from the first amplification are diluted and used as a template for a second COLD-PCR amplification reaction [8,30–32].
Figure 3. Enrichment observed in amplicons produced by conventional PCR, full-COLD-PCR, fast-COLD-PCR and ice-COLD-PCR.
Data are presented for approximately 3% mutation abundance of Tm-increasing, Tm-equivalent and Tm-reducing mutations.
COLD: Coamplification at lower denaturation temperature; Tm: Melting temperature. Reproduced with permission from [28].
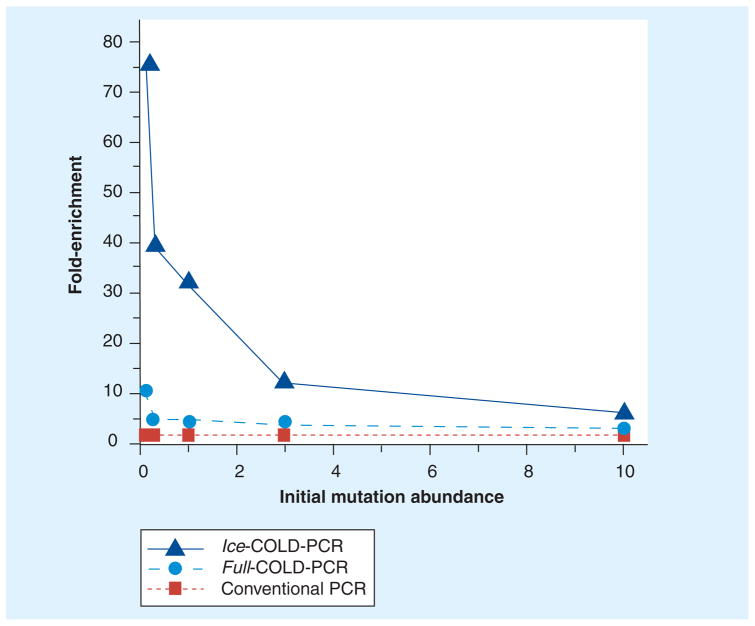
Throughout our evaluation of mutation enrichment by COLD-PCR platforms, we have observed a trend for the mutation enrichment related to the initial abundance of the mutation or variant fraction. As presented in Figure 4, we have determined that the degree of mutation enrichment is not only highly dependent upon platform type and mutant type, but it is also influenced by the initial starting abundance of the mutation [28]. Thus, in COLD-PCR platforms that exhibit lower starting mutation abundances (i.e., 0.1% mutant) there is a greater enrichment than there is for the relatively higher mutant fractions (i.e., 10% mutant).
Figure 4. Enrichment potential for three PCR amplification strategies: conventional PCR, full-COLD-PCR and ice-COLD-PCR.
Enrichment is inversely related to initial mutation abundance for a melting temperature-equivalent (G>C) mutation, whereas higher enrichment potential is exhibited in lower mutation abundances. COLD: Coamplification at lower denaturation temperature.
Data taken from [28].
Designing a COLD-PCR reaction
Coamplification at lower denaturation temperature-PCR is relatively simple in its design and application, although each amplicon requires some optimization and validation. Primer design for COLD-PCR follows the same general rules applied in conventional PCR, requiring high accuracy to avoid mispriming and nonspecific amplification (such as pseudogenes); however, each primer set should be examined empirically such that the annealing temperature does not exceed 65.0°C in the full- or ice-COLD-PCR platforms. If primers anneal during the 70.0°C hybridization step, they will interfere with heteroduplex formation and limit enrichment. Mutation enrichment via COLD-PCR is most efficient for relatively short amplicons (<200 bp); presumably because singlebase mismatches have a larger impact on the Tm within relatively short PCR products [26]. Depending upon sequence composition and the mutation position, the degree of enrichment may vary; however, in all cases appreciable enrichment can be achieved and substantially improve the detection limits of downstream assays.
In general, it is recommended that the PCR amplicon possesses a single melting domain to ensure equivalent enrichment efficiency along the length of the amplicon. The amplicon melting profile can be predicted from DNA melting software, such as MeltSim [33,101] or the Poland algorithm [34,102]. The presence of a single melting domain can be confirmed experimentally via post-PCR melting profiles generated by real-time thermocyclers.
The Tm of the amplicon can also be determined from a post-PCR real-time melting curve analysis. A real-time PCR should be performed using standard thermocycling conditions and reagents, including a fluorescent dye; wild-type DNA (or the major allele) should be used as the template for the determination of amplicon Tm. The Tm typically varies between polymerase systems owing to different buffer composition, as well as the dyes that are used; one should always re-evaluate the Tm if new polymerase systems or fluorescent dyes are to be used.
To experimentally determine the appropriate Tc of an amplicon, DNA containing known mutations, either from cell lines or clinical samples, can be diluted with wild-type DNA to a 1:10 ratio. The optimization of the Tc should be performed in a step-wise fashion; for example, the following denaturation temperatures can be tested: standard denaturation temperature for the polymerase system (typically ~95°C); amplicon Tm; amplicon Tm-0.5°C; amplicon Tm-1.0°C; and amplicon Tm-1.5°C. Generally, Sanger sequencing can be used to determine the degree to which the mutant allele has been enriched. The denaturation temperature that reproducibly produces good quality PCR amplicons with strong enrichment should be selected as the Tc. To date, optimization of our COLD-PCR reactions has revealed that a Tc at 1.0°C below the Tm typically produces high-quality PCR amplicons with strong mutant enrichment.
Integrating COLD-PCR with current molecular methodologies
To assess COLD-PCR enrichment, many different assays can be applied, either in combination with COLD-PCR (real-time COLD-PCR [35]) or downstream, in order to analyze enriched genetic variations. The assay of choice will depend upon the sensitivity of the detection that is required and upon the availability of the application’s equipment. Suggested applications include, but are not limited to, the following approaches: Sanger sequencing, pyrosequencing, restriction fragment length polymorphism analysis, MALDI-TOF genotyping, denaturing high-performance liquid chromatography, TaqMan® real-time PCR, and high-resolution melting (HRM) analysis.
Sequence-based applications are highly advantageous approaches for qualitative identification of both known and unknown mutations, as well as methodological validation of enrichment achieved through COLD-PCR amplification. As previously mentioned, while Sanger sequencing is convenient, the conventional sensitivity of mutation detection is typically limited to detecting approximately 10–20% mutant fractions. Similarly, pyrosequencing can be applied for the identification of both known and unknown mutation scanning. While using conventional methods, the detection sensitivity of pyrosequencing is limited to approximately 5–10% mutant in wild-type DNA [36]. Despite the increased sensitivity that this approach provides, the equipment and reagents are expensive, sequence reads are relatively short and it is not widely available. For both sequence-based approaches, COLD-PCR routinely demonstrates the ability to identify much lower mutant fractions, down to 0.1% (Figures 5A & B), however, the enrichment potential is highly dependent upon the type of mutation being analyzed, the initial mutation abundance and the COLD-PCR platform utilized [8,24,28,37].
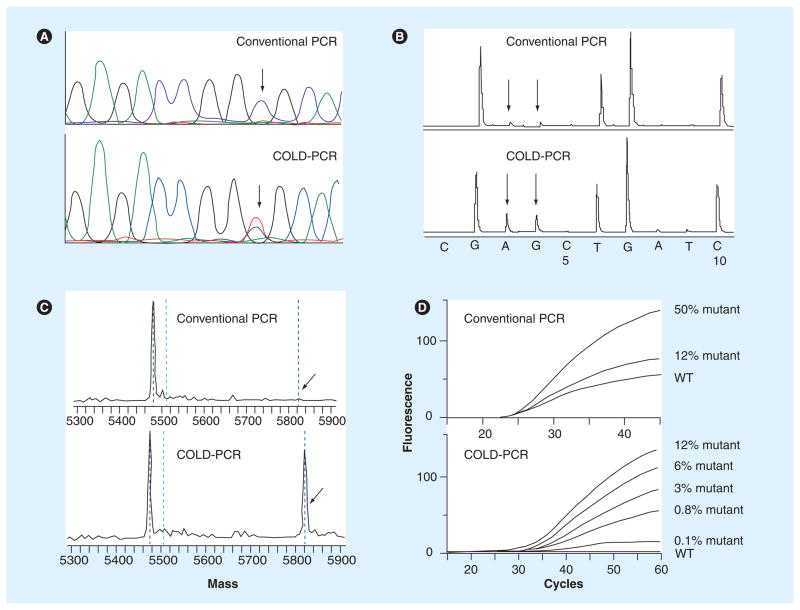
Figure 5. COLD-PCR improves mutation detection in downstream assays.
(A) Sanger sequencing detects low-abundance mutations after COLD-PCR. A 10% abundance of a C>T mutation in WT DNA was amplified by both conventional PCR and COLD-PCR. Sanger sequence chromatograms were evaluated for both approaches, respectively (conventional PCR is presented in the upper panel; COLD-PCR is presented in the lower panel); an approximate sixfold mutation enrichment by COLD-PCR is evident. (B) COLD-PCR improves detection via pyrosequencing. A 33-fold dilution of a G>A mutation in WT DNA was amplified by both conventional and COLD-PCR. The mutant is only visible in the COLD-PCR amplicons. (C) COLD-PCR improves the sensitivity of MALDI-TOF genotyping technologies. A G>A mutation was amplified by both conventional and COLD-PCR, and amplicons were genotyped using MALDI-TOF. The G>A mutation was undetectable in conventional PCR amplicons (upper panel); however, it was detectable in COLD-PCR amplicons (lower panel). (D) COLD-PCR improves the sensitivity of TaqMan® genotyping technologies [35]. Serial dilutions of the human H1975 cell line (containing the T790M mutation in EGF receptor exon 20) in WT DNA were screened with conventional and COLD-PCR TaqMan genotyping for the T790M mutation. Conventional PCR TaqMan genotyping for the T790M mutation is presented in the upper panel; COLD-PCR TaqMan genotyping for the T790M mutation is presented in the lower panel.
COLD: Coamplification at lower denaturation temperature; WT: Wild-type.
Many genotyping approaches can be combined with COLD-PCR to analyze known mutations. For example, mass spectrometry- based MALDI-TOF genotyping technologies can be applied in a high-throughput manner. For analyzing conventional PCR amplicons, the detection sensitivity is limited to approximately 5% mutant in wild-type DNA [38]. Our results indicate that mutation enrichment of approximately 10- to 100-fold can be achieved by replacing conventional PCR with fast-COLD-PCR prior to MALDI-TOF (Figure 5C), thus suggesting that somatic mutations originally at frequencies of as little as 0.1–0.5% in a mixture of wild-type DNA can be detected [24].
Similarly, for known mutations, we have demonstrated that a 0.3% mutant mix, amplified by either fast- or full-COLD-PCR exhibits approximately 6- to 11-fold mutant enrichment and, when subjected to digestion by a restriction enzyme (for restriction fragment length polymorphism analysis), the mutant fraction can be easily identified using denaturing high- performance liquid chromatography analysis [24]. In comparison, a 10% mutant mix amplified by conventional PCR remains difficult to identify [24]. Another common method for analyzing and quantifying known mutations is real-time PCR using TaqMan probes. While the current mutation detection sensitivity limit is approximately 10% mutant among wild-type alleles [39,40], we have demonstrated an improvement in method sensitivity by incorporating COLD-PCR in place of conventional PCR. We present data for TaqMan amplification that demonstrates the ability to detect a 0.8% mutant abundance among wild-type alleles with one round of real-time COLD-PCR (Figure 5D). A recent study revealed the detection of one mutant allele in a mixture of 2000 wild-type alleles after enrichment by two consecutive rounds of fast-COLD-PCR, with each round of COLD-PCR resulting in 15- to 30-fold enrichment of the T790M EGF receptor resistance mutation in lung cancer [35].
High-resolution melting is another approach that we have recently evaluated as it is advantageous for the detection and enrichment of both known and unknown mutations and variants. HRM is a highly sensitive and high-throughput approach for mutation scanning [41,42]. Post-PCR melting can be completed in just a few minutes; aberrant melting profiles are indicative of a mutant sequence. While this approach is highly sensitive and often able to detect a mutant fraction (generally 3–10% depending upon the nature of the amplicon) there still remains an inability to specifically identify unknown mutations after an aberrant melting profile has been detected. We have demonstrated that applying COLD-PCR in place of conventional PCR improves the detection sensitivity of HRM screening, allowing for the detection of mutant mixtures at frequencies of as little as 0.1–1.0% (Figure 6) [37]. Recent applications of COLD-PCR combined with HRM have revealed comparable levels of mutation enrichment and improved levels of mutation detection sensitivity [30–32,43].
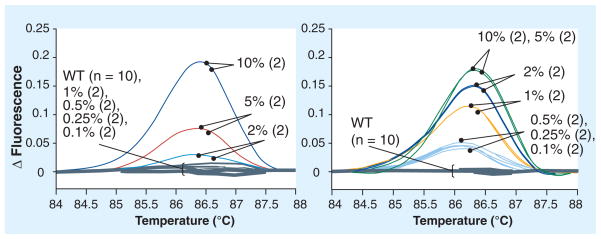
Figure 6. High-resolution melt analysis of TP53 exon 6 amplification products produced via conventional and coamplification at lower denaturation temperature (COLD)-PCR.
The amplicons were produced from genomic DNA serial dilutions of the human cell line SNU-182 (c.644G>T, p.S215I) in WT DNA. While the 2% mutant abundance is the lower limit of detection in the conventional PCR amplicons, COLD-PCR enriches the mutant fraction so that an initial mutant abundance as low as 0.1% can now be detected.
WT: Wild-type.
Data taken from [37].
We are continually evaluating the incorporation of COLD-PCR with molecular diagnostic applications for increased sensitivity in minor allele detection, with a particular interest in identifying unknown mutations. Simultaneously, we are developing new COLD-PCR approaches to improve the degree of mutation enrichment throughout the course of COLD-PCR.
Expert commentary
DNA mutations present at low abundance in clinical specimens contain profuse amounts of diagnostic and prognostic information in fields such as cancer, prenatal diagnosis and infectious diseases. For example, in clinical samples from infiltrating and multifocal cancer types, mutation-containing cancer cells are greatly outnumbered by an excess of normal cells. Low-abundance DNA mutations in heterogeneous specimens from precancerous or cancerous tissue biopsies, sputum, urine, stool or circulating DNA released in blood, can cause drug resistance and can be clinically significant biomarkers of disease progression. However, utilizing the clinical and diagnostic potential of such rare mutations is often limited by the accuracy and sensitivity of the molecular techniques and by the methods employed. To date, these variants have been difficult to detect, especially when their discrete identification is unknown. Several methods can enrich low-abundance mutations at predetermined positions during PCR amplification; however, when the position and type of such mutations on the DNA sequence is unknown a priori there are very few approaches that can enrich these unknown mutations so that they can be identified by downstream technologies [23].
Coamplification at lower denaturation temperature-PCR resolves many of these current limitations. Few other methodologies have been developed, despite increasing interest in understanding full mutational spectrums and landscapes. Methods that focus on single-molecule amplification or analysis, such as digital PCR-based applications, are increasing in use. However, digital PCR requires the analysis of a very large number of samples in order to detect mutants occurring at very low frequencies relative to the wild-type DNA, is mainly directed towards detection of mutations at known positions and is currently difficult to apply in routine applications with conventional thermocyclers. With the onset of micro- and nano-fluidics, these technologies, along with multiplexed analyses, are becoming more feasible.
With the remarkable achievements in next-generation sequencing, particularly in the reduced cost and increased availability, there is tremendous potential in more fully understanding the true low-level mutation spectrum of diseases such as cancer, or of genetic mosaicism. Most relevant to our applications with COLD-PCR is the recognition that primary, secondary and metastatic tumors are discordant, and that pre-existing low-abundance mutations in primary tumors may be critical in determining how diseases progress and evolve to metastasis.
Five-year view
Throughout the development of COLD-PCR, we have investigated its potential application in combination with a variety of molecular diagnostic tools. We anticipate that we will continue to pair COLD-PCR with other technologies and that COLD-PCR will continue to improve the sensitivity of detection methods. Given our recent observation that lower mutant fractions exhibit higher enrichment potential in COLD-PCR reactions, we hope to further investigate this trend in our optimization of the COLD-PCR platforms and downstream analyses. This trend suggests that much lower initial mutation abundances can be enriched beyond those which we have demonstrated to date. Our 5-year goal is to further improve mutation-enrichment potential, such that detection sensitivities are enhanced appreciably, and such that we improve the ability to detect minor allele variants in the early stages of disease progression.
The ideal trajectory of the COLD-PCR technique would be its incorporation into a diagnostic and clinical setting. Identifying the presence of rare mutations within a cancer patient at a very early stage could significantly impact the course of personalized medicine, as well as determine patient response, disease relapse or recurrence, metastatic evolution and ultimately patient survival. As the COLD-PCR approach provides a method to screen specimens where variants are present in low representation, we anticipate that COLD-PCR can be routinely applied to screen patient specimens (such as bodily fluids) so that diagnoses can be performed in a routine, noninvasive manner. This approach would be advantageous, not only for screening cancer patients, but also for the analysis of fetal DNA within an overwhelming abundance of maternal DNA, or even for screening for signs of recurrence or the development of resistance in infectious diseases. While massively parallel sequencing methods, as well as single-cell micro-fluidic genomic screening approaches, are gaining popularity in medical genetics, these approaches remain both costly to perform and to analyze with regard to both finances and time. We believe that COLD-PCR could be highly advantageous in non-invasive screening because it is simple and inexpensive to perform and analyze. Furthermore, the combination of COLD-PCR with massively parallel sequencing methods can be synergistic, thus enabling next-generation sequencing to attain a much improved ability to identify low-level DNA variants.
Key issues
Low-abundance mutations and variants are often present in cancer. These low abundances may be the result of disease evolution through different clones within the same tumor, disease progression, treatment resistance, tissue pathology and specimen source.
Many low-abundance mutations lie below the sensitivity and selectivity of molecular assays and, as such, limit the accuracy of the assay analysis; such scenarios make it especially difficult to accurately identify unknown mutations that can be clinically relevant.
Coamplification at lower denaturation temperature (COLD)-PCR is a PCR-based application that preferentially enriches the amplification of mutants and variants throughout the course of thermocycling, irrespective of the type and position of mutations.
COLD-PCR can be applied in the place of conventional PCR, and analyzed either in real time or via most downstream molecular applications.
COLD-PCR currently has three platforms: full-COLD-PCR, fast-COLD-PCR and ice-COLD-PCR. An appropriate platform can be utilized depending upon the desired level of enrichment and the type of variant(s) to be evaluated.
COLD-PCR exploits a small but critical difference in amplicon melting temperature.
Performing PCR at a lower denaturation temperature, the critical denaturation temperature (Tc), results in selective amplification and robust enrichment of variant alleles and mutations. Tc is usually equal to the melting temperature (Tm)−1, where Tm is the experimentally determined melting temperature of the amplicon
Acknowledgments
Financial & competing interests disclosure
This work was supported by T32-CA009078 from the National Cancer Institute and National Institutes of Health grants CA-138280 and CA-111994. COLD-PCR technology is patent-pending, and assigned to the Dana Farber Cancer Institute (MA, USA). Certain portions of the COLD-PCR technology have been licensed to Transgenomic Inc. (NE, USA). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Disclaimer
The contents of this manuscript are the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Loeb LA, Bielas JH, Beckman RA, Bodmer IW. Cancers exhibit a mutator phenotype: clinical implications. Cancer Res. 2008;68(10):3551–3557. doi: 10.1158/0008-5472.CAN-07-5835. [DOI] [PubMed] [Google Scholar]
- 2.Campbell PJ, Pleasance ED, Stephens PJ, et al. Subclonal phylogenetic structures in cancer revealed by ultra-deep sequencing. Proc Natl Acad Sci USA. 2008;105(35):13081–13086. doi: 10.1073/pnas.0801523105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenman C, Stephens P, Smith R, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446(7132):153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merlo LMF, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6(12):924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 5.Bielas JH, Loeb KR, Rubin BP, True LD, Loeb LA. Human cancers express a mutator phenotype. Proc Natl Acad Sci USA. 2006;103(48):18238–18242. doi: 10.1073/pnas.0607057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafner C, Knuechel R, Stoehr R, Hartmann A. Clonality of multifocal urothelial carcinomas: 10 years of molecular genetic studies. Int J Cancer. 2002;101(1):1–6. doi: 10.1002/ijc.10544. [DOI] [PubMed] [Google Scholar]
- 7.Kwiatkowska J, Wigowska-Sowinska J, Napierala D, Slomski R, Kwiatkowski DJ. Mosaicism in tuberous sclerosis as a potential cause of the failure of molecular diagnosis. N Engl J Med. 1999;340(9):703–707. doi: 10.1056/NEJM199903043400905. [DOI] [PubMed] [Google Scholar]
- 8•.Li J, Milbury CA, Li C, Makrigiorgos GM. Two-round coamplification at lower denaturation temperature-PCR (COLD-PCR)- based sanger sequencing identifies a novel spectrum of low-level mutations in lung adenocarcinoma. Hum Mutat. 2009;30(11):1583–1590. doi: 10.1002/humu.21112. Two consecutive rounds of coamplification at lower denaturation temperature (COLD)-PCR for enrichment of extremely low-abundance mutations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delaney D, Diss TC, Presneau N, et al. GNAS1 mutations occur more commonly than previously thought in intramuscular myxoma. Mod Pathol. 2009;22(5):718–724. doi: 10.1038/modpathol.2009.32. [DOI] [PubMed] [Google Scholar]
- 10.Fukui T, Ohe Y, Tsuta K, et al. Prospective study of the accuracy of EGFR mutational analysis by high-resolution melting analysis in small samples obtained from patients with non-small cell lung cancer. Clin Cancer Res. 2008;14(15):4751–4757. doi: 10.1158/1078-0432.CCR-07-5207. [DOI] [PubMed] [Google Scholar]
- 11.Shah SP, Morin RD, Khattra J, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461(7265):809–813. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 12.Prat E, del Rey J, Camps J, et al. Genomic imbalances in urothelial cancer: intratumor heterogeneity versus multifocality. Diag Mol Pathol. 2008;17(3):134–140. doi: 10.1097/PDM.0b013e31815ce4e6. [DOI] [PubMed] [Google Scholar]
- 13.Thomas RK, Nickerson E, Simons JF, et al. Sensitive mutation detection in heterogeneous cancer specimens by massively parallel picoliter reactor sequencing. Nat Med. 2006;12(7):852–855. doi: 10.1038/nm1437. [DOI] [PubMed] [Google Scholar]
- 14.Emmert-Buck MR, Bonner RF, Smith PD, et al. Laser capture microdissection. Science. 1996;274(5289):998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- 15.Erickson HS, Gannot G, Tangrea MA, Chuaqui RF, Gillespie JW, Emmert-Buck MR. High throughput screening of normal and neoplastic tissue samples. Comb Chem High Throughput Screen. 2010;13(3):253–267. doi: 10.2174/138620710790980540. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 17.Sjoholm MIL, Hoffmann G, Lindgren S, Dillner J, Carlson J. Comparison of archival plasma and formalin-fixed paraffin-embedded tissue for genotyping in hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2005;14(1):251–255. [PubMed] [Google Scholar]
- 18.Pinzani P, Salvianti F, Pazzagli M, Orlando C. Circulating nucleic acids in cancer and pregnancy. Methods. 2010;50(4):302–307. doi: 10.1016/j.ymeth.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Engelman JA, Mukohara T, Zejnullahu K, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116(10):2695–2706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diehl F, Li M, Dressman D, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci USA. 2005;102(45):16368–16373. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc Natl Acad Sci USA. 2003;100(3):776–781. doi: 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Milbury CA, Li J, Makrigiorgos GM. PCR-based methods for the enrichment of minority alleles and mutations. Clin Chem. 2009;55(4):632–640. doi: 10.1373/clinchem.2008.113035. Brief review of currently available PCR-based methods for mutation enrichment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Li J, Wang L, Mamon H, Kulke MH, Berbeco R, Makrigiorgos GM. Replacing PCR with COLD-PCR enriches variant DNA sequences and redefines the sensitivity of genetic testing. Nat Med. 2008;14(5):579–584. doi: 10.1038/nm1708. Principal publication revealing COLD-PCR. [DOI] [PubMed] [Google Scholar]
- 25•.Li J, Makrigiorgos GM. COLD-PCR: a new platform for highly improved mutation detection in cancer and genetic testing. Biochem Soc Trans. 2009;37:427–432. doi: 10.1042/BST0370427. Brief review of COLD-PCR. [DOI] [PubMed] [Google Scholar]
- 26.Lipsky RH, Mazzanti CM, Rudolph JG, et al. DNA melting analysis for detection of single nucleotide polymorphisms. Clin Chem. 2001;47(4):635–644. [PubMed] [Google Scholar]
- 27.Palais B. Quantitative heteroduplex analysis. Clin Chem. 2007;53(6):1001–1003. doi: 10.1373/clinchem.2007.087072. [DOI] [PubMed] [Google Scholar]
- 28••.Milbury CA, Li J, Makrigiorgos GM. Ice-COLD-PCR enables rapid amplification and robust enrichment for low-abundance unknown DNA mutations. Nucleic Acids Res. 2011;39(1):e2. doi: 10.1093/nar/gkq899. Principal publication revealing ice-COLD-PCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Milbury CA, Chen CC, Mamon H, Liu P, Santagata S, Makrigiorgos GM. Multiplex amplification coupled with COLD-PCR and high resolution melting enables identification of low-prevalence mutations in cancer samples with low DNA content. J Mol Diag. 2011 doi: 10.1016/j.jmoldx.2010.10.008. (In Press). Approach for screening low-DNA-content specimens with COLD-PCR and high-resolution melting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Song C, Milbury CA, Li J, Liu P, Zhao M, Makrigiorgos GM. Rapid and sensitive detection of KRAS mutation after COLD-PCR enrichment and high resolution melting analysis. Diag Mol Pathol. 2010 doi: 10.1097/PDM.0b013e3181fde92f. (In Press). COLD-PCR approach for screening KRAS mutations in low- or heterogeneous-DNA-content specimens. [DOI] [PubMed] [Google Scholar]
- 31.Boisselier B, Marie Y, Labussière M, et al. COLD PCR HRM: a highly sensitive detection method for IDH1 mutations. Hum Mutat. 2010;31(12):1360–1365. doi: 10.1002/humu.21365. [DOI] [PubMed] [Google Scholar]
- 32.Kristensen LS, Daugaard IL, Christensen M, Hamilton-Dutoit S, Hager H, Hansen LL. Increased sensitivity of KRAS mutation detection by highresolution melting analysis of COLD-PCR products. Hum Mutat. 2010;31(12):1366–1373. doi: 10.1002/humu.21358. [DOI] [PubMed] [Google Scholar]
- 33.Blake RD, Bizzaro JW, Blake JD, et al. Statistical mechanical simulation of polymeric DNA melting with MELTSIM. Bioinformatics. 1999;15(5):370–375. doi: 10.1093/bioinformatics/15.5.370. [DOI] [PubMed] [Google Scholar]
- 34.Steger G. Thermal denaturation of double-stranded nucleic acids: prediction of temperatures critical for gradient gel electrophoresis and polymerase chain reaction. Nucleic Acids Res. 1994;22(14):2760–2768. doi: 10.1093/nar/22.14.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Li J, Wang L, Janne PA, Makrigiorgos GM. Coamplification at lower denaturation temperature-PCR increases mutation-detection selectivity of TaqMan-based real-time PCR. Clin Chem. 2009;55(4):748–756. doi: 10.1373/clinchem.2008.113381. Principal application pairing COLD-PCR with real-time TaqMan® analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogino S, Kawasaki T, Brahmandam M, et al. Sensitive sequencing method for KRAS mutation detection by pyrosequencing. J Mol Diag. 2005;7(3):413–421. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Milbury CA, Li J, Makrigiorgos GM. COLD-PCR-enhanced high-resolution melting enables rapid and selective identification of low-level unknown mutations. Clin Chem. 2009;55(12):2130–2143. doi: 10.1373/clinchem.2009.131029. Principal application of COLD-PCR in combination with high-resolution melting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood LD, Parsons DW, Jones S, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318(5853):1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 39.Wilkening S, Hemminki K, Thirumaran RK, et al. Determination of allele frequency in pooled DNA: comparison of three PCR-based methods. Biotechniques. 2005;39(6):853–858. doi: 10.2144/000112027. [DOI] [PubMed] [Google Scholar]
- 40.De La Vega FM, Lazaruk KD, Rhodes MD, Wenz MH. Assessment of two flexible and compatible SNP genotyping platforms: TaqMan® SNP Genotyping Assays and the SNPlex™Genotyping System. Mutat Res. 2005;573(1–2):111–135. doi: 10.1016/j.mrfmmm.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Erali M, Voelkerding KV, Wittwer CT. High resolution melting applications for clinical laboratory medicine. Exp Mol Pathol. 2008;85(1):50–58. doi: 10.1016/j.yexmp.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gundry CN, Vandersteen JG, Reed GH, Pryor RJ, Chen J, Wittwer CT. Amplicon melting analysis with labeled primers: a closed-tube method for differentiating homozygotes and heterozygotes. Clin Chem. 2003;49(3):396–406. doi: 10.1373/49.3.396. [DOI] [PubMed] [Google Scholar]
- 43.Mancini I, Santucci C, Sestini R, et al. The use of COLD-PCR and high-resolution melting analysis improves the limit of detection of KRAS and BRAF mutations in colorectal cancer. J Mol Diagn. 2010;12(5):705–711. doi: 10.2353/jmoldx.2010.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.MeltSim: The DNA Melting Simulator. www.bioinformatics.org/meltsim/wiki.
- 102.Institut für Physikalische Biologie. www.biophys.uni-duesseldorf.de/local/POLAND/poland.html.