Abstract
Background
The detrimental effects of smoking on risk of myocardial infarction and stroke are well documented, but less information is available regarding peripheral artery disease (PAD), particularly among women.
Objective
To prospectively assess the association of current smoking status, cumulative smoking exposure and smoking cessation with incident symptomatic PAD in women
Design
Prospective cohort study
Setting
U.S. female health care professionals in the Women's Health Study
Participants
39825 women free of cardiovascular disease were prospectively followed for a median of 12.7 years.
Measurements
Incident symptomatic PAD (n=178). Cox proportional hazards models were used to compare PAD risk among never (n=20336) and former smoking (n=14263) women, women who smoked <15 cigarettes/day (n=1967) and women who smoked ≥15 cigarettes/day (n=3259).
Results
Age-adjusted incidences across smoking categories were 0.12, 0.34, 0.95 and 1.63 per 1000 person-years of follow-up. Multivariable adjustment had little impact on this risk gradient; adjusted hazard ratios (HRs) (95% confidence intervals (CIs)) were 3.14 (2.01–4.90), 8.93 (5.02–15.89) and 16.95 (10.77–26.67) compared with never-smokers. Additional adjustment for high sensitivity C-reactive protein and soluble intercellular adhesion molecule 1 among women with available blood samples (n=28314, 117 events) attenuated risk estimates with HRs of 5.58 (2.61–11.93) and 9.52 (5.17–7.53) for women smoking <15 and ≥15 cigarettes/day, respectively. We found a strong dose-response relationship for lifetime exposure such that the fully adjusted HR’s for 0 (reference), <10, 10–30 and ≥30 pack years were 2.52 (1.49–4.25), 6.75 (4.33–10.52) and 11.09 (6.94–17.72). Compared to current smokers, the adjusted HRs (95% CIs) for smoking abstinence of <10 years, 10–20 years, >20 years and lifelong abstinence were 0.39 (0.24–0.66), 0.28 (0.17–0.46), 0.16 (0.10–0.26) and 0.08 (0.05–0.12).
Limitation
The use of symptomatic PAD as the primary a priori end point excludes asymptomatic disease.
Conclusion
Among initially healthy women, smoking is a potent risk factor for symptomatic PAD, an effect partially explained by subclinical inflammation. Smoking cessation substantially reduces PAD risk, but an increased occurrence of PAD persists even among former smokers who maintain abstinence.
Primary Funding Source
National Heart, Lung, and Blood Institute and National Cancer Institute
Keywords: Peripheral artery disease, cardiovascular disease, smoking, inflammation, women
INTRODUCTION
Despite the reduction in smoking prevalence achieved through recent aggressive tobacco control initiatives, one in five US adults (22.9% of men, 18.2% of women) currently report cigarette smoking (1) and the smoking prevalence is even higher in most European countries (2). Tobacco use therefore remains one of the leading preventable causes of morbidity and mortality worldwide (3–5). Indeed, a British study estimated a 10 year decline in life expectancy among male smokers compared to lifelong smoking abstinence in this population (6). A similar devastating effect of smoking on vascular, cancer and respiratory diseases has been documented in women (7).
Despite this vast body of literature, detailed analyses evaluating smoking as a risk factor for peripheral artery disease (PAD) have been limited, as most prior studies did not focus on smoking as the main exposure variable (8–14). In one of the few prospective studies providing a more comprehensive assessment, smoking >25 pack years of cigarettes in a lifetime was associated with an adjusted hazard ratio (HR) (95% confidence interval (CI)) for PAD of 2.72 (1.13–6.53) compared to lifelong abstinence. However, risk estimates were based on only 64 PAD events, with less than half of these occurring in women (13). Furthermore, the effect of smoking cessation on PAD incidence is unknown and whether smoking confers heightened risk through vascular inflammation has not been studied in detail (13).
In order to address these important issues, we evaluated the relationship of smoking and smoking cessation with symptomatic PAD defined as intermittent claudication or lower extremity artery revascularization in a large cohort of initially healthy women.
METHODS
Participants
All study subjects participated in the Women’s Health Study, a completed randomized trial evaluating the risks and benefits of low dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer. Details of the study design have been described previously (15–17). Briefly, beginning in 1993, 39,876 female health professionals in the United States who were 45 years or older and free of cardiovascular disease, cancer or other major illnesses were randomized to receive 100 mg aspirin every other day, 600 IU vitamin E every other day, both agents or placebo. Information on baseline variables was collected using mailed questionnaires. Follow-up questionnaires asking participants about study outcomes and other information were sent every six months during the first year and every 12 months thereafter. Self-reported PAD was systematically confirmed through participant interview and medical record review as described below.
For the purpose of this study, we excluded 15 participants with confirmed prerandomization PAD, and 36 participants with missing baseline information on smoking. Thus, the final study population for the present analysis consisted of 39825 women. Of these subjects, 28314 (71.1%) provided baseline blood specimens on which measurements of high sensitivity C-reactive protein and soluble intercellular adhesion molecule 1 (sICAM-1) were available for analysis (18). Written informed consent was obtained from all participants. The study was approved by the institutional review board of Brigham and Women’s Hospital, Boston, and was monitored by an external data and safety monitoring board.
Definition of Smoking Status
On the baseline questionnaire, participants answered the question “Have you smoked 100 cigarettes or more in your lifetime?” with “no”, “yes, currently smoke”, or “yes, smoked in past but quit”. Current smokers were queried regarding the average number of cigarettes they smoked per day at study entry (None, 1–4, 5–14, 15–24, 25–35, 36–44, or 45+ cigarettes per day). Participants answered the same questionnaire again at months 12, 24, 48, 72, and 96 and at the end of the randomized portion of the study, and again 3 more times during the observational follow up study. According to these questions we classified women into never smokers, past smokers, current smokers smoking <15 cigarettes per day and current smokers smoking ≥15 cigarettes per day. Participants were asked how many total years they had smoked (<5, 5–9, 10–19, 20–29, 30–39, 40–49 or 50+ years) and the number of cigarettes they had smoked per day across 8 age categories ranging from <15 to 70+ years. Pack years of cigarette smoking were calculated by multiplying the midpoint of the total number of years smoked category times the average number of cigarettes smoked per day on the baseline questionnaire. For women who reported being former smokers at enrollment, we calculated the number of years since smoking cessation by subtracting the midpoint of the age category when they reported quitting smoking from the age at enrollment.
Laboratory analyses
Blood samples were stored in liquid nitrogen (−150° to −180° C) until the time of the analysis. All blood analyses were performed in a core laboratory certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization Program. High-density lipoprotein cholesterol (HDL-C) was ascertained with direct measurement assays (Roche Diagnostics, Indianapolis, Ind). Plasma levels of high-sensitivity C-reactive protein were measured with a validated immunoturbidimetric method (Denka Seiken, Niigata, Japan). The interassay coefficients of variation using two levels of control materials ranged from 1.07 to 5.20%. sICAM-1 was measured by quantitative sandwich Enzyme Linked Immunosorbent Assay (R&D Systems, Minneapolis, Minn) with a reproducibility of 8.89% and 6.39% at concentrations of 171.8 and 289.1 ng/mL, respectively.
Outcome Ascertainment
Participants are surveyed annually for multiple health outcomes, including symptomatic PAD events, defined as intermittent claudication and/or peripheral artery surgery inclusive of catheter-based interventions. Case confirmation occurred by telephone interview with a cardiovascular physician blinded to the participants’ smoking status. The presence of vascular claudication was established using the Edinburgh Claudication Questionnaire. This instrument is a modified version of the World Health Organization/Rose Claudication Questionnaire, which has previously been validated in a community outpatient setting with 92% sensitivity and 99% specificity for physician-diagnosed intermittent claudication (19). In addition, we obtained medical records to assess the concordance of reported symptoms with diagnostic testing when available. Reports of peripheral arterial surgery or peripheral angioplasty were confirmed after review of operative notes or procedural reports, respectively. Each case was reviewed and validated based on response to the claudication questionnaire and medical record documentation of diagnostic procedures or vascular intervention. Of 695 self-reported PAD events occurring as of November 23, 2007, 178 events were confirmed utilizing these methods. Among disconfirmed events, venous disease, lower extremity arthritis, lumbar disc disease, and peripheral neuropathy were the main causes of non-ischemic leg pain. Only confirmed events were considered in the current analysis.
Statistical analyses
Baseline characteristics were age-adjusted using direct standardization for categorical variables and general linear models for continuous variables, and compared according to smoking status at study entry (never, past, current <15 cigarettes per day, current ≥15 cigarettes per day) using 3 df tests of general linear models for continuous variables and Mantel-Haenszel chi square tests for categorical variables. Individual person-years of follow-up were calculated from the date of return of the baseline questionnaire to the date of incident PAD, loss to follow-up, death or November 23, 2007, whichever came first. We then constructed a series of multivariable Cox proportional hazards models to estimate HRs and 95% CIs for incident PAD according to baseline smoking status, adjusted for age, history of hypertension, history of diabetes, history of hypercholesterolemia, body mass index, alcohol consumption and physical activity. We also constructed time-varying Cox models in which a participant’s baseline smoking status was updated according to information provided on subsequent questionnaires. We then assessed the dose-response relationship between lifelong smoking exposure and incident PAD using pack years of smoking (categorized as 0, <10, 10–29 and ≥30 pack years) with adjustment for the same variables described above. Because prior studies described an earlier age of menopause in smokers compared with nonsmokers (20) , we performed a stratified analysis according to postmenopausal status. Effect modification according to menopausal status and randomized treatment assignments was assessed using multiplicative interaction terms and likelihood ratio tests. The incidence of PAD over time across categories of pack years was estimated using Kaplan-Meier survival curves and compared with the log-rank test.
The impact of smoking cessation was evaluated by comparing the hazards of symptomatic PAD among women who indicated smoking cessation <10 years ago, 10–20 years ago and >20 years ago to that of current smokers using HRs and 95% CIs. Former smokers who did not report when they quit smoking (n=241, 1.7%) were excluded from these analyses.
In order to gain further insights in the relationship between smoking and incident PAD, and identify variables that may potentially mediate such an association, we constructed another series of Cox models among the 28314 women with available blood measures. Because of our prior findings, we focused on high-sensitivity C-reactive protein and sICAM-1, for these analyses (21, 22). Biomarkers were log-transformed to normalize the variable distribution and better meet the assumption of linearity in risk.
Tests for linear trend were performed using integer scores across categories. The proportional hazards assumption was examined by including a time by smoking interaction term into the model (23). No violation of this assumption was detected. We used a complete-case analysis in multivariate models without imputation for missing data. No exposure of interest had more than 2.1% missing data (Table 1 footnote). The sample size analyzed in each of the multivariable adjusted models is presented with the results of those models in Tables 2, 3, and 4. In the primary analysis, no multivariable model excluded more than 865 participants (2.2%) due to missing data. All analyses were carried out using SAS version 9.1 (SAS Institute Inc, Cary, NC). A two-tailed P value <0.05 was considered to indicate statistical significance.
Table 1.
Age-Adjusted Baseline Characteristics of the Study Population According to Smoking Status
| Smoking Status | |||||
|---|---|---|---|---|---|
| All women (n=39825)* | Never (n=20336) |
Past (n=14263) |
Current, <15 cigs/day (n=1967) |
Current, ≥15 cigs/day (n=3259) |
P value‡ |
| Age, years | 54.5 (54.4– 54.6) |
55.0 (54.9–55.1) | 54.1 (53.8–54.4) | 53.8 (53.6–54.0) | <0.001 |
| History of hypertension, % | 26.4 | 25.5 | 23.2 | 25.5 | 0.021 |
| History of diabetes, % | 2.9 | 2.9 | 2.7 | 3.4 | 0.193 |
| Body mass index, kg/m2 | 26.1 (26.0– 26.2) |
26.2 (26.1–26.2) | 25.3 (25.1–25.6) | 25.5 (25.3–25.7) | <0.001 |
| Exercise frequency in times/week, % | <0.001 | ||||
| Rarely/never | 37.2 | 35.1 | 44.9 | 55.1 | |
| <1 | 19.9 | 19.1 | 21.2 | 22.2 | |
| 1–3 | 32.1 | 33.3 | 27.7 | 18.9 | |
| >3 | 10.8 | 12.6 | 6.2 | 3.8 | |
| Highest education level, % | <0.001 | ||||
| Less than a bachelor’s degree | 54.4 | 56.0 | 69.0 | 74.1 | |
| Bachelor’s degree | 24.0 | 23.7 | 18.8 | 16.5 | |
| Master’s degree or doctorate | 21.6 | 20.3 | 12.2 | 9.4 | |
| Alcohol consumption, % | <0.001 | ||||
| Rarely/never | 53.2 | 34.2 | 40.3 | 45.1 | |
| 1–3 drinks per month | 13.1 | 13.4 | 12.5 | 13.1 | |
| 1–6 drinks per week | 27.7 | 38.0 | 33.0 | 26.3 | |
| ≥1 drink per day | 6.0 | 14.4 | 14.2 | 15.6 | |
| History of hypercholesterolemia, % | 29.2 | 29.4 | 28.8 | 31.0 | 0.022 |
| Menopausal status | 52.9 | 54.6 | 58.9 | 59.7 | <0.001 |
| Hormone replacement therapy | 41.8 | 43.2 | 36.9 | 37.7 | <0.001 |
| Randomized to Aspirin | 50.0 | 50.2 | 49.1 | 49.1 | 0.48 |
| Randomized to Vitamin E | 49.9 | 50.4 | 49.6 | 49.4 | 0.77 |
|
Subcohort with available blood samples (n=28314)† |
n=14631 | n=10389 | n=1255 | n=2039 | |
| Total cholesterol, mmol/L [mg/dL] | 5.51 (5.48– 5.53) [212.6 (211.5–213.6)] |
5.52 (5.49–5.55) [213.1 (212.0– 214.3)] |
5.64 (5.57–5.70) [217.6 (215.1– 220.1)] |
5.72 (5.66–5.77) [220.6 (218.6– 222.7)] |
<0.001 |
| LDL cholesterol, mmol/L [mg/dL] | 3.21 (3.19– 3.23) [123.9 (123.0–124.8)] |
3.17 (3.15–3.20) [122.5 (121.6– 123.5)] |
3.30 (3.25–3.36) [127.6 (125.4– 129.7)] |
3.38 (3.33–3.43) [130.5 (128.7– 132.3)] |
<0.001 |
| HDL cholesterol, mmol/L [mg/dL] | 1.34 (1.34– 1.35) [51.9 (51.5–52.3)] |
1.37 (1.36–1.38) [53.0 (52.6–53.4)] |
1.28 (1.26–1.30) [49.4 (48.6–50.2)] |
1.22 (1.20–1.23) [46.9 (46.3–47.5)] |
<0.001 |
| hsCRP, nmol/L [mg/L] | 18.7 (18.2– 19.3) [1.97 (1.91–2.03)] |
19.1 (18.4–19.7) [2.00 (1.94–2.07)] |
18.7 (17.4–20.0) [1.96 (1.83–2.11)] |
24.8 (23.4–26.3) [2.60 (2.5–2.8)] |
<0.001 |
| ICAM, µg/L | 345.9 (344.0– 347.8) |
348.5 (346.5–350.6) | 397.0 (392.1–402.0) | 457.0 (452.3–461.7) | <0.001 |
Data are means (95% confidence intervals) or percentages
Missing data, n (%): Age, 0 (0); history of hypertension, 9 (0.02); history of diabetes 0 (0); body mass index, 818 (2.1); exercise frequency, 20 (0.05); highest education level, 670 (1.7%); alcohol consumption, 10 (0.03); history of hypercholesterolemia, 16 (0.04); menopausal status, 71 (0.2); hormone replacement therapy, 78 (0.2); randomized to aspirin 0 (0); randomized to vitamin E 0 (0).
Missing data in the subcohort with available blood samples, n (%): total cholesterol, 404 (1.4%); LDL cholesterol, 403 (1.4); HDL cholesterol, 404 (1.4); hsCRP, 403 (1.4); ICAM, 551 (1.9).
P values were calculated from 3 df tests of general linear models for continuous variables and Mantel-Haenszel χ2 tests for categorical variables
LDL = Low-density lipoprotein, HDL = High-density lipoprotein, hsCRP = high sensitivity C-reactive protein, sICAM-1= soluble intercellular adhesion molecule 1
Table 2.
Risk of Incident PAD According to Smoking Status
| Smoking Status |
|||||
|---|---|---|---|---|---|
| Never | Past | Current, <15 cigs/day |
Current, ≥15 cigs/day |
P trend* | |
| Participants | 20336 | 14263 | 1967 | 3259 | |
| Events / Person-years of follow-up | 30 / 260855 | 63 / 182532 | 22 / 24440 | 63 / 39502 | - |
| Age-adjusted incidence rate† | 0.12 | 0.34 | 0.95 | 1.63 | - |
| Baseline examination | |||||
| Age adjusted model (n=39825) | 1.0 (Referent) | 2.95 (1.91–4.55) | 8.76 (5.05–15.21) | 16.51 (10.66–25.57) | <0.001 |
| Multivariable model 1 (n=39803)‡ | 1.0 (Referent) | 2.96 (1.92–4.58) | 9.26 (5.33–16.09) | 16.53 (10.66–25.62) | <0.001 |
| Multivariable model 2 (n=38960) § | 1.0 (Referent) | 3.14 (2.01–4.90) | 8.93 (5.02–15.89) | 16.95 (10.77–26.67) | <0.001 |
| Smoking updated during follow-up | |||||
| Age adjusted model (n=39825) | 1.0 (Referent) | 2.97 (1.94–4.55) | 11.28 (6.62–19.21) | 20.99 (13.29–33.15) | <0.001 |
| Multivariable model 1 (n=39803) ‡ | 1.0 (Referent) | 2.98 (1.95–4.56) | 11.89 (6.97–20.28) | 21.19 (13.40–33.50) | <0.001 |
| Multivariable model 2 (n=38960) § | 1.0 (Referent) | 3.16 (2.04–4.89) | 11.94 (6.90–20.65) | 21.08 (13.10–33.91) | <0.001 |
| Subcohort with available blood samples (n=28314) | |||||
| Participants | 14631 | 10389 | 1255 | 2039 | |
| Events / Person-years of follow-up | 21 / 188625 | 43 / 133470 | 14 / 15742 | 39 / 24878 | - |
| Age-adjusted incidence rate† | 0.11 | 0.32 | 1.02 | 1.57 | - |
| Age adjusted model (n=28314) | 1.0 (Referent) | 2.85 (1.69–4.80) | 8.98 (4.56–17.68) | 16.45 (9.65–28.05) | <0.001 |
| Multivariable model 1 (n=27347) ║ | 1.0 (Referent) | 2.89 (1.70–4.91) | 7.12 (3.46–14.63) | 13.46 (7.38–23.42) | <0.001 |
| Multivariable model 2 (n=27159) ¶ | 1.0 (Referent) | 2.83 (1.64–4.86) | 5.58 (2.61–11.93) | 9.52 (5.17–17.53) | <0.001 |
Data are HR (95% CI)
P for trend across categories of cigarette smoking
Incidence rates are per 1000 person years of observation
Additionally adjusted for history of hypertension, history of diabetes, and history of hypercholesterolemia
Additionally adjusted for body mass index, alcohol consumption, and physical activity
Additionally adjusted for history of hypertension, history of diabetes, body mass index, alcohol consumption, physical activity and measured total and high-density lipoprotein cholesterol
Additionally adjusted for high sensitivity C-reactive protein and soluble intercellular adhesion molecule 1
PAD = Peripheral artery disease
Table 3.
Risk of Incident PAD According to Pack Years of Smoking
| Smoking Exposure Status |
|||||
|---|---|---|---|---|---|
| N=39227 | 0 | < 10 pack years | 10 to < 30 pack years | ≥ 30 pack years | P-trend* |
| Events / Person-years of follow-up | 30 / 260855 | 28 / 110538 | 67 / 94648 | 49 / 33887 | - |
| Age-adjusted incidence rate† | 0.12 | 0.28 | 0.72 | 1.33 | - |
| Age adjusted model (n=39227) | 1.0 (Referent) | 2.30 (1.38–3.86) | 6.43 (4.18–9.89) | 11.06 (7.01–17.43) | <0.001 |
| Multivariable model 1 (n=39803)‡ | 1.0 (Referent) | 2.33 (1.39–3.90) | 6.54 (4.25–10.06) | 10.76 (6.82–16.97) | <0.001 |
| Multivariable model 2 (n=38960) § | 1.0 (Referent) | 2.52 (1.49–4.25) | 6.75 (4.33–10.52) | 11.09 (6.94–17.72) | <0.001 |
Data are HR (95% CI)
P for trend across categories of cigarette exposure
Incidence rates are per 1000 person-years of observation
Additionally adjusted for history of hypertension, history of diabetes, and history of hypercholesterolemia
Additionally adjusted for body mass index, alcohol consumption, and physical activity
PAD = Peripheral artery disease
Role of the sponsor
The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and in the decision to submit the manuscript for publication.
RESULTS
At study entry, 20336 (51.1%) women were never smokers, 14263 (35.8%) former smokers, 1967 (4.9%) currently smoked <15 cigarettes per day, and 3259 (8.2%) currently smoked ≥15 cigarettes per day. Age adjusted baseline characteristics according to smoking status are shown in Table 1. Currently smoking women were younger, had a lower body mass index, exercised less frequently, had a lower education level and consumed more alcohol compared with never smoking women. Among women with available blood samples, current smokers had higher levels of total cholesterol, lower levels of HDL-cholesterol and higher levels of high-sensitivity C-reactive protein and sICAM-1 (Table 1).
During a median (interquartile range) follow-up of 12.7 (12.4–13.8) years, 178 symptomatic PAD events occurred. Age-adjusted incidence rates revealed a strong risk gradient for incident PAD across the four smoking categories (Table 2). While never smoking women had a very low incidence of symptomatic PAD (0.12 events per 1000 person-years of follow-up), the risk was more than tenfold higher among women who actively smoked ≥15 cigarettes per day at study entry (1.63 events per 1000 person-years of follow-up), corresponding to a 16.5 fold increased relative hazard of PAD in age-adjusted Cox regression models. Former smokers had a 3-fold increased risk of PAD compared with never smokers. Multivariable adjustment for traditional risk factors, menopausal status, or the use of hormone replacement therapy had little impact on these effects. Furthermore, neither menopausal status nor hormone replacement therapy was associated with incident PAD in these models (data not shown). Finally, consistent findings were obtained when analyses were performed separately for women with intermittent claudication only and those undergoing invasive procedures (supplementary Table).
Updating smoking status during follow-up using time varying covariates further strengthened the association for active smoking, such that currently smoking <15 cigarettes per day conveyed an almost 12-fold increased risk and currently smoking ≥15 cigarettes per day was associated with a more than 20-fold increased risk of symptomatic PAD (Table 2). When subjects were censored for a diagnosis of cardiovascular disease prior to PAD, similar results were obtained (data not shown). However, in analyses that examined differences according to baseline menopausal status, the risk of symptomatic PAD in smokers appeared stronger among premenopausal than postmenopausal women. Specifically, compared with never smoking women, HRs across smoking categories were 1.98 (0.78–5.04), 8.57 (2.79–26.36) and 26.73 (12.0–59.54) for premenopausal women and 3.50 (2.10–5.86), 9.04 (4.61–17.37) and 12.82 (7.29–22.55) for postmenopausal women (P for interaction 0.024). No statistically significant evidence for an interaction between smoking and either aspirin or Vitamin E therapy was observed (P for interaction 0.186 and 0.40, respectively).
The relationship between smoking status and risk of symptomatic PAD within the subcohort of 28314 women who provided a blood sample was similar to the relationship observed in the entire cohort (Table 2). The risk estimates for each category of smoking were slightly attenuated with the addition of baseline total and HDL cholesterol levels to the regression models. Adding high-sensitivity C-reactive protein and sICAM-1 to the multivariable models attenuated risk estimates for current smoking more substantially, however currently smoking <15 cigarettes per day maintained a more than 5-fold increased risk (HR 5.58 (2.61–11.93)), and currently smoking ≥15 cigarettes per day was associated with an almost 10-fold increased risk of symptomatic PAD during follow-up (HR 9.52 (5.17–17.53)). By contrast, the increased risk among former smokers was not appreciably affected by the inclusion of high-sensitivity C-reactive protein or sICAM-1 (Table 2).
There was a strong dose-response relationship between lifelong smoking exposure defined by pack years of smoking and risk of symptomatic PAD, as shown in Table 3 and the supplementary Figure. In age-adjusted models, HRs (95% CIs) among women whose lifetime exposure was <10, 10–29 and ≥30 pack years were 2.30 (1.38–3.86), 6.43 (4.18–9.89) and 11.06 (7.01–17.43) compared with never smoking women (P for linear trend <0.001). Multivariable adjustment did not attenuate this relationship. Again, there was no appreciable change in our results when subjects were censored for a diagnosis of cardiovascular disease prior to PAD (data not shown).
Among women who quit smoking before study entry, 2906 did so within 10 years of study entry, 4475 between 10 and 20 years before study entry and 6641 women quit smoking >20 years ago. Smoking cessation markedly decreased the risk of PAD compared with active smoking and longer smoking abstinence was associated with an additional reduction of this risk, as shown in the Figure. Fully adjusted HRs (95% CIs) for smoking abstinence of <10 years, 10–20 years, >20 years and lifelong non-smoking were 0.39 (0.24–0.66), 0.28 (0.17–0.46), 0.16 (0.10–0.26) and 0.08 (0.05–0.12) (P for linear trend <0.001) (Figure).
Figure.
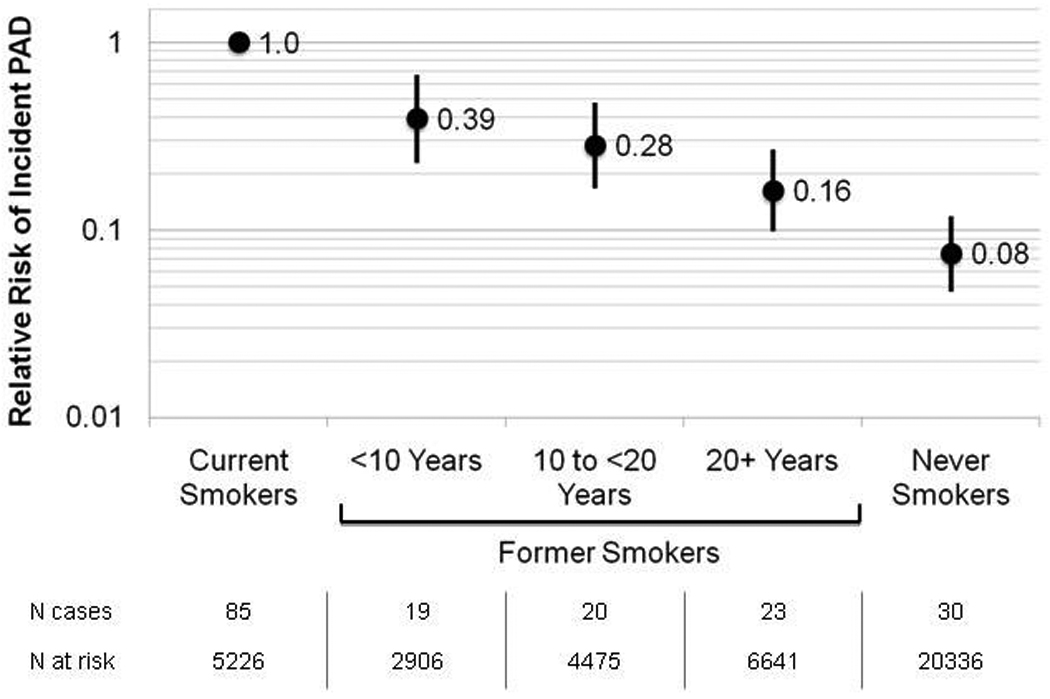
Hazard Ratio for Incident PAD According to Time Since Smoking Cessation
Data are hazard ratios adjusted for age, hypertension, diabetes, hypercholesterolemia, body mass index, alcohol consumption and physical activity. Current smokers represent the reference group. Error bars represent 95 percent confidence intervals. PAD = Peripheral artery disease
DISCUSSION
At least two major findings are derived from this large-scale prospective study conducted among initially healthy women. First, smoking is a potent risk factor for the occurrence of symptomatic PAD, even after accounting for a number of established risk factors and markers of subclinical inflammation. Second, smoking cessation is associated with a substantial reduction in the risk of PAD, highlighting the importance of efforts to promote smoking cessation.
While prior prospective studies consistently showed an increased risk of PAD among smokers, few analyses specifically assessed smoking as main exposure variable and the strength of the relationship varied widely across reports, with relative risks for current smoking ranging from 1.6 – 10.2 (8–14). Our data represent one of the strongest associations for current smoking reported thus far, a finding which may be explained by our focus on symptomatic as opposed to subclinical disease (9) and our evaluation in an exclusively female, otherwise relatively young and low risk population. Even within this relatively homogenous population, the risk of PAD associated with smoking is higher among younger, premenopausal women. A higher relative risk conferred by current smoking among younger and relatively low risk individuals has been previously reported with regard to myocardial infarction where the odds ratio for non fatal myocardial infarction was 3.53 (3.23–3.86) in younger as compared to 2.55 (2.35–2.76) in older individuals (P for interaction <0.001) (24). Whether the strength of the association is also influenced by gender should be assessed in other mixed-gender cohorts.
We also demonstrate a strong dose response relationship between life-long smoking exposure and subsequent PAD, confirming prior studies that were suggestive of such an association (10, 13). While our data show no particular threshold below which smoking does not confer an increased risk, a particularly steep risk increase emerged among women who indicated at least 10 pack years of smoking exposure, underscoring the importance of smoking as a determinant of PAD and a priority for intervention.
Also of importance from a public health perspective is our finding that smoking cessation is associated with a dramatic reduction in incident PAD. Prior investigations in this context have focused on total cardiovascular morbidity and mortality. For example, in the Nurses’ Health Study, the risk of vascular death among women who stopped smoking was gradually reduced with an increasing duration of smoking abstinence and reached the relative risk of never smokers after approximately 20 years (7). While we also found a gradual decrease in risk with an increasing duration of smoking abstinence, even women who had quit smoking >20 years prior to enrolling in our study had a higher risk of developing symptomatic PAD than never smoking women. This residual risk may relate to chronic adverse effects of smoking on the peripheral vasculature. Still, for patients and their physicians, the current findings suggest that long-term smoking cessation substantially reduces the risk of symptomatic PAD. However, the residual PAD risk even among smokers who have been abstinent for at least 20 years underscores the importance of primary efforts for smoking prevention.
There was no strong confounding of the adverse effects of smoking by any of the traditional risk factor variables included in the multivariable models. Adding high-sensitivity C-reactive protein and sICAM-1 as markers of subclinical inflammation somewhat attenuated the coefficients for actively smoking women (Table 2) suggesting that these factors may in part mediate the adverse smoking effects. Nevertheless, despite multivariable adjustment, we found that the increased risk associated with smoking remains largely unexplained, which is consistent with one of the few prior studies on this issue (13).
Strengths of the present study include the prospective design, large sample size, long-term follow-up with a large number of confirmed endpoints, and the homogeneity of our study participants, which may reduce confounding. Our results should also be considered in the context of several potential limitations. First, our study included mainly Caucasian women, and our findings may not be generalizable to other groups. Second, as our study is observational, unmeasurable or residual confounding may be present. Third, the use of symptomatic PAD as the primary a priori end point by definition excludes subclinical disease, which may have otherwise been detected through abnormal pulse examination or ankle-brachial index (25). However, we believe our data to be not only relevant from a mechanistic perspective but also of clinical importance, because claudication and revascularization of an ischemic limb are the principal clinical manifestations of PAD. Importantly, events included in this analysis were confirmed by a validated claudication questionnaire, cardiovascular physician interview and medical record review. Our focus on symptomatic disease may also have reduced the likelihood of endpoint misclassification, as would the characteristics of our study population. Participants were health professionals and therefore less likely to encounter barriers to medical care, which may otherwise have led to misdiagnosis or underdiagnosis among all smoking exposure groups. Whether or not women with PAD are more likely to have atypical leg symptoms or more often be asymptomatic is controversial (26, 27), especially as some studies suggest a lower (less stringent) ABI cut-off for PAD diagnosis may be more appropriate for women (28, 29). Nonetheless, if smoking is even modestly associated with PAD, misclassification of women with atypical PAD symptoms as non-cases would, if anything, tend to bias our results towards the null by including relatively more smokers in the non-PAD group. Finally, in this study we did not consider exposure to second-hand smoke, which has been shown to be an important risk factor for PAD in women (30).
In conclusion, this large prospective study underscores the importance of smoking as a risk factor for the development of symptomatic PAD. While this association appears partly mediated by inflammation, the majority of excess risk conferred by smoking remains unexplained. Although smoking cessation dramatically reduces the risk of PAD, an increased disease risk remains even among long term abstinent women, highlighting the importance of both prevention of smoking initiation and efforts to promote long-term abstinence.
Supplementary Material
Acknowledgments
Financial Support
The Women's Health Study was supported by grants from the National Heart, Lung, and Blood Institute (HL-43851, HL-58755, HL-082740, and HL-075771), the National Cancer Institute (CA-47988), and the Donald W. Reynolds Foundation.
Dr. Conen has received within the last five years investigator-initiated research funding from the Swiss Heart Foundation, the Mach Gaensslen Foundation, the University of Basel, Boehringer Ingelheim and Novartis.
Dr. Everett is the recipient of an investigator-initiated award from Roche Diagnostics for research on unrelated biomarkers in cardiovascular disease.
Dr. Kurth has received within the last five years investigator-initiated research funding from the French National Research Agency, the US National Institutes of Health, Merck, and the Migraine Research Foundation. Further, he is a consultant to i3 Drug Safety and World Health Information Science Consultants, LLC; he has received honoraria from Genzyme, Merck, and Pfizer for educational lectures.
Dr. Creager reports being the Simon C. Fireman Scholar in Cardiovascular Medicine at Brigham and Women's Hospital and having received research funding support from the National Heart, Lung, and Blood Institute.
Dr. Ridker reports having received research funding support from multiple not-for-profit entities including the National Heart, Lung, and Blood Institute, the National Cancer Institute, the American Heart Association, the Doris Duke Charitable Foundation, the Leducq Foundation, the Donald W Reynolds Foundation, and the James and Polly Annenberg La Vea Charitable Trusts. Dr Ridker also reports having received investigator-initiated research support from multiple for-profit entities including Astra-Zeneca, Novartis, Pharmacia, Roche, Sanofi-Aventis, and Abbott, as well as non-financial research support from Amgen.
Dr. Pradhan was supported within the last five years by a career development award (HL-082740) and reports having received research support from Sanofi-Aventis.
Footnotes
Disclosures
Paul M Ridker is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease.
REFERENCES
- 1.Heyman KM, Barnes PM, Schiller JS. Early Release of Selected Estimates Based on Data from the 2008 National Health Interview Survey. Hyattsville, MD: National Center for Health Statistics; 2009. [Google Scholar]
- 2.World Health Organisation. [Accessed 19th January 2011];European health for all statistical database. 2010 http://www.euro.who.int/hfadb.
- 3.World Health Organization. WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER Package. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 4.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362:847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- 5.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 6.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299:2037–2047. doi: 10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantin B, Moorjani S, Dagenais GR, Lupien PJ. Lipoprotein(a) distribution in a French Canadian population and its relation to intermittent claudication (the Quebec Cardiovascular Study) Am J Cardiol. 1995;75:1224–1228. doi: 10.1016/s0002-9149(99)80767-x. [DOI] [PubMed] [Google Scholar]
- 9.Hooi JD, Kester AD, Stoffers HE, Overdijk MM, van Ree JW, Knottnerus JA. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol. 2001;153:666–672. doi: 10.1093/aje/153.7.666. [DOI] [PubMed] [Google Scholar]
- 10.Ingolfsson IO, Sigurdsson G, Sigvaldason H, Thorgeirsson G, Sigfusson N. A marked decline in the prevalence and incidence of intermittent claudication in Icelandic men 1968–1986: a strong relationship to smoking and serum cholesterol--the Reykjavik Study. J Clin Epidemiol. 1994;47:1237–1243. doi: 10.1016/0895-4356(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 11.Kollerits B, Heinrich J, Pichler M, Rantner B, Klein-Weigel P, Wolke G, et al. Intermittent claudication in the Erfurt Male Cohort (ERFORT) Study: its determinants and the impact on mortality. A population-based prospective cohort study with 30 years of follow-up. Atherosclerosis. 2008;198:214–222. doi: 10.1016/j.atherosclerosis.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Murabito JM, D'Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 13.Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20:344–353. doi: 10.1053/euhj.1998.1194. [DOI] [PubMed] [Google Scholar]
- 14.Tapp RJ, Balkau B, Shaw JE, Valensi P, Cailleau M, Eschwege E. Association of glucose metabolism, smoking and cardiovascular risk factors with incident peripheral arterial disease: the DESIR study. Atherosclerosis. 2007;190:84–89. doi: 10.1016/j.atherosclerosis.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 16.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A–I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 19.Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101–1109. doi: 10.1016/0895-4356(92)90150-l. [DOI] [PubMed] [Google Scholar]
- 20.McKinlay SM, Bifano NL, McKinlay JB. Smoking and age at menopause in women. Ann Intern Med. 1985;103:350–356. doi: 10.7326/0003-4819-103-3-350. [DOI] [PubMed] [Google Scholar]
- 21.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008;117:823–831. doi: 10.1161/CIRCULATIONAHA.107.719369. [DOI] [PubMed] [Google Scholar]
- 22.Conen D, Rexrode KM, Creager MA, Ridker PM, Pradhan AD. Metabolic syndrome, inflammation, and risk of symptomatic peripheral artery disease in women: a prospective study. Circulation. 2009;120:1041–1047. doi: 10.1161/CIRCULATIONAHA.109.863092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox DR. Regression models and life tables. J Roy Stat Soc B. 1972;34:187–220. [Google Scholar]
- 24.Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647–658. doi: 10.1016/S0140-6736(06)69249-0. [DOI] [PubMed] [Google Scholar]
- 25.Ankle Brachial Index Collaboration. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality. A meta analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med. 1999;159:387–392. doi: 10.1001/archinte.159.4.387. [DOI] [PubMed] [Google Scholar]
- 27.Vavra AK, Kibbe MR. Women and peripheral arterial disease. Womens Health (Lond Engl) 2009;5:669–683. doi: 10.2217/whe.09.60. [DOI] [PubMed] [Google Scholar]
- 28.Aboyans V, Criqui MH, McClelland RL, Allison MA, McDermott MM, Goff DC, Jr, et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA) J Vasc Surg. 2007;45:319–327. doi: 10.1016/j.jvs.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 29.Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation. 1995;91:1472–1479. doi: 10.1161/01.cir.91.5.1472. [DOI] [PubMed] [Google Scholar]
- 30.He Y, Lam TH, Jiang B, Wang J, Sai X, Fan L, et al. Passive smoking and risk of peripheral arterial disease and ischemic stroke in Chinese women who never smoked. Circulation. 2008;118:1535–1540. doi: 10.1161/CIRCULATIONAHA.108.784801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


