Abstract
The relationship between accommodative lag and annual myopia progression was investigated using linear models in 592 myopic children wearing a full refractive correction in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study. The mean (± SD) age and spherical equivalent refractive error at baseline were 10.4 ± 1.8 years and −2.13 ± 1.24 D, respectively. The mean annual progression of myopia was −0.45 ± 0.32 D, and the mean accommodative lag (for a 4-D Badal stimulus) was 1.59 ± 0.63 D. Neither lag at the beginning nor at the end of a yearly progression interval was associated with annual myopia progression (all p ≥ 0.12). These data suggest that foveal hyperopic retinal blur during near viewing may not drive juvenile-onset myopia progression.
Keywords: myopia, accommodative lag, myopia progression, children, refractive error
Introduction
The ability of optical defocus to control eye growth is supported by animal studies in which positive and negative lenses predictably influence axial elongation (Irving, Sivak, & Callender, 1992; Norton, Siegwart, & Amedo, 2006; Schaeffel, Glasser, & Howland, 1988; Smith & Hung, 1999; Wildsoet & Wallman, 1995). Hyperopic retinal blur due to a high lag of accommodation during near viewing activities has been proposed as a cause of juvenile-onset myopia progression in humans (Charman, 1999; Goss, Hampton, & Wickham, 1988; Goss & Rainey, 1999). Reports disagree about whether accommodative lag is elevated before the onset of juvenile myopia. Gwiazda et al. reported elevated lag in pre-myopic children two years before myopia onset (Gwiazda, Thorn, & Held, 2005). Conversely, data from the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study indicated that accommodative lag in pre-myopic children is not elevated until the year after the onset of myopia (Mutti, Mitchell, Hayes, Jones, Moeschberger, Cotter, Kleinstein, Manny, Twelker, Zadnik, & CLEERE Study Group, 2006). A study of young adults who became myopic reported lower accommodative lag both before and after the onset of myopia compared to those who were either emmetropic or already myopic (Rosenfield, Desai, & Portello, 2002). While there is no consensus regarding accommodative lag prior to the onset of myopia, the literature clearly indicates that myopic adults and children have an elevated accommodative lag (Abbott, Schmid, & Strang, 1998; Bullimore, Gilmartin, & Royston, 1992; Gwiazda, Thorn, Bauer, & Held, 1993; McBrien & Millodot, 1986). A correlation has also been reported between myopia progression in children and decreases in the area under the curve of the accommodative response function (Gwiazda, Bauer, Thorn, & Held, 1995).
As referenced above, in 2006 the CLEERE Study reported that accommodative lag was elevated in myopic children but did not evaluate whether this elevated lag of accommodation was also related to the annual rate of myopia progression (Mutti et al., 2006). The hypothesis for a relationship between accommodative lag and myopia progression is that the higher the level of hyperopic defocus from greater accommodative lag, the faster the rate of myopia progression (Irving, Callender, & Sivak, 1991). Studies of the association between accommodative lag and myopia progression conflict; one reports no association in children (Weizhong, Zhikuan, Wen, Xiang, & Jian, 2008), one reports that elevated accommodative lag is associated with myopia progression in adults (Allen & O’Leary, 2006), and another reports that lower accommodative lag is associated with myopia progression in adults (Rosenfield et al., 2002). Differences in age, measurement methods, and accommodative demand levels among these three studies make comparisons problematic. The effects of the differences in population and/or study design are unknown. The purpose of this paper is to investigate the relationship between the degree of accommodative lag and the rate of juvenile-onset myopia progression in the large, ethnically diverse sample of children wearing refractive corrections enrolled in the CLEERE Study.
Methods
The CLEERE Study is a US-based, longitudinal cohort study following school-aged children at multiple sites and is an extension of the Orinda Longitudinal Study of Myopia (OLSM). The project began in 1989 in Orinda, CA, a predominantly white community. To expand ethnic representation, four sites were added that targeted specific ethnic groups: Eutaw, Alabama (African Americans); Irvine, California (Asians); Houston, Texas (Hispanics); and Tucson, Arizona (Native Americans). CLEERE measured the development of ocular components annually and examined risk factors for prevalent and incident myopia. The Institutional Review Board at each institution (The Ohio State University; the University of California, Berkeley; the University of Alabama at Birmingham; Southern California College of Optometry; the University of Houston; and the University of Arizona) reviewed and approved the study protocol and informed consent documents according to the tenets of the Declaration of Helsinki. Written informed consent was provided by children’s parents, and verbal assent was obtained from children before they were tested.
Accommodative response while wearing full refractive correction was measured annually in myopic children wearing refractive correction in US grades 1 through 8 (typically 6 to 14 years of age) between 1995 and 2005. Refractive error was also measured annually. Myopia was defined as −0.75 D or more myopia in each meridian as measured by cycloplegic autorefraction. In the CLEERE Study, accommodation was measured while the child wore a full refractive correction only if the child wore his or her own correction to the visit. If a child did not wear his or her own correction to a visit, accommodation was measured without refractive correction at that visit. Because the accommodative demand while viewing the same target is not equal for a fully corrected myope versus an uncorrected myope, only accommodative lag measurements collected when the child wore refractive correction to the visit have been included in this dataset. To be included in this analysis, children had to have at least two consecutive annual myopic study visits (so that an annual progression rate could be calculated) and have at least one accommodative lag measurement with a temporally-linked myopia progression rate. A total of 592 children were eligible for inclusion in the analyses.
Accommodative response was measured monocularly (right eye) with a 4-D Badal letter stimulus using methods described in detail previously (Mutti, Jones, Moeschberger, & Zadnik, 2000; Mutti et al., 2006). The accommodative stimulus was a 4 by 4 grid of letters with each letter and the space between letters subtending 38.75 minutes of arc (20/155 equivalent) with luminance of 30 to 50 cd/m2. Children were continuously instructed to keep the letters clear during the measurement. Letters of this size were chosen because they represent a size typical of text in children’s books. Letters similar in size have been shown previously to detect the expected differences in accommodative response between emmetropic and myopic children (Gwiazda et al., 1993). The 4-D Badal stimulus detects significant differences in accommodative lag between emmetropic and myopic children and detects changes in accommodative lag associated with the onset of myopia in children (Mutti et al., 2006). The left eye was occluded by an infrared filter during the accommodative measurements. The accommodation value for each visit was the mean of five measurements.
To determine the full refractive correction used for accommodative testing for spectacle wearers, at least five autorefractor readings were made of the right eye after removing the child’s spectacles; a minimum of three readings was acceptable for younger children (6 to 7 years old) because of their reduced attention span. Accommodation was relaxed for the measurement of distance refractive error by moving the target on a Badal lens track away from the child’s eye until the child reported sustained blur. Autorefractor measurements were made using either the Canon R-1 autorefractor (1995 – 2000; Canon USA, Lake Success, NY; no longer manufactured) or the Grand Seiko WR-5100K autorefractor (2001 – 2005; Grand Seiko Co., Hiroshima, Japan). Both the Canon R-1 autorefractor (Zadnik, Mutti, & Adams, 1992) and the Grand Seiko WR-5100K autorefractor (Choong, Chen, & Goh, 2006) have been previously validated. The sphere and, if necessary, the cylindrical trial lens correction were placed in a trial clip over the right eye when measuring accommodative response. The trial lens correction was modified as needed to keep the overrefraction at the 0-D stimulus level within ±0.50 D for sphere and −1.00 D or less for cylinder. The stimulus levels and trial lens values were adjusted for lens effectivity (Mutti et al., 2000). Children wearing contact lenses wore their contact lenses for testing, and the overrefraction requirements described above were followed.
Accommodative lag values were statistically corrected for the minor difference due to the change in autorefractor in 2001 described previously (Mutti et al., 2006). A correction factor was applied for each study site that was determined by fitting a linear model of lag as a function of age for each emmetropic child in the CLEERE dataset with lag data measured on both the Canon autorefractor before 2001 and the Grand Seiko in 2001 or later. The intercept of the line, but not the slope of the line, was allowed to change for study years before 2001. The site-specific correction was applied to all lag data measured with the Canon autorefractor at that site. This correction is the estimated change in lag measurements due to the change in autorefractors. Emmetropic children in the CLEERE dataset were used to estimate the change in lag due to the change in autorefractor because emmetropic children both in the CLEERE dataset (Mutti et al., 2006) and elsewhere (Gwiazda et al., 2005) have been shown to have a consistent level of lag.
Refractive error measurements were made using cycloplegic autorefraction (either the Canon R-1 [1995 to 2000] or the Grand Seiko WR-5100K [2001 to 2005] autorefractor) while the child fixated a reduced Snellen target on a Badal lens track. If children had an iris color grade of one or two (Seddon, Sahagian, Glynn, Sperduto, & Gragoudas, 1990), they were tested 30 minutes after instilling one drop of 0.5% proparacaine and two drops of 1% tropicamide. If children had an iris color grade darker than grade two, they were tested 30 minutes after instilling one drop or 0.5% proparacaine, one drop of 1% tropicamide, and one drop of 1% cyclopentolate (Kleinstein, Mutti, Manny, Shin, & Zadnik, 1999). Using a standardized protocol, ten autorefractor measurements were made (Zadnik, Mutti, Friedman, & Adams, 1993). Myopia progression was defined as the annual change in cycloplegic, spherical equivalent refractive error, where the child’s refractive error in the prior year was subtracted from the present year’s value, making progression negative in sign.
Two definitions of accommodative lag were considered. “Lag Before” was defined as the accommodative lag at the beginning of each yearly interval. “Lag After” was defined as the accommodative lag at the end of each yearly interval. Because foveal hyperopic retinal blur is a frequently cited explanation for an association between accommodative lag and axial elongation in juvenile-onset myopia progression (Charman, 1999; Gilmartin, 2004; Goss et al., 1988; Goss & Rainey, 1999; Gwiazda et al., 1995), the “Lag Before” variable is arguably the most appropriate accommodative lag definition and was chosen as the independent variable for the primary analysis. Table 1 shows the number of years of progression data that were contributed by myopic children wearing refractive correction for each of these two definitions of accommodative lag.
Table 1.
Number of years of myopia progression data contributed by children wearing refractive correction for the “Lag Before” and the “Lag After” definitions of accommodative lag. Minor differences between the numbers of children contributing data to the “Lag Before” and “Lag After” model were due to differences between the year of myopia onset and the year that the child first wore correction, not wearing correction to every myopic visit, or missed visits.
| Years of Progression Data | Lag Before | Lag After |
|---|---|---|
| 1 | 209 (40.2%) | 197 (34.4%) |
| 2 | 130 (25.0%) | 149 (26.0%) |
| 3 | 97 (18.7%) | 111 (19.4%) |
| 4 | 51 (9.8%) | 54 (9.4%) |
| 5 | 23 (4.4%) | 45 (7.9%) |
| 6 | 8 (1.5%) | 14 (2.4%) |
| 7 | 2 (0.4%) | 2 (0.3%) |
| Total | 520 (100%) | 572 (100%) |
Near phoria was measured in prism diopters (pd) using an alternate cover test with prism neutralization. During near phoria testing the child viewed an accommodative target (small letter) held at 40 cm. Cover testing was performed with the correction that the child wore to the CLEERE examination visit.
Near work was assessed using an annual survey that was completed by each child’s parents, as previously described (Jones, Sinnott, Mutti, Mitchell, Moeschberger, & Zadnik, 2007; Mutti, Mitchell, Moeschberger, Jones, & Zadnik, 2002). The survey asked, “During the school year, how many hours per week (outside of regular school hours) would you estimate this child performs the following activities?” The parent was asked to provide an answer for the following tasks: “Studies or reads for school assignments; reads for fun (pleasure); watches television; uses a computer/plays video games; and engages in outdoor and/or sports activities.” A composite variable (diopter hours) was calculated that weights near activities by their accommodative effort. Diopter hours was calculated as follows: 3 x (hours studying + hours reading for pleasure) + 2 x (video/computer hours) + (hours watching television).
Multilevel, repeated-measures linear regression models were fitted to determine the effect of accommodative lag on the annual refractive error change (SAS, version 9.1; SAS Institute, Cary, NC). Covariates evaluated in the models were age, gender, ethnicity, study site, near phoria, near work, and a factor to indicate annual myopia progression data that were measured during the transition between autorefractor models.
Results
Of the 592 children in the analyses, 500 contributed data to both the “Lag Before” and the “Lag After” models, 20 contributed data only to the “Lag Before” model, and 72 contributed data only to the “Lag After” model. The racial and gender distributions of the 592 children are shown in Table 2. More observations were available for the “Lag After” model than for the “Lag Before” model, and incident myopic children are one reason for the greater number of observations in the “Lag After” model. It was not uncommon for incident myopic children to not yet have correction. After being identified as myopic at a visit, many children subsequently received their first spectacle correction and wore that correction to the next annual visit. In this case, there was a spectacle-corrected “Lag After” value to associate with the year of progression, but no corrected “Lag Before” value because the child had not yet been prescribed glasses. Additionally, annual accommodative lag measures were first made in 1996; therefore, myopic children with progression data available from 1995 to 1996 did not have a “Lag Before” value available.
Table 2.
Racial and gender distribution of the 592 myopic children who contributed data to the analyses.
| Native American | Asian | African American | Hispanic | White | Other | Total | |
|---|---|---|---|---|---|---|---|
| Male | 15 (33%) | 71 (44%) | 29 (39%) | 72 (36%) | 42 (41%) | 5 (63%) | 234 (40%) |
| Female | 30 (67%) | 89 (56%) | 45 (61%) | 130 (64%) | 61 (59%) | 3 (38%) | 358 (60%) |
| Total | 45 (8%) | 160 (27%) | 74 (13%) | 202 (34%) | 103 (17%) | 8 (1%) | 592 (100%) |
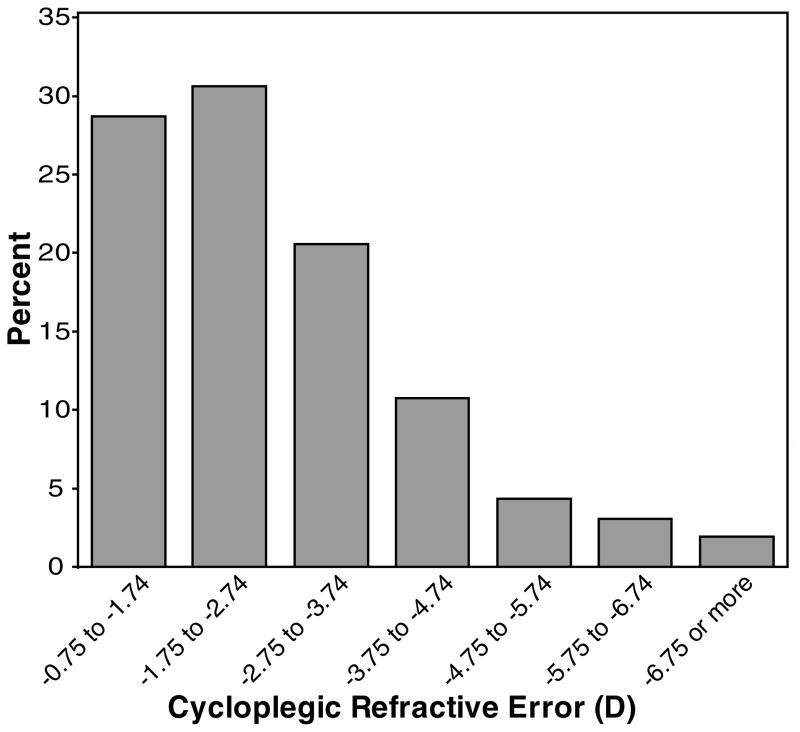
At the first myopic study visit that was included in this analysis, the mean age (± SD) was 10.4 ± 1.8 years (range: 6.2 to 15.2 years), near phoria was 0.8 pd exophoria ± 5.9 pd, and mean spherical equivalent refractive error was −2.13 ± 1.24 D (range: −0.80 to −8.76 D). Figure 1 displays a histogram of spherical equivalent refractive error at the beginning of each yearly progression interval for all observations. Almost 60% of the observations are for a spherical equivalent refractive error that is less myopic than −2.75 D, thus a substantial proportion of children with low myopia remains in the sample despite the requirement that children wore refractive correction to be included in the analysis.
Figure 1.
Histogram displaying the distribution of cycloplegic, spherical equivalent refractive error at the beginning of each yearly progression interval. (Note: Children with more than one year of progression data are represented more than once.)
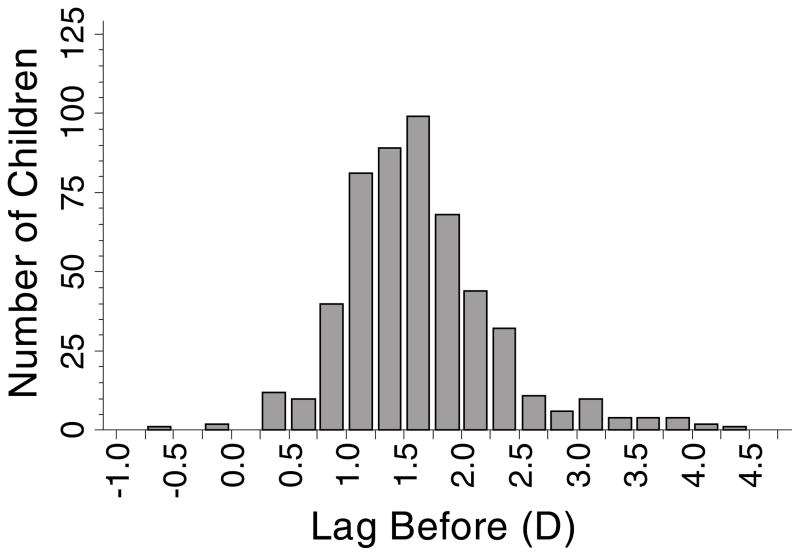
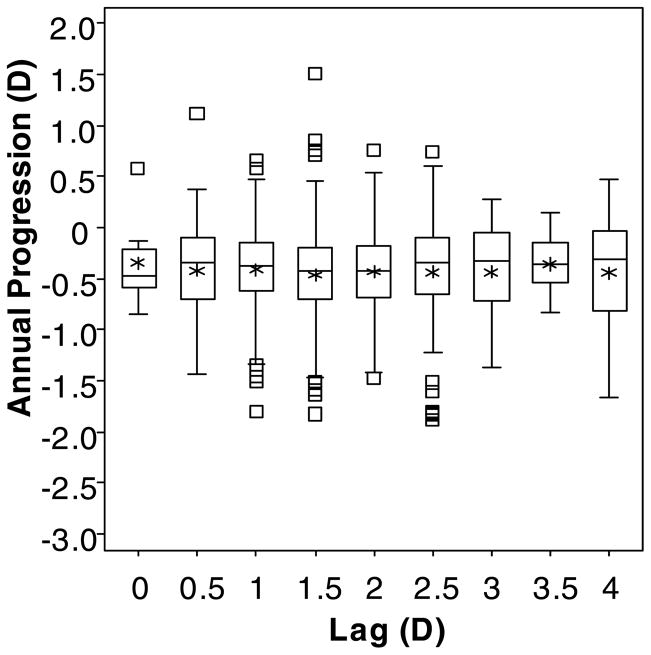
Using the “Lag Before” definition of accommodative lag, 520 children contributed data at 1141 observations. Their mean annual change in myopia (± SD) was −0.42 ± 0.34 D, and their mean lag of accommodation (± SD) was 1.64 ± 0.67 D (range: lead of −0.56 D to lag of 4.25 D; Figure 2). “Lag Before” was not associated with annual myopia progression (β = 0.002 D; although not significant, the positive sign of the slope coefficient (β) from the model represents less myopia progression per D of lag; p = 0.91; 95% CI: −0.032 to 0.036). Figure 3 shows box plots indicating the lack of association between the annual amount of progression and the amount of accommodative lag at the beginning of a one-year progression interval.
Figure 2.
Histogram showing the distribution of accommodative lag for the 520 children who contributed data to the “Lag Before” model.
Figure 3.
Box plots of the annual change in spherical equivalent refractive error by the amount of accommodative lag for a 4-D Badal stimulus (rounded to the nearest 0.5 D) for the “Lag Before” model. Upper and lower box boundaries represent the 75th and 25th percentiles, respectively. Within each box, the horizontal line represents the median, and the asterisk represents the mean. The upper and lower whiskers mark the most extreme observations within 1.5 interquartile range units above the 75th and below the 25th percentile, respectively, and square symbols represent those observations falling outside of this range.
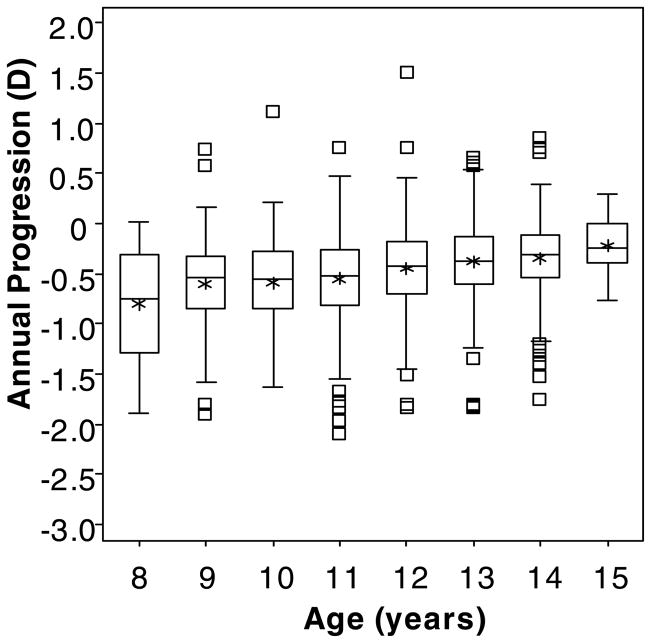
In the “Lag Before” model, older age was marginally associated with a slightly decreased annual change in myopia (β = 0.044 D less myopic progression per year of age; p = 0.052; 95% CI: 0.000 to 0.089). Figure 4 shows box plots indicating the slight upward tendency toward less annual progression with greater age. More exophoria at near was associated with increased myopia progression, although the coefficient was small in magnitude (β = −0.004 D more myopic progression per pd of exophoria; p = 0.046; 95% CI: 0.000 to 0.008); i.e., more esophoria at near was slightly protective against myopia progression. The annual myopia progression rate was greater in girls compared to boys (β = −0.061 D more progression in girls; p = 0.045; 95% CI: −0.120 to −0.002).
Figure 4.
Box plots of the annual change in spherical equivalent refractive error by age in years. (Note: Children with more than one year of progression data are included in more than one age bin.) Upper and lower box boundaries represent the 75th and 25th percentiles, respectively. Within each box, the horizontal line represents the median, and the asterisk represents the mean. The upper and lower whiskers mark the most extreme observations within 1.5 interquartile range units above the 75th and below the 25th percentile, respectively, and square symbols represent those observations falling outside of this range.
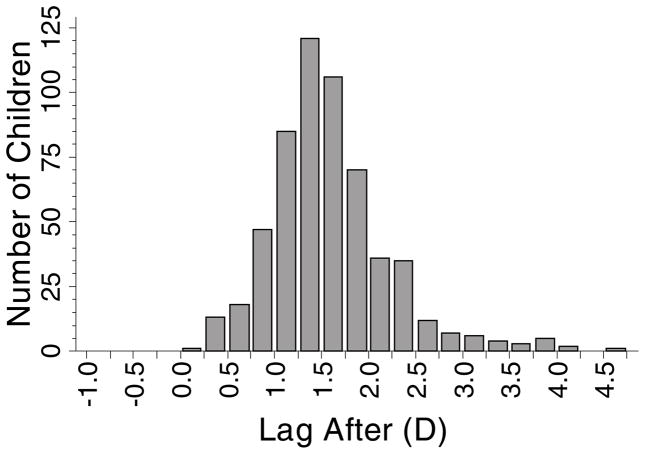
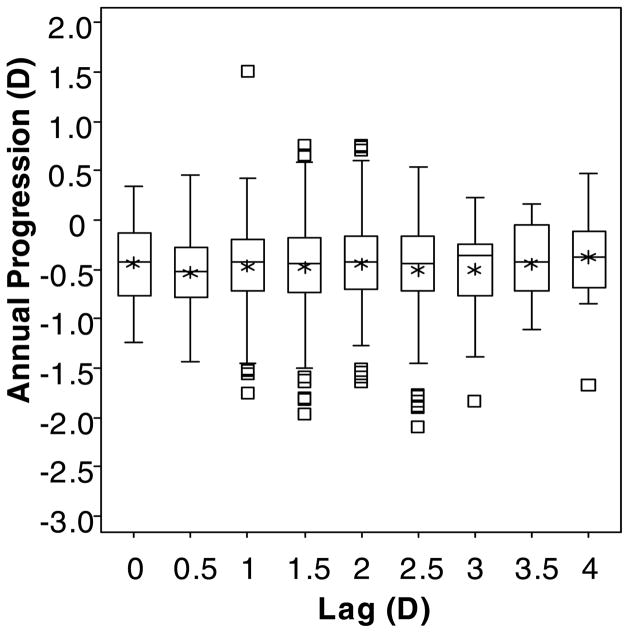
Using the “Lag After” definition of accommodative lag, 572 children contributed data at 1367 observations. Their mean annual change in myopia (± SD) was −0.46 ± 0.32 D, and their mean lag of accommodation (± SD) was 1.59 ± 0.64 D (range: lag of 0.24 D to lag of 4.64 D; Figure 5). “Lag After” was not associated with annual myopia progression (β = 0.025 D; although not significant, the positive sign of the coefficient represents less myopia progression per D of lag; p = 0.12; 95% CI: −0.007 to 0.056). Figure 6 shows box plots indicating the lack of association between the annual amount of progression and the amount of accommodative lag at the end of a one-year progression interval.
Figure 5.
Histogram showing the distribution of accommodative lag for the 572 children who contributed data to the “Lag After” model.
Figure 6.
Box plots of the annual change in spherical equivalent refractive error by the amount of accommodative lag for a 4-D Badal stimulus (rounded to the nearest 0.5 D) for the “Lag After” model. Upper and lower box boundaries represent the 75th and 25th percentiles, respectively. Within each box, the horizontal line represents the median, and the asterisk represents the mean. The upper and lower whiskers mark the most extreme observations within 1.5 interquartile range units above the 75th and below the 25th percentile, respectively, and square symbols represent those observations falling outside of this range.
In the “Lag After” model, older age was not associated with a decreased annual change in myopia (β = 0.021 D in the direction of less myopia progression per year of age; p = 0.27; 95% CI: −0.016 to 0.058). More exophoria at near was again associated with slightly increased myopia progression, (β = −0.005 D more progression per pd of exophoria; p = 0.01; 95% CI: 0.001 to 0.009). Girls had an annual progression rate that was greater than boys (β = −0.080 D more progression in girls; p = 0.003; 95% CI: − 0.132 to −0.028).
Models for both “Lag Before” and “Lag After” were built to examine any interaction between near work and accommodative lag (i.e., whether having a higher accommodative lag was related to greater myopia progression for a child who performs more near work). There was no interaction between near work (diopter-hours) and accommodative lag, and there was no effect of near work on myopia progression (all p > 0.05; data not shown). Models were also used to investigate whether the amount of myopia at the beginning of each year of observed myopia progression interacted with accommodative lag (i.e., whether a higher accommodative lag was related to more myopia progression in children with higher compared to lower amounts of myopia). No interactions were found, and no effect of refractive error at the beginning of a year of progression on myopia progression was found (all p > 0.05; data not shown).
All analyses presented above were performed using accommodative lag data that were corrected to account for the change in autorefractors in 2001 (as noted in the methods section). For completeness, the analyses were repeated using the uncorrected lag data. Using the uncorrected accommodative lag data, accommodative lag was still not associated with increased myopia progression (data not shown).
Discussion
Although previously reported CLEERE Study results indicated that accommodative lag was not elevated in children who become myopic until the year after myopia onset (Mutti et al., 2006), the CLEERE dataset had not been used prior to this report to investigate whether the elevated lag of accommodation observed after myopia onset was related to the annual rate of myopia progression.
In the present analyses, accommodative lag by either definition was not associated with juvenile-onset myopia progression. The 95% confidence intervals for lag at the beginning and at the end of a year of progression were clustered tightly around zero, which demonstrates that the sample size was more than ample to detect a clinically meaningful association if one existed. The most negative coefficient from the 95% confidence intervals for lag was from the Lag Before model. The mean yearly increase in myopia of −0.42 D found in the Lag Before sample is close to the typically observed progression rate of −0.50 D per year (Fulk, Cyert, & Parker, 2000; Gwiazda, Hyman, Hussein, Everett, Norton, Kurtz, Leske, Manny, Marsh-Tootle, & Scheiman, 2003). If the most negative coefficient from the 95% confidence intervals (−0.032) were the true accommodative lag value, the mean accommodative lag (1.64 D) would only account for −0.05 D (1.64 D lag x −0.032 D progression per D of lag) of the −0.42 D observed mean annual progression. Even if the most extreme relationship between accommodative lag and myopia progression that was not excluded by the observed data were in fact true, accommodative lag would still not account for a clinically meaningful amount of the myopia progression observed.
Because this is a negative result, we evaluated whether accommodative lag measurement error could have masked a meaningful relationship between lag and myopia progression. In a simple regression (i.e., one predictor and one outcome), the more error there is in the predictor (i.e., accommodative lag), the closer the estimated slope gets to zero, which is referred to as regression dilution (Frost & Thompson, 2000). We assessed the effect of regression dilution on our slope estimate for the relationship between lag and myopia progression using the intraclass correlation coefficient (ICC) of accommodative lag. An unbiased estimate of the true slope can be determined by multiplying the estimate of the slope from a simple regression by the inverse of the ICC (Frost & Thompson, 2000). Accommodative lag at each visit was an average of five measurements, and each set of five measurements was used to calculate the ICC for the accommodative lag measurement. The ICC estimate of 0.73 was used to correct the estimated slope from a simple regression of myopia progression on accommodative lag. The simple regression of myopia progression on accommodative lag (which controls for no covariates, unlike the analyses in the Results) was not statistically significant (β = −0.021 D progression per D of lag; 95% CI: −0.063 to 0.020). After correction, the unbiased slope (−0.021 × 0.73−1) is −0.030 D progression per D of lag (95% CI: −0.087 to 0.026), which was still not statistically significant. Based on this theoretical analysis, the absence of any relationship between accommodative lag and myopia progression was not due to measurement error.
This absence of an association between accommodative lag and juvenile-onset myopia progression is consistent with the results presented by Weizhong et al. (2008), which is the only published longitudinal report in children that examined the association between accommodative lag and myopia progression. They followed 62 Chinese myopic 7- to 13-year-old children for one year and found no correlation between either the initial accommodative lag and myopia progression or the average of the initial and final accommodative lag and myopia progression. Advantages of this CLEERE analysis are that the sample size is nearly ten times that of Weizhong et al. and includes many subjects with more than one year of data. Contrary to these results in children, Allen and O’Leary (2006) reported an association between higher accommodative lag and faster myopia progression in adults 18 to 22 years old (R2= 0.13). Based on closer examination of their myopia progression and accommodative lag data for the 35 adults in Figure 3 of their manuscript, it is possible that the result was influenced by one outlier with both the largest lag and the greatest progression (Allen & O’Leary, 2006). Rosenfield et al. found a significant relationship between accommodative lag and myopia progression (R2 = 0.25) in adults age 21 to 27 years; however, their subjects with the greatest myopia progression had the lowest accommodative lag (Rosenfield et al., 2002). This result contradicts the conventional hypothesis that hyperopic foveal blur causes myopia progression.
One might ask whether a child must do more near work for accommodative lag to affect myopia progression. We found that the amount of near work that a child performs had no influence on the lack of relationship between accommodative lag and the rate of myopia progression (i.e., no interaction between lag and near work). These data indicate that environmental influences do not interact with accommodative lag to accelerate myopia progression and suggest that accommodative lag may be the result of myopia development, rather than its cause (Mutti et al., 2006).
The circumstances under which children in this study wore their refractive correction were “real world,” and it is not known whether children wore their correction full time. If a child did not always wear his or her correction, accommodative lag when performing near work would be expected to be less than the lag that we measured with full correction. Though we have no way of knowing whether children removed their correction when reading or during other activities, reduced lag when not wearing correction that is randomly distributed across all refractive errors could attenuate the estimated relationship between lag and myopia progression. It is more likely that children with more myopia wore their correction full time. If this was true, one might expect progression to be related to lag in higher myopes, whereas lower myopes with less or inconsistent exposure to lag due to part-time correction might not have their myopic progression be related to lag. This difference should be detectable as a significant interaction between refractive error and lag (i.e., accommodative lag would be more important in myopia progression when children have higher amounts of myopia). Our analysis of this issue found no such interaction. Refractive error at the visit before each yearly progression interval was also not related to annual myopia progression. Despite this finding, the level of refractive error can be important in the management of a myopic child; the amount of myopia has recently been reported as an important factor in determining whether children wearing progressive addition lenses (PALs) experience a significant reduction in myopia progression (Gwiazda, Hyman, Norton, Hussein, Marsh-Tootle, Manny, Wang, & Everett, 2004). Factors that influenced the rate of myopia progression in our study were older age, which slowed progression (“Lag Before” model), and female gender, which was associated with faster progression.
Clinical trials designed to slow the progression of myopia using bifocal spectacles have found either modest or non-significant treatment effects (Edwards, Li, Lam, Lew, & Yu, 2002; Fulk et al., 2000; Gwiazda et al., 2003; Hasebe, Ohtsuki, Nonaka, Nakatsuka, Miyata, Hamasaki, & Kimura, 2008). The permanence of the bifocal treatment effect, and therefore the role of accommodative lag in myopia progression, are still unknown. While the Correction of Myopia Evaluation Trial (COMET) found a statistically significant PAL treatment effect of 0.20 D after three years (Gwiazda et al., 2003), the PAL treatment effect after five years (0.13 D) was not statistically significant (Gwiazda, Hyman, Everett, Norton, Kurtz, Manny, Marsh-Tootle, Scheiman, Dong, & The COMET Group, 2006). A study in which myopic children wearing single vision lenses were undercorrected, creating myopic retinal blur at distance and optically resulting in an add for near vision tasks, resulted in an unexpected statistically significant acceleration in myopia progression of −0.23 D over two years compared to children wearing full correction (Chung, Mohidin, & O’Leary, 2002). A subsequent 18-month undercorrection study did not find a statistically significant difference in myopia progression for undercorrected children compared to fully-corrected children (Adler & Millodot, 2006). Our finding of no relationship between accommodative lag and myopia progression seems in agreement with these small, transient, and inconsistent effects. Based on our results, if accommodative lag does contribute to myopia progression, the contribution appears to be very small and not clinically meaningful for myopic children in general.
Correction of accommodative lag may be more relevant to sub-groups of myopic children. There is evidence from COMET that PALs are more effective when children have a high lag of accommodation (Gwiazda et al., 2003), especially when the child also has near esophoria and low myopia (Gwiazda et al., 2004). Although the subgroup of COMET children with both high lag of accommodation and near esophoria had the greatest three-year PAL treatment effect (0.64 D), the five-year PAL treatment effect was reduced to 0.49 D in these same children (Gwiazda et al., 2006; Gwiazda et al., 2004). The results of COMET2 will shed more light on the effects of PALs, phoria, and accommodative lag on myopia progression in this subgroup of myopic children (ClinicalTrials.gov Identifier: NCT00320593).
The temporal and cognitive effects of accommodative lag were not investigated in this study nor was the effect of monocular versus binocular accommodative testing. Previous research indicates that extended near work would, at most, slightly reduce the amount of accommodative lag, making it unlikely that temporal aspects of accommodative lag influenced the outcome of this study. Studies have reported either no change in accommodative lag after 30 minutes of reading in adults (Shapiro, Kelly, & Howland, 2005) or a slight decrease in accommodative lag of about 0.25 D after 20 minutes of watching cartoons in myopic children (Sreenivasan, Irving, & Bobier, 2009) and emmetropic adults (Sreenivasan, Irving, & Bobier, 2008). Similarly, more cognitively intense near activities would, at most, be expected to slightly reduce the amount of accommodative lag measured compared to the task of keeping letters clear used in the present study (Kruger, 1980; Winn, Gilmartin, Mortimer, & Edwards, 1991).
We also did not evaluate accommodative lag binocularly in CLEERE; however, previous research has shown that the difference between binocular and monocular accommodative lag is minimal. In emmetropic adults, emmetropic children, and myopic children, monocular conditions either resulted in no significant change in accommodative lag or in a slight increase in accommodative lag of up to 0.25 D compared to binocular viewing conditions (Seidel, Gray, & Heron, 2005; Sreenivasan et al., 2008; Sreenivasan et al., 2009). Although the accommodative testing conditions in CLEERE may have slightly increased the amount of accommodative lag, this should only have enhanced our ability to detect a relationship between lag and the progression of myopia.
The lag data presented here were measured using a Badal target rather than a real-space target. Although a study of adolescents and young adults found no difference between accommodation to Badal targets and real targets (Stark & Atchison, 1994), most studies have reported that children accommodate less accurately to minus-lens induced blur (Anderson, Glasser, Stuebing, & Manny, 2009; Chen & O’Leary, 2000; Gwiazda et al., 1993) and Badal targets (Mutti et al., 2006) than to real targets. CLEERE measured accommodative lag monocularly using both the 4-D Badal target reported in these analyses and a 4-D real-space target, and our data show that the Badal target resulted in accommodative lag values in myopic children that were generally about 0.10 D greater than with the real target (a difference that is slightly less than 10% of the average lag measured with the two targets) (Mutti et al., 2006). Testing with a real target would have most likely resulted in more accurate accommodation to the target; however, the Badal target was chosen for these analyses because it performed better than the 4-D real target at consistently detecting the elevation in lag found in myopic versus emmetropic children. Although there is no way to know what a child’s accommodative lag was at times other than the study visit, which is true of any clinical measurement, it is reassuring that the 4-D Badal stimulus detects the greater lag found in myopic versus emmetropic children and the increase in lag associated with the onset of myopia.
Surprisingly, although the effect size was not clinically meaningful, exophoria at near was associated with myopia progression. In the model with “Lag Before,” each prism diopter of exophoria at near was associated with −0.004 D more myopic progression. If a child had 10 prism diopters of exophoria at near, it would only account for −0.04 D of his or her annual progression of myopia. This finding is contrary to the commonly held view that esophoria is associated with more rapid myopia progression (Fulk et al., 2000; Fulk, Cyert, & Parker, 2002; Goss, 1990; Goss & Jackson, 1996; Gwiazda et al., 2004). When considered in a clinical context, this association is inconsequential.
The absence of any association between accommodative lag and annual juvenile-onset myopia progression leads to speculation as to what might underlie the small bifocal treatment effects that have been reported (Edwards et al., 2002; Fulk et al., 2002; Gwiazda et al., 2003; Hasebe et al., 2008). One proposed alternate theory is that ocular mechanical factors may restrict growth of the equator and result in axial elongation in children at risk for myopia with factors that produce a larger than normal eye size (Mutti et al., 2006). In this model, ciliary-choroidal tension increases as the eye grows until it reaches a limit and prevents further equatorial expansion of the globe and compensatory changes in crystalline lens power. When equatorial expansion is halted, there is accelerated axial growth and myopia development. This could explain observations in myopic subjects such as increased ciliary body thickness (Bailey, Sinnott, & Mutti, 2008), reduced accommodative response (Abbott et al., 1998; Bullimore et al., 1992; Gwiazda et al., 1993; McBrien & Millodot, 1986), and an increase in the relatively prolate shape of the globe (Atchison, Pritchard, & Schmid, 2006; Atchison, Pritchard, Schmid, Scott, Jones, & Pope, 2005; Logan, Gilmartin, Wildsoet, & Dunne, 2004; Millodot, 1981; Mutti, Hayes, Mitchell, Jones, Moeschberger, Cotter, Kleinstein, Manny, Twelker, Zadnik, & CLEERE Study Group, 2007; Seidemann, Schaeffel, Guirao, Lopez-Gil, & Artal, 2002). Chronic reduction of accommodative response when wearing a bifocal could reduce ciliary-choroidal tension leading to a small reduction in myopia progression.
Another recent theory of myopia progression in children is that peripheral retinal defocus affects eye growth and may play an important role in determining refractive error, even if central retinal images are in focus (Smith, Hung, & Huang, 2009). By decreasing the amount of accommodative lag during near work, bifocal spectacles may also decrease the amount of peripheral hyperopic retinal defocus resulting in a reduced rate of myopia progression. This hypothesis could explain the recent, positive results of pilot studies of rigid, corneal reshaping lenses worn overnight to control myopia progression because increased spherical aberration may decrease the amount of peripheral hyperopic retinal defocus (Cho, Cheung, & Edwards, 2005; Walline, Jones, & Sinnott, 2009). More research is needed on the effect of peripheral defocus on myopia progression in children. Based on the current analysis, hypotheses other than high accommodative lag at the fovea warrant exploration as explanations for juvenile-onset myopia progression.
Acknowledgments
Grant Support: NIH/NEI Grants U10-EY008893, R24-EY014792, K12-EY015447, the Ohio Lions Eye Research Foundation, and the EF Wildermuth Foundation. American Optometric Foundation (AOF) Ezell Fellowship sponsored by the AOF Presidents’ Circle (to DAB).
Appendix
The CLEERE Study Group (as of September 2008)
Clinical Centers
Franklin Primary Health Center, Inc.:Sandral Hullett (Principal Investigator, 1997–2006), Robert N. Kleinstein (Co-Investigator, 1997–2006), Janene Sims (Optometrist, 1997–2001 and 2004–2006), Raphael Weeks (Optometrist, 1999–2006), Sandra Williams (Study Coordinator, 1999–2006), LeeAndra Calvin (Study Coordinator, 1997–1999), Melvin D. Shipp (Co-Investigator, 1997–2004).
University of California, Berkeley School of Optometry, Berkeley, CA: Nina E. Friedman (Principal Investigator, 1999–2001), Pamela Qualley (Study Coordinator, 1997–2001), Donald O. Mutti (Principal Investigator, 1996–1999), Karla Zadnik (Optometrist, 1996–2001).
University of Houston College of Optometry: Ruth E. Manny (Principal Investigator, 1997–2006), Suzanne M. Wickum (Optometrist, 1999–2006), Ailene Kim (Optometrist, 2003–2006), Bronwen Mathis (Optometrist, 2002–2006), Mamie Batres (Study Coordinator, 2004–2006). Sally Henry (Study Coordinator, 1997–1998), Janice M. Wensveen (Optometrist, 1997–2001), Connie J. Crossnoe (Optometrist, 1997–2003), Stephanie L. Tom (Optometrist, 1999–2002), Jennifer A. McLeod (Study Coordinator, 1998–2004), Julio C. Quiralte (Study Coordinator, 1998–2005).
Southern California College of Optometry, Fullerton, CA: Susan A. Cotter (Principal Investigator, 2004–2006, Optometrist, 1997–2004), Julie A. Yu (Principal Investigator, 1997–2004; Optometrist 2005–2006), Raymond J. Chu (Optometrist, 2001–2006), Carmen N. Barnhardt (Optometrist 2004–2006), Jessica Chang (Optometrist, 2005–2006), Kristine Huang (Optometrist, 2005–2006), Rebecca Bridgeford (Study Coordinator, 2005–2006), Connie Chu (Optometrist, 2004–2005), Soonsi Kwon (Optometrist, 1998–2004), Gen Lee (Study Coordinator, 1999–2003), John Lee (Optometrist, 2000–2003), Robert J. Lee (Optometrist, 1997–2001), Raymond Maeda (Optometrist, 1999–2003), Rachael Emerson (Study Coordinator, 1997–1999); Tracy Leonhardt (Study Coordinator, 2003–2004).
University of Arizona, Department of Ophthalmology and Vision Science, Tucson, AZ: J. Daniel Twelker (Principal Investigator, 2000-present), Dawn Messer (Optometrist, 2000-present), Denise Flores (Study Coordinator, 2000–2007), Rita Bhakta (Optometrist, 2000–2004), Katie Garvey (Optometrist, 2005–2008), Amanda Mendez Roberts (Optometrist, 2008-present).
Resource Centers
Chairman’s Office, The Ohio State University College of Optometry, Columbus, OH: Karla Zadnik (Chairman, 1997-present), Jodi M. Malone (Study Coordinator, 1997-present)
Videophakometry Reading Center, The Ohio State University College of Optometry, Columbus, OH: Donald O. Mutti (Director, 1997-present), Huan Sheng (Reader, 2000–2006), Holly Omlor (Reader, 2003–2006), Meliha Rahmani (Reader, 2004-present), Jaclyn Brickman (Reader, 2002–2003), Amy Wang (Reader, 2002–2003), Philip Arner (Reader, 2002–2004), Samuel Taylor (Reader, 2002–2003), Myhanh T. Nguyen (Reader, 1998–2001), Terry W. Walker (Reader, 1997–2001), Vidhya Subramanian (Reader, 2006-present).
Optometry Coordinating Center, The Ohio State University College of Optometry, Columbus, OH: Lisa A. Jones-Jordan (Director, 1997-present), Linda Barrett (Data Entry Operator, 1997–2007), John Hayes (Biostatistician, 2001–2007), G. Lynn Mitchell (Biostatistician, 1998-present), Melvin L. Moeschberger (Consultant, 1997-present), Loraine Sinnott (Biostatistician, 2005-present), Pamela Wessel (Program Coordinator, 2000-present), Julie N. Swartzendruber (Program Coordinator, 1998–2000).
Project Office, National Eye Institute, Rockville, MD: Donald F. Everett.
Committees
Executive Committee: Karla Zadnik (Chairman), Lisa A. Jones, Robert N. Kleinstein, Ruth E. Manny, Donald O. Mutti, J. Daniel Twelker, Susan A. Cotter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott ML, Schmid KL, Strang NC. Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthalmic Physiol Opt. 1998;18(1):13–20. [PubMed] [Google Scholar]
- Adler D, Millodot M. The possible effect of undercorrection on myopic progression in children. Clin Exp Optom. 2006;89(5):315–321. doi: 10.1111/j.1444-0938.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Allen PM, O’Leary DJ. Accommodation functions: co-dependency and relationship to refractive error. Vision Res. 2006;46(4):491–505. doi: 10.1016/j.visres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Glasser A, Stuebing KK, Manny RE. Minus lens stimulated accommodative lag as a function of age. Optom Vis Sci. 2009;86(6):685–694. doi: 10.1097/OPX.0b013e3181a7294f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison DA, Pritchard N, Schmid KL. Peripheral refraction along the horizontal and vertical visual fields in myopia. Vision Res. 2006;46(8–9):1450–1458. doi: 10.1016/j.visres.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Pritchard N, Schmid KL, Scott DH, Jones CE, Pope JM. Shape of the retinal surface in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2005;46(8):2698–2707. doi: 10.1167/iovs.04-1506. [DOI] [PubMed] [Google Scholar]
- Bailey MD, Sinnott LT, Mutti DO. Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci. 2008;49(10):4353–4360. doi: 10.1167/iovs.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullimore MA, Gilmartin B, Royston JM. Steady-state accommodation and ocular biometry in late-onset myopia. Doc Ophthalmol. 1992;80(2):143–155. doi: 10.1007/BF00161240. [DOI] [PubMed] [Google Scholar]
- Charman WN. Near vision, lags of accommodation and myopia. Ophthalmic Physiol Opt. 1999;19(2):126–133. doi: 10.1046/j.1475-1313.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- Chen AH, O’Leary DJ. Free-space accommodative response and minus lens-induced accommodative response in pre-school children. Optometry. 2000;71(7):454–458. [PubMed] [Google Scholar]
- Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30(1):71–80. doi: 10.1080/02713680590907256. [DOI] [PubMed] [Google Scholar]
- Choong YF, Chen AH, Goh PP. A comparison of autorefraction and subjective refraction with and without cycloplegia in primary school children. Am J Ophthalmol. 2006;142(1):68–74. doi: 10.1016/j.ajo.2006.01.084. [DOI] [PubMed] [Google Scholar]
- Chung K, Mohidin N, O’Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res. 2002;42(22):2555–2559. doi: 10.1016/s0042-6989(02)00258-4. [DOI] [PubMed] [Google Scholar]
- Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Invest Ophthalmol Vis Sci. 2002;43(9):2852–2858. [PubMed] [Google Scholar]
- Frost C, Thompson SG. Correcting for regression dilution bias: comparison of methods for a single predictor variable. J R Stat Soc Ser A Stat Soc. 2000;163(2):173–189. [Google Scholar]
- Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77(8):395–401. doi: 10.1097/00006324-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE. A randomized clinical trial of bifocal glasses for myopic children with esophoria: results after 54 months. Optometry. 2002;73(8):470–476. [PubMed] [Google Scholar]
- Gilmartin B. Myopia: precedents for research in the twenty-first century. Clin Exp Ophthalmol. 2004;32(3):305–324. doi: 10.1111/j.1442-9071.2004.00831.x. [DOI] [PubMed] [Google Scholar]
- Goss DA. Variables related to the rate of childhood myopia progression. Optom Vis Sci. 1990;67(8):631–636. doi: 10.1097/00006324-199008000-00014. [DOI] [PubMed] [Google Scholar]
- Goss DA, Hampton MJ, Wickham MG. Selected review on genetic factors in myopia. J Am Optom Assoc. 1988;59(11):875–884. [PubMed] [Google Scholar]
- Goss DA, Jackson TW. Clinical findings before the onset of myopia in youth: 3. Heterophoria. Optom Vis Sci. 1996;73(4):269–278. doi: 10.1097/00006324-199604000-00009. [DOI] [PubMed] [Google Scholar]
- Goss DA, Rainey BB. Relationship of accommodative response and nearpoint phoria in a sample of myopic children. Optom Vis Sci. 1999;76(5):292–294. doi: 10.1097/00006324-199905000-00016. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Bauer J, Thorn F, Held R. A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vision Res. 1995;35(9):1299–1304. doi: 10.1016/0042-6989(94)00238-h. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, Leske MC, Manny R, Marsh-Tootle W, Scheiman M. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44(4):1492–1500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34(3):690–694. [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Held R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci. 2005;82(4):273–278. doi: 10.1097/01.opx.0000159363.07082.7d. [DOI] [PubMed] [Google Scholar]
- Gwiazda JE, Hyman L, Everett D, Norton T, Kurtz D, Manny R, Marsh-Tootle W, Scheiman M, Dong L The COMET Group. Five-year results from the correction of myopia evaluation trial (COMET) Invest Ophthalmol Vis Sci. 2006;47:E-abstract 1166. [Google Scholar]
- Gwiazda JE, Hyman L, Norton TT, Hussein ME, Marsh-Tootle W, Manny R, Wang Y, Everett D. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest Ophthalmol Vis Sci. 2004;45(7):2143–2151. doi: 10.1167/iovs.03-1306. [DOI] [PubMed] [Google Scholar]
- Hasebe S, Ohtsuki H, Nonaka T, Nakatsuka C, Miyata M, Hamasaki I, Kimura S. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci. 2008;49(7):2781–2789. doi: 10.1167/iovs.07-0385. [DOI] [PubMed] [Google Scholar]
- Irving EL, Callender MG, Sivak JG. Inducing myopia, hyperopia, and astigmatism in chicks. Optom Vis Sci. 1991;68(5):364–368. doi: 10.1097/00006324-199105000-00007. [DOI] [PubMed] [Google Scholar]
- Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12(4):448–456. [PubMed] [Google Scholar]
- Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48(8):3524–3532. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstein RN, Mutti DO, Manny RE, Shin JA, Zadnik K. Cycloplegia in African-American children. Optom Vis Sci. 1999;76(2):102–107. doi: 10.1097/00006324-199902000-00017. [DOI] [PubMed] [Google Scholar]
- Kruger PB. The effect of cognitive demand on accommodation. Am J Optom Physiol Opt. 1980;57(7):440–445. doi: 10.1097/00006324-198007000-00006. [DOI] [PubMed] [Google Scholar]
- Logan NS, Gilmartin B, Wildsoet CF, Dunne MC. Posterior retinal contour in adult human anisomyopia. Invest Ophthalmol Vis Sci. 2004;45(7):2152–2162. doi: 10.1167/iovs.03-0875. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Millodot M. The effect of refractive error on the accommodative response gradient. Ophthalmic Physiol Opt. 1986;6(2):145–149. [PubMed] [Google Scholar]
- Millodot M. Effect of ametropia on peripheral refraction. Am J Optom Physiol Opt. 1981;58(9):691–695. doi: 10.1097/00006324-198109000-00001. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Hayes JR, Mitchell GL, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K CLEERE Study Group. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48(6):2510–2519. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Jones LA, Moeschberger ML, Zadnik K. AC/A ratio, age, and refractive error in children. Invest Ophthalmol Vis Sci. 2000;41(9):2469–2478. [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K CLEERE Study Group. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006;47(3):837–846. doi: 10.1167/iovs.05-0888. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children’s refractive error. Invest Ophthalmol Vis Sci. 2002;43(12):3633–3640. [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47(11):4687–4699. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield M, Desai R, Portello JK. Do progressing myopes show reduced accommodative responses? Optom Vis Sci. 2002;79(4):268–273. doi: 10.1097/00006324-200204000-00014. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28(5):639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Sahagian CR, Glynn RJ, Sperduto RD, Gragoudas ES. Evaluation of an iris color classification system. The Eye Disorders Case-Control Study Group. Invest Ophthalmol Vis Sci. 1990;31(8):1592–1598. [PubMed] [Google Scholar]
- Seidel D, Gray LS, Heron G. The effect of monocular and binocular viewing on the accommodation response to real targets in emmetropia and myopia. Optom Vis Sci. 2005;82(4):279–285. doi: 10.1097/01.opx.0000159369.85285.21. [DOI] [PubMed] [Google Scholar]
- Seidemann A, Schaeffel F, Guirao A, Lopez-Gil N, Artal P. Peripheral refractive errors in myopic, emmetropic, and hyperopic young subjects. J Opt Soc Am A Opt Image Sci Vis. 2002;19(12):2363–2373. doi: 10.1364/josaa.19.002363. [DOI] [PubMed] [Google Scholar]
- Shapiro JA, Kelly JE, Howland HC. Accommodative state of young adults using reading spectacles. Vision Res. 2005;45(2):233–245. doi: 10.1016/j.visres.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39(8):1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49(19):2386–2392. doi: 10.1016/j.visres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan V, Irving EL, Bobier WR. Binocular adaptation to near addition lenses in emmetropic adults. Vision Res. 2008;48(10):1262–1269. doi: 10.1016/j.visres.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Sreenivasan V, Irving EL, Bobier WR. Binocular adaptation to +2 D lenses in myopic and emmetropic children. Optom Vis Sci. 2009;86(6):731–740. doi: 10.1097/OPX.0b013e3181a59d78. [DOI] [PubMed] [Google Scholar]
- Stark LR, Atchison DA. Subject instructions and methods of target presentation in accommodation research. Invest Ophthalmol Vis Sci. 1994;35(2):528–537. [PubMed] [Google Scholar]
- Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol. 2009;93(9):1181–1185. doi: 10.1136/bjo.2008.151365. [DOI] [PubMed] [Google Scholar]
- Weizhong L, Zhikuan Y, Wen L, Xiang C, Jian G. A longitudinal study on the relationship between myopia development and near accommodation lag in myopic children. Ophthalmic Physiol Opt. 2008;28(1):57–61. doi: 10.1111/j.1475-1313.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35(9):1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- Winn B, Gilmartin B, Mortimer LC, Edwards NR. The effect of mental effort on open- and closed-loop accommodation. Ophthalmic Physiol Opt. 1991;11(4):335–339. [PubMed] [Google Scholar]
- Zadnik K, Mutti DO, Adams AJ. The repeatability of measurement of the ocular components. Invest Ophthalmol Vis Sci. 1992;33(7):2325–2333. [PubMed] [Google Scholar]
- Zadnik K, Mutti DO, Friedman NE, Adams AJ. Initial cross-sectional results from the Orinda Longitudinal Study of Myopia. Optom Vis Sci. 1993;70(9):750–758. doi: 10.1097/00006324-199309000-00012. [DOI] [PubMed] [Google Scholar]