Abstract
The cervical mucus plug (CMP) differs from the cervical secretions of non-pregnant women, and is the ultimate sealant of the uterine cavity during pregnancy. Although several studies have analyzed biochemical properties of large glycoproteins in the CMP, comprehensive information about its protein composition is yet unavailable. We hypothesized that protein profiling of the CMP could provide key clues to its physiological functions in pregnancy. For this purpose, five CMPs obtained from women in labor at term were analyzed by LC-MS/MS. Out of 291 total proteins identified, 137 were detected in two or more samples, which included S100A8, S100A9, and complement proteins (C3, C4a, C4b, C6, C8g). Several proteins, which have not been described in the cervical mucus of non-pregnant women or in cervicovaginal fluids, such as CD81 antigen and pregnancy zone protein, were also identified. Gene ontology analysis of identified proteins showed significant enrichment of 28 biological processes such as ‘activation of plasma proteins involved in acute inflammatory response’ and ‘positive regulation of cholesterol esterification’. We report the proteome of CMPs from pregnant women at term for the first time, and the overall findings strongly suggest an important role for the CMP in the maintenance of pregnancy and parturition.
Keywords: Cervical mucus plug, Pregnancy, Proteomics
1 Introduction
Cervical mucus is involved in the regulation of sperm transfer [1] and also acts as a barrier to infection. The biological properties of this mucus change during the menstrual cycle. Mucus glycoproteins (mucins) are most abundant near ovulation due to high secretion, but the abundance of other proteins increases during the luteal phase [2]. Wolf et al proposed that the variable viscoelastic properties of cervical mucus are determined by the carbohydrate composition and/or structure. They also suggested that mucin concentration, rather than composition, changes in response to alterations in the hormonal milieu [3]. During pregnancy, sustained elevation of maternal plasma progesterone induces cervical mucus to form a more viscous structure called ‘the cervical mucus plug’ (CMP) [4,5]. The CMP is a unique and sizable (approximately 10 gm) structure that forms physical and biochemical barriers for the microorganisms present in the vagina [6]. It has been demonstrated that mucin proteins in the CMP, such as MUC1, MUC2, MUC5AC, and MUC5B, profoundly inhibit the activity of human immunodeficiency virus type I [7]. The biological and physical integrity of the CMP is therefore critical for a successful pregnancy because its disruption can lead to ascending intra-amniotic infection, one of the leading causes of preterm birth [6,8].
Transcriptome and proteome analyses are two effective and proven measures used to address global biological characteristics and functions of tissue or cells in reproductive science [9–11]. Protein mixtures of considerable complexity can now be routinely characterized using the mass spectrometry-based proteomics [12]. For biological samples that lack cellular elements, however, the transcriptomic approach is of very limited value. Therefore, proteome analysis becomes a far more feasible option in assessing the biological functions of serum and secretions [13,14]. Previous studies have elegantly shown the characteristics of mucins and the presence of selected anti-microbial peptides in the CMPs [4,12,15]. Protein profiles of the cervical mucus of non-pregnant women have been studied. Andersch-Bjőrkman et al have reported comprehensive proteomic and glycomic analyses of the cervical mucus obtained from 12 non-pregnant women relative to time in the menstrual cycle (before, during, and after ovulation) using LC-FT-ICR and MS/MS, and Panicker et al applied SELDI-TOF MS [16,17].
During pregnancy, the uterine cervix shows changes that differentiate it from that of non-pregnant women, and it undergoes physiological remodeling throughout pregnancy [18]. Functional integrity of the cervix is critical to both maintenance of pregnancy and uneventful parturition. Since the cervix has a protective role, it is not surprising that women with a short cervix are at increased risk of intra-amniotic infection related to preterm birth [19,20]. Based on the integral role the CMP has on uterine cervical function, we hypothesized that there are proteins in the CMP that are not present in the cervical mucus of non-pregnant women. For this purpose, the CMPs obtained from five women were profiled by a proteomic approach. These data were then used for bioinformatics analyses to better understand the biological function of the CMP.
2 Materials and methods
2.1 Patient materials
Cervical mucus plugs were obtained from five women who underwent vaginal delivery at term in the Aarhus University Hospital, Skejby, Denmark. The gestational age at delivery ranged between 39+0 weeks and 41+0 weeks. The CMPs were either obtained digitally during routine vaginal exploration or shed spontaneously when a cervical dilatation of 2–10 cm allowed for their detection. The characteristic visco-elastic and sticky appearance of the CMP makes it easy for an experienced midwife to recognize and distinguish it from blood, amniotic fluids, and vaginal fluids (Figure 1). All samples were stored at −80 °C until analysis. As the CMP passes through the birth canal, minor surface contamination with these fluids cannot be completely avoided. To minimize the influence of such contamination on study results, the center portions of the CMPs were cut out from the center before protein extraction. The samples were obtained before rupture of membranes in four women, and one woman had premature rupture of membranes before collection of the CMP. All patients provided written informed consent, and the Institutional Review Boards of the participating institutions approved collection and use of the CMPs for research purposes.
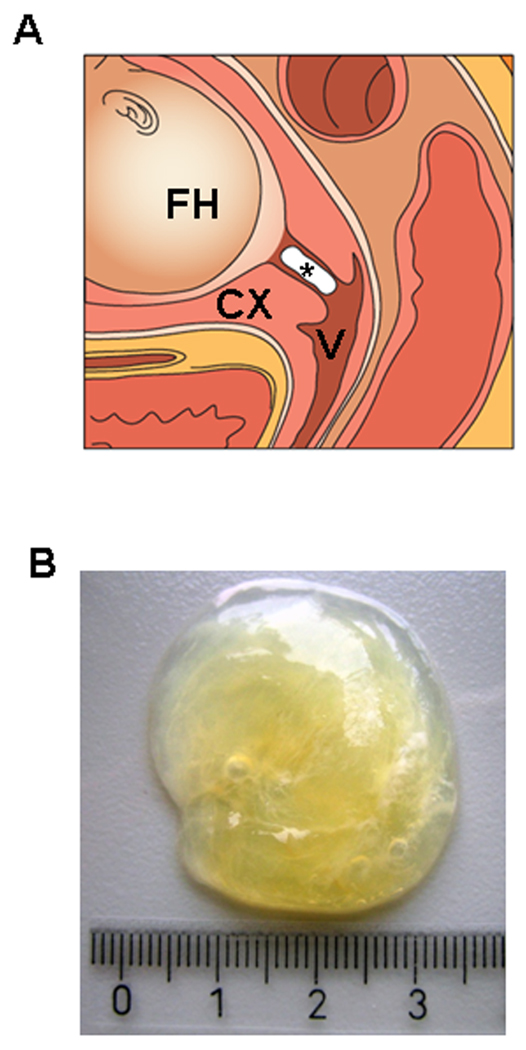
Figure 1.
Illustration and gross characteristics of the cervical mucus plug (CMP) in the cervical canal. (A) In this schematic representation, the CMP fills the endocervical canal, and its uterine pole is in contact with the decidua, while the vaginal pole faces the cervicovaginal compartment. *: cervical mucus plug, FH: fetal head, CX: uterine cervix, V: vagina. (B) CMP obtained at term showing its highly viscous nature.
2.2 Protein isolation
Frozen CMPs were homogenized by mortar and pestle in liquid nitrogen. Proteins were isolated with T-PER® Tissue Protein Extraction Reagent (Pierce) containing the Halt™ Protease and Phosphatase Inhibitor Cocktail (Pierce). The samples in T-PER buffer were kept on ice for 30 min with intermittent vortexing and then centrifuged at 16,000g for 15 min at 4°C. This procedure produced a consistent banding pattern as determined by SDS-PAGE (Figure 2). Proteins were quantified by BCA™ Protein Assay (Pierce) with a BSA standard solution (Pierce).
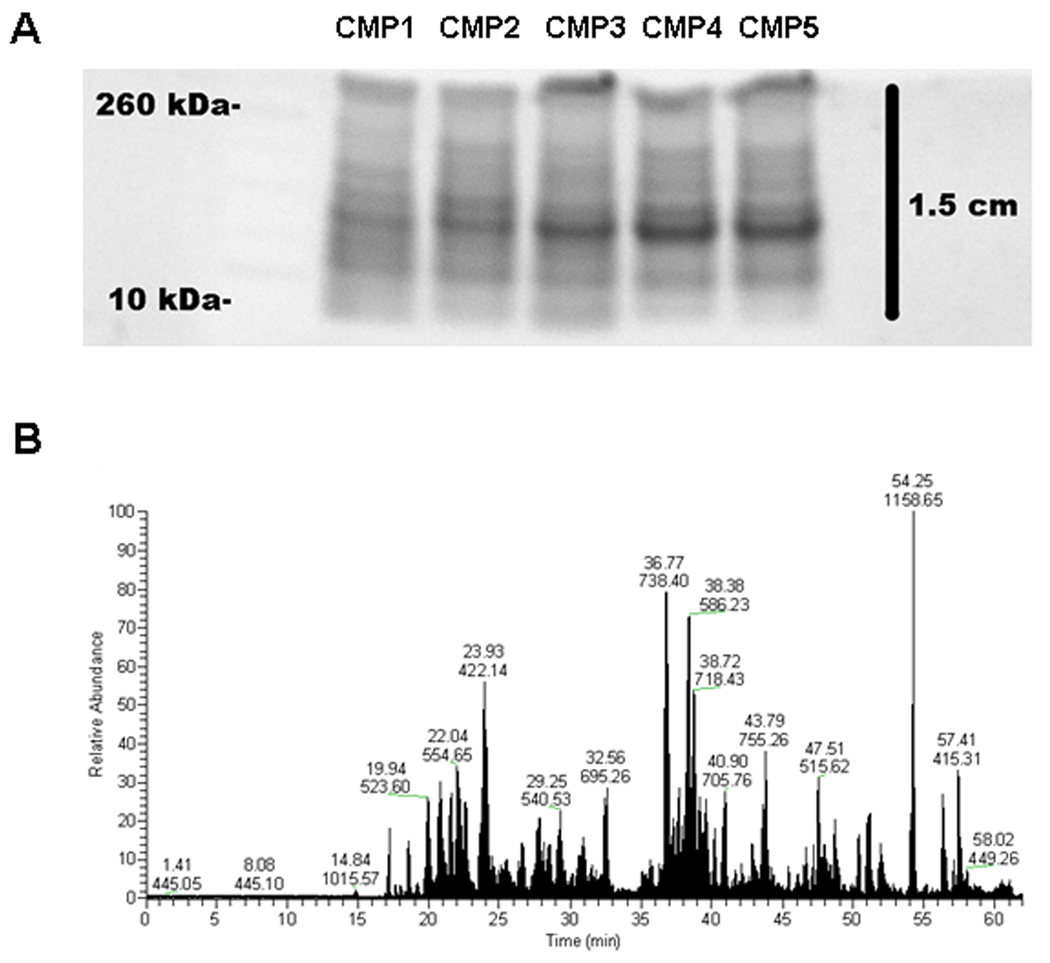
Figure 2.
Approach for protein profiling of cervical mucus plugs (CMP). (A) Protein (20 µg) extracted from each CMP was separated by SDS-PAGE and stained with SYPRO Ruby (reverse image). Each lane was cut into 12 slices for subsequent trypsin digestion and LC-MS/MS analyses. (B) Representative chromatogram obtained from sample 1, slice #4. Graph shows the relative base peak intensity (y-axis) over time (x-axis). Numbers over abundant peaks represent retention time (in minutes, top) and mass-to-charge ratio (m/z, bottom).
2.3 LC-MS/MS
Protein samples (20 µg) were separated by SDS-PAGE and stained with SYPRO Ruby (Invitrogen). Each lane was divided into 12 slices. The slices were excised, cut into 1 mm3 cubes, and transferred to a 96-well plate. Reduction with 10 mM tris(2-carboxyethyl)phosphine, alkylation with 50 mM iodoacetamide, and digestion with 0.05 µg trypsin were performed in-gel in 50 mM ammonium bicarbonate buffer. Peptides were eluted from the gel pieces using 0.1% formic acid and acetonitrile (1:1 volume), followed by acetonitrile alone. Volatile buffers and salts were removed by vacuum drying the peptides, resolubilizing in water, and redrying.
Peptides were resuspended in 2% acetonitrile, 0.1% formic acid, and 0.005% trifluoroacetic acid. Peptides were separated by reverse phase chromatography [Paradigm MS4 HLPC (Michrom) and 0.1 × 150 mm, 200 Ǻ Magic C18AQ column (Michrom)] using a 5–32% acetonitrile gradient with 0.1% formic acid over 45 min at 500 nl/min. Peptides were ionized with the ADVANCE ion source (Michrom) and introduced into an LTQ-XL mass spectrometer (Thermo Fisher Scientific). Data dependent selection was performed with the top five most abundant peaks over a 5×103 threshold selected for MS2 fragmentation by collision induced dissociation (isolation width = 2 Da; normalized collision energy = 30; activation Q = 0.25; activation time = 30). Dynamic exclusion was turned on (repeat = 2 over 5 s; list size = 50; duration = 20 s).
Data analyses were performed using Proteome Discoverer (ver. 1.1.0.263; Thermo Fisher Scientific) that incorporated the Mascot search algorithm (ver. 2.3.01; Matrix Science). MS2 spectra from all gel slices per lane were combined in a ‘MudPIT’ type analysis and searched against a human IPI protein database (ver. 3.71) in both forward and reverse directions to calculate the false discovery rate (FDR). Search criteria included +2 and +3 peptide charges, 1 missed tryptic cleavage, precursor tolerance of 1.8 Da, fragment tolerance of 1 Da, dynamic modification of methionine (+16 Da), and static modification of cysteine (+57 Da). Identified proteins contained at least two unique peptides which scored above a 5% FDR threshold. Spectral counts were calculated by normalizing the number of assigned spectra for each protein to the total number of assigned spectra for each sample.
2.4 Immunoblotting and densitometry
SDS-PAGE was performed with 15 µg of proteins extracted from each CMP used in the LC-MS/MS analysis. Immunoblots were incubated with glycodelin-A (Progestagen-associated endometrial protein; Santa Cruz Biotechnology Inc.) or matrix metalloproteinase-9 antibodies (Life Science). HRP-conjugated rabbit IgG (Santa Cruz Biotechnology Inc.) was used for secondary binding. Chemiluminescence was detected using a ChemiGlow West kit (Alpha Innotech Corporation). As the CMPs do not contain proteins that can be used as loading controls to normalize immunoblotting results, the protein staining intensity of Coomassie Brilliant Blue staining was used for densitometric analysis (FluorChem™ SP sensitometry, Alpha Innotech Corporation). To determine the relationship between spectral counts and densitometric values, the Spearman rank correlation test was done using the SPSS Version 15.0 (SPSS Inc.).
2.5 Bioinformatics analysis
One of the first approaches to characterize sets of genes/proteins was to test whether the given set of genes/proteins enrich (over-represent) one or more gene ontologies. In this study, we characterized the set of proteins detected in two or more of the five CMP samples. This procedure was performed by initially mapping each detected protein to gene Entrez identifiers using the mapping provided by EBI (ftp://ftp.ebi.ac.uk/pub/databases/IPI/current/). Then we applied a Gene Ontology (GO) enrichment analysis method described elsewhere [21] and implemented in the GOstats package [22]. Briefly, each GO term having one or more hits in the list of proteins present in the CMP samples were tested for enrichment using as reference the list of all known human genes. Nominal p-values and odds ratios for over-representation of each GO term were obtained using the hyperGTest function of the GOstats package (version 1.7.4), testing for over-representation only and using node conditioning. The FDR algorithm [23] was applied on the nominal p values obtained from the hyperGTest function to account for multiple terms being simultaneously tested for enrichment. All analyses were performed using the R statistical language and environment (www.r-project.org) version 2.11.
With the list of genes corresponding to the proteins detected in two or more of the five CMP samples, the network was built using the MetaCore™ (GeneGO, Inc., MI) in order to gain information about how they interact. The “direct interactions” algorithm was used to identify a network of genes interacting directly via all known mechanisms (e.g. binding, phosphorylation). A filter was applied to construct tissue-specific (uterus or placenta) gene networks separately, based on the Applied Biosystems database accessible within Metacore.
3 Results
3.1 Identification of proteins in cervical mucus plug
Proteins were reduced/alkylated/digested in-gel and extracted peptides were analyzed by LC-MS/MS with a linear ion trap mass spectrometer. A stained SDS-PAGE gel showing the reproducibility of the protein extraction procedure and a representative ion chromatogram of peptides separated by reverse phase chromatography are displayed in Figure 2. We identified a total of 291 proteins in five CMPs (Supplementary Table 1), and 137 (47.1 %) of those proteins were found in two or more CMP samples (Table 1).
Table 1.
Proteins identified by liquid chromatography-tandem mass spectrometry
| Description | MW (kDa) |
Found in CVF (PW) |
Found in CVF (HW) |
Found in CM |
Found in amnion |
Found in AF |
|---|---|---|---|---|---|---|
| 21 kDa protein | 20.8 | d | ||||
| Actin, cytoplasmic 1 | 41.7 | d | f | h | h | |
| Actinin, alpha 1 isoform c | 102.6 | |||||
| Afamin | 69.0 | d | f | i,j | ||
| Alpha-1-acid glycoprotein 1 | 23.5 | a,c | d | g | h,j | |
| Alpha-1-antichymotrypsin | 47.6 | a,b | d | f,g | i | |
| Alpha-1-antitrypsin | 46.7 | a,b,c | d | f,g | h,i | |
| Alpha-1B-glycoprotein | 54.2 | f | h,i,j | |||
| Alpha-2-macroglobulin | 163.2 | d | f | i | ||
| Alpha-2-macroglobulin-like protein 1 | 161.0 | |||||
| Alpha-enolase | 47.1 | e | g | h | ||
| Aminopeptidase N | 109.5 | h | ||||
| Angiotensinogen | 53.1 | f | i,j | |||
| Annexin A1 | 38.7 | a,b | d,e | f,g | h | |
| Annexin A2 | 38.6 | a,b | d,e | f,g | h | |
| Annexin A3 | 36.4 | a,c | d,e | f,g | h | |
| Annexin A5 | 35.8 | c | e | f,g | h | |
| Antileukoproteinase | 14.3 | a | d | g | j | |
| Antithrombin-III | 52.6 | d | f | i,j | ||
| Apolipoprotein A-I | 30.8 | a | e | f,g | h | h,i,j |
| Apolipoprotein A-II | 11.2 | i,j | ||||
| Apolipoprotein A-IV | 45.4 | f | h,i | |||
| Apolipoprotein B-100 | 515.2 | i | ||||
| Azurocidin | 26.9 | d | f | |||
| Carbonic anhydrase 1 | 28.9 | f,g | h | |||
| Catalase | 59.7 | a | d,e | f | ||
| Cathelicidin antimicrobial peptide precursor | 19.6 | g | ||||
| Cathepsin G | 28.8 | a,b | d | f | ||
| CD81 antigen | 25.8 | |||||
| Ceruloplasmin | 122.1 | a | d,e | f,g | i,j | |
| Chloride intracellular channel protein 1 | 26.9 | e | f | h | ||
| Chondroadherin | 40.5 | |||||
| Clusterin | 52.3 | d | f | i,j | ||
| Cofilin-1 | 18.5 | f | h | |||
| Collagen alpha-2(I) chain | 129.2 | d | j | |||
| Complement C3 | 187.0 | a | d,e | f,g | i,j | |
| Complement C4-A | 192.7 | a | g | i,j | ||
| Complement C5 | 188.2 | |||||
| Complement component 4B preproprotein | 192.6 | a | d | f | i,j | |
| Complement component 6 precursor | 105.7 | d | f | |||
| Complement component C8 gamma chain | 22.3 | |||||
| Complement factor B | 85.5 | d | g | h,i,j | ||
| Complement factor H | 139.0 | d | f | j | ||
| Cystatin-A | 11.0 | a,c | d,e | f,g | j | |
| Cysteine-rich secretory protein 3 | 27.6 | a | d | f | ||
| Cytidine deaminase | 16.2 | d | ||||
| DKFZp686C11235 | 52.1 | |||||
| DKFZp686C15213 | 51.1 | |||||
| DKFZp686D0114 | 30.5 | |||||
| DKFZp686I04196 | 46.0 | |||||
| DKFZp686M24218 | 52.4 | |||||
| Fatty acid-binding protein, epidermal | 15.2 | a,b,c | d,e | f,g | h | |
| Ferritin | 11.2 | f | ||||
| Fibronectin | 259.0 | c | d | f | i,j | |
| Fibulin-1 | 74.4 | d | f | j | ||
| Galectin-3-binding protein | 65.3 | d | f | |||
| Glutaredoxin-1 | 11.8 | d | ||||
| Glyceraldehyde 3-phosphate dehydrogenase | 28.6 | a,b,c | d,e | f,g | ||
| Haptoglobin | 46.7 | a,c | d,e | f,g | h | |
| Hemoglobin subunit alpha | 15.2 | b | d | f,g | h | h |
| Hemoglobin subunit beta | 16.0 | d | g | |||
| Hemoglobin subunit delta | 16.0 | d | f | |||
| Hemopexin | 51.6 | a | d | f,g | i,j | |
| Histone H2A type 2-C | 14.0 | a | ||||
| Histone H4 | 11.4 | a,b,c | d | g | ||
| Ig gamma-3 chain C region | 41.3 | j | ||||
| Ig kappa chain C region | 25.4 | a,b | d | g | h | |
| Ig kappa chain V-I region OU | 11.8 | g | ||||
| Ig kappa chain V-II region TEW | 12.3 | a | g | |||
| Ig kappa chain V-III region B6 | 11.6 | g | ||||
| Ig kappa chain V-IV region Len | 12.6 | a | d | g | ||
| Ig mu chain C region | 49.3 | a,b,c | ||||
| IGFBP1 | 22.9 | h,i,j | ||||
| IgGFc-binding protein | 571.6 | d | g | |||
| Immunoglobulin J chain | 18.1 | a | d | f | ||
| Inter-alpha (globulin) inhibitor H2 | 105.2 | i | ||||
| Inter-alpha (globulin) inhibitor H4 isoform 2 | 99.8 | f | i | |||
| Inter-alpha-trypsin inhibitor heavy chain H1 | 101.3 | |||||
| Keratin 13 isoform b | 45.8 | f | ||||
| Keratin, type I cytoskeletal 10 | 58.8 | f | ||||
| Keratin, type I cytoskeletal 14 | 51.5 | |||||
| Keratin, type I cytoskeletal 16 | 51.2 | f | ||||
| Keratin, type I cytoskeletal 9 | 62.0 | f | i | |||
| Keratin, type II cytoskeletal 1 | 66.0 | b | f | h | h,i | |
| Keratin, type II cytoskeletal 2 epidermal | 65.8 | f | ||||
| Keratin, type II cytoskeletal 5 | 62.3 | f | ||||
| Leukocyte elastase inhibitor | 42.7 | a,b | d | h | ||
| Long palate, lung and nasal epithelium carcinoma-associated protein 1 | 52.4 | a | ||||
| Lumican | 38.4 | i,j | ||||
| Lysozyme C | 16.5 | a,b,c | d,e | f,g | j | |
| Mammaglobin-B | 10.9 | |||||
| Matrix metalloproteinase-9 (MMP-9) | 78.4 | a | d | f | ||
| MUC1 isoform T9 | 12.1 | |||||
| Mucin-16 | 2351.2 | d | ||||
| Mucin-5AC | 526.3 | |||||
| Mucin-5B | 590.4 | a,c | d | j | ||
| Myeloblastin | 27.8 | a | d | g | ||
| Myeloperoxidase | 73.8 | a,b | d | f | ||
| Myosin-reactive immunoglobulin heavy chain variable region | 13.2 | d | i | |||
| Myosin-reactive immunoglobulin light chain variable region | 11.5 | d | h | |||
| Neutrophil defensin 1 | 10.2 | a | d | g | ||
| Neutrophil elastase | 28.5 | |||||
| Neutrophil gelatinase-associated lipocalin | 22.6 | a,b,c | d,e | g | j | |
| Peptidoglycan recognition protein | 21.7 | |||||
| Pigment epithelium-derived factor | 46.3 | i | ||||
| Plasma protease C1 inhibitor | 55.1 | d | ||||
| Plasma serine protease inhibitor | 45.7 | d | f | i | ||
| Plasminogen | 90.5 | a | d | i,j | ||
| Plastin-2 | 70.2 | d | g | |||
| Polymeric immunoglobulin receptor | 83.2 | a,b | d | f | i,j | |
| Pregnancy zone protein | 163.8 | |||||
| Profilin-1 | 15.0 | a,c | d,e | f,g | h | h |
| Progestagen-associated endometrial protein (Glycodelin-A) | 14.9 | j | ||||
| Prolactin-inducible protein | 16.6 | d | ||||
| Prominin 1 isoform 4 | 92.7 | f | ||||
| Protein AMBP | 39.0 | j | ||||
| Protein S100-A11 | 11.7 | d | f,g | |||
| Protein S100-A8 | 10.8 | a,b,c | d | f,g | h | |
| Protein S100-A9 | 13.2 | a,b,c | d | f,g | h,i | |
| Resistin | 11.4 | d | ||||
| Retinol binding protein 4, plasma | 22.9 | |||||
| Rheumatoid factor D5 light chain | 12.8 | |||||
| Ribonuclease T2 | 29.5 | d | ||||
| Secretoglobin family 1D member 2 | 9.9 | |||||
| Serotransferrin | 77.0 | a,c | d | h | h,j | |
| Serpin B3 | 44.5 | d,e | g | |||
| Serum albumin | 69.3 | a | e | g | h,i,j | |
| Serum paraoxonase/arylesterase 1 | 39.7 | f | ||||
| Sulfhydryl oxidase 1 | 66.8 | d | ||||
| Thioredoxin, isoform CRA_b | 9.4 | a | e | h | j | |
| TIMP metallopeptidase inhibitor 1 | 16.0 | |||||
| Transaldolase | 37.5 | a | d | g | ||
| Transcobalamin-1 | 48.2 | d | ||||
| Transthyretin | 15.9 | d | f,g | i,j | ||
| Vitamin D-binding protein | 52.9 | a,c | d,e | h,i,j | ||
| Vitronectin | 54.3 | a | f,g | i,j | ||
| Zymogen granule protein 16 homolog B | 22.7 |
Identified proteins in the list were found in at least 2 of 5 CMPs. These proteins were also found in other studies of CVF, amnion, or AF:
ref (27);
ref (28);
ref (29);
ref (30);
ref (31);
ref (16);
ref (17);
ref (32);
ref (33);
ref (34).
MW: theoretical molecular weight, CMP: cervical mucus plug, CVF(PW): cervicovaginal fluid of pregnant women, CVF(HW): cervicovaginal fluid of non-pregnant women, CM: cervical mucus, AF: amniotic fluid
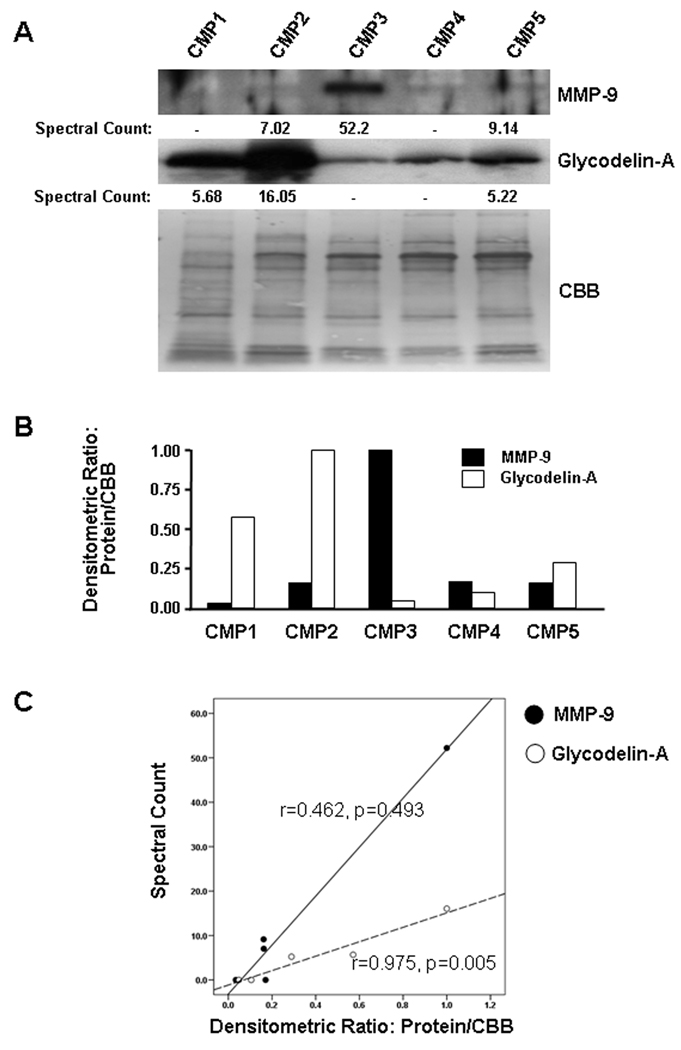
Matrix metalloproteinase-9 (MMP-9) and glycodelin-A (progestagen-associated endometrial protein) were selected as representative proteins for the relative quantitative evaluation of the spectral counting data by immunoblotting. These proteins each had a wide range of spectra counted between samples and, therefore, presented valid targets to demonstrate a range of abundance in the five samples. Each of the proteins was detected by mass spectrometry in three of the samples with an 8 fold range in spectra counted for the MMP-9 and a 3 fold range for glycodelin-A. MMP-9 and glycodelin-A are also biologically important in the rupture of the chorioamniotic membranes/cervical ripening and suppression of CD8+ cytotoxic T cell activity, respectively [24–26]. Glycodelin-A was detected in all five samples by immunoblotting indicating that the immunodetection of that protein was more sensitive than the mass spectrometry-based analysis (Figure 3A). There was a good correlation between spectral counts and the relative intensity of the bands obtained by densitometry for glycodelin-A (r=0.975, p=0.005) but not for MMP-9 (r=0.462, p=0.493) (Figure 3B and 3C).
Figure 3.
Validation of LC-MS/MS data by immunoblotting. (A) Immunoblotting using specific antibodies to show abundance of MMP-9 and glycodelin-A (progestagen-associated endometrial protein) in each CMP sample. Spectral counts of MMP-9 and glycodelin-A on LC-MS/MS analysis of each sample were also provided. Spectral counts, obtained from LC-MS/MS analysis, were calculated by normalizing the number of assigned spectra for each protein to the total number of assigned spectra for each LC-MS/MS run. CBB: Coomassie Brilliant Blue (B) Densitometric analysis of MMP-9 and glycodelin-A abundance in each CMP. As there is no housekeeping gene product, which can be used as a loading control for CMP samples, the density of all Coomassie Brilliant Blue stained bands on electro-blotted PVDF membrane were used as a normalizer for densitometric comparison of each sample for MMP-9 and glycodelin-A. (C) There is a good correlation between spectral counts and densitometric values for glycodelin-A (r=0.975, p=0.005) but not for MMP-9 (r=0.462, p=0.493).
The CMP contained a variety of protein molecules with known immunological functions such as complement proteins (C3, C4a, C5), antimicrobial peptides (azurocidin, cathelicidin antimicrobial peptide, neutrophil defensin, peptidoglycan recognition protein), and mucins (mucin-16, mucin-5AC, mucin-5B). Twenty-two proteins have not been described before in the related biological samples such as the cervical mucus of non-pregnant women, cervicovaginal fluid, and amniotic fluid (Table 1), which included CD81 antigen, mammaglobin-B, TIMP metallopeptidase inhibitor 1, and pregnancy zone protein.
3.2 Gene ontology analysis of CMP proteins
Gene ontology analysis of the proteins identified in the CMPs demonstrated significant enrichment of 28 biological processes such as ‘activation of plasma proteins involved in acute inflammatory response’, ‘inflammatory response’, and both ‘classical pathway’ and ‘alternative pathway’ of complement activation (Table 2). Of note, biological processes associated with cholesterol metabolism such as ‘positive regulation of cholesterol esterification (Odds Ratio (OR) =147.9)’, ‘low-density lipoprotein particle remodeling (OR=88.7)’, ‘sterol esterification (OR=63.4), ‘regulation of steroid metabolic process (OR=24.3), and ‘reverse cholesterol transport (OR=40.3)’ were enriched. These were associated with the presence of angiotensinogen, apoplipoprotein A-I, apoplipoprotein A-II, apolipoprotein A-IV, apoplipoprotein B-100, and clusterin.
Table 2.
Gene ontology analysis of human CMP proteome
| Biological Process | Odds Ratio | Adjusted P value |
|---|---|---|
| Activation of plasma proteins involved in acute inflammatory response | 45.7 | 2.21E-12 |
| Inflammatory response | 10.5 | 1.84E-10 |
| Regulation of immune system process | 10.5 | 2.77E-09 |
| Activation of immune response | 21.1 | 5.57E-09 |
| Humoral immune response | 21.6 | 3.17E-08 |
| Complement activation, classical pathway | 45.8 | 9.10E-08 |
| Complement activation, alternative pathway | 75.1 | 2.08E-06 |
| Protein processing | 13.7 | 1.27E-05 |
| Immune effector process | 16.8 | 1.28E-05 |
| Macromolecular complex remodeling | 45.0 | 2.15E-05 |
| Protein metabolic process | 5.2 | 9.55E-05 |
| Positive regulation of cholesterol esterification | 147.9 | 0.000191 |
| Acute-phase response | 27.0 | 0.000269 |
| Immunoglobulin mediated immune response | 16.8 | 0.000493 |
| Low-density lipoprotein particle remodeling | 88.7 | 0.000676 |
| Epidermis development | 8.2 | 0.001041 |
| Sterol esterification | 63.4 | 0.001742 |
| Lymphocyte mediated immunity | 13.2 | 0.002141 |
| Protein-lipid complex assembly | 55.4 | 0.002588 |
| Regulation of steroid metabolic process | 24.3 | 0.004095 |
| Cellular iron ion homeostasis | 23.3 | 0.004906 |
| Adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains | 11.5 | 0.004935 |
| Reverse cholesterol transport | 40.3 | 0.006951 |
| Cell motion | 5.1 | 0.009758 |
| Immune response | 4.6 | 0.012670 |
| Hyaluronan metabolic process | 82.5 | 0.020769 |
| Regulation of body fluid levels | 7.2 | 0.027727 |
| Negative regulation of cellular metabolic process | 7.9 | 0.047098 |
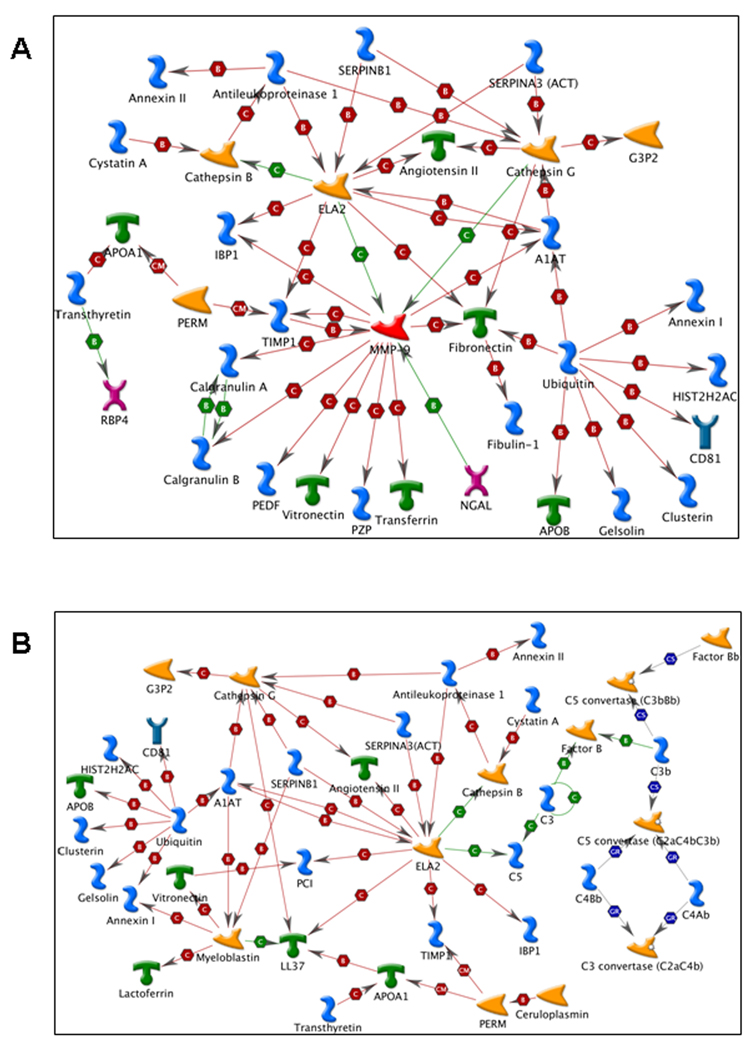
3.3 Metacore analysis of CMP proteins
The list of genes detected in the CMP samples contained a cluster of highly connected genes. MMP-9 and neutrophil elastase (ELA2) enzymes were important hubs in the network specific to the placental tissue (Figure 4A), while ELA2 was also a hub in the network specific to the uterus (Figure 4B).
Figure 4.
 Generic enzyme
Generic enzyme Generic binding protein
Generic binding protein Receptor ligand
Receptor ligand Generic protease
Generic protease Metalloprotease
Metalloprotease Generic receptor
Generic receptor Transporter
Transporter A complex or a group
A complex or a group Positive effect
Positive effect Negative effect
Negative effect Unspecified effect
Unspecified effect Binding
Binding Cleavage
Cleavage Covalent modifications
Covalent modifications Group relation
Group relation Complex subunit
Complex subunit
4 Discussion
This study reports the proteome of the CMP from normal pregnant women at term for the first time. The primary finding is that several proteins documented in the amniotic fluid and the chorioamniotic membranes are found in the CMP in addition to a variety of proteins involved in the immune response such as complements and neutrophil defensins. When we compared the list of identified CMP proteins with proteins previously reported in proteomic analyses of the cervical mucus of non-pregnant women [16,17], cervicovaginal fluid [27–31], the amnion [32], and the amniotic fluid [32–34], 81 were described in the cervical mucus, 52 in the cervicovaginal fluid of pregnant women, 71 in the cervicovaginal fluid of non-pregnant women, 20 in the amnion, and 56 in the amniotic fluid. The results of gene ontology (GO) analysis of enriched biological processes using proteins detected in the CMPs share similarities with those of cervicovaginal fluid in a study by Shaw et al such as cell motion (cellular movement) and immune response [30].
The results of the present study further underscore the functional significance of the CMP as a critical ‘gate-keeper’ based on its physiological and immunologic properties [6,35,36]. Mucus glycoproteins (mucins) are the core proteins of the CMP, provide the structural framework, and inhibit diffusion of large molecules and bacteria [4]. CMP proteins such as lactoferrin, lysozyme, neutrophil defensin, haptoglobin, and leukocyte elastase inhibitor are involved in host defense, and also have been found in cervical mucus, cervicovaginal fluid, and amniotic fluid [16,17,27–34,37–40]. Peptidoglycan recognition protein was also detected in our analysis. In addition to this catalogue of anti-microbial peptides, the T-cell inhibitory molecule, glycodelin-A, which is largely produced by decidua, and pregnancy zone protein were also detected. Pregnancy zone protein belongs to the alpha 2 macroglobulin family, and can bind vascular endothelial growth factor, placental growth factor, and glycodelin-A, all of which have critical biological importance in pregnancy [41]. Pregnancy zone protein is an effective carrier of glycodelin-A, and these two molecules showed synergistic inhibitory effects on T cell proliferation and IL-2 production [42]. Therefore, the CMP appears to protect the intrauterine fetal compartments from both the spread of microbial pathogens and the maternal anti-fetal immune response.
The basic difference between the CMP of pregnant women and the cervical mucus of non-pregnant women is that the CMP is in direct contact with the supracervical region of the chorioamniotic membranes which contain amniotic fluid. As expected, several proteins documented in the amniotic fluid and the amnion were identified in the CMP. Resistin and retinol binding protein 4 were present in the CMPs, and we previously have shown these two adipokines are increased in amniotic fluid in the presence of intra-amniotic infection/inflammation [43–45]. IGFBP1 is also detected in the amniotic fluid, and its 13.5 kD fragment is detected in cases with intra-amniotic infection [46]. It is also noteworthy that apolipoprotin A-II and apoplipoprotein B-100 are components of cholesterol metabolism (positive regulation of cholesterol esterification, low-density lipoprotein particle remodeling, sterol esterification, reverse cholesterol transport), which have been demonstrated in the amniotic fluid but not in the cervical mucus or the cervicovaginal fluid. Therefore, proteins in the amniotic fluid seem to be easily integrated into the CMP. In this context, the CMP is an ideal venue through which the changes in the intrauterine compartment could be coordinated with the uterine cervix throughout pregnancy. Furthermore, the findings strongly suggest a possibility that the CMP can be used for the diagnosis of certain genetic and metabolic diseases of the fetus.
Smith-Lemli-Opitz syndrome (SLOS) is a rare form of autosomal recessive malformation syndrome. It results from deficiency of 7-dehydrocholesterol reductase, due to mutation of the gene on chromosome 11 [47]. This leads to defective cholesterol synthesis and elevation of 7-dehydrocholesterol in plasma and tissues [48]. Prenatal diagnosis of SLOS has been made by measuring the amniotic fluid concentration of 7-dehydrocholesterol in the first trimester [49]. There have been efforts to integrate the risk assessment of SLOS as a part of screening for Down’s syndrome in the second trimester of pregnancy [50,51]. Interestingly, Anagnostopoulos et al have demonstrated decrease in apolipoprotein A-I and retinol binding protein in the second trimester amniotic fluid samples obtained from cases of Klinefelter syndrome (karyotype: 47, XXY) [52], and a study by Wang et al has shown that amniotic fluid apolipoprotein A-I is decreased in trisomy 18 (Edwards syndrome) and trisomy 21 (Down syndrome), while glycodelin-A is increased in trisomy 18 cases [53]. All of these observations support a potential for diagnostic application of the CMP in genetic disorders.
There are major limitations of this study, and some aspects of the study design must be considered while evaluating the data presented here. The study samples were restricted to normal term in labor cases. However, the sampling of intact CMPs is difficult in the majority of cases, and the collection of CMPs from pregnancy complications such as preterm labor is not feasible. Therefore, a comparative analysis of normal and abnormal pregnancies could not be made. Another point of discussion is the protein isolation procedure. Mucins provide the structural framework of the CMP, and the extraction of mucins for analysis is a challenging procedure. Therefore, guanidinium chloride has been generally used to extract mucins [7,54–56]. As a reducing agent was not used in our protein isolation protocol, the mucins remain cross-linked to each other to a substantial degree and so are not represented to the extent that they are present in the CMP. Therefore, our approach might not be relevant for comparative studies of mucins among different groups.
5 Conclusions
We report the proteome of the cervical mucus plug, and overall findings suggest a vital role of this highly viscous and elastic structure both as a gatekeeper and as a reservoir of active biological mediators affecting cervical properties in human pregnancy. We propose that the CMP itself functions as a biological suppository, which affects many cellular and molecular events occurring in the uterine cervical regions.
Supplementary Material
Acknowledgements
This work was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. The Proteomics Facility Core of the Institute of Environmental Health Sciences at Wayne State University is supported by National Institute of Environmental Health Sciences grant P30-ES006639.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Chantler E, Sharma R, Sharman D. Changes in cervical mucus that prevent penetration by spermatozoa. Symp Soc Exp Biol. 1989;43:325–336. [PubMed] [Google Scholar]
- 2.Van Kooij RJ, Roelofs HJ, Kathmann GA, Kramer MF. Human cervical mucus and its mucous glycoprotein during the menstrual cycle. Fertil Steril. 1980;34:226–233. [PubMed] [Google Scholar]
- 3.Wolf DP, Sokoloski JE, Litt M. Composition and functioin of human cervical mucus. Biochim Biophys Acta. 1980;630:545–558. doi: 10.1016/0304-4165(80)90009-4. [DOI] [PubMed] [Google Scholar]
- 4.Becher N, Waldorf KA, Hein M, Uldbjerg N. The cervical mucus plug: structured review of the literature. Acta Obstet Gynecol Scand. 2009;88:502–513. doi: 10.1080/00016340902852898. [DOI] [PubMed] [Google Scholar]
- 5.Becher N, Hein M, Danielsen CC, Uldbjerg N. Matrix metalloproteinases and their inhibitors in the cervical mucus plug at term of pregnancy. Am J Obstet Gynecol. 2004;191:1232–1239. doi: 10.1016/j.ajog.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187:137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 7.Habte HH, de Beer C, Lotz ZE, Tyler MG, Schoeman L, Kahn D, Mall AS. The inhibition of the Human Immunodeficiency Virus type 1 activity by crude and purified human pregnancy plug mucus and mucins in an inhibition assay. Virol J. 2008;5:59. doi: 10.1186/1743-422X-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 9.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Mittal P, Vaisbuch E, Gonzalez JM, Chaiworapongsa T, li-Fehmi R, Dong Z, Than NG, Kim CJ. The molecular basis for sonographic cervical shortening at term: identification of differentially expressed genes and the epithelial-mesenchymal transition as a function of cervical length. Am J Obstet Gynecol. 2010;203:472.e1–472.e14. doi: 10.1016/j.ajog.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 10.Paidas MJ, Krikun G, Huang SJ, Jones R, Romano M, Annunziato J, Barnea ER. A genomic and proteomic investigation of the impact of preimplantation factor on human decidual cells. Am J Obstet Gynecol. 2010;202:459.e1–459.e8. doi: 10.1016/j.ajog.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson JM, Vandre DD, Ackerman WE. Placental proteomics: a shortcut to biological insight. Placenta. 2009 Suppl A:S83–S89. doi: 10.1016/j.placenta.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh GM, Rogalski JC, Klockenbusch C, Kast J. Mass spectrometry-based proteomics in biomedical research: emerging technologies and future strategies. Expert Rev Mol Med. 2010;12:e30. doi: 10.1017/S1462399410001614. [DOI] [PubMed] [Google Scholar]
- 13.Pereira L, Reddy AP, Alexander AL, Lu X, Lapidus JA, Gravett MG, Nagalla SR. Insights into the multifactorial nature of preterm birth: proteomic profiling of the maternal serum glycoproteome and maternal serum peptidome among women in preterm labor. Am J Obstet Gynecol. 2010;202:555.e1–555.e10. doi: 10.1016/j.ajog.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Hitti J, Lapidus JA, Lu X, Reddy AP, Jacob T, Dasari S, Eschenbach DA, Gravett MG, Nagalla SR. Noninvasive diagnosis of intraamniotic infection: proteomic biomarkers in vaginal fluid. Am J Obstet Gynecol. 2010;203:32.e1–32.e8. doi: 10.1016/j.ajog.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol. 2001;185:586–592. doi: 10.1067/mob.2001.116685. [DOI] [PubMed] [Google Scholar]
- 16.Andersch-Björkman Y, Thomsson KA, Holmen Larsson JM, Ekerhovd E, Hansson GC. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics. 2007;6:708–716. doi: 10.1074/mcp.M600439-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Panicker G, Lee DR, Unger ER. Optimization of SELDI-TOF protein profiling for analysis of cervical mucous. J Proteomics. 2009;71:637–646. doi: 10.1016/j.jprot.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab. 2010;21:353–361. doi: 10.1016/j.tem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, Berry SM, Bujold E, Camacho N, Sorokin Y. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, Dong Z, Yeo L, Mittal P, Yoon BH, Romero R. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433.e1–433.e8. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draghici S, Khatri P, Martins RP, Ostermeier GC, Krawetz SA. Global functional profiling of gene expression. Genomics. 2003;81:98–104. doi: 10.1016/s0888-7543(02)00021-6. [DOI] [PubMed] [Google Scholar]
- 22.Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics. 2007;23:257–258. doi: 10.1093/bioinformatics/btl567. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 24.Soni C, Karande AA. Glycodelin A suppresses the cytolytic activity of CD8+ T lymphocytes. Mol Immunol. 2010;47:2458–2466. doi: 10.1016/j.molimm.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Stygar D, Wang H, Vladic YS, Ekman G, Eriksson H, Sahlin L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod. 2002;67:889–894. doi: 10.1095/biolreprod.102.005116. [DOI] [PubMed] [Google Scholar]
- 26.Yonemoto H, Young CB, Ross JT, Guilbert LL, Fairclough RJ, Olson DM. Changes in matrix metalloproteinase (MMP)-2 and MMP-9 in the fetal amnion and chorion during gestation and at term and preterm labor. Placenta. 2006;27:669–677. doi: 10.1016/j.placenta.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Dasari S, Pereira L, Reddy AP, Michaels JE, Lu X, Jacob T, Thomas A, Rodland M, Roberts CT, Jr, Gravett MG, Nagalla SR. Comprehensive proteomic analysis of human cervical-vaginal fluid. J Proteome Res. 2007;6:1259–1268. doi: 10.1021/pr0605419. [DOI] [PubMed] [Google Scholar]
- 28.Klein LL, Jonscher KR, Heerwagen MJ, Gibbs RS, McManaman JL. Shotgun proteomic analysis of vaginal fluid from women in late pregnancy. Reprod Sci. 2008;15:263–273. doi: 10.1177/1933719107311189. [DOI] [PubMed] [Google Scholar]
- 29.Pereira L, Reddy AP, Jacob T, Thomas A, Schneider KA, Dasari S, Lapidus JA, Lu X, Rodland M, Roberts CT, Jr, Gravett MG, Nagalla SR. Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J Proteome Res. 2007;6:1269–1276. doi: 10.1021/pr0605421. [DOI] [PubMed] [Google Scholar]
- 30.Shaw JL, Smith CR, Diamandis EP. Proteomic analysis of human cervico-vaginal fluid. J Proteome Res. 2007;6:2859–2865. doi: 10.1021/pr0701658. [DOI] [PubMed] [Google Scholar]
- 31.Tang LJ, De SF, Odreman F, Venge P, Piva C, Guaschino S, Garcia RC. Proteomic analysis of human cervical-vaginal fluids. J Proteome Res. 2007;6:2874–2883. doi: 10.1021/pr0700899. [DOI] [PubMed] [Google Scholar]
- 32.Park SJ, Yoon WG, Song JS, Jung HS, Kim CJ, Oh SY, Yoon BH, Jung G, Kim HJ, Nirasawa T. Proteome analysis of human amnion and amniotic fluid by two-dimensional electrophoresis and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proteomics. 2006;6:349–363. doi: 10.1002/pmic.200500084. [DOI] [PubMed] [Google Scholar]
- 33.Michaels JE, Dasari S, Pereira L, Reddy AP, Lapidus JA, Lu X, Jacob T, Thomas A, Rodland M, Roberts CT, Jr, Gravett MG, Nagalla SR. Comprehensive proteomic analysis of the human amniotic fluid proteome: gestational age-dependent changes. J Proteome Res. 2007;6:1277–1285. doi: 10.1021/pr060543t. [DOI] [PubMed] [Google Scholar]
- 34.Michel PE, Crettaz D, Morier P, Heller M, Gallot D, Tissot JD, Reymond F, Rossier JS. Proteome analysis of human plasma and amniotic fluid by Off-Gel isoelectric focusing followed by nano-LC-MS/MS. Electrophoresis. 2006;27:1169–1181. doi: 10.1002/elps.200500680. [DOI] [PubMed] [Google Scholar]
- 35.Hein M, Petersen AC, Helmig RB, Uldbjerg N, Reinholdt J. Immunoglobulin levels and phagocytes in the cervical mucus plug at term of pregnancy. Acta Obstet Gynecol Scand. 2005;84:734–742. doi: 10.1111/j.0001-6349.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 36.Eggert-Kruse W, Botz I, Pohl S, Rohr G, Strowitzki T. Antimicrobial activity of human cervical mucus. Hum Reprod. 2000;15:778–784. doi: 10.1093/humrep/15.4.778. [DOI] [PubMed] [Google Scholar]
- 37.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, Romero R. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007;20:15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, Blackwell S, Whitty J, Berman S, Redman M, Yoon BH, Sorokin Y. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 39.Pacora P, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH, Romero R. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol. 2000;183:904–910. doi: 10.1067/mob.2000.108882. [DOI] [PubMed] [Google Scholar]
- 40.Svinarich DM, Wolf NA, Gomez R, Gonik B, Romero R. Detection of human defensin 5 in reproductive tissues. Am J Obstet Gynecol. 1997;176:470–475. doi: 10.1016/s0002-9378(97)70517-9. [DOI] [PubMed] [Google Scholar]
- 41.Tayade C, Esadeg S, Fang Y, Croy BA. Functions of alpha 2 macroglobulins in pregnancy. Mol Cell Endocrinol. 2005;245:60–66. doi: 10.1016/j.mce.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Skornicka EL, Kiyatkina N, Weber MC, Tykocinski ML, Koo PH. Pregnancy zone protein is a carrier and modulator of placental protein-14 in T-cell growth and cytokine production. Cell Immunol. 2004;232:144–156. doi: 10.1016/j.cellimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, Erez O, Vaisbuch E, Edwin SS, Than NG, Camacho N, Pacora P, Rogers W, Hassan SS. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21:902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero R, Kusanovic JP, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, Moser A, Tam S, Leszyk J, Master SR, Juhasz P, Pacora P, Ogge G, Gomez R, Yoon BH, Yeo L, Hassan SS, Rogers WT. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:261–280. doi: 10.3109/14767050903067386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Erez O, Than NG, Kim SK, Dong Z, Gotsch F, Mittal P, Chaiworapongsa T, Pacora P, Yeo L, Hassan SS, Romero R. Retinol binding protein 4: an adipokine associated with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:111–119. doi: 10.3109/14767050902994739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bujold E, Romero R, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, Gomez R, Espinoza J, Vaisbuch E, Mee KY, Edwin S, Pisano M, Allen B, Podust VN, Dalmasso EA, Rutherford J, Rogers W, Moser A, Yoon BH, Barder T. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern Fetal Neonatal Med. 2008;21:697–713. doi: 10.1080/14767050802053289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loeffler J, Utermann G, Witsch-Baumgartner M. Molecular prenatal diagnosis of Smith-Lemli-Opitz syndrome is reliable and efficient. Prenat Diagn. 2002;22:827–830. doi: 10.1002/pd.419. [DOI] [PubMed] [Google Scholar]
- 48.Cunniff C, Kratz LE, Moser A, Natowicz MR, Kelley RI. Clinical and biochemical spectrum of patients with RSH/Smith-Lemli-Opitz syndrome and abnormal cholesterol metabolism. Am J Med Genet. 1997;68:263–269. [PubMed] [Google Scholar]
- 49.Mills K, Mandel H, Montemagno R, Soothill P, Gershoni-Baruch R, Clayton PT. First trimester prenatal diagnosis of Smith-Lemli-Opitz syndrome (7-dehydrocholesterol reductase deficiency) Pediatr Res. 1996;39:816–819. doi: 10.1203/00006450-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Craig WY, Haddow JE, Palomaki GE, Kelley RI, Kratz LE, Shackleton CH, Marcos J, Stephen TG, Macrae AR, Nowaczyk MJ, Kloza EM, Irons MB, Roberson M. Identifying Smith-Lemli-Opitz syndrome in conjunction with prenatal screening for Down syndrome. Prenat Diagn. 2006;26:842–849. doi: 10.1002/pd.1518. [DOI] [PubMed] [Google Scholar]
- 51.Palomaki GE, Bradley LA, Knight GJ, Craig WY, Haddow JE. Assigning risk for Smith-Lemli-Opitz syndrome as part of 2nd trimester screening for Down's syndrome. J Med Screen. 2002;9:43–44. doi: 10.1136/jms.9.1.43. [DOI] [PubMed] [Google Scholar]
- 52.Anagnostopoulos AK, Kolialexi A, Mavrou A, Vougas K, Papantoniou N, Antsaklis A, Kanavakis E, Fountoulakis M, Tsangaris GT. Proteomic analysis of amniotic fluid in pregnancies with Klinefelter syndrome foetuses. J Proteomics. 2010;73:943–950. doi: 10.1016/j.jprot.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Wang TH, Chao AS, Chen JK, Chao A, Chang YL, Cheng PJ, Chang SD, Wang HS. Network analyses of differentially expressed proteins in amniotic fluid supernatant associated with abnormal human karyotypes. Fertil Steril. 2009;92:96–107. doi: 10.1016/j.fertnstert.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 54.Grgic O, Matijevic R, Vasilj O. Qualitative glandular cervical score as a potential new sonomorphological parameter in screening for preterm delivery. Ultrasound Med Biol. 2006;32:333–338. doi: 10.1016/j.ultrasmedbio.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Carlstedt I, Lindgren H, Sheehan JK. The macromolecular structure of human cervical-mucus glycoproteins. Studies on fragments obtained after reduction of disulphide bridges and after subsequent trypsin digestion. Biochem J. 1983;213:427–435. doi: 10.1042/bj2130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheehan JK, Carlstedt I. The effect of guanidinium chloride on the behaviour of human cervical-mucus glycoproteins. Evidence for unfolding regions of ordered structure in 6M-guanidinium chloride. Biochem J. 1984;221:499–504. doi: 10.1042/bj2210499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.