Abstract
The neurophysiological basis for binocular control of eye movements in primates has been characterized by a scientific controversy that has its origin in the historical conflict of Hering and Helmholtz in the 19th century. This review focuses on two hypotheses, linked to that conflict, that seek to account for binocular coordination: Hering’s Law versus uniocular control of each eye. In an effort to manage the length of the review, the focus is on extracellular single unit studies of premotor eye movement cells and extraocular motoneurons. In the latter half of the 20th century, these studies provided a wealth of neurophysiological data pertaining to the control of vergence and conjugate eye movements. The data were initially supportive of Hering’s Law. More recent data, however, have provided support for uniocular control of each eye consistent with Helmholtz’ original idea. The controversy is far from resolved. New anatomical descriptions of the disparate inputs to multiply and singly innervated extraocular muscle fibers challenge the concept of a “final common pathway” since they suggest there may be separate groups of motoneurons involved in vergence and conjugate control of eye position. These data provide a new challenge for interpretation of uniocular premotor control networks and how they cooperate to produce coordinated eye movements.
Introduction
Accurate control of eye movements is an essential component of visual behavior. In primates, eye movements are coordinated so as to precisely aim each eye’s fovea at an object in the visual field. Depending on the distance and eccentricity of the visual target, the lines of sight of the two eyes must be parallel when distant objects are viewed (conjugate gaze) or must intersect (converge) at a near target’s location. Failure to achieve binocular coordination results in diplopia and loss of stereo acuity. Dating from the late 19th century, the neural basis of binocular control of eye movement has been mired in controversy. The most widely accepted hypothesis has been Hering’s Law (Hering, 1977), which states that there are separate neural controllers for conjugate and vergence gaze shifts and that each eye receives an identical neural command from each controller (Figure 1A).
Figure 1.
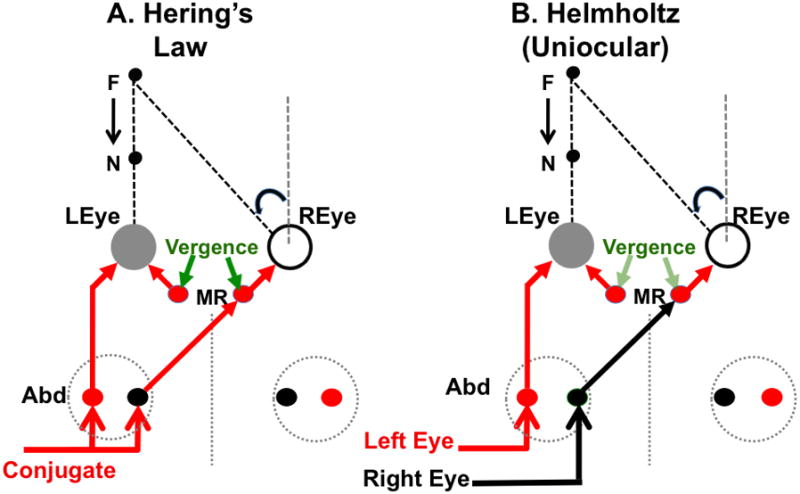
Binocular coordination of eye movements. A. Hering’s Law. Separate vergence (green arrows) and conjugate (red and black arrows) commands are summed by medial rectus motoneurons (MR) of the right eye. B. Helmholtz. Uniocular motor commands innervate each eye. The diagrams illustrate Meuller’s Paradigm where the visual target is aligned with one eye (left eye in the figure). If the target location changes from far to near (black arrows), the right eye must execute a disjunctive uniocular saccade in the nasal direction in order to refixate the target; however, the left eye’s position should not change. According to Hering’s Law (A), the medial rectus motoneurons of both eyes receive the same excitatory vergence innervation (green arrows). However, the vergence command for adduction of the left eye is opposed by a conjugate command for abduction (leftward eye movement, red arrow). Ideally, these commands cancel and the left eye would remain stationary. In the right eye, the conjugate (red arrow) and vergence commands are additive; both innervations produce adduction and the right eye rotates uniocularly to the left. According to the uniocular (Helmholtz) hypothesis (B), each eye receives its own motor command. For the aligned target arrangement illustrated in this figure, the left eye command (red arrow) would (ideally) be zero and the right eye command (black arrow) would produce the required leftward rotation. The uniocular hypothesis assumes there is a disparity vergence signal (light green arrows) that adjusts the vergence angle during fixations to maintain binocular correspondence. See the text for further details.
In The Theory of Binocular Vision, Hering said
“As far as their movements in the service of the sense of sight are concerned, both eyes will be handled as a single organ. To the mobilizing will it is irrelevant that this organ really consists of two separate parts, because it is not necessary to move each part separately; rather one and the same impulse of the will directs both eyes simultaneously, as one can direct a pair of horses single reins.”
Alternatively, Helmholtz (1962) argued that binocular coordination is a learned behavior based on independent neural control of each eye (Figure 1B).
“… the connection existing between the two eyes is not an obligatory anatomical mechanism, but is rather something which can be altered by the mere influence of our own volition; and that the only restriction consists in controlling the intent of our will, so far as its sole purpose is distinct and single vision.”
“it may be shown that the regularity of these associations [between the movements of the two eyes] is simply a matter of training.”
These hypotheses are difficult to distinguish behaviorally because the movement of each eye (ER, EL) can always be represented as a linear sum or difference, respectively, of conjugate (Econj) and vergence (Everg) components common to each eye.
| Eq. 1 |
| Eq. 2 |
For much of the 20th century Hering’s view prevailed and substantial behavioral evidence was amassed in its support (reviewed in Howard & Rogers, 1996) ( see also Turner & Benschop, 1994 for the philosophical and historical aspects of the Hering-Helmholtz controversy). The critical observations were that symmetric vergence movements are much slower than conjugate gaze shifts (Rashbass & Westheimer, 1961a) and that they appear to occur independently of one another (Rashbass & Westheimer, 1961b). In the latter part of the century, however, technical improvements in recording and analyzing eye movements allowed several studies to challenge Hering’s Law. The peak velocities of conjugate saccades and disparity vergence are proportional to the size of an eye movement (Rashbass & Westheimer, 1961a) (“main sequence”, Bahill, Clark, & Stark, 1975; for a review, see Leigh & Zee, 2006). However, during disjunctive saccadic gaze shifts between near and far targets, the vergence and conjugate components of the eye movements appear to interact non-linearly; the vergence exhibits higher peak velocity and the conjugate saccade lower peak velocity than if either component had occurred by itself (Enright, 1984; Erkelens, Steinman, & Collewijn, 1989; Kenyon, Ciuffreda, & Stark, 1980; Maxwell & King, 1992; Ono, Nakamizo, & Steinbach, 1978). Figure 2 shows an example of saccade-vergence interaction during a disjunctive eye movement (from near to far) made by a monkey (Maxwell and King, 1992). Changes in either vergence or saccadic peak velocity (as predicted by the main sequence relationships) would not be expected if independent vergence and version inputs added linearly as implied by Hering’s Law. One solution to this problem is to postulate separate vergence and conjugate commands, but assume that they interact to produce the trajectories of disjunctive saccades (Zee, Fitzgibbon, & Optican, 1992).
Figure 2.
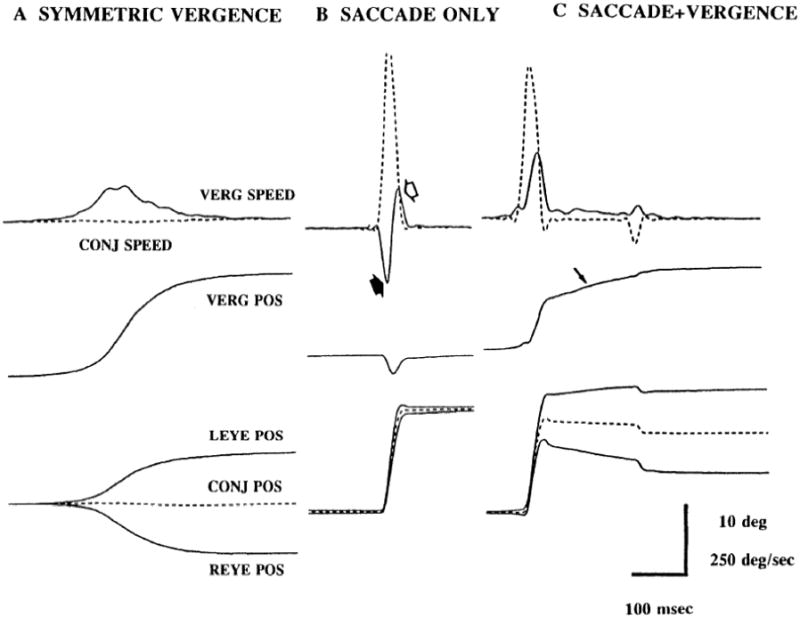
Non-addictively of saccades and vergence. A. Saccade-free symmetric convergence. B. Conjugate saccade. C. Saccade + Vergence. In panel C where the saccade and vergence occur together, peak vergence speed (upper traces, solid lines) is greater than in panel A and peak saccade speed (upper traces, dashed lines) is less than in panel B. From Maxwell and King, 1992.
A study of disjunctive nystagmus in achiasmatic Belgian sheep dogs provided additional behavioral evidence that the movements of the two eyes are not rigidly yoked by anatomy (Dell’Osso, 1994; Dell’Osso & Williams, 1995). In these animals, each eye appeared to move independently in amplitude and direction suggesting an anatomical substrate for uniocular movement. Similar to the canine data reported by Dell’Osso, Zhou and King (1997) showed that binocular control of eye position in nonhuman primates vanishes with sleep and uniocular saccades or slow drifts in any direction occur frequently (see Figure 6).
Figure 6.
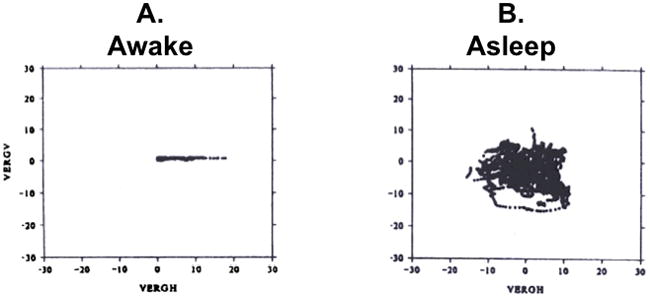
Binocular control of eye position in the monkey. A. Awake state. B. Sleep state. In both panels, the plotted data points are spontaneous ocular fixations. The ordinate is vertical vergence and the abscissa is horizontal vergence. When the monkey is asleep, vertical and horizontal vergence are uncorrelated and may take on positive and negative values. When the animal is awake, vertical vergence is zero and horizontal vergence is positive (convergent eye positions). From Zhou and King, 1997.
Neurophysiological evidence for Hering’s law and possible explanations for its failures were not obtained until the technical development of single unit extracellular recording in alert animals (Evarts, 1968; Fuchs & Luschei, 1970a). With the advent of extracellular recording, the neural information encoded by spike trains of extraocular motoneurons and premotor eye movement neurons could be described and analyzed quantitatively in relation to eye movements. Data from these experiments suggest that binocular coordination is neither entirely consistent with Hering’s Law nor with Helmholtz’ assumption of independent controllers for each eye. This review will focus on these recent neurophysiological findings, their interpretation, and current unresolved questions.
Hering’s Law: Evidence for Neural Control of Vergence
Hering’s Law requires that each eye be innervated by common neural commands that encode the vergence and conjugate components of an eye movement. Mays (1984) described recordings from neurons near the oculomotor nucleus (“peri-oculomotor region”) that encode vergence. These cells exhibited tonic discharge rates proportional to vergence eye position (left minus right eye position) and, for some cells, vergence eye velocity. The discharge rates of cells encoding vergence were not modulated during conjugate gaze shifts; in fact, many of them were silent when the monkey viewed distant targets and the vergence angle was zero. A representative example of a peri-oculomotor neuron during asymmetric vergence is shown in Figure 3A. During this task, a visual target is moved toward the monkey. In response, the animal executes a symmetric convergence movement; both eyes rotate nasally (traces labeled HR and HL). Figure 3B shows that the discharge rate of the neuron is proportional to the vergence angle of the eyes (Zhang, Mays, & Gamlin, 1992). Zhang et al. (1992) used antidromic stimulation to demonstrate that the axons of these cells projected to the ipsilateral medial rectus cell column but not the contralateral cell column. Although they do not project bilaterally to medial rectus motoneurons, the encoded vergence signal is likely to be the same for either eye, so these neurons are assumed to convey a common vergence motor command in accord with Hering’s Law. Judge and Cumming (1986) confirmed Mays’ original finding of peri-oculomotor cells with vergence related activity. Importantly, however, they showed that many of them also encoded a signal related to lens accommodation and were, therefore, involved in the “near response” triad of pupillary constriction, lens accommodation and ocular vergence. Zhang et al. (1992) were able to show that the mean accommodative response, averaged across “near response” cells antidromically activated from the medial rectus cell column, was effectively zero; thus the ensemble of neurons that project to medial rectus motoneurons convey a net signal related solely to vergence. The nomenclature, “near response”, implies a motor response to visual sensation (e.g., disparity or blur); consistent with this interpretation, Judge and Cumming (1986) also showed that most near response cells responded similarly to changes in vergence angle during monocular as well as binocular viewing; vision was not required for a cells’ response (Figure 3C).
Figure 3.
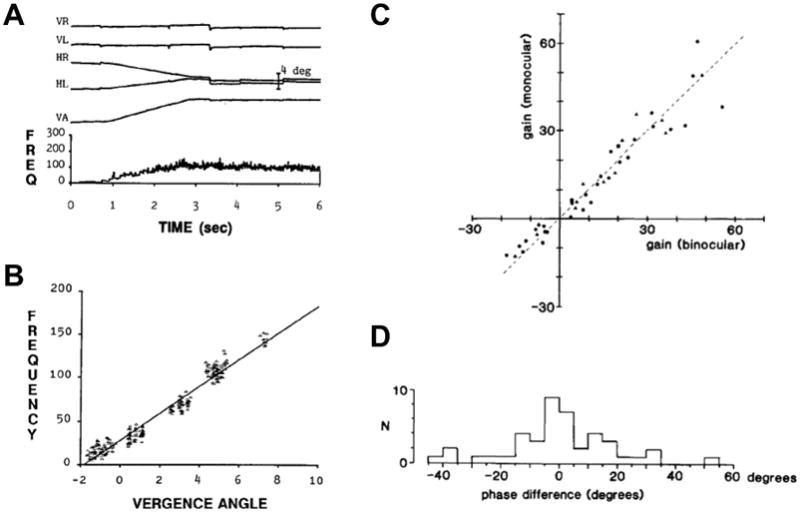
The behavior of a near response cell. A. The firing rate of the near response cell (bottom trace) increased for a ~6 degree convergence eye movement (VA = vergence angle). The top four traces are the horizontal and vertical eye positions for left and right eyes (HL, VL, HR, VR). B Isa scatter plot of steady firing rate as a function of convergence angle. C. Sensitivity of 43 near-response cells compared in normal binocular viewing (abscissa) and monocular viewing (ordinate). D. Histogram of the number of near-response cells (N) with various differences in phase between normal and monocular viewing. Panel A is from Zhang et al. 1991; panel B is from Judge and Cumming, 1986.
These studies left unresolved the source of the inputs to near response cells; are they from pathways historically associated with the near response (e.g., visual pathways in the pretectum, Büttner-Ennever, Cohen, Horn, & Reisine, 1996a) or perhaps from eye movement related cells in the pontine or mesencephalic reticular formations or the cerebellum? For example, later studies suggest that at least part of the vergence signal encoded by these cells might reflect a difference signal derived from neural integrators associated with each eye (King & Zhou, 2002; McConville, Tomlinson, King, Paige, & Na, 1994).
Hering’s Law: Evidence for Neural Control of Conjugacy
Inspired by Bender’s seminal study of clinical lesions of the brainstem in humans (Bender, 1964), studies in monkeys showed that lesions of the paramedian pontine reticular formation (PPRF) cause an ipsilateral palsy of conjugate gaze (Goebel, Komatsuzaki, Bender, & Cohen, 1971; Henn, Lang, Hepp, & Reisine, 1984). Single unit studies described neurons in the PPRF near the abducens nucleus that exhibit eye movement related activity during ipsilateral saccades and fixations (“burst neurons”, Cohen & Henn, 1972; Hepp & Henn, 1983; Keller, 1974; Luschei & Fuchs, 1972) that project to the abducens nucleus (Hikosaka, Igusa, Nakao, & Shimazu, 1978; Langer, Kaneko, Scudder, & Fuchs, 1986). Based on the lesion and physiological data, the PPRF is known as the “conjugate gaze center” wherein a common motor command for conjugate horizontal eye movements is assembled and conveyed to motoneurons of both eyes.
Baker and Highstein (1975) and Highstein and Baker (1978) described a pathway in the medial longitudinal fasciculus (MLF) that links “internuclear” neurons in the abducens nuclei to medial rectus motoneurons of the opposite eye. Recordings from MLF fibers showed that the axons of internuclear fibers encode horizontal eye position and velocity signals that are similar to those of abducens motoneurons (King, Lisberger, & Fuchs, 1976; Pola & Robinson, 1978). Gamlin et al. (1989) later confirmed this result by recording antidromically identified abducens internuclear cells. The dendritic trees of abducens motoneurons and internuclear neurons are comingled within the abducens nucleus, an arrangement that suggests they share common inputs. Thus, synaptic inputs to the abducens nucleus could activate lateral rectus motoneurons of the ipsilateral eye and simultaneously activate internuclear neurons. Since internuclear neurons, via the MLF pathway, activate medial rectus motoneurons of the opposite eye, this pathway facilitates conjugate movements of the two eyes. Anatomically and functionally, the internuclear pathway is in apparent accord with Hering’s hypothesis (see Figure 1A). During disjunctive eye movements, however, abducens motoneurons and internuclear neurons also encode a signal related to the vergence component of the eye movement, an unexpected finding that is in apparent conflict with Hering’s Law (Gamlin et al., 1989).
Lesions of the MLF in monkeys (Evinger, Fuchs, & Baker, 1977) severed the internuclear pathway that links ipsilateral internuclear neurons with contralateral medial rectus motoneurons. The experimental lesion caused bilateral paralysis of adduction during conjugate eye movements but preserved adduction related to slow vergence movements consistent with mesencephalic near response cells being a source of a vergence signal to medial rectus motoneurons.
Similar deficits are observed in human clinical studies where infarcts or conduction failures caused by multiple sclerosis disrupt signal transmission in the MLF (“internuclear ophthalmoplegia, INO”, Leigh & Zee, 2006). If a target aligned with one eye is rapidly stepped toward or away from the subject (Mueller’s Paradigm, Figure 1), disjunctive saccades and vergence are produced. According to Hering’s Law, separate rapid vergence and conjugate saccadic commands are summated on medial rectus motoneurons of the adducting (non-aligned) eye (right eye, Figure 1A). Chen et al. (2010) hypothesized that, peak adduction velocity of the affected eye of patients with INO should be greater during disjunctive saccades compared to conjugate saccades because mesencephalic vergence burst neurons (Mays, Porter, Gamlin, & Tello, 1986) are able to drive the adductive component of the disjunctive eye movement (Figure 1A, pathway indicated by green arrow). During conjugate saccades, the INO interrupts or weakens the ascending conjugate command (Figure 1A, red pathway). However, Chen et al. failed to support this hypothesis, as they found no evidence for a substantial contribution of the hypothesized vergence velocity pulse from mesencephalic near response cells. Instead, they suggested that disjunctive saccadic pulses produced by monocular burst neurons (as in Figure 1B) provided a better explanation of their data (Chen et al., 2010).
Helmholtz: Evidence for Monocular Control of Each Eye
During a study in my laboratory of how viewing distance influenced the gain of the vestibulo-ocular reflex, we were surprised to find that vestibular neurons (position-vestibular-pause, PVP) encode uniocular eye position; that is, they encode the orbital position of the left or right eye but not conjugate eye position (McConville et al., 1994) as previously assumed (Fuchs & Kimm, 1975). PVP cells are excitatory second order neurons in the vestibular ocular reflex and synapse directly onto contralateral abducens motoneurons (Scudder & Fuchs, 1992). The eye position signal is assumed to be created in a neural network that integrates eye velocity commands produced, for example, by PPRF burst neurons (Robinson, 1973). The integrator circuit includes neurons in the nucleus prepositus hypoglossi (NPH) and the medial vestibular nucleus where the PVP cells are located (Baker, 1977; Baker & Berthoz, 1975; McFarland & Fuchs, 1992). Thus, the discovery of distinct populations of vestibular neurons that encode uniocular eye position implies that the oculomotor integrator is also uniocular. This conclusion appears to conflict with Hering’s Law, but it is possible that the uniocular responses of PVP neurons represent central summation of separate conjugate and vergence eye movement commands. For example, vergence velocity neurons in the midbrain and PPRF burst cells (representing the conjugate gaze command) might project to left and right eye integrator networks to produce the uniocular signal. To test this hypothesis, we recorded from PPRF burst neurons and NPH neurons during disjunctive saccades. Figure 4A shows that 79% of the pontine burst neurons encoded a uniocular saccadic command with about 50% preferring the ipsilateral eye (“ocular selectivity”, Zhou & King, 1998). These data were later confirmed by van Horn et al., who found a similar proportion of monocular burst neurons (2008). Similarly, 78% of our recorded NPH neurons encoded monocular eye position (Figure 4B), a finding that was later confirmed by Sylvestre, et al. (2003).
Figure 4.
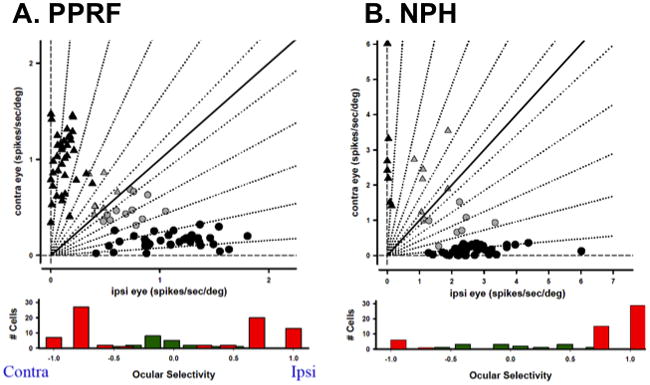
Monocular encoding of eye movement. A, Upper panel, regression coefficients for saccadic burst neurons in the PPRF. Black symbols represent Cells with monocular discharge patterns; grey symbols represent binocular units. Circles represent cells with ipsilateral (ipsi) eye preference, and triangles represent cells with contralateral (contra) eye preference. Lower panel, distribution of ocular selectivity. Red bars correspond to monocular neurons, green barsto binocular neurons. B. NPH neurons, format similar to A. Panel A is after Zhou and King, 1998.
Within the PPRF, neurons that encode left and right eye saccadic velocity are comingled. Thus, lesions of the PPRF destroy left and right eye neurons and result in conjugate gaze palsy. Similarly, microstimulation evokes ipsilateral conjugate saccades since both populations of cells are activated.
Uniocular encoding of eye movement position and velocity is a serious challenge to the concept of a “conjugate” gaze center and to Hering’s hypothesis. We created a model based on uniocular eye movement signals and showed that it could simulate correctly the waveforms characteristic of disjunctive saccades (King and Zhou 2002). Busettini and Mays (2005) and Kumar (2006) used simulations to show that disjunctive saccade dynamics could still be accounted for by an interaction of conjugate and vergence saccadic commands. However, Van Horn et al. (2008) used a careful quantitative analysis to show that the uniocular saccadic command produced by PPRF burst neurons is sufficient to produce disjunctive saccades with correct dynamics without an additional vergence contribution. The failure to find evidence for a mesencephalic vergence pulse in INO patients (Chen et al. 2010) supports Van Horn et al. and the concept of separate right and left eye controllers.
Extraocular Motoneuron Discharge Patterns
Motoneurons are traditionally considered the “final common pathway” so regardless of how premotor commands are organized, one would expect an extraocular motoneuron to encode a signal related to the eye it innervates. The discharge pattern of extraocular motoneurons was first described in 1970 (Fuchs & Luschei, 1970b; Robinson, 1970; Schiller, 1970). Two broad findings emerged from these seminal studies: first, motoneuron firing rate is characterized by a linear sum of terms related to eye position, eye velocity and eye acceleration. Second, all motoneurons appeared to have the same discharge pattern (called “burst-tonic”, tonic discharge proportional to orbital position and a burst of spikes during saccades) and were assumed to participate in all types of eye movements: saccadic, pursuit, or fixation. However, recordings were not made during vergence so there remained a possibility that a separate set of motoneurons might drive vergence eye movements. Keller and Robinson (1972) recorded abducens motoneuron activity during accommodative vergence and reported that changes in discharge rate during vergence in their recorded cell population were similar to changes in discharge rate produced during conjugate eye movements. Subject to the important caveat that they may not have sampled smaller motoneurons, Keller and Robinson concluded that their data supported summation of independent vergence and conjugate commands, the “net result appearing as activity in a shared final common path” (Keller and Robinson, 1972).
The well-replicated observation that abducens motoneurons and internuclear neurons encode signals related to vergence (Gamlin et al. 1989; Keller and Robinson 1972; Mays and Porter 1984) is unexpected. The vergence signal is inappropriate as it causes abducens motoneurons and internuclear neurons to discharge at higher rates for an eye position achieved by convergence than they would for the same eye position achieved by a conjugate movement (Gamlin et al 1989). Zhou and King reinterpreted these data (1998) after recording abducens motoneuron axons during a smooth pursuit task based on Mueller’s paradigm (“monocular pursuit”). When the monkey pursues a target aligned with an eye (Figure 5A), only the un-aligned eye’s position changes (blue trace). In this condition, the firing rate of a motoneuron associated with the un-aligned (moving) eye changes with the position and velocity of the eye (Figure 5A, left panel). Surprisingly, when the target is aligned with the other eye (Figure 5A, right panel), the firing rate of the motoneuron is still modulated, even though the innervated (aligned) eye is stationary. Sylvestre and Cullen (2002) and Van Horn and Cullen (2009) reported similar findings that supported the interpretation of Zhou and King.
Figure 5.
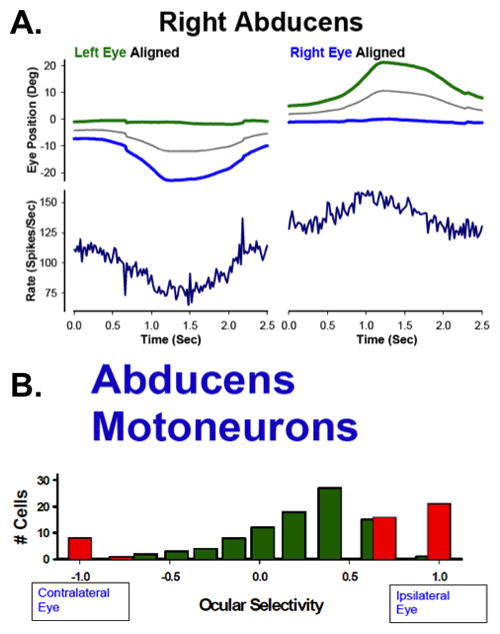
Discharge pattern of an abducens motoneuron axon during disjunctive smooth pursuit. A. Left panel: Mueller’s paradigm with left eye aligned. The motoneuron’s firing rate is modulated with movement of the ipsilateral (right) eye. Right panel: Mueller’s paradigm with right eye aligned. The motoneuron’s firing rate is paradoxically modulated with movement of the contralateral (left) eye. B. Ocular selectivity of identified abducens motoneurons. After Zhou and King, 1998.
The analysis of motoneuron activity during asymmetric vergence and disjunctive saccades into “right” and “left” eye related activity (Figure 5B) is consistent with the apparent monocular encoding scheme of the premotor brainstem circuitry. The actual finding, however, is similar to that of Mays and Porter since the change in contralateral eye position is also a change in vergence. However, the monocular pursuit paradigm focuses attention on the paradoxical nature of the result; if one assumes that abducens nerve activity translates into muscle force, then that force must be countered by activity of the agonist muscle (medial rectus co-contraction) or it must somehow be ineffective in rotating the eye. Miller et al. (2002) recorded medial and lateral rectus muscle force using miniature transducers implanted on the muscle tendons during a similar pursuit task that also elicited asymmetric vergence. Their data failed conclusively to provide evidence for co-contraction; if anything, muscle forces actually declined slightly. Despite the elegance and appeal of linear push-pull models relating motoneuron discharge rate to eye movement (for a review of this topic, see Robinson, 1981), the “missing force” during asymmetric vergence movements suggests there is a fundamental problem in understanding the conversion of motoneuron discharges into muscle force and with the assumption of a “final common path” in binocular control of eye movements.
Discussion
Hering versus Helmholtz
The controversy between Helmholtz and Hering reflected the empiricist versus nativist philosophical viewpoints held respectively by each man and his followers. Helmholtz argued that the “connection existing between the two eyes is not an obligatory anatomical mechanism, but is something that can be altered by the mere influence of our own volition” (Helmholtz 1962). He described his own experiences of double vision when drowsy as examples of volitional control; “if the movements of the eye were coordinated by some anatomical mechanical contrivance, it might be expected to function with even less resistance in the state of drowsiness, when the energy of the will is in abeyance”. Consistent with Helmholtz’ observations, eye movements in monkeys are uncoordinated during sleep, and uniocular movements in any direction may occur resulting in large divergent vertical and horizontal eye positions (Zhou & King, 1997). Figure 6A shows vertical vergence angle plotted against horizontal vergence angle in the awake monkey. The vertical vergence is nearly zero at all times and the horizontal vergence is always convergent (positive). When the monkey is asleep, horizontal and vertical vergence angles are uncorrelated and take on positive and negative values (Figure 6B). The apparently random slow eye movements characteristic of sleep suggest that the oculomotor integrator does not function during sleep and that binocular coordination is, in turn, dependent on integrator function and the exercise of volitional control (Schreyer, Büttner-Ennever, Tang, Mustari, & Horn, 2009).
An important aspect of Helmholtz’ view of binocular coordination is that it necessarily is a learned behavior. Conjugate saccade accuracy is under adaptive control and has been intensively studied over the past 2 decades (for review, see Iwamoto & Kaku, 2010). In contrast, the accuracy of disjunctive saccades has not been systematically studied. The data reviewed above, however, suggest that the same burst generator circuits that produce conjugate saccades also produce the saccadic component of each eye’s movement during disjunctive saccades (Figure 1B and Chen et al. 2010). If this assertion is true, then any adaptive mechanism that corrects conjugate saccade components should also correct disjunctive saccade components monocularly in each eye. This assertion requires reconsideration of how and where each eye’s saccade amplitude is encoded. The superior colliculus is believed to play a major role in determining conjugate saccade amplitude and direction (Iwamoto and Kaku 2010). However, efforts to document its role in producing disjunctive saccades have not been successful (Walton & Mays, 2003). Other studies have found, however, associated vergence responses in cells located in the rostral superior colliculus (in monkey, Chaturvedi & Van_Gisbergen, 2000; in cat, Suzuki, Suzuki, & Ohtsuka, 2004). The primate’s central mesencephalic reticular formation (cMRF) may also play a role in determining monocular saccade metrics (Cromer & Waitzman, 2006; Cromer & Waitzman, 2007) since many cells in the cMRF encode uniocular components of disjunctive saccades similar to cells in the PPRF (van Horn et al. 2008) (Waitzman, Van Horn, & Cullen, 2008). Additional studies are needed to investigate the role of the cerebellum, superior colliculus and cMRF in generating disjunctive saccades and in controlling the accuracy of the components in each eye.
Is Hering Off the Hook?
The discovery that uniocular burst cells in the PPRF are sufficient to produce the vergence changes associated with disjunctive saccades would appear to get Hering off the hook (King and Zhou 2002; van Horn et al. 2008; Mays, 1998). Significant questions remain, however, about the role of the mesencephalic near response neurons and the sources of their inputs. These cells do, in fact, provide a tonic vergence signal common to both eyes. However, one might hypothesize that mesencephalic vergence signal has two, functionally different sources: one source may be the oculomotor integrators that encode each eye’s position (King & Zhou, 2000; King & Zhou, 2002); the other sources are visual disparity signals conveyed by pretectal pathways (Judge and Cumming 1986; Büttner-Ennever, Cohen, Horn, & Reisine, 1996b). A signal encoding the vergence angle of the eyes could be constructed by subtraction of the monocular left and right eye position signals encoded by NPH neurons (Zhou and King 1998; King and Zhou 2002). This vergence signal would be proportional to the vergence angle produced by the PPRF saccade burst generator, since the outputs of the burst cells are integrated within the NPH. The left minus right difference signal would contribute to the tonic discharge of near response cells that project to medial rectus motoneurons (Zhang et al. 1991) or could, via the internuclear pathway or vestibular pathways, contribute to the vergence related discharge of medial rectus motoneurons. Alternatively, retrograde tracer studies have provided evidence for separate populations of “slow” extraocular motoneurons that synapse on multiply innervated muscle fibers and “fast” motoneurons that synapse on singly innervated fast-twitch muscle fibers. Slow motor units might selectively encode eye position signals for disparity driven vergence and fixation whilst fast motoneurons might encode rapid eye movement signals for saccades and the VOR (Horn et al., 2008; Ugolini et al., 2006). The anatomical evidence supports different inputs to these two groups of motoneurons. For example, separate vergence signals from the NPH and peri-oculomotor region might preferentially synapse on distinct populations of “slow” motoneurons in the abducens and oculomotor nuclei. Functionally, these inputs could provide a fixation signal for conjugate eye position and a disparity driven vergence signal to fine-tune vergence eye position in association with disjunctive saccades. Direct inputs from PPRF burst cells would preferentially innervate “fast” motoneurons (the cells most commonly recorded in the extraocular motor nuclei). It is beyond the scope of this review to discuss the complexity of extraocular muscles. However, the suggestion that the multiply innervated “slow” fibers are predominantly concerned with vergence is a clear departure from the notion that the extraocular motoneuron is a final common pathway for all types of eye movement. Because of their small size, slow motoneurons were probably under sampled or missed entirely in extracellular recording studies, so we have no direct confirmation that they actually convey a signal related to vergence and/or conjugate eye position (e.g., do they encode vergence or uniocular eye position?). Therefore, until there are additional data, Hering may still be on the hook, at least for disparity driven vergence and binocular fixation.
Acknowledgments
Wu Zhou for his many intellectual contributions and unflagging enthusiasm for understanding how eye movements are binocularly controlled. A special acknowledgement of the late Dave Tomlinson’s very important contributions to understanding monocular eye position signals encoded by vestibular neurons. This work was supported by NIH R21 DC009293.
Bibliography
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Mathematical Biosciences. 1975;24(3–4):191–204. doi: 10.1016/00255564(75)90075-9. [DOI] [Google Scholar]
- Baker R. The nucleus prepositus hypoglossi. Eye movements: ARVO symposium; 1976.1977. [Google Scholar]
- Baker R, Berthoz A. Is the prepositus hypoglossi nucleus the source of another vestibulo-ocular pathway? Brain Research. 1975;86(1):121–7. doi: 10.1016/0006-8993(75)90643-5. [DOI] [PubMed] [Google Scholar]
- Baker R, Highstein SM. Physiological identification of interneurons and motoneurons in the abducens nucleus. Brain Research. 1975;91(2):292–8. doi: 10.1016/0006-8993(75)90551-x. [DOI] [PubMed] [Google Scholar]
- Bender MB. The oculomotor system. Academic Medicine. 1964;39(9):868. [Google Scholar]
- Busettini C, Mays LE. Saccade-Vergence interactions in macaques. II. Vergence enhancement as the product of a local feedback vergence motor error and a weighted saccadic burst. Journal of Neurophysiology. 2005;94(4):2312–30. doi: 10.1152/jn.01337.2004. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Cohen B, Horn AK, Reisine H. Efferent pathways of the nucleus of the optic tract in monkey and their role in eye movements. The Journal of Comparative Neurology. 1996a;373(1):90–107. doi: 10.1002/(SICI)1096-9861(19960909)373:1<90::AID-CNE8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Cohen B, Horn AK, Reisine H. Pretectal projections to the oculomotor complex of the monkey and their role in eye movements. The Journal of Comparative Neurology. 1996b;366(2):348–59. doi: 10.1002/(SICI)1096-9861(19960304)366:2<348::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Van_Gisbergen JA. Stimulation in the rostral pole of monkey superior colliculus: Effects on vergence eye movements. Experimental Brain Research. 2000;132(1):72–8. doi: 10.1007/s002219900221. [DOI] [PubMed] [Google Scholar]
- Chen AL, Ramat S, Serra A, King SA, Leigh RJ. The role of the medial longitudinal fasciculus in horizontal gaze: Tests of current hypotheses for saccade-vergence interactions. Experimental Brain Research. Experimentelle Hirnforschung. Expérimentation Cérébrale; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, Henn V. Unit activity in the pontine reticular formation associated with eye movements. Brain Research. 1972;46:403–10. doi: 10.1016/0006-8993(72)90030-3. [DOI] [PubMed] [Google Scholar]
- Cromer JA, Waitzman DM. Neurones associated with saccade metrics in the monkey central mesencephalic reticular formation. The Journal of Physiology. 2006;570(Pt 3):507–23. doi: 10.1113/jphysiol.2005.096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer JA, Waitzman DM. Comparison of saccade-associated neuronal activity in the primate central mesencephalic and paramedian pontine reticular formations. Journal of Neurophysiology. 2007;98(2):835–50. doi: 10.1152/jn.00308.2007. [DOI] [PubMed] [Google Scholar]
- Dell’Osso LF. Evidence suggesting individual ocular motor control of each eye (muscle) Journal of Vestibular Research : Equilibrium & Orientation. 1994;4(5):335–45. [PubMed] [Google Scholar]
- Dell’Osso LF, Williams RW. Ocular motor abnormalities in achiasmatic mutant belgian sheepdogs: Unyoked eye movements in a mammal. Vision Research. 1995;35(1):109–16. doi: 10.1016/0042-6989(94)e0045-m. [DOI] [PubMed] [Google Scholar]
- Enright JT. Changes in vergence mediated by saccades. Journal of Physiology (London) 1984;350:9–31. doi: 10.1113/jphysiol.1984.sp015186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkelens CJ, Steinman RM, Collewijn H. Ocular vergence under natural conditions. II. Gaze shifts between real targets differing in distance and direction. Proceedings Royal Society of London, Series B Biological Sciences. 1989;236(1285):441–65. doi: 10.1098/rspb.1989.0031. [DOI] [PubMed] [Google Scholar]
- Evarts EV. A technique for recording activity of subcortical neurons in moving animals. Electroencephalogr Clin Neurophysiol. 1968;24(1):83–6. doi: 10.1016/0013-4694(68)90070-9. [DOI] [PubMed] [Google Scholar]
- Evinger LC, Fuchs AF, Baker R. Bilateral lesions of the medial longitudinal fasciculus in monkeys: Effects on the horizontal and vertical components of voluntary and vestibular induced eye movements. Experimental Brain Research. 1977;28(1–2):1–20. doi: 10.1007/BF00237082. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Kimm J. Unit activity in vestibular nucleus of the alert monkey during horizontal angular acceleration and eye movement. Journal of Neurophysiology. 1975;38(5):1140–1161. doi: 10.1152/jn.1975.38.5.1140. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Luschei ES. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. Journal of Neurophysiology. 1970a;33(3):382–92. doi: 10.1152/jn.1970.33.3.382. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Luschei ES. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. Journal of Neurophysiology. 1970b;33(3):382–92. doi: 10.1152/jn.1970.33.3.382. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, Gnadt JW, Mays LE. Abducens internuclear neurons carry an inappropriate signal for ocular convergence. Journal of Neurophysiology. 1989;62(1):70–81. doi: 10.1152/jn.1989.62.1.70. [DOI] [PubMed] [Google Scholar]
- Goebel HH, Komatsuzaki A, Bender MB, Cohen B. Lesions of the pontine tegmentum and conjugate gaze paralysis. Archives of Neurology. 1971;24:431–440. doi: 10.1001/archneur.1971.00480350065007. [DOI] [PubMed] [Google Scholar]
- Helmholtz H. Helmholtz’s treatise on physiological optics. New York: Dover; 1962. [Google Scholar]
- Henn V, Lang W, Hepp K, Reisine H. Experimental gaze palsies in monkeys and their relation to human pathology. Brain : A Journal of Neurology. 1984;107(Pt 2):619–36. doi: 10.1093/brain/107.2.619. [DOI] [PubMed] [Google Scholar]
- Hepp K, Henn V. Spatio-Temporal recoding of rapid eye movement signals in the monkey paramedian pontine reticular formation (PPRF) Experimental Brain Research. Experimentelle Hirnforschung. Expérimentation Cérébrale. 1983;52(1):105–20. doi: 10.1007/BF00237155. [DOI] [PubMed] [Google Scholar]
- Hering E. The theory of binocular vision. New York: Plenum Press; 1977. [Google Scholar]
- Highstein SM, Baker R. Excitatory termination of abducens internuclear neurons on medial rectus motoneurons: Relationship to syndrome of internuclear ophthalmoplegia. Journal of Neurophysiology. 1978;41(6):1647–61. doi: 10.1152/jn.1978.41.6.1647. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Igusa Y, Nakao S, Shimazu H. Direct inhibitory synaptic linkage of pontomedullary reticular burst neurons with abducens motoneurons in the cat. 3–4. Vol. 33. Experimental Brain Research. Experimentelle Hirnforschung. Expérimentation Cérébrale; 1978. pp. 337–52. [DOI] [PubMed] [Google Scholar]
- Horn AK, Eberhorn A, Härtig W, Ardeleanu P, Messoudi A, Büttner-Ennever JA. Perioculomotor cell groups in monkey and man defined by their histochemical and functional properties: Reappraisal of the edinger-westphal nucleus. The Journal of Comparative Neurology. 2008;507(3):1317–35. doi: 10.1002/cne.21598. [DOI] [PubMed] [Google Scholar]
- Howard IP, Rogers BJ. Oxford Psychology Series, No. 29: Binocular vision and stereopsis. New York: Oxford University Press; 1996. [Google Scholar]
- Iwamoto Y, Kaku Y. Saccade adaptation as a model of learning in voluntary movements. Experimental Brain Research. Experimentelle Hirnforschung Expérimentation Cérébrale. 2010;204(2):145–62. doi: 10.1007/s00221-010-2314-3. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Cumming BG. Neurons in the monkey midbrain with activity related to vergence eye movement and accommodation. Journal of Neurophysiology. 1986;55(5):915–30. doi: 10.1152/jn.1986.55.5.915. [DOI] [PubMed] [Google Scholar]
- Keller EL. Participation of medial pontine reticular formation in eye movement generation in monkey. Journal of Neurophysiology. 1974;37(2):316–32. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- Keller EL, Robinson DA. Abducens unit behavior in the monkey during vergence movements. Vision Research. 1972;12(3):369–82. doi: 10.1016/0042-6989(72)90082-x. [DOI] [PubMed] [Google Scholar]
- Kenyon RV, Ciuffreda KJ, Stark L. Unequal saccades during vergence. American Journal of Optometry and Physiological Optics. 1980;57:586–594. doi: 10.1097/00006324-198009000-00009. [DOI] [PubMed] [Google Scholar]
- King WM, Zhou W. New ideas about binocular coordination of eye movements: Is there a chameleon in the primate family tree? The Anatomical Record (New Anat) 2000;261(4):153–161. doi: 10.1002/1097-0185(20000815)261:4<153::AID-AR4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- King WM, Zhou W. Neural basis of disjunctive eye movements. Annals of the New York Academy of Sciences. 2002;956:273–283. doi: 10.1111/j.1749-6632.2002.tb02826.x. [DOI] [PubMed] [Google Scholar]
- King WM, Lisberger SG, Fuchs AF. Responses of fibers in medial longitudinal fasciculus (MLF) of alert monkeys during horizontal and vertical conjugate eye movements evoked by vestibular or visual stimuli. Journal of Neurophysiology. 1976;39(6):1135–49. doi: 10.1152/jn.1976.39.6.1135. [DOI] [PubMed] [Google Scholar]
- Kumar AN, Han YH, Kirsch RF, Dell’Osso LF, King WM, Leigh RJ. Tests of models for saccade-vergence interaction using novel stimulus conditions. Biological Cybernetics. 2006;95(2):143–57. doi: 10.1007/s00422-006-0073-9. [DOI] [PubMed] [Google Scholar]
- Langer T, Kaneko CR, Scudder CA, Fuchs AF. Afferents to the abducens nucleus in the monkey and cat. Journal of Comparative Neurology. 1986;245(3):379–400. doi: 10.1002/cne.902450307. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. 4. Vol. 70. New York: Oxford University Press; 2006. Contemporary Neurology Series. [Google Scholar]
- Luschei ES, Fuchs AF. Activity of brain stem neurons during eye movements of alert monkeys. Journal of Neurophysiology. 1972;35(4):445–61. doi: 10.1152/jn.1972.35.4.445. [DOI] [PubMed] [Google Scholar]
- Maxwell JS, King WM. Dynamics and efficacy of saccade-facilitated vergence eye movements in monkeys. Journal of Neurophysiology. 1992;68(4):1248–60. doi: 10.1152/jn.1992.68.4.1248. [DOI] [PubMed] [Google Scholar]
- Mays L. Has hering been hooked? [News] Nat Med. 1998;4(8):889–90. doi: 10.1038/nm0898-889. [DOI] [PubMed] [Google Scholar]
- Mays LE. Neural control of vergence eye movements: Convergence and divergence neurons in midbrain. Journal of Neurophysiology. 1984;51(5):1091–1108. doi: 10.1152/jn.1984.51.5.1091. [DOI] [PubMed] [Google Scholar]
- Mays LE, Porter JD, Gamlin PD, Tello CA. Neural control of vergence eye movements: Neurons encoding vergence velocity. Journal of Neurophysiology. 1986;56(4):1007–21. doi: 10.1152/jn.1986.56.4.1007. [DOI] [PubMed] [Google Scholar]
- McConville K, Tomlinson RD, King WM, Paige G, Na EQ. Eye position signals in the vestibular nuclei: Consequences for models of integrator function. Journal of Vestibular Research. 1994;4(5):391–400. [PubMed] [Google Scholar]
- McFarland JL, Fuchs AF. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques. Journal of Neurophysiology. 1992;68(1):319–332. doi: 10.1152/jn.1992.68.1.319. [DOI] [PubMed] [Google Scholar]
- Miller JM, Bockisch CJ, Pavlovski DS. Missing lateral rectus force and absence of medial rectus co-contraction in ocular convergence. Journal of Neurophysiology. 2002;87(5):2421–33. doi: 10.1152/jn.00566.2001. [DOI] [PubMed] [Google Scholar]
- Ono H, Nakamizo S, Steinbach M. Non-Additivity of vergence and saccadic eye movement. Vision Research. 1978;18:735–739. doi: 10.1016/0042-6989(78)90152-9. [DOI] [PubMed] [Google Scholar]
- Pola J, Robinson DA. Oculomotor signals in medial longitudinal fasciculus of the monkey. Journal of Neurophysiology. 1978;41(2):245–59. doi: 10.1152/jn.1978.41.2.245. [DOI] [PubMed] [Google Scholar]
- Rashbass C, Westheimer G. Disjunctive eye movements. Journal of Physiology (London) 1961a;159:339–360. doi: 10.1113/jphysiol.1961.sp006812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass C, Westheimer G. Independence of conjugate and disjunctive eye movements. Journal of Physiology (London) 1961b;159:361–364. doi: 10.1113/jphysiol.1961.sp006813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. Oculomotor unit behavior in the monkey. Journal of Neurophysiology. 1970;33(3):393–403. doi: 10.1152/jn.1970.33.3.393. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Oculomotor control system. Invest Ophthalmol. 1973;12(3):164–6. [PubMed] [Google Scholar]
- Robinson DA. Control of eye movements. In: Brookhart JM, Mountcastle VB, editors. Handbook of physiology section 1: The nervous system volume II motor control. part 2. Bethesda: American Physiological Society; 1981. pp. 1275–320. [Google Scholar]
- Schiller PH. The discharge characteristics of single units in the oculomotor and abducens nuclei of the unanesthetized monkey. Experimental Brain Research. Experimentelle Hirnforschung Expérimentation Cérébrale. 1970;10(4):347–62. doi: 10.1007/BF02324764. [DOI] [PubMed] [Google Scholar]
- Schreyer S, Büttner-Ennever JA, Tang X, Mustari MJ, Horn AK. Orexin-A inputs onto visuomotor cell groups in the monkey brainstem. Neuroscience. 2009;164(2):629–40. doi: 10.1016/j.neuroscience.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. Journal of Neurophysiology. 1992;68(1):244–64. doi: 10.1152/jn.1992.68.1.244. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Suzuki Y, Ohtsuka K. Convergence eye movements evoked by microstimulation of the rostral superior colliculus in the cat. Neuroscience Research. 2004;49(1):39–45. doi: 10.1016/j.neures.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Cullen KE. Dynamics of abducens nucleus neuron discharges during disjunctive saccades. Journal of Neurophysiology. 2002;88(6):3452–68. doi: 10.1152/jn.00331.2002. [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Choi JT, Cullen KE. Discharge dynamics of oculomotor neural integrator neurons during conjugate and disjunctive saccades and fixation. Journal of Neurophysiology. 2003;90(2):739–54. doi: 10.1152/jn.00123.2003. [DOI] [PubMed] [Google Scholar]
- Turner RS, Benschop R. In the eye’s mind: Vision and the helmholtz-hering controversy. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Ugolini G, Klam F, Doldan Dans M, Dubayle D, Brandi AM, Büttner-Ennever J, Graf W. Horizontal eye movement networks in primates as revealed by retrograde transneuronal transfer of rabies virus: Differences in monosynaptic input to” slow” and “fast” abducens motoneurons. Journal of Comparative Neurology. 2006;498(6):762–785. doi: 10.1002/cne.21092. [DOI] [PubMed] [Google Scholar]
- Van Horn MR, Cullen KE. Dynamic characterization of agonist and antagonist oculomotoneurons during conjugate and disconjugate eye movements. Journal of Neurophysiology. 2009;102(1):28–40. doi: 10.1152/jn.00169.2009. [DOI] [PubMed] [Google Scholar]
- Van Horn MR, Sylvestre PA, Cullen KE. The brain stem saccadic burst generator encodes gaze in three-dimensional space. Journal of Neurophysiology. 2008;99(5):2602–16. doi: 10.1152/jn.01379.2007. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Van Horn MR, Cullen KE. Neuronal evidence for individual eye control in the primate cmrf. Progress in Brain Research. 2008:143–150. doi: 10.1016/S0079-6123(08)00619-5. [DOI] [PubMed] [Google Scholar]
- Walton MM, Mays LE. Discharge of saccade-related superior colliculus neurons during saccades accompanied by vergence. Journal of Neurophysiology. 2003;90(2):1124–39. doi: 10.1152/jn.00877.2002. [DOI] [PubMed] [Google Scholar]
- Zee DS, Fitzgibbon EJ, Optican LM. Saccade-Vergence interactions in humans. Journal of Neurophysiology. 1992;68(5):1624. doi: 10.1152/jn.1992.68.5.1624. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mays LE, Gamlin PDR. Characteristics of near response cells projecting to the oculomotor nucleus. Journal of Neurophysiology. 1992;67:944–960. doi: 10.1152/jn.1992.67.4.944. [DOI] [PubMed] [Google Scholar]
- Zhou W, King WM. Binocular eye movements not coordinated during REM sleep. Experimental Brain Research. 1997;117(1):153–60. doi: 10.1007/s002210050209. [DOI] [PubMed] [Google Scholar]
- Zhou W, King WM. Premotor commands encode monocular eye movements. Nature. 1998;393(6686):692–5. doi: 10.1038/31489. [DOI] [PubMed] [Google Scholar]


