Abstract
The fully substituted hydroisobenzofuran core of the massileunicellins containing 8 contiguous stereocenters was prepared in 12 steps from (S)-(+)-carvone. Noteworthy elements of the synthesis include a one-step oxidative rearrangement/epoxidation, a novel stereoselective directed reduction of a keto diol, and a directed hydrogenation of a congested tetrasubstituted alkene.
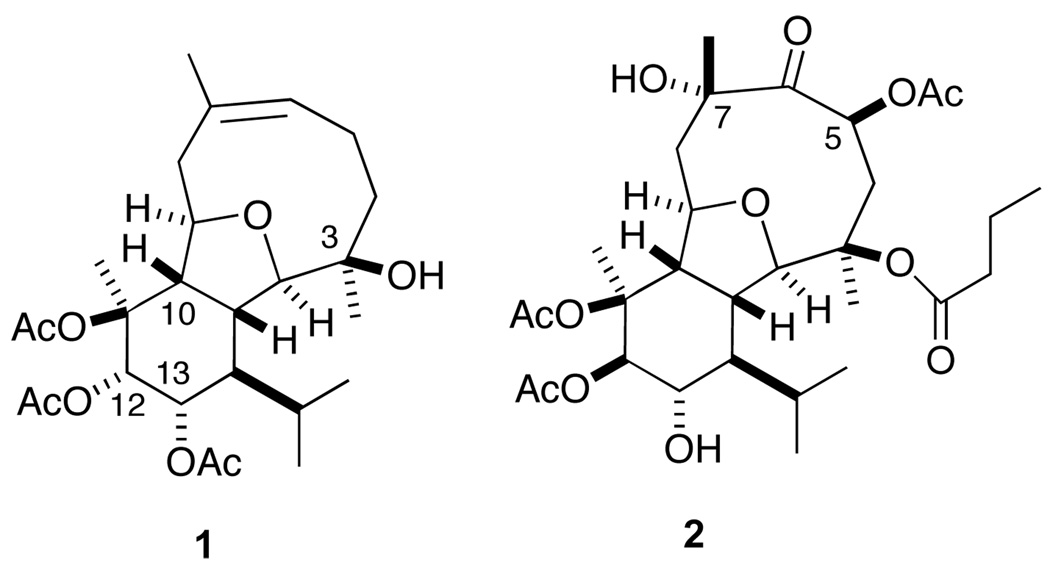
In 1999 and 2000, Pietra et al reported the structures of a series of eleven highly oxidized eunicellin diterpenes, designated massileunicellins, from the gorgonian coral Eunicella cavolinii (Figure 1).1 In 2009, Sheu et al disclosed the structures of a related set of natural products from Klyxum simplex that they named the simplexins.2,3 These compounds are the most densely functionalized and highly oxidized members of the eunicellin diterpene family.4 All of the massileunicellins and most of the simplexins contain 9 contiguous stereocenters, while simplexins C-I possess two additional oxygen-containing stereocenters at C5 and C7. While there have been a number of total, formal and partial syntheses of various 2,11-cyclized cembranoids,5,6 there have been no reports outside our own concerning the synthesis of massileunicellins (or simplexins).7
Figure 1.
Massileunicellin C (1) and simplexin F (2).
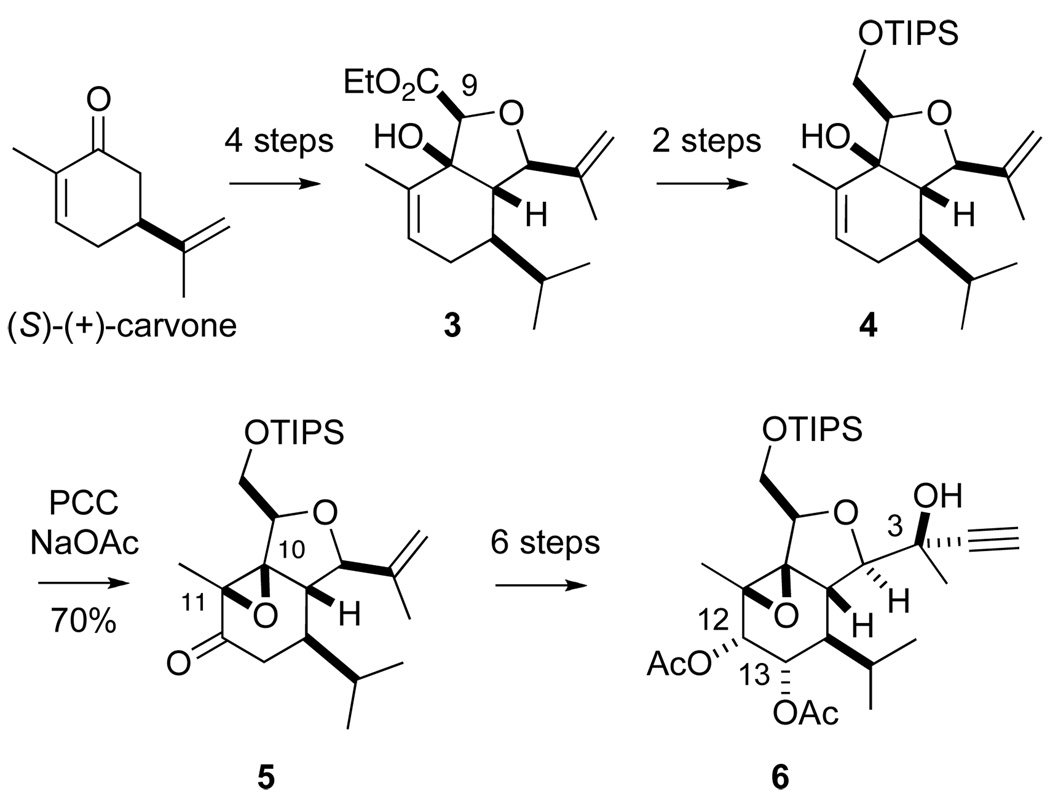
In our previous report, we described an aldol/cycloaldol approach to the massileunicellins that assembled hydroisobenzofuran 3 in 4 steps from (S)-(+)-carvone (Scheme 1). We also described an unusual oxidative rearrangement of 3° allylic alcohol 4 epoxy ketone 5. This oxidation, originally disclosed by Sundaraman and Herz, had not been employed in synthesis since their 1977 report.8
Scheme 1.
Initial approach toward the massileunicellins.
The Herz oxidation has the advantage of installing the requisite β-C-O bond at C11 (4 to 5, Scheme 1). However, it would be necessary at some point to reduce the C10 C-O bond to the alkane oxidation state (cf. 1 and 2, Figure). In our earlier report, we elected to postpone addressing that issue in order to focus on installing the C3, C12 and C13 stereocenters. We describe herein the details of a modified approach that takes advantage of the Herz oxidation, but resolves the issue of the C10 oxidation state.
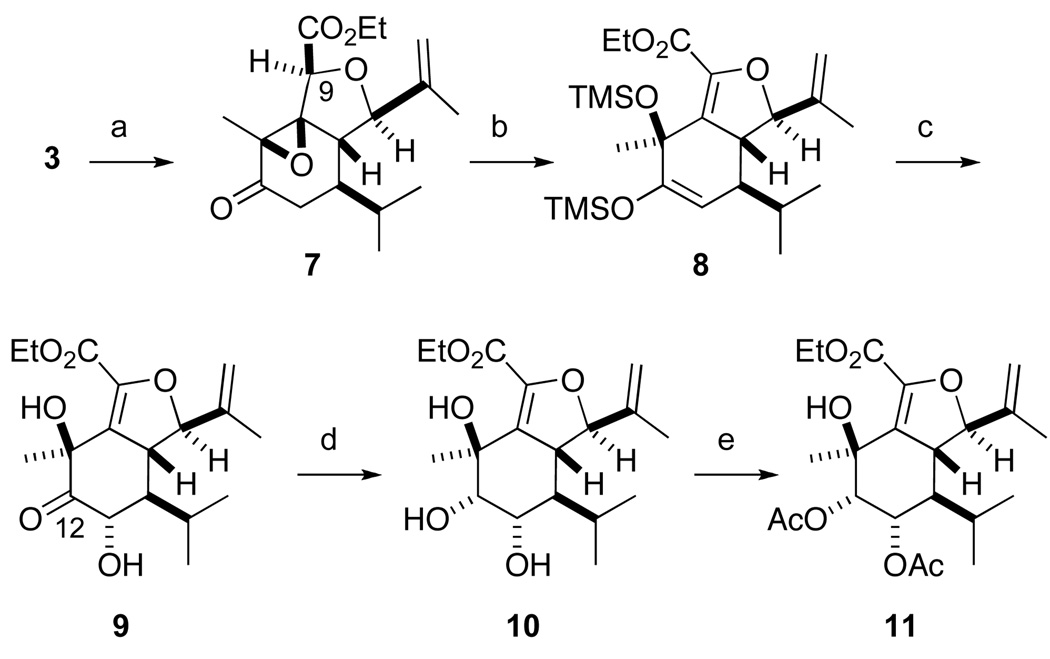
In our modified synthesis, we retain the C9 ester rather than reducing it (cf. Scheme 1). In the course of optimizing the Herz oxidation of β-hydroxy ester 3, we found that Collins’ reagent converted allylic alcohol 3 to epoxy ketone 7 in high yield (Scheme 2). Exposure of epoxide 7 to excess KHMDS/TMSCl simultaneously opened the epoxide and converted the C12 ketone to an enol silane to give enol silane 8. Rubottom oxidation9 of the enol silane was effected using in situ generated dioxirane10,11 to furnish keto diol 9 without competitive oxidation of the other alkenes.
Scheme 2.
Reagents and conditions: (a) CrO3, pyr, silica gel, CH2Cl2, −78 °C to rt, 90%; (b) KHMDS, TMSCl, ether, −78 °C. (c) Oxone®, EDTA, NaHCO3, CH3CN, H2O, acetone, rt; Bu4NF, THF, 0 °C, 83% from 7. (d) Me4NBH(OAc)3, AcOH, CH3CN, −40 to −20 °C, 95%; (e) Ac2O, DMAP, NEt3, CH2Cl2, −10 °C, 80%.
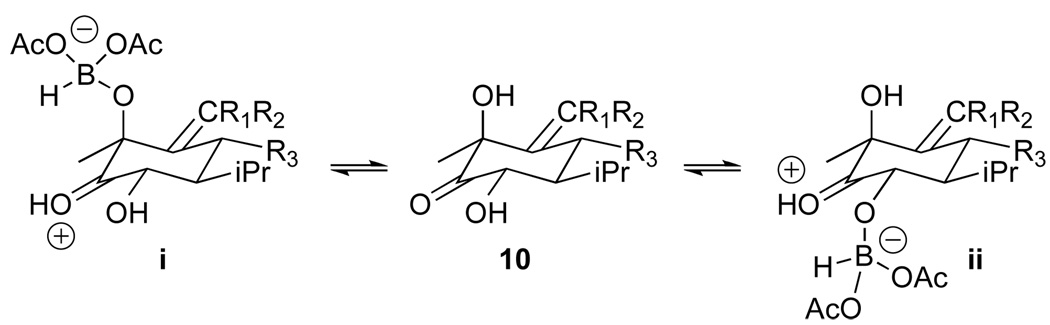
We next sought to employ a hydroxyl directed reduction to convert the hindered C12 ketone of ketodiol 9 to triol 10. Reductions of cyclic and acyclic β-hydroxy ketones with Me4NHB(OAc)3 are believed to occur through internal delivery of the hydride resulting from exchange of acetate of the borohydride reagent for the substrate hydroxyl group.12 Directed reduction of cyclic α-hydroxy ketones presumably occurs via a similar pathway (Scheme 3).13 Keto diol 9 could give rise to either the desired α-C12 configuration via equatorial delivery of hydride from axial hydride complex i or the undesired β-C12 configuration via axial delivery from equatorial complex ii.
Scheme 3.
Presumed borohydride intermediates. The dihydrofuran ring is abbreviated for simplicity.
Examination of molecular and computer models of i and ii suggested to us that the transition state for delivery of hydride from equatorial hydride complex ii would be considerably more strained than for axial complex i.14 We reasoned that prior protection of the C13 hydroxy group would hence be unneccessary. In the event, treatment of keto diol 9 with Me4NHB(OAc)3 gave rise to triol 10 as a single diastereomer in excellent yield. Selective acylation of the two 2° hydroxyl groups yielded diacetate 11.
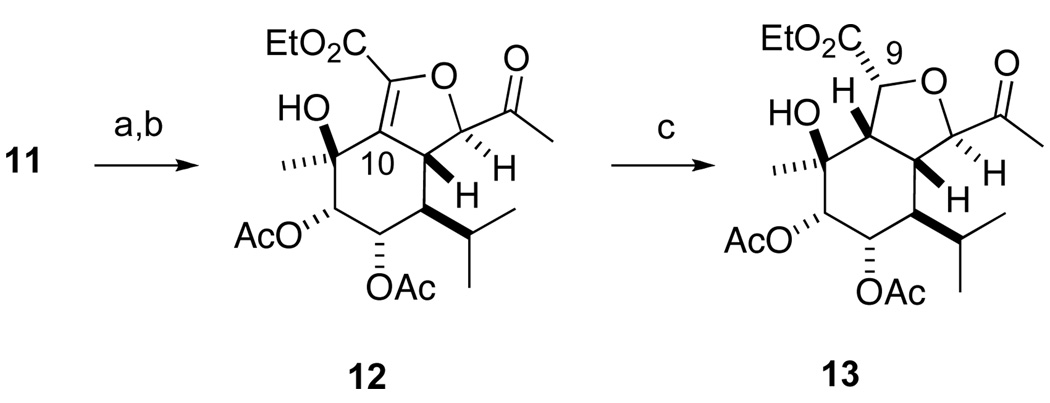
The next step in the synthesis was to unmask the C2 ketone via oxidative cleavage of the C2 isopropenyl group (Scheme 4). The ozonolytic approach that we employed previously was clearly obviated by the presence of the C9–C10 alkene. Fortunately, oxidative cleavage of the alkene via the intermediate diol was successful. Both one-step15 and two-step procedures were examined, with the two-step procedure giving similar overall yield while simplifying purification. The chiral (DHQD)2PHAL ligand proved optimal for the dihydroxylation of diene 11, although the diastereoselectivity of the reaction is presumably irrelevant. Oxidative cleavage of the intermediate diol yielded methyl ketone 12.
Scheme 4.
Reagents and conditions: (a) OsO4, (DHQD)2PHAL, K3Fe(CN)6, MeSO2NH2, K3CO3, t-BuOH, H2O, 4 °C; (b) NaIO4, THF, H2O, rt, 85% from 11; (c) [Ir(COD)PCy3Py]PF6 (17 mol %), CH2Cl2, H2 (50 psi), 12 h, rt, 100%.
A hydrogenation directed by the C11 hydroxyl group would install the requisite configuration at C10.16 Although this would also presumably generate the α-C9 ester epimer, we expected that epimerization to the β-configuration would be thermodynamically favored.17
While diastereoselective directed hydrogenation using Ir(I) and Rh(I) catalysts is well established,16 the multiple Lewis basic functional groups that were present in alkene 12 was a potential cause for concern. Although ketones, esters and alcohols are all known to serve as directing groups, we expected that the allylic C11 hydroxyl (possibly reinforced by the C2 ketone) would be more a effective directing group than the C12 and C13 esters. In practice, we found that use of Crabtree’s catalyst18 at moderate H2 pressure (50 psi) gave high yield of the desired hydroisobenzofuran 13.
In summary, we have prepared the fully substituted isobenzofuran core of the massileunicellins in 12 steps from (S)-(+)-carvone, albeit with the epi-C9 configuration. We note that the synthesis employed no protecting groups beyond the adventitious silylation of the C11 hydroxyl group in the course of the Rubottom oxidation protocol. Further, all transformations were effected on multigram scale with the exception of the dihydroxyation step, for which a 1.5 g scale was optimal. We are currently examining adjusting the C9 configuration and the completion of the synthesis. These studies will be disclosed in due course.
Supplementary Material
Acknowledgments
We thank the NIH (GM59402, CA125602 and RR15569), and the Arkansas Biosciences Institute for support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found in the online version.19
References and notes
- 1.(a) Mancini I, Guella G, Zibrowius H, Laurent D, Pietra F. Helvetica Chimica Acta. 1999;82:1681–1689. [Google Scholar]; (b) Mancini I, Guella G, Zibrowius H, Pietra F. Helv. Chim. Acta. 2000;83:1561–1575. [Google Scholar]
- 2.Wu S-L, Su J-H, Wen Z-H, Hsu C-H, Chen B-W, Dai C-F, Kuo Y-H, Sheu J-H. J. Nat. Prod. 2009;72:994–1000. doi: 10.1021/np900064a. [DOI] [PubMed] [Google Scholar]
- 3. Unfortunately, the name choice will likely lead to some confusion, since the name simplexin was previously given to an unrelated diterpenoid isolated from the desert rice flower Pimelea simplex found in Australia: Freeman PW, Ritchie E, Taylor WC. Aust. J. Chem. 1979:2495–2506.
- 4.(a) Bernardelli P, Paquette LA. Heterocycles. 1998;49:531–556. [Google Scholar]; (b) Wahlberg I, Eklund A-M. Prog. Chem. Org. Nat. Prod. 1992;60:1–141. [Google Scholar]
- 5.Ellis JM, Crimmins MT. Chem. Rev. 2008;108:5278–5298. doi: 10.1021/cr078117u. [DOI] [PubMed] [Google Scholar]
- 6.Campbell MJ, Johnson JS. J. Am. Chem. Soc. 2009;131:10370–10371. doi: 10.1021/ja904136q. [DOI] [PubMed] [Google Scholar]
- 7.Chai Y, McIntosh MC. Tetrahedron Lett. 2004;45:3269–3272. doi: 10.1016/j.tetlet.2010.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundararaman P, Herz W. J. Org. Chem. 1977;42:813–819. [Google Scholar]
- 9.Rubottom GM, Vazquez MA, Pelegrina DR. Tetrahedron Lett. 1974:4319–4322. [Google Scholar]
- 10.Adam W, Saha-Moeller CR, Zhao C-G. Org. React. 2002;61:219–516. [Google Scholar]
- 11.While we initially employed the distilled solution of dioxirane in acetone for the oxidation, we later found that the enol silane did not hydrolyze during its oxidation when buffered conditions were used.
- 12.Evans DA, Chapman KT, Carreira EM. J. Am. Chem. Soc. 1988;110:3560–3578. [Google Scholar]
- 13.(a) Bailey S, Teerawutgulrag A, Thomas EJ. J. Chem. Soc., Chem. Commun. 1995:2519–2520. [Google Scholar]; (b) Helliwell M, Karim S, Parmee ER, Thomas EJ. Organic Biomol. Chem. 2005:3636–3653. doi: 10.1039/b508670a. [DOI] [PubMed] [Google Scholar]; (c) Bailey S, Helliwell M, Teerawutgulrag A, Thomas EJ. Organic Biomol. Chem. 2005:3654–3677. doi: 10.1039/b508675b. [DOI] [PubMed] [Google Scholar]
- 14.Evans et al noted that equatorially disposed hydroxyl groups were slower than axial hydroxyls in reducing β–ketones in cyclohexanones (ref 11). They suggested that the directed reduction would have to proceed through a higher energy twist-boat conformation in the former case.
- 15.Yu W, Mei Y, Kang Y, Hua Z, Jin Z. Org. Lett. 2004;6:3217–3219. doi: 10.1021/ol0400342. [DOI] [PubMed] [Google Scholar]
- 16.For a recent review, see: Yamagishi T. In: Handbook of Homogeneous Hydrogenation. de Vries JG, Elsevier CJ, editors. Vol. 2. Weinheim: Wiley-VCH; 2007. pp. 631–712.
- 17.A related epimerization of the C2 stereocenter was reported by Paquette et al in their synthesis of sclerophytin A: Bernardelli P, Moradei OM, Friedrich D, Yang J, Gallou F, Dyck BP, Doskotch RW, Lange T, Paquette LA. J. Am .Chem. Soc. 2001;123:9021–9032. doi: 10.1021/ja011285y.
- 18.Crabtree RH, Davis MW. J. Org. Chem. 1986;51:2655–2661. [Google Scholar]
- 19.NMR data for selected compounds:3: 1H-NMR (270 MHz, CDC13) δ 5.56 (m, 1H), 5.19 (s, 1H), 4.94 (s, 1H), 4.15–4.28 (m, 4H), 3.18 (s, 1H), 2.26 (t, J=7.92 Hz, 1H), 1.9 (m, 2H), 1.75-1.9 (m, 4H), 1.7 (s, 3H), 1.3 (m, 1H), 1.21 (t, J=7.1 Hz, 3H), 0.90 (d, J=6.73 Hz, 3H), 0.76 (d, J=6.73 Hz, 3H). 13C-NMR (67 MHz, CDCl3) δ 170.77, 144.63, 133.11, 113.83, 86.17, 83.46, 81.88, 61.42, 51.42, 40.52, 27.22, 23.42, 21.49, 17.97, 17.61, 14.11.7: 1H-NMR (270 MHz, CDCl3) δ 5.08 (s, 1H), 5.02 (s, 1H), 4.36 (s, 1H), 4.1–4.3 (m, 3H), 2.64 (d, J=9.5 Hz, 1H), 2.59 (m, 1H), 2.52 (d, J=11.5 Hz, 1H), 2.00 (dd, J=2.5, 13.0 Hz, 1H), 1.92 (s, 3H), 1.67–1.82 (m, 2H), 1.44 (s, 3H), 1.26 (t, J=7.12 Hz, 3H), 0.79 (d, J=6.73 Hz, 3H), 0.75 (d, J=6.73 Hz, 3H). 13C-NMR (67 MHz, CDCl3) δ 207.46, 168.61, 143.34, 117.43, 89.34, 78.81, 74.50, 63.34, 61.56, 45.41, 41.37, 32.52, 28.05, 20.84, 16.07, 15.47, 14.23, 11.45.9: 1H-NMR (270 MHz, CDCl3) δ 5.02 (s, 1H), 4.97 (s, 1H), 4.75 (d, J=6.93 Hz, 1H), 4.66 (dd, J=4.75, 10.98 Hz, 1H), 4.2 (m, 2H), 3.3 (m, 2H), 3.16 (s, 1H), 1.95 (m, 1H), 1.79 (s, 3H), 1.68 (t, J=10.98 Hz, 1H), 1.58 (s, 3H), 1.30 (t, J=7.12 Hz, 3H), 1.07 (d, J=6.93 Hz, 3H), 0.97 (d, J=6.93 Hz, 3H). 13C-NMR (67 MHz, CDCl3) δ̣ 208.93, 161.59, 143.29, 142.88, 120.59, 114.75, 88.89, 73.37, 72.42, 62.29, 56.27, 46.13, 27.54, 22.58, 21.67, 16.66, 16.37, 14.04.10: 1H-NMR (270 MHz, CDCl3) δ 5.14 (s, 1H), 5.05 (s, 1H), 4.96 (s, 1H), 4.56 (d, J=11.48 Hz, 1H), 4.28 (m, 2H), 4.05 (s, 1H), 3.95 (t, J=5.34 Hz, 1H), 3.62 (s, 1H), 3.08 (t, J=12.47 Hz, 1H), 3.00 (s, 1H), 1.62–1.89 (m, 5H), 1.49 (s, 3H), 1.29 (t, J=7.12 Hz, 3H), 0.97 (d, J=6.93 Hz, 3H), 0.63 (d, J=6.73 Hz, 3H). 13C-NMR (67 MHz, CDCl3) δ 162.49, 142.10, 140.68, 134.81, 117.14, 91.63, 74.03, 70.50, 67.29, 62.21, 50.74, 43.75, 26.51, 23.34, 21.39, 16.62, 15.95, 14.17.11: 1H-NMR (270 MHz, CDCl3) δ 5.31 (dd, J=4.35, 7.62 Hz, 1H), 5.00 (m, 2H), 4.97 (s, 1H), 4.65 (d, J=9.90 Hz, 1H), 4.25 (m, 2H), 3.18 (dd, J=9.90, 12.27 Hz, 1H), 2.01 (s, 3H), 1.96 (s, 3H), 1.76–1.91 (m, 2H), 1.74 (s, 3H), 1.48 (s, 3H), 1.29 (t, J=7.13 Hz, 3H), 0.84 (d, J=7.13 Hz, 3H), 0.74 (d, J=6.73 Hz, 3H). 13C-NMR (67 MHz, CDCl3) δ 170.20, 169.89, 162.05, 142.57, 141.10, 116.06, 90.50, 74.19, 69.23, 68.18, 62.08, 48.43, 45.17, 26.61, 23.87, 21.33, 21.09, 20.85, 16.51, 15.76, 14.11.12: 1H-NMR (270 MHz, CDC13) δ 5.36 (dd, J=3.56, 9.7 Hz, rew1H), 5.27 (s, 1H), 5.06 (d, J=3.56 Hz, 1H), 4.3 (m, 2H), 3.36 (dd, J=7.52, 12.07 Hz, 1H), 2.27 (s, 3H), 2.04 (s, 3H), 1.97 (s, 3H), 1.72–1.83 (m, 2H), 1.40 (s, 3H), 1.28 (t, J=7.12 Hz, 3H), 0.93 (d, J=6.93 Hz, 3H), 0.82 (d, J=6.73 Hz, 1H). 13C-NMR (67 MHz, CDCl3) δ 207.53, 170.17, 170.02, 161.34, 140.67, 75.09, 69.65, 62.07, 47.03, 46.69, 27.20, 25.93, 24.00, 21.90, 21.07, 20.85, 16.07, 14.06.13: 1H-NMR (270 MHz, CDCl3) δ 5.37 (dd, J=3.17, 6.14 Hz, 1H), 5.00 (d, J=3.17 Hz, 1H), 4.75 (d, J=6.43 Hz, 1H), 4.58 (d, J=8.11 Hz, 1H), 4.3 (m, 2H), 2.77 (t, J=6.93 Hz, 1H), 2.52 (s, 1H), 2.45 (app q, J=7.52 Hz, 1H), 2.23 (s, 3H), 2.07 (s, 3H), 2.05 (s, 3H), 2.0 (m, 1H), 1.8 (m, 1H), 1.46 (s, 3H), 1.34 (t, J=7.32 Hz, 3H), 0.99 (d, J=6.93 Hz, 3H), 0.96 (t, J=6.93 Hz, 3H). 13C-NMR (67 MHz, CDC13) δ 209.33, 171.36, 170.49, 169.85, 87.29, 80.26, 74.28, 71.66, 71.02, 61.62, 51.97, 43.33, 41.98, 29.90, 25.89, 22.70, 22.50, 21.25, 21.00, 18.39, 14.22.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.