Abstract
Mice with a deletion of the hypothalamic basic helix-loop-helix transcription factor Nhlh2 display adult onset obesity, implicating Nhlh2 in the neuronal circuits regulating energy availability. Nhlh2 colocalises with the hypothalamic thyrotrophin-releasing hormone (TRH) neurones in the paraventricular nucleus (PVN) and pro-opiomelanocortin (POMC) neurones in the arcuate nucleus. We show that Nhlh2 expression is significantly reduced in response to 24-h food deprivation in the arcuate nucleus, PVN, lateral hypothalamus, ventromedial hypothalamus (VMH) and dorsomedial hypothalamus (DMH). Food intake for 2 h following deprivation stimulates Nhlh2 expression in the arcuate nucleus and the PVN, and leptin injection following deprivation results in increased Nhlh2 expression in the arcuate nucleus, PVN, lateral hypothalamus, VMH, and DMH. Hypothalamic Nhlh2 expression in response to leptin injection is maximal by 2 h. Following leptin injection, Nhlh2 mRNA colocalises in POMC neurones in the arcuate nucleus and TRH neurones in the PVN. Nhlh2 mRNA expression in POMC neurones in the arcuate nucleus and TRH neurones in the PVN is reduced with energy deprivation and is stimulated with food intake and leptin injection. Modulation of POMC expression in response to changes in energy availability is not affected in mice with a targeted deletion of Nhlh2. However, deletion of Nhlh2 does result in loss of normal TRH mRNA expression in mice exposed to food deprivation and leptin stimulation. These data implicate Nhlh2 as a regulatory target of the leptin-mediated energy availability network of the hypothalamus, and TRH as a putative downstream target of Nhlh2.
Keywords: nescient helix-loop-helix 2, NSCL-2, Hen2, POMC, TRH, leptin
In the balance between energy intake and energy expenditure, genetic, physiological and behavioural factors coordinate to maintain a stable body weight. Energy surplus and energy deprivation signals are managed by the central and peripheral nervous systems, the gut, the pancreas, and other tissues. Following food intake or other modulators of energy availability, the paraventricular (PVN) and arcuate nuclei of the hypothalamus respond to signals, such as leptin, insulin and changes in blood glucose, with changes in gene regulation and subsequent neuropeptide release to regulate energy status in the body (1, 2).
Nescient helix-loop-helix 2 (Nhlh2) is a basic helix-loop-helix (bHLH) transcription factor expressed in the adult hypothalamus (3). Mice containing a targeted deletion of Nhlh2 (N2KO) display adult-onset obesity, which becomes evident by 12 weeks of age (4). Unlike other mouse obesity models, N2KO mice gain weight due to reduced spontaneous physical activity, which precedes body weight gain and mild hyperphagia in older animals (5). Nhlh2 colocalises with pro-thyrotrophin releasing hormone (TRH) neurones in the PVN and pro-opiomelanocortin (POMC) neurones in the arcuate nucleus (3). TRH and POMC mRNA and peptide levels fluctuate with energy status, with high mRNA and peptide expression following leptin injection or food intake, and reduced expression following food deprivation (6–8). The processing enzyme, Prohormone Convertase I (PC1), that converts the proneuropeptide form of pro-TRH and POMC to fully active peptides, follows a similar expression pattern in each of these regions (8, 9). N2KO mice have up to a 60% reduction in hypothalamic PC1 levels, effecting processing and expression levels of both POMC and TRH mature peptides (3).
Only a few of the neuronal transcription factors involved in energy balance regulatory pathways have been identified and fewer still have been studied with respect to the energy availability signals governing their mRNA expression (10). The phenotype of the N2KO mice and colocalisation of Nhlh2 in POMC and TRH neurones led us to ask whether Nhlh2 expression was modulated in response to energy availability and if the modulation has an effect on potential downstream target genes of this transcription factor. Here, we demonstrate that accumulation of Nhlh2 mRNA occurs in response to food intake and leptin injection following food deprivation. In addition, we show that the effect of leptin stimulation on Nhlh2 mRNA expression is rapid and long-lasting. We demonstrate that changes in energy availability modulate the expression of Nhlh2 within TRH positive cells in the PVN and POMC positive cells in the arcuate nucleus and that Nhlh2 is required for correct TRH mRNA expression in the PVN. However, POMC mRNA levels are not affected by the absence of Nhlh2 in N2KO mice. These data identify Nhlh2 as a target of leptin signals, and TRH as a putative Nhlh2-regulated gene.
Materials and methods
Animals
All work involving animals was done in compliance with the Institutional Animal Care and Use Committee at the University of Massachusetts, Amherst. Breeding and genotyping of this line of mice have been described elsewhere (3). Wild-type (WT) and N2KO mice were maintained under a 12 : 12 h light/dark cycle with food available ad lib (4.5% crude fat). For all experiments, only male mice were used to eliminate the need for oestrous cycle analysis in female mice. All mice were euthanised by CO2 asphyxiation at 13.00 h to standardise hormone and steroid levels that fluctuate hourly.
Serum leptin analysis
Upon euthanisation, blood was obtained by exsanguinations. Serum was stored at −80 °C until all samples were available for leptin analysis via ELISA (Quantikine M Mouse Leptin Immunoassay, R & D Systems, Minneapolis, MN, USA).
In situ hybridisation
WT mice were euthanised under differing states of energy availability [ad lib fed (ad lib), food deprived for 24 h (deprived), food deprived for 24 h + 2 h food return (deprived + food), food deprived for 24 h + 2 h phosphate buffered saline (PBS) injection (deprived + PBS), food deprived for 24 h + 2 h leptin (3 mg/kg body weight in PBS) injection (deprived + leptin)]. Brains were isolated by dissection and a hypothalamic tissue block was made by trimming between the optic chiasm and the mammillary bodies. The block was fresh-frozen on dry ice and sectioned on a cryostat at 12 µm. Sections containing the PVN were determined visually based on the position of densely grouped cells surrounding the third ventricle. Sections containing the arcuate nucleus were identified based on the position of a dense group of cells in the region of the median eminence of the hypothalamus. Lateral hypothalamus, ventromedial hypothalamus (VMH), and dorsal medial hypothalamus (DMH) regions were determined by dense groupings of cells in reference to the third ventricle and the arcuate nucleus and PVN. The cRNA probes (riboprobes) were prepared using linearised plasmid according to the manufacturer’s directions using the Promega T3/T7/SP6 Riboprobe kit (Promega Corporation, Madison, WI, USA). The cRNA probe for mouse Nhlh2 has been described previously (3). The methods for prehybridisation and hybridisation of the slides have also been reported previously (3). Approximately 2 × 106 c.p.m. of 33P-labelled probe was diluted into 25 µl of hybridisation buffer and added to the centre of each tissue section affixed to slides. The slides were hybridised for 16–18 h at 52 °C in the Boekel Slide Moat (Feasterville, PA, USA). After several posthybridisation washes to remove unbound probes, the slides were exposed to phosphorimager screens overnight. The intensity of the signal was used to determine the length of exposure to emulsion. The slides were then dipped in Hypercoat LM-1 liquid emulsion (Amersham Biosciences, Bucks, UK) and developed using Kodak products (Kodak, Rochester, NY, USA). Six mice were tested under each energy availability condition in all five regions of the hypothalamus examined. In the PVN, seven mice were tested under ad lib, deprived + PBS, and deprived + leptin conditions.
Dual-label in situ hybridisation
The procedure for dual-label in situ hybridisation (ISH) is identical to the procedure for ISH with the following exceptions: the POMC and TRH probes were prepared according to the manufacturer’s directions (Promega T3/T7/SP6 Riboprobe kit) with digoxigenin (DIG)-uridine triphosphate added in place of the 33P-uridine triphosphate described above. The cRNA probes for mouse POMC and mouse TRH have been described previously (3). The Nhlh2 riboprobe was prepared using 33P-uridine triphosphate as described above. Approximately 2 × 106 c.p.m. of 33P-labelled probe and 10–25 ng of DIG-labelled probe in 25 µl of hybridisation buffer were added to the centre of each slide and hybridised for 16–18 h at 52 °C. The slides were washed as described previously (11). The DIG-labelled probe was detected using an anti-DIG-horseradish peroxidase-conjugated antibody and developed with diaminobenzidine according to the directions in the TSA Biotin Amplification System (Perkin Elmer Life Sciences, Boston, MA, USA). After detection of the nonradioactive signals, the slides were developed as described above. Three mice were tested under each energy availability condition.
Quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PC)R
WT and N2KO mice were euthanised under differing states of energy availability [ad lib, deprived, deprived + food, deprived + PBS, deprived + leptin (3 mg/kg body weight, in PBS)]. Brains were isolated by dissection. A hypothalamic block was isolated by cutting the centre millimetre of brain in a 2 mm Mouse Brain Matrix (Zivic Laboratories Inc., Pittsburgh, PA, USA). RNA was isolated via guanidine isothiocyanate preparation. The brain segment was put into 4 m guanidine isothiocyanate buffer and homogenised. Samples were layered over 5.7 m caesium chloride buffer and spun for 18 h at 120 000 g at 20 °C. The supernatant was discarded and RNA was resuspended in DEPC water. RNA was then DNAse treated. cDNA was created using reverse transcriptase in a magnesium buffer (Eppendorf, Hamburg, Germany) for 1 h at 42 °C. Quantitative real-time reverse transcription PCR (QPCR) was performed at the University of Massachusetts – Amherst Genomics & Bioinformatics Facility (Amherst, MA, USA) using Mouse Nhlh2 (used with WT mice brain tissue only) (catalogue number PPH09284A) and Mouse β-actin (used with WT and N2KO mice brain tissue) (catalogue number PPM02945A), Mouse POMC1 (used with WT and N2KO mice brain tissue) (catalogue number PPM37114A), and Mouse TRH (used with WT and N2KO mice brain tissue) (catalogue number PPM24656A) primer sets (Superarray Bioscience Corporation, Frederick, MD, USA) and the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA). mRNA levels of each gene of interest (Nhlh2, POMC and TRH) were normalised against β-actin. β-actin levels remain stable during changes in energy availability in WT mice and in KO mice, and are constant between WT and N2KO animals (data not shown). Normalised levels of mRNA were measured in triplicate per individual mouse from which sample means were calculated for each mouse. Five mice per experimental group were averaged and data is reported as the fold-difference (in log) from the ad lib fed experimental group. For each mRNA amplified, melting curve analysis was done to confirm the presence of a single amplicon. A reaction from WT ad lib animals was used to subclone and sequence duplicate amplicons and confirm correct amplification of the target mRNA.
Nhlh2 expression time-course
Following 24 h of food deprivation, WT mice were injected with vehicle (PBS) or leptin (3 mg/kg body weight) in PBS. Mice were euthanised at 15 min, 30 min, 1 h, 2 h, 4 h and 8 h following leptin injection. Brains were collected, RNA was isolated, and QPCR was performed according to the methods reported above. Five mice were tested for each time point and each mouse mRNA was run three times. As stated above, Nhlh2, POMC and TRH mRNA levels were normalised against β-actin. Each time point was reported as fold difference (in log) from PBS injected samples whose values were set to one.
Quantification of results and statistical analysis of data
Based on previous findings (3), ISH and dual-label ISH quantification concentrated on rostral regions of the PVN and arcuate nucleus. For ISH, digitalised images were taken with an Olympus Q-colour3 digital camera using an Olympus BH-2 microscope (Olympus, Melville, NY, USA) with the same exposure time, brightness and contrast for the same comparison groups. ISH images (× 20 and × 40 magnification) were greyscale-digitalised and quantified using the Bioquant Classic system (BIOQUANT Image Analysis Corporation, Nashville, TN, USA) where the same threshold was used for comparison sets. The signal-to-noise ratio was adjusted, and the total number of positive pixels per unit area (cells) (pixel density) was calculated using at least 50 single cell measurements for each brain region. Each experimental condition in each region of the hypothalamus was tested in a minimum of six animals and the sample means were reported with standard error. The significance was tested using one-way anova with Tukey’s post-hoc analysis. Significance was set at the 95% and, if appropriate, the 99% confidence level. The details on optimisation of quantitative analysis using Bioquant software have been published previously (11).
For dual-label ISH, images were taken similar to ISH images. Images (× 20 and × 40 magnification) were greyscale-digitalised and quantified using the ImageJ system (Public Domain, Developed at the National Institute of Mental Health, Bethesda, MD, USA.) where the same threshold was used for comparison sets. The colour images were used as reference for the location of POMC and TRH stained cells. Nhlh2 expression was quantified by measuring the total number of positive pixels per unit area (cells) (pixel density) over POMC stained (arcuate nucleus) and TRH stained (PVN) cells only using at least 50 single cell measurements. In the PVN and the arcuate nucleus, six animals were tested under ad lib and deprived condition and three animals were tested under food return and leptin injected conditions. The significance was tested using one-way anova with Tukey’s post-hoc analysis. Significance was set at the 95% and, if appropriate, the 99% confidence level.
For QPCR, sample means were calculated for each mouse mRNA performed in triplicate. Averages were calculated from five mice in each experimental group and standard error is reported. For QPCR, significance was tested using one-way anova with Tukey’s post-hoc tests, except for the altered expression of TRH mRNA, where data underwent a two-way anova with Tukey’s post-hoc tests. Significance was set at the 95% and, if appropriate, the 99% confidence level for all analyses.
Results
Hypothalamic Nhlh2 levels respond to changes in energy availability
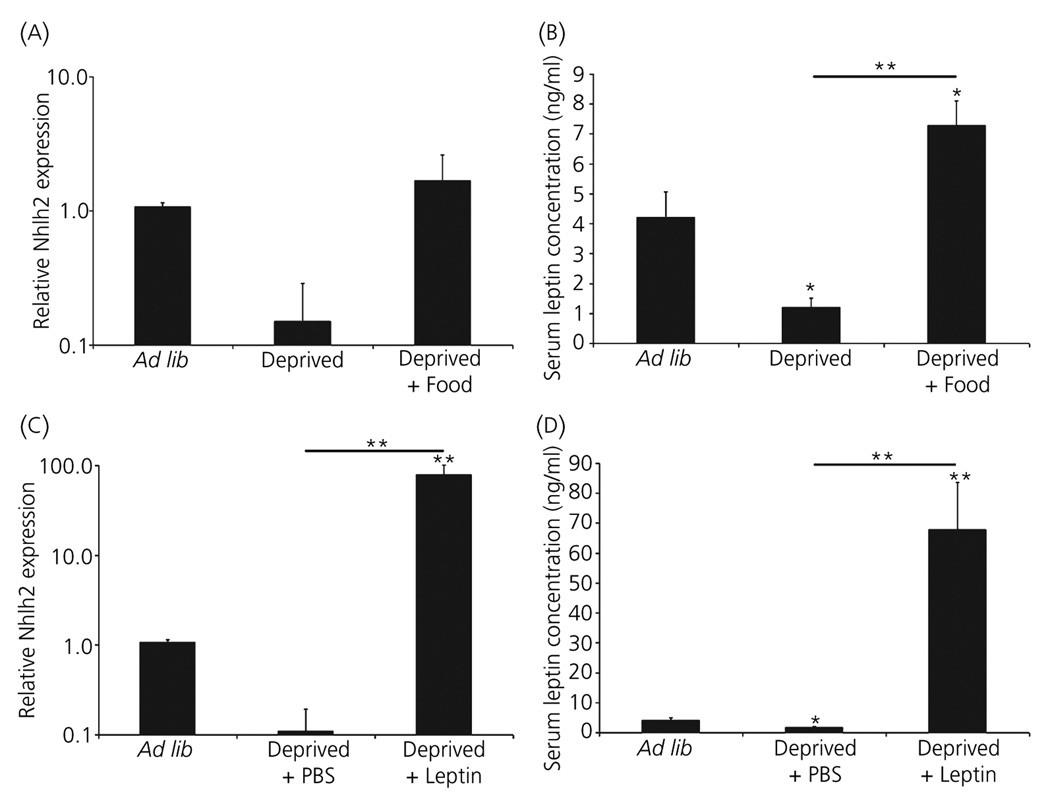
To ask whether hypothalamic Nhlh2 mRNA expression is modulated with energy availability, QPCR was performed on hypothalamic blocks of tissue isolated from mice undergoing a food deprivation and refeeding challenge. The Nhlh2 PCR product was verified by both a melting curve analysis and subcloning and sequencing of the PCR fragment (data not shown). Using whole hypothalamic RNA, Nhlh2 levels in mice undergoing a 24-h food deprivation showed no difference in expression levels between any of the tested conditions (Fig. 1A, note the log scale on left). However, serum leptin concentration in deprived animals showed a significant decline compared to ad lib fed animals. Serum leptin concentration in deprived + food animals were significantly increased by 173% over ad lib levels (P < 0.001; Fig. 1B).
Fig. 1.
Hypothalamic Nhlh2 expression changes with respect to energy status. Whole hypothalamic RNA was isolated from wild-type animals that were given (A,B) ad lib access to food (Ad lib), food deprived for 24 h (Deprived), or food deprived for 24 h followed by 2 h of ad lib food (Deprived + -Food), and given (C,D) ad lib access to food (Ad lib), food deprived for 24 h, given a phosphate buffered saline (PBS) injection and euthanised after 2 h (Deprived + PBS) or food deprived for 24 h, given a leptin injection and euthanised after 2 h (Deprived + Leptin). (A,C) Relative expression levels of Nhlh2 RNA via quantitative real-time reverse transcription polymerase chain reaction using primers to Nhlh2 normalised to β-actin and (B,D) corresponding serum leptin levels. The results are expressed as mean ± SEM (n = 5 animals per condition) (A–D). *P ≤ 0.05, **P ≤ 0.01. Horizontal lines indicate significance between food deprived and either food return or leptin injected groups. All other asterisks indicate significance compared to ad lib fed animals.
Mice were then subjected to food deprivation followed by leptin injection (deprived + leptin). Leptin injection resulted in a significant 93-fold increase in Nhlh2 expression when compared to ad lib mice (P ≤ 0.001; Fig. 1C, note the log scale on left). Accordingly, the deprived + leptin mice showed the expected rise in serum leptin levels (Fig. 1D). This, together with the data from the mice in the food return paradigm, indicates that the relative rise and fall of Nhlh2 mRNA levels were concordant with the rise and fall of serum leptin levels.
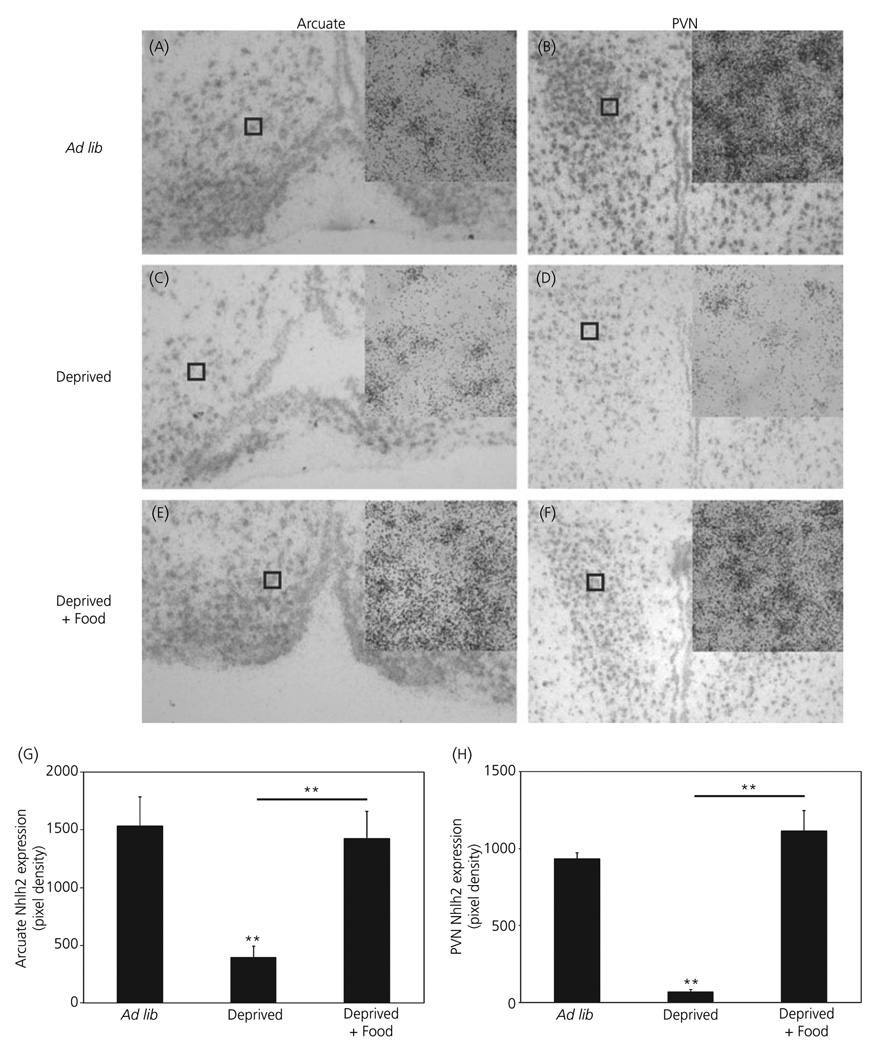
In situ hybridisation was used to identify the hypothalamic nuclei in which Nhlh2 mRNA modulation occurs in response to food deprivation, re-feeding and leptin injection. In the arcuate nucleus and the PVN, Nhlh2 mRNA levels were significantly reduced following food deprivation (Fig. 2A–D,G–H; P ≤ 0.01). Nhlh2 mRNA levels in mice given food for 2 h following 24-h food deprivation were significantly increased over deprived mice in the arcuate nucleus (Fig. 2C,E,G; P ≤ 0.01) and in the PVN (Fig. 2D,F,H; P ≤ 0.001).
Fig. 2.
Expression of Nhlh2 in the hypothalamus responds to food intake. A 33P-labelled cRNA probe to Nhlh2 was used to label hypothalamic sections of brains from wild-type mice under different states of energy availability [Ad lib: A, arcuate nucleus; B, paraventricular nucleus (PVN)], energy deficit (24-h food deprivation, Deprived: C, arcuate nucleus; D, PVN) and energy availability following an energy deficit (24-h food deprivation followed by 2 h of ad lib access to food, Deprived + Food: E, arcuate nucleus; F, PVN) (× 10 magnification with × 40 magnification inset). Nhlh2 expression density in the arcuate nucleus (G) or PVN (H) displayed as total pixel density. The results are expressed as mean ± SEM (n = 6 animals per condition in both the arcuate nucleus and PVN) (A–H). **P ≤ 0.01. Horizontal lines indicate significance between food deprived and food return groups. All other asterisks indicate significance compared to ad lib fed animals.
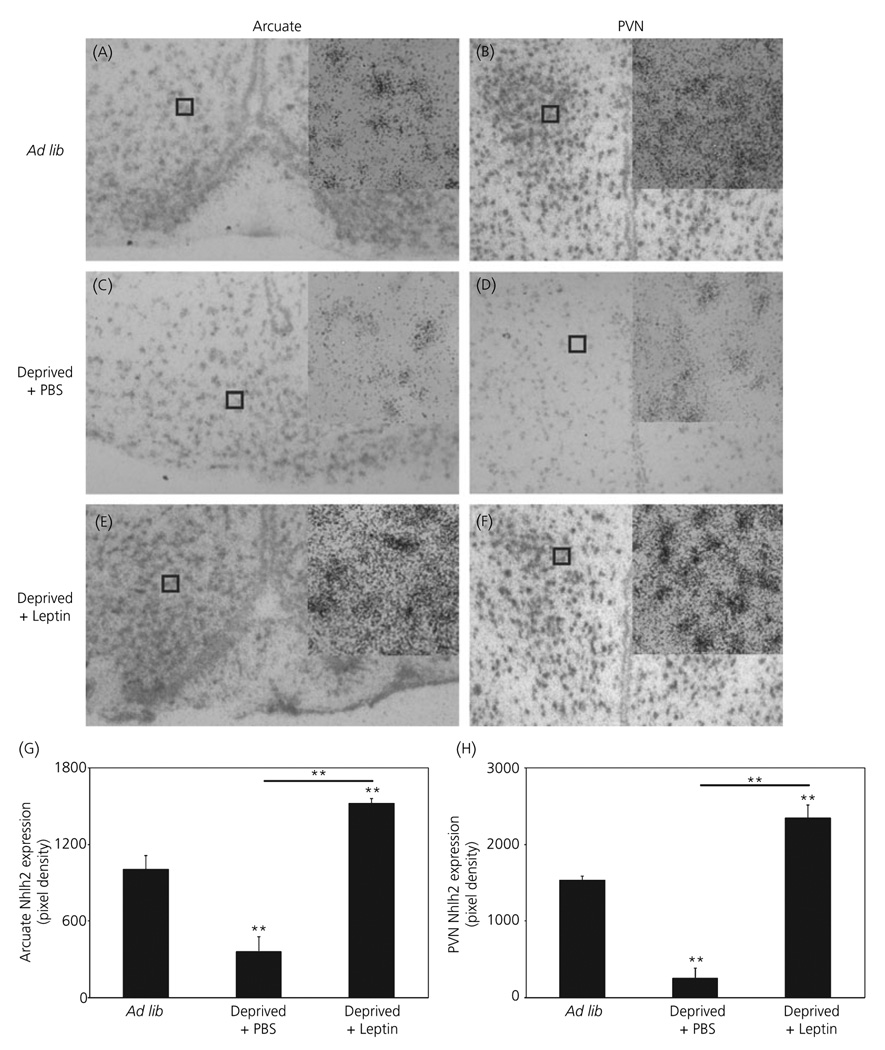
A similar Nhlh2 mRNA pattern was seen between ad lib fed, deprived + PBS animals, and deprived + leptin injected animals in arcuate nucleus and PVN (Fig. 3A–F). In deprived animals given a leptin injection, leptin increases Nhlh2 message levels in both the arcuate nucleus and PVN above ad lib mouse levels (Fig. 3). Quantification of these results shows that in the arcuate nucleus there is a statistically significant 4.2-fold increase of Nhlh2 mRNA in deprived + leptin mice over deprived + PBS mice (P ≤ 0.01) and a 1.5-fold increase over ad lib mice (Fig. 3G; P ≤ 0.01). In the PVN, Nhlh2 expression in deprived + leptin mice significantly increases 9.2-fold over deprived + PBS mice (P ≤ 0.001) and 1.5-fold over ad lib mice (Fig. 3H; P ≤ 0.01).
Fig. 3.
Expression of Nhlh2 in the hypothalamus responds to leptin injection. A 33P-labelled cRNA probe to Nhlh2 was used to label hypothalamic sections of brains from wild-type mice under different states of energy availability [Ad lib: A, arcuate nucleus; B, paraventricular nucleus (PVN)], energy deficit [24-h food deprivation with phosphate buffered saline (PBS) injection, Deprived + PBS: C, arcuate nucleus; D, PVN] and energy availability following an energy deficit (24-h food deprivation, injection of leptin 2 h prior to sacrifice, Deprived + Leptin: E, arcuate nucleus; F, PVN) (× 10 magnification with × 40 magnification inset). Nhlh2 expression density in the arcuate nucleus (G) or PVN (H) displayed as total pixel density. The results are expressed as mean ± SEM [n = 6 animals per condition in the arcuate nucleus) (A,C,E,G) and n = 7 animals per condition in the PVN (B,D,F,H)]. **P ≤ 0.01. Horizontal lines indicate significance between food deprived and leptin injected groups. All other asterisks indicate significance compared to ad lib fed animals.
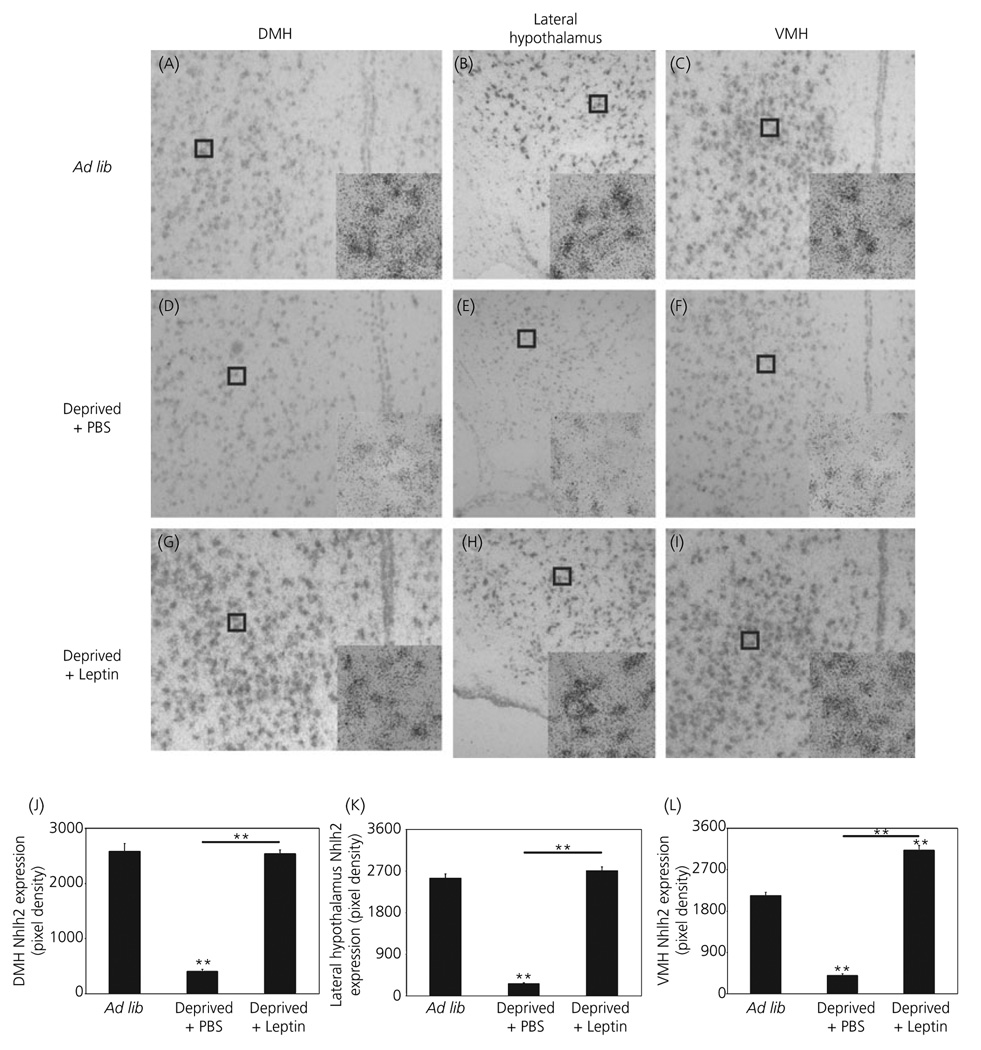
Hypothalamic Nhlh2 expression is not limited to just the arcuate nucleus and PVN but extends to the dorsal medial hypothalamus (DMH), lateral hypothalamus and the ventromedial hypothalamus (VMH) (3). These areas of the brain also see significant fluctuations in Nhlh2 mRNA expression in response to leptin injection (Fig. 4A–I). In all three regions, Nhlh2 mRNA levels are significantly reduced in deprived + PBS animals compared to ad lib animals (Fig. 4J–L; DMH: p ≤ 0.001, lateral hypothalamus: P ≤ 0.001, and VMH: P ≤ 0.001). In the VMH only, Nhlh2 mRNA levels of deprived + leptin animals were 1.5-fold higher than ad lib animals (Fig. 4L; P ≤ 0.001). Compared to deprived + PBS animals, Nhlh2 expression increases 6.3-fold in the DMH (P ≤ 0.001), 10.2-fold in the lateral hypothalamus (P ≤ 0.001) and 7.8-fold in the VMH (P ≤ 0.001) in deprived + leptin animals (Fig. 4J–L).
Fig. 4.
Expression of Nhlh2 in other hypothalamic nuclei is responsive to leptin injection. A 33P-labelled cRNA probe to Nhlh2 was used to label hypothalamic sections of brains from wild-type (WT) mice under different states of energy availability [Ad lib: A, dorsomedial hypothalamus (DMH); B, lateral hypothalamus; C, ventromedial hypothalamus (VMH); Deprived + phosphate buffered saline (PBS): D, DMH; E, lateral hypothalamus; F, VMH; Deprived + Leptin: G, DMH; H, lateral hypothalamus; I, VMH] (× 10 magnification with × 40 magnification, inset). (J–L) Nhlh2 expression density in DMH (J), lateral hypothalamus (K) and VMH (L) of WT mice under different states of energy availability displayed as pixel density. The results are expressed as mean ± SEM (n = 6 animals per condition in all three regions of the hypothalamus) (A–L). **P ≤ 0.01. Horizontal lines indicate significance between food deprived and leptin injected groups. All other asterisks indicate significance compared to ad lib fed animals.
Leptin stimulated Nhlh2 expression precedes POMC + TRH mRNA increases
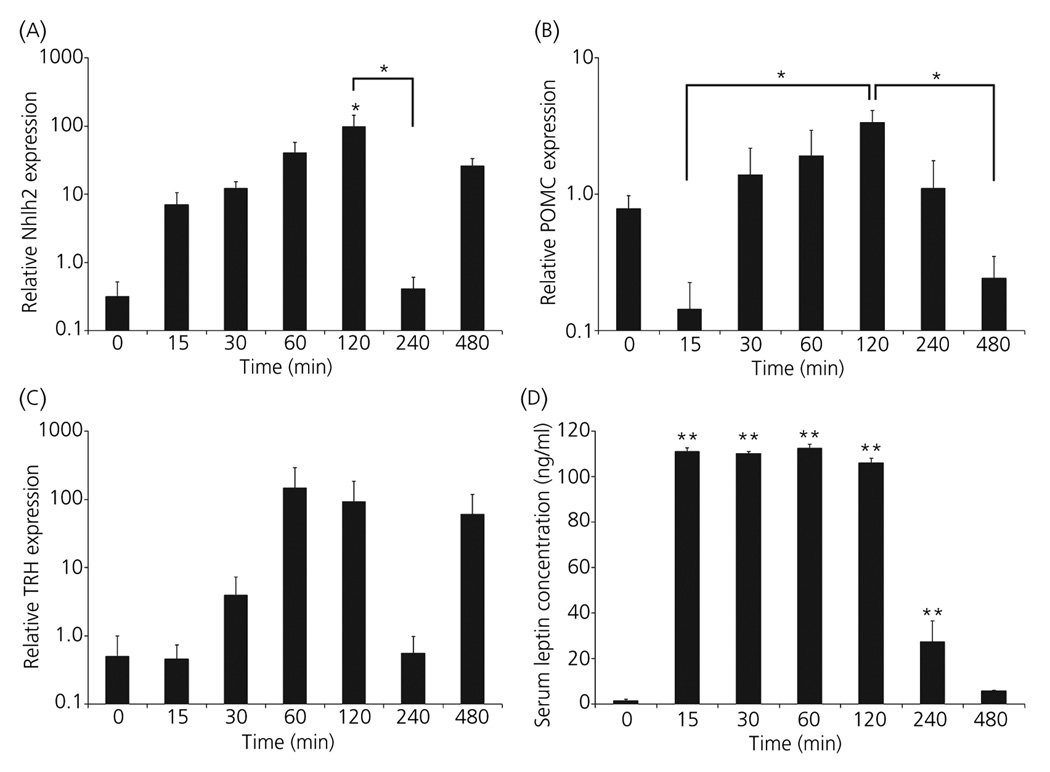
We next sought to understand the timing of Nhlh2 expression in vivo and compare it to known leptin target genes, POMC and TRH. WT mice were injected with leptin following a 24-h fast and then euthanised at various time points following the injection, starting at 15 min and ending maximally at 8 h. The peak in Nhlh2 expression occurs 2 h after leptin injection (P ≤ 0.05; Fig. 5A). There is a drop in Nhlh2 expression at 4 h, which is not significantly different than PBS-injected controls, but is significant compared to Nhlh2 expression at 2 h (P ≤ 0.05).
Fig. 5.
Increase in Nhlh2 mRNA expression is rapid following a leptin injection of deprived mice. (A) Nhlh2 mRNA expression at the indicated time points following leptin injection was quantified using quantitative real-time reverse transcription polymerase chain reaction (QPCR) and normalised to β-actin expression. (B) pro-opiomelanocortin (POMC) and (C) thyrotrophin-releasing hormone (TRH) mRNA expression at the indicated time points following leptin injection was quantified using QPCR and normalised to β-actin expression. (D) Serum leptin concentration levels were analysed via enzyme-linked immunosorbent assay at the indicated time points following leptin injection (n = 5 animals per time point) (A–D). *P ≤ 0.05, **P ≤ 0.01. Horizontal lines indicate significance between two time points. All other asterisks indicate significance compared to time point zero.
For POMC mRNA induction by leptin, one-way anova indicated an overall effect of leptin treatment on POMC expression (P ≤ 0.05), but only POMC levels at 2 h was significantly increased compared to levels at 15 min and 8 h (Fig. 5B; P ≤ 0.05). A similar trend is seen for TRH mRNA, but the effect of leptin treatment in a one-way anova analysis of the data indicates no overall significance (Fig. 5C). Serum leptin levels are significantly increased until 8 h post leptin injection where they drop to levels of PBS-injected control animals (Fig. 5D).
Differential Nhlh2 expression pattern extends to POMC neurones but is not essential to POMC expression in response to energy status
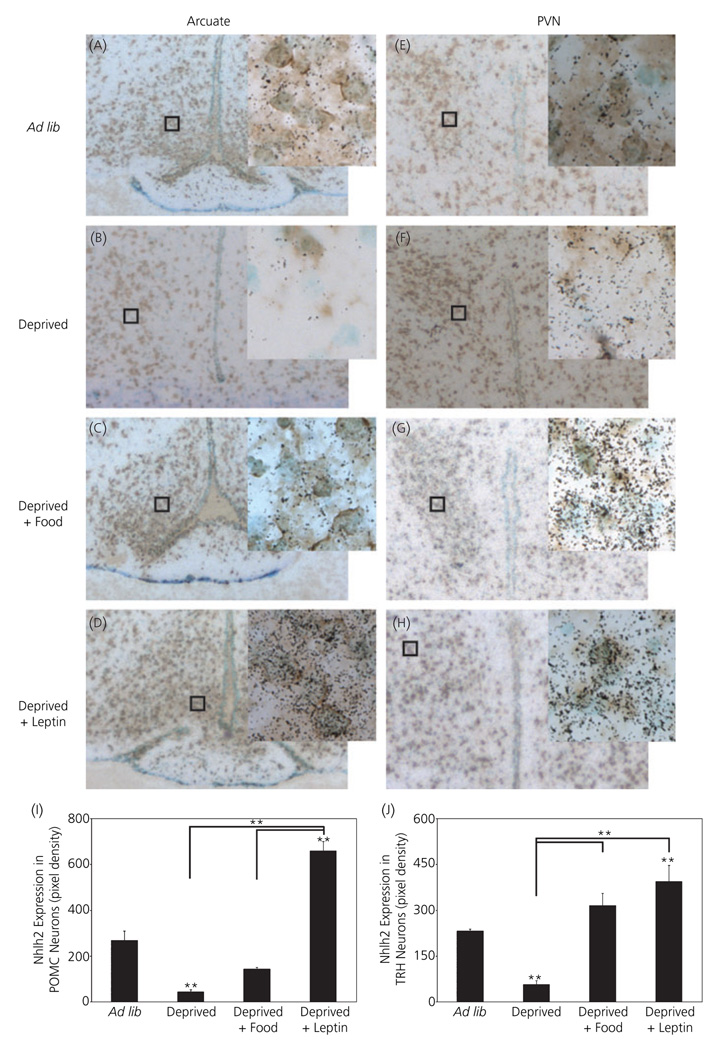
To determine whether energy availability-dependent changes in Nhlh2 occurred in specific neurone types, dual-label ISH was used to label Nhlh2 neurones and POMC neurones in WT mice. In the arcuate nucleus, Nhlh2 mRNA expression was significantly reduced in POMC neurones following food deprivation compared to ad lib Nhlh2 mRNA expression (Fig. 6A,B,I; P ≤ 0.01). Nhlh2 mRNA levels in POMC neurones in mice given food for 2 h following 24-h food deprivation were not significantly different from mRNA levels in ad lib mice, but were significantly lower than levels in deprived + leptin mice (Fig. 6A–D,I; P ≤ 0.01). Deprived + leptin mice Nhlh2 mRNA levels in POMC neurones were also significantly higher than deprived mice (Fig. 6I; P ≤ 0.01).
Fig. 6.
Nhlh2 is coexpressed with pro-opiomelanocortin (POMC) neurones in the arcuate nucleus and thyrotrophin-releasing hormone (TRH) neurones in the paraventricular nucleus (PVN) under states of variable energy status. Dual label in situ hybridisation of hypothalamic sections of brains from wild-type mice were compared under states of energy availability (Ad lib: A, arcuate nucleus; E, PVN), energy deficit (Deprived: B, arcuate nucleus; F, PVN) and energy availability following an energy deficit (Deprived + Food return: C, arcuate nucleus; G, PVN; Deprived + Leptin: D, arcuate nucleus; H, PVN). Animals deprived given an injection of phosphate buffered saline (a control group for leptin injection) were measured, were identical to the deprived group and therefore not shown (× 10 magnification with × 40 magnification, inset). Silver grains (black) show Nhlh2 expression. Brown stain indicates POMC (A–D) or TRH (E–H) expression. (I,J) Nhlh2 expression density over POMC stained cells in the arcuate nucleus (I) and over TRH stained cells in the PVN (J) under different states of energy availability displayed as pixel density. The results are expressed as mean ± SEM (n = 6 animals under ad lib and deprived condition and n = 3 animals under food return and leptin injected conditions). **P ≤ 0.01. Horizontal lines indicate significance between deprived, food deprived and leptin injected groups. All other asterisks indicate significance compared to ad lib fed animals.
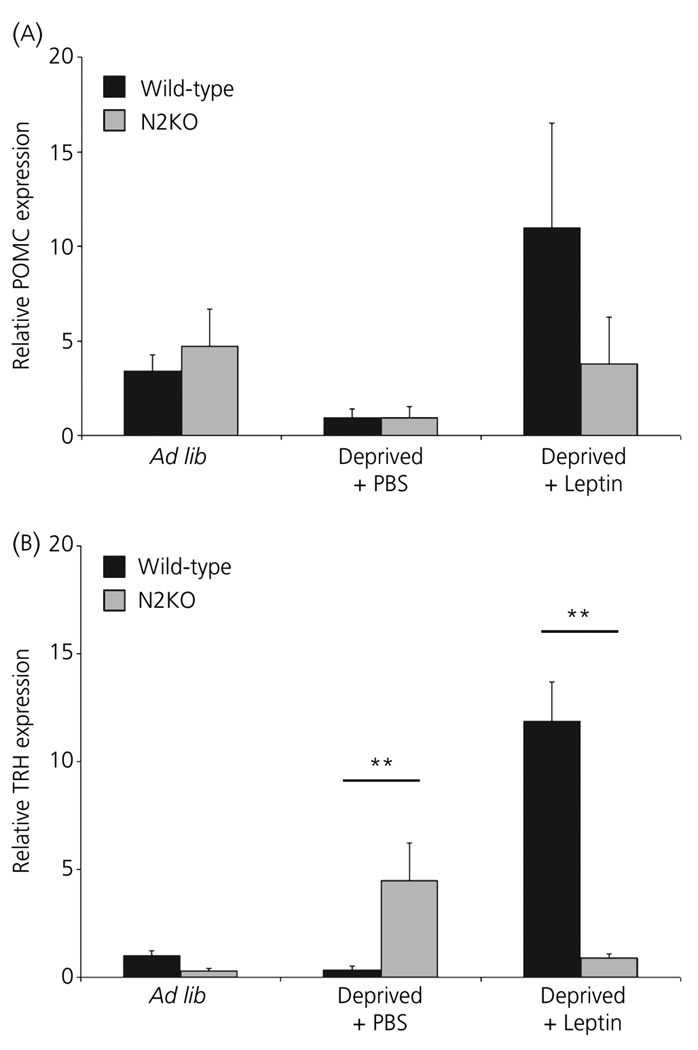
To determine if Nhlh2 plays a role in POMC mRNA expression under the conditions of food deprivation and leptin injection, POMC mRNA was measured via QPCR in WT mice and mice with a targeted deletion of the Nhlh2 gene (N2KO mice). Significant differences were not found between the two groups of mice under each condition (Fig. 7A), indicating that Nhlh2 is coexpressed in POMC neurones deletion but Nhlh2 does not affect the expression of POMC mRNA under these conditions.
Fig. 7.
Altered expression of thyrotrophin-releasing hormone (TRH) mRNA in mice with a targeted deletion of Nhlh2. Whole hypothalamic RNA was isolated from wild-type (black bars) and N2KO (grey bars) animals that were given ad lib access to food (Ad lib), food deprived for 24 h with a phosphate buffered saline (PBS) injection 2 h prior to euthanisation (Deprived + PBS), food deprived for 24 h with injection leptin given 2 h prior to euthanisation (Deprived + Leptin). Relative expression levels of pro-opiome-lanocortin (POMC) (A) or TRH (B) via QPCR using appropriate primers and normalised to β-actin mRNA expression (n = 3 animals per genotype and condition). ** P ≤ 0.01.
Differential Nhlh2 expression pattern extends to TRH neurones and is required for the correct expression of TRH in response to energy status
To determine whether energy availability-dependent changes in Nhlh2 occurred in TRH neurones in the PVN, dual-label ISH was used to label Nhlh2 neurones and TRH neurones in WT mice. Similar to POMC/Nhlh2 coexpression analysis, Nhlh2 mRNA levels in TRH neurones were significantly decreased in mice following 24-h food deprivation or food deprivation with PBS injection (data not shown) when compared to ad lib, deprived + food and deprived + leptin animals (Fig. 6E–H,J; P ≤ 0.001). Compared to ad lib mice, Nhlh2 mRNA levels in TRH neurones were significantly increased in deprived + leptin animals (Fig. 6E,H,J; P ≤ 0.001).
To determine if Nhlh2 is required for TRH mRNA expression under these conditions, QPCR was performed on hypothalamic mRNA from WT and N2KO mice. Unlike the results for POMC expression, normal TRH expression requires Nhlh2 expression (Fig. 7B). Using ISH and antipeptide antibodies, we had previously reported that TRH expression in N2KO mice fed ad lib were reduced by 40% compared to WT mice (3). Using QPCR, we find an overall genotype by treatment effect (P < 0.001) with post-hoc analysis indicating a significant 4.5-fold increase in TRH mRNA levels in deprived N2KO mice compared to deprived WT mice (Fig. 7B; P ≤ 0.01), and a loss of normal leptin induction of TRH message in N2KO mice (Fig. 7B; P ≤ 0.01). These data demonstrate the necessity of Nhlh2 for proper TRH mRNA expression in response to leptin-mediated signals.
Discussion
The present study identifies the Nhlh2 transcription factor as a hypothalamic target of peripheral energy balance signals, including food intake, food deprivation and leptin. These results support a role for the Nhlh2 transcription factor in responding to and then regulating genes involved in body weight control. We show that ad lib fed animals have moderate levels of Nhlh2 expression in the arcuate nucleus, PVN, lateral hypothalamus, VMH and DMH. Nhlh2 mRNA expression drops significantly in all nuclei with food deprivation. Re-feeding restores Nhlh2 mRNA expression to ad lib mRNA levels in the arcuate nucleus and in the PVN, whereas leptin injection results in a significant 93-fold increase in hypothalamic Nhlh2 expression compared to ad lib levels using QPCR and induction in all hypothalamic nuclei examined by ISH. These data suggest that Nhlh2 gene expression responds positively to increased energy availability and negatively to reduced energy availability.
Previous work from our laboratory demonstrated that adult-onset obesity in N2KO mice is accompanied by reduced PC1 levels, which lead to reduced αMSH and mature TRH peptide in the N2KO mice (3, 4). Based on these findings and data from another laboratory showing lower levels of Nhlh2 mRNA in ob/ob and db/db mice and in WT mice deprived for 24 h (12), we hypothesised that Nhlh2 mRNA expression could be responsive to a return of energy availability. Our data showing that Nhlh2 expression in the PVN is reduced in WT mice deprived for 24 h correlates with previous observations (12). We also found that Nhlh2 mRNA levels are significantly affected by food deprivation in the PVN and that Nhlh2 expression is significantly elevated by 2 h post leptin injection, which is in contrast to a previous report (12). We believe that some of the differences lie in the time points and methods used in the two studies. In the present study, we have extensively looked at Nhlh2 expression with QPCR in response to leptin injection at several time points over 8 h following 24-h food deprivation. We have found that the 4-h time point represents a low level of Nhlh2 expression but, when all of the time points are examined, we see levels of Nhlh2 increase as early as 15 min post leptin injection, with a significant rise by 2 h postinjection followed by a significant reduction in Nhlh2 mRNA levels at 4 h. The 8-h time point seems to indicate a re-stimulation of Nhlh2 mRNA, although later time points would be necessary to confirm this.
The pattern of Nhlh2 expression correlates well with the pattern of circulating leptin hormone levels in serum suggesting a tight link between leptin signalling and Nhlh2 promoter activation. The primary signal transduction pathway used by leptin receptor in the hypothalamus involves the recruitment of janus kinase 2 (JAK2), which transphosphorylates other JAK2 proteins and the leptin receptor. This complex provides a docking site for STAT3 transcription factor, which is also subsequently phosphorylated by JAK2 (13). STAT3 then dimerises and translocates to the nucleus to regulate expression of genes, such as SOCS3, PC1, TRH and POMC (8, 14–17). The rapid expression of these genes following leptin stimulation, usually within 30 min to 1 h is similar to what we have shown for Nhlh2, suggesting that Nhlh2 is a target for STAT3-mediated transcriptional regulation. In addition, STAT3 could be acting through an alternate mechanism, such as through protein–protein interactions with other transcription factors, such as AP-1 (c-fos/c-jun), CREB or Sp1 (15, 18). The mouse Nhlh2 promoter contains five putative STAT binding motifs, which are conserved with respect to both sequence and spacing with the human Nhlh2 promoter (2), and a putative AP-1 motif (19). STAT3 has been shown to regulate transcription through an AP-1 site on the pituitary-specific POMC promoter (18). Thus, we predict that further analysis of the role of STAT3 and other potential transcription regulators could identify both direct and indirect methods for leptin-stimulated expression of the Nhlh2 gene.
We had previously reported that Nhlh2 is expressed in POMC neurones in the arcuate nucleus and in TRH neurones in the PVN (3) and now can show that these neurones respond significantly to leptin injection with increased Nhlh2 expression. In addition, we demonstrate the deletion of Nhlh2 in mice does not affect the expression of POMC mRNA in the arcuate nucleus under varying conditions of energy availability. However, the deletion of Nhlh2 reduced TRH mRNA expression in ad lib N2KO mice and unexpectedly caused a significant rise in TRH expression under conditions of food deprivation. These data provide solid evidence that Nhlh2 is a target of energy availability signals in the arcuate nucleus and PVN and support our hypothesis that Nhlh2 function is necessary for mediating and integrating signals that communicate energy status to the hypothalamus, particularly in TRH neurones.
Relative amounts of TRH peptide and mRNA in the PVN and POMC-derived peptides and POMC mRNA in the arcuate nucleus are substantially reduced in the fasted animals relative to fed or leptin injected animals (6, 8). Investigation of the putative link between the Nhlh2 transcription factor and POMC and TRH mRNA levels using N2KO mice shows that POMC mRNA levels are not affected by the absence of Nhlh2 during changes in energy availability. This is concordant with previous findings that showed POMC mRNA levels were similar between ad lib WT and N2KO mice (3). Further investigation in this previous study demonstrated a reduction in αMSH levels and PC1 mRNA and protein levels in N2KO mice (3). Together, these data and the findings in this report suggest that Nhlh2 plays a role in the post-transcriptional regulation of POMC through its affect on PC1. Our findings in the PVN agree with the previous report that TRH mRNA is reduced in ad lib fed N2KO mice (3). Surprisingly, N2KO mice in an energy deprived state show increased levels of TRH mRNA, greater than ad lib fed N2KO mice. The level of TRH mRNA expression in leptin injected N2KO mice is reduced compared deprived N2KO mice but significantly increased over ad lib N2KO. These data suggest a role for Nhlh2 in TRH regulation, which could be direct through binding to an Ebox motif on the TRH promoter and/or indirect through the action of αMSH from the arcuate nucleus binding to melanacortin-4-receptors (MCR4) in the PVN. An obvious prediction is that manipulation of PC1 levels independent of Nhlh2 would lead to problems with body weight regulation through downstream effects on POMC, TRH and other neuropeptides requiring peptide processing by PC1. To that end, PC1 heterozygous mice make approximately the same amount of PC1 as N2KO animals and also show adult-onset obesity by 15 weeks of age (20). Another mouse model containing a mutation in PC1 which reduces its enzymatic activity by 50% leads to adult-onset obesity on a high fat diet (21). Overall, these data allow us to begin to integrate how leptin and food-mediated signals result in changes in transcriptional regulation of Nhlh2 target genes leading to downstream effects on neuropeptide processing. These data appear to suggest that even a 50% reduction in PC1 expression mediated either through homozygous deletion of the Nhlh2 gene, heterozygous deletion of the PC1 gene, or mutation of PC1 protein lead to downstream changes in body weight regulation.
Investigation into other regions of the hypothalamus that express Nhlh2 that include the VMH, lateral hypothalamus, and DMH, show that energy availability also affects Nhlh2 mRNA expression in these regions. These areas of the brain are involved in communicating hunger and satiety signals. Although previous work from our laboratory has shown that N2KO mice are not hyperphagic prior to the onset of obesity (5), exploration of the genes expressed in these hypothalamic nuclei should reveal some interesting gene targets. The DMH has previously been implicated in the regulation of thermogenesis (22), suggesting a role for Nhlh2 in body temperature regulation.
In summary, Nhlh2 is a target of leptin signalling in both POMC and TRH neurones in mice and is required for correct expression of TRH mRNA in response to energy balance perturbations. The data presented here demonstrate that up-regulation of the Nhlh2 transcription factor is one of the first steps in beginning the cascade of gene regulatory changes that occurs in response to increased energy availability. Extending this to humans, it is possible that mutations that affect either the expression of Nhlh2 in response to leptin or the function of the protein could lead to adult-onset obesity. Studies linking gene regulatory pathways with exercise behaviour and food intake have direct implications to human obesity, since sedentary lifestyles and unrestricted diets are major contributors to the obesity epidemic in the US and abroad.
Acknowledgements
This work was supported by a grant from the National Institute of Diabetes and Digestive Disorders #DK-59903. K.R.V. was supported by a fellowship from the Centre for Neuroendocrine Studies Training grant (T32 MH47538). The authors thank Ms Dana Fox, Mr James Cormier, Mr Robert Daniels and Mr David Sharlin for refinement of techniques and analysis of data, Mr Christopher Coyle for careful reading of the manuscript, and Ms Alison Bardwell and Mr Christopher Coyle for excellent technical assistance.
References
- 1.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 2.Good DJ. How tight are your genes? Transcriptional and posttranscriptional regulation of the leptin receptor, NPY, and POMC genes. Horm Behav. 2000;37:284–298. doi: 10.1006/hbeh.2000.1587. [DOI] [PubMed] [Google Scholar]
- 3.Jing E, Nillni EA, Sanchez VC, Stuart RC, Good DJ. Deletion of the Nhlh2 transcription factor decreases the levels of the anorexigenic peptides alpha melanocyte-stimulating hormone and thyrotropin-releasing hormone and implicates prohormone convertases I and II in obesity. Endocrinology. 2004;145:1503–1513. doi: 10.1210/en.2003-0834. [DOI] [PubMed] [Google Scholar]
- 4.Good DJ, Porter FD, Mahon KA, Parlow AF, Westphal H, Kirsch IR. Hypogonadism and obesity in mice with a targeted deletion of the Nhlh2 gene. Nat Genet. 1997;15:397–401. doi: 10.1038/ng0497-397. [DOI] [PubMed] [Google Scholar]
- 5.Coyle CA, Jing E, Hosmer T, Powers JB, Wade G, Good DJ. Reduced voluntary activity precedes adult-onset obesity in Nhlh2 knockout mice. Physiol Behav. 2002;77:387–402. doi: 10.1016/s0031-9384(02)00885-5. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 7.Bjorbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez VC, Goldstein J, Stuart RC, Hovanesian V, Huo L, Munzberg H, Friedman TC, Bjorbaek C, Nillni EA. Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J Clin Invest. 2004;114:357–369. doi: 10.1172/JCI21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilaweera KN, Barrett P, Mercer JG, Morgan PJ. Precursor-protein convertase 1 gene expression in the mouse hypothalamus: differential regulation by ob gene mutation, energy deficit and administration of leptin, and coexpression with prepro-orexin. Neuroscience. 2003;119:713–720. doi: 10.1016/s0306-4522(02)00869-2. [DOI] [PubMed] [Google Scholar]
- 10.Burnside AS, Good DJ. Mind over matter: transcriptional regulation of body weight by hypothalamic neurons. In: Pandalai SG, editor. Recent Research Developments in Molecular and Cellular Biology. Kerala: Research Signpost; 2004. pp. 23–39. [Google Scholar]
- 11.Petersen SL, McCrone S, Keller M, Shores S. Effects of estrogen and progesterone on luteinizing hormone-releasing hormone messenger ribonucleic acid levels: consideration of temporal and neuroanatomical variables. Endocrinology. 1995;136:3604–3610. doi: 10.1210/endo.136.8.7628399. [DOI] [PubMed] [Google Scholar]
- 12.Nilaweera KN, Ellis C, Barrett P, Mercer JG, Morgan PJ. Hypothalamic bHLH transcription factors are novel candidates in the regulation of energy balance. Eur J Neurosci. 2002;15:644–650. doi: 10.1046/j.1460-9568.2002.01894.x. [DOI] [PubMed] [Google Scholar]
- 13.Hegyi K, Fulop K, Kovacs K, Toth S, Falus A. Leptin-induced signal transduction pathways. Cell Biol Int. 2004;28:159–169. doi: 10.1016/j.cellbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 15.Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjoorbaek C, Elmquist JK, Flier JS, Hollenberg AN. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest. 2001;107:111–120. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 17.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 18.Bousquet C, Zatelli MC, Melmed S. Direct regulation of pituitary proopiomelanocortin by STAT3 provides a novel mechanism for immunoneuroendocrine interfacing. J Clin Invest. 2000;106:1417–1425. doi: 10.1172/JCI11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipkowitz S, Gobel V, Varterasian ML, Nakahara K, Tchorz K, Kirsch IR. A comparative structural characterization of the human NSCL-1 and NSCL-2 genes. Two basic helix-loop-helix genes expressed in the developing nervous system. J Biol Chem. 1992;267:21065–21071. [PubMed] [Google Scholar]
- 20.Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C, Laurent V, Lindberg I, Ugleholdt R, Holst JJ, Steiner DF. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci USA. 2002;99:10293–10298. doi: 10.1073/pnas.162352599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd DJ, Bohan S, Gekakis N. Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Hum Mol Genet. 2006;15:1884–1893. doi: 10.1093/hmg/ddl111. [DOI] [PubMed] [Google Scholar]
- 22.Dimicco JA, Zaretsky D. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2006;292:R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]