Abstract
OBJECTIVES
To assess how influenza vaccination coverage in children is related to pneumonia and influenza (P&I) in US seniors and if these associations are modified by sociodemographic factors.
DESIGN
We abstracted approximately 5 million hospitalization records from the Centers for Medicare and Medicaid Services for four influenza years, 2002–2006. We estimated a single year age distribution of rates of P&I hospitalization by state for each influenza season and observed an exponential acceleration in the P&I rates with age for each influenza season. State-and season-specific P&I rate accelerations were regressed against the percentage of vaccinated children, seniors, or both using mixed effects models.
SETTING
United States population, 2002–2006
PARTICIPANTS
US population aged 65 and above
MEASUREMENTS
State-level influenza annual vaccination coverage data in children and seniors were obtained from the National Immunization Survey and the Behavioral Risk Factor Surveillance System, respectively.
RESULTS
Child influenza vaccination coverage was negatively associated with age acceleration in P&I, whereas influenza vaccination in the seniors themselves was not significantly associated with P&I in seniors.
CONCLUSION
Vaccination of children against influenza may induce herd immunity against influenza for seniors and has the potential to be more beneficial to seniors than the existing policy to prevent influenza by vaccinating seniors themselves.
Keywords: vaccination, influenza, herd immunity, children, elderly
INTRODUCTION
Influenza and associated diseases are among the leading causes of death for older adults in the US. In the general population, there were nearly 1.7 million annual hospitalizations attributable to pneumonia and influenza (P&I), with 65% of those cases having P&I as the primary diagnosis.1 Of the 1.7 million annual hospitalizations for influenza-associated diseases, most (1.2 million) are from the 65+ population. Older adults consequently experience the most severe complications from these diseases compared to all other population subgroups. The P&I mortality rate is 22.1 per 100,000 in the older population aged 65–99 years (hereafter ‘older population’), nearly 100 times higher than the group with the next highest rate—0.3 per 100,000 in children under one year of age. From 2000 to 2002, there were over 1.2 million hospitalizations in the 65+ population with any-listed diagnosis of pneumonia.2 The population aged 85 and above experience the highest rates of hospitalization from P&I—629 per 100,000 person-years.1 P&I morbidity increases nearly exponentially with age in the older population,3 likely resulting from declining immunological function with age.4 The aging and growth of the older population will likely magnify the impact of P&I in this vulnerable population.
Influenza is easily spread from person to person. One means of controlling influenza is to vaccinate those individuals who are most likely to spread the infection first, particularly in environments where vaccination is not obligatory or where vaccine supply is limited. The primary means of controlling influenza, which is largely preventable, is through annual vaccination.5 Vaccine safety in children has been well documented, for both inactivated vaccine6 and live attenuated vaccine7 in children ages 9–17. In children, influenza vaccine has been shown to be highly efficacious in clinical settings, with efficacy ranging between 65 and 96%.8 For the older population, however, vaccine efficacy is consistently lower (12%9–68%10). Clinical efficacy of the influenza vaccine is higher in older adults under age 75 than in those above age 75,11 which is consistent with the observed declines in immune function with age.12
Recent research suggests potential pathways for transmission of influenza virus between children and the older population. Influenza vaccination coverage in children was associated with decreased all-cause mortality and mortality from P&I in the older population in Japan,13 in addition to protecting children themselves from death.14 Mass influenza vaccination of children was shown to significantly reduce influenza-like illness in children, and influenza-related diseases in older adults.15 These results suggest herd immunity, which occurs when vaccinating one population subgroup reduces exposure in another group,16 and has been demonstrated in other infectious diseases, including Haemophilus influenzae type b,17 measles,18 and oral cholera.19 A community with customary vaccination practices experienced substantially higher influenza incidence in the adult population than in a neighboring community in which 85% of schoolchildren were vaccinated against influenza.20 Modest, but significant reductions in influenza rates in older adults aged 50–64 occurred where schoolchildren were immunized against seasonal influenza compared to the control school,21 suggesting that such a vaccination strategy may impart indirect protection to older adults, beyond the contribution of vaccination of older adults themselves.
The objective of this study is to assess the potential for herd immunity in the older population against P&I-associated hospitalization using the most complete database of hospitalization records of older adults. We assess associations between vaccination coverage in children and P&I hospitalizations in the older population during the period 2002–2006. We also examine the effect of influenza vaccination coverage in the older population on P&I outcomes in the older population.
METHODS
Data Sources
Outcome variables
Data from the Centers for Medicare and Medicaid Services (CMS) containing all Medicare-eligible hospitalizations in the United States from July 2002 through June 2006 were utilized for this analysis. All claims records of hospitalizations associated with P&I (ICD-9CM codes 480 – 487)22 were abstracted from CMS databases for each of four influenza years, defined as July through June of the following year for the population aged 65 to 99. For each state and influenza year the records were summed and arranged as a single-year age distribution. Population counts were obtained for the population aged 65–99 for single-years of age and influenza year from the US Census Bureau Population Estimates Program. From hospitalization and population counts, the P&I rates were estimated by single-year of age, state, and season.
P&I rates increase nearly exponentially with age in the older population, therefore the main outcome variable is defined as the log of the rate of increase in disease rates with age.3 This variable, called the P&I rate acceleration, is a relative measure of disease burden in older adults. This measure takes advantage of having disease rates by single-year of age and captures the relationship between age and disease rates, which is important as the burden of P&I is highest in the oldest old. The standard approach of using age-adjusted rates prioritizes age groups with the highest populations, which is generally the younger portion of older adults, given that population size decreases with increasing age. Using P&I rate acceleration, however, effectively weights each age equally reflecting P&I patterns across all ages simultaneously in the 65+ population, including those at the highest risk of disease.23
Exposure Variables
Data on state-level influenza vaccination coverage in children were obtained from the National Immunization Program, a program of the Centers for Disease Control and Prevention. The National Immunization Program conducts annual telephone surveys on influenza vaccination in children age 19 to 35 months old through the National Immunization Survey (NIS). It is used to estimate immunization coverage on the national and state levels, although in this analysis, only the state influenza vaccination coverage will be used. Influenza vaccination coverage in older adults was extracted from the Behavioral Risk Factor Surveillance System (BRFSS) database, a telephone survey administered by CDC to assess prevalence, of many disease and disease risk factors in the U.S. and its territories. Vaccination status was asked of study participants aged 65+ and aggregated state-level influenza vaccination coverage was abstracted for this analysis for each year from 2002 to 2006.
Statistical Analysis
Summary statistics were obtained for all variables, including influenza vaccination coverage in the 65+ population, and influenza vaccination coverage in children at the state level. Pearson correlations were then estimated between each of the exposure-outcome pairs of variables for all states by season, and for all seasons by state. To model the relationship between vaccination coverage and P&I outcomes, linear mixed-effects models were used with state as the random effect to assess the role of state-level covariates in explaining between-state variation.
Base Model
For each model, parameter estimates were obtained for unadjusted models, and for models adjusted for three sociodemographic characteristics: median state income24 log of population density,25 and percent of institutionalized population aged 65+, given that the BRFSS is a telephone survey. Based on the observed autocorrelations, first-order autoregressive covariance structures were used in all models, to account for the observed temporal correlations that were strongest for a one-season lag, and weakened progressively as the lag increased. The equation used is:
for each state i in influenza season j. β1 represents the coefficients of the explanatory variable influenza vaccination coverage in older adults. β2 represents the coefficients from influenza vaccination coverage in children. βi is the vector of effects of the potential confounding variables matrix Xi for each state i in the adjusted models. The random effect of state is captured in the model and represented by the αi intercept term, and the residual error term is represented by εij. The temporal correlation between a pair of measurements on the same state decreases toward zero as the seasonal lag increases, as described above. Since the observation times are equally spaced, each element of the variance-covariance matrix corrected for decay in autocorrelation ρ can be expressed as
where ρ is equal to exp(-ϕ), and ϕ is a constant representing the rate of decline and k is the seasonal lag, measured in seasons, k = 0 to 3, for all models. Statistical analysis was performed using SAS version 9.0 (Cary, N.C.), and relevant figures were produced in SPSS version 17.0 (Chicago, Ill.).
RESULTS
Summary statistics are displayed in Table 1. The results illustrate several notable features of the data. Overall, influenza comprises approximately 2% of the P&I rate. Influenza vaccination coverage in children increased steadily during the four years examined. The relationship between vaccination coverage and P&I rate acceleration are shown in Figure 1. This graph illustrates the association between P&I rate acceleration and vaccination coverage in children and illustrate the substantial state-by-state variation in level of disease burden and vaccination coverage over time. Pearson correlations between age-acceleration and vaccination coverage in children and older adults were 0.064 (p = 0.373) and 0.302 (p < 0.001), respectively, when examining all states and seasons. Single-year correlations ranged from 0.205 to 0.358 for vaccination coverage in children, and 0.029–0.457 for older adults.
Table 1.
Summary statistics for key outcome and explanatory variables, 2002–06
| Mean (SD) | 2002–2003 | 2003–2004 | 2004–2005 | 2005–2006 | Overall |
|---|---|---|---|---|---|
| Pneumonia and influenza rate* | 39.4 (9.7) | 42.9 (10.4) | 44.2 (10.7) | 40.5 (9.5) | 41.8 (10.2) |
| Pneumonia rate* | 39.2 (9.6) | 41.5 (9.9) | 42.8 (10.0) | 39.8 (9.2) | 40.8 (9.7) |
| Influenza rate* | 0.2 (0.2) | 1.4 (0.8) | 1.5 (1.0) | 0.9 (0.4) | 1.0 (0.8) |
| Pneumonia and influenza rate in 65–74* | 20.5 (5.6) | 23.0 (6.3) | 23.3 (6.3) | 21.7 (5.7) | 22.1 (6.1) |
| Pneumonia and influenza rate in 75–84* | 45.5 (11.2) | 49.0 (11.8) | 50.6 (12.2) | 46.0 (11.0) | 47.8 (11.7) |
| Pneumonia and influenza rate in 85+* | 98.3 (21.9) | 104.3 (23.4) | 106.8 (23.3) | 96.5 (20.2) | 101.5 (22.5) |
| Percent of all P&I cases that are influenza | 0.6 (0.3) | 3.2 (1.4) | 3.2 (1.7) | 2.1 (0.8) | 2.3 (1.6) |
| P&I rate acceleration (× 100)** | 7.6 (0.5) | 7.4 (0.5) | 7.5 (0.7) | 7.3 (0.7) | 7.4 (0.6) |
| Pneumonia rate acceleration (× 100)** | 7.6 (0.5) | 7.4 (0.5) | 7.4 (0.7) | 7.3 (0.7) | 7.4 (0.6) |
| Influenza rate acceleration (× 100)** | 9.0 (1.7) | 7.7 (1.5) | 8.6 (1.2) | 8.1 (1.4) | 8.4 (1.5) |
| Influenza vaccination coverage in children (%) | 8.3 (4.8) | 18.8 (7.7) | 32.6 (9.5) | 33.5 (10.5) | 23.3 (13.4) |
| Influenza vaccination coverage in 65+ (%) | 69.4 (4.2) | 69.9 (4.2) | 67.3 (5.0) | 67.2 (4.6) | 68.5 (4.6) |
Per 1,000 older adults
The disease rate acceleration coefficient is a measure of the increase (on the log scale) in hospitalization rates for each singe-year increase in age for the population aged 65+.
Figure 1.
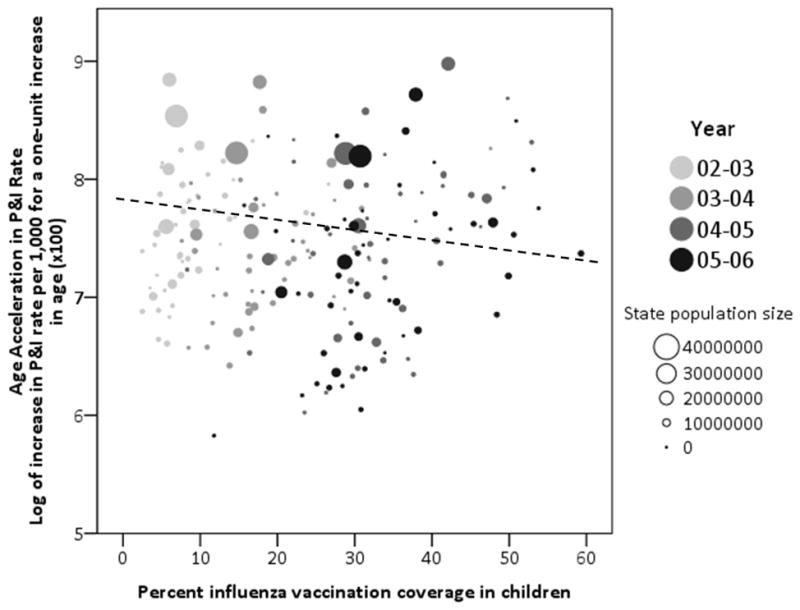
Relationship between age acceleration of pneumonia and influenza rates and influenza vaccination coverage in children by influenza year, 2002–2006.
The dashed line depicts the association of pneumonia and influenza rate acceleration and percent vaccination coverage in children.
The results of P&I rate acceleration modeling are shown in Table 2. For each outcome, Model 1 has influenza vaccination coverage in older adults (65+) as its explanatory variable, Model 2 included influenza vaccination coverage in children as its main explanatory variable, and Model 3 contained influenza vaccination coverage in both children and older adults as explanatory variables. For influenza, vaccination coverage in either children or older adults was not significantly associated with age acceleration in disease rates, with one exception: in the unadjusted model, influenza vaccination coverage of older adults was significantly and positively associated with age acceleration in disease rates. For the pneumonia-only and P&I models, states with higher levels of influenza vaccination of children had significantly lower age acceleration in rates than states with lower levels of influenza vaccination of children. Influenza vaccination coverage in older adults, however, was not significantly associated with influenza, pneumonia, or combined P&I age acceleration rates in the models that were adjusted for confounders.
Table 2.
Parameter estimates (and standard errors) representing the effects of vaccination coverage on pneumonia and influenza rate acceleration‡ from linear mixed effects models
| Rate acceleration | |||
|---|---|---|---|
| Influenza vaccine coverage in: | Unadjusted | Adjusted† | |
| Influenza | |||
| Model 1 | Older adults | 0.057 (0.028)* | 0.044 (0.032) |
|
| |||
| Model 2 | Children | −0.006 (0.006) | −0.007 (0.006) |
|
| |||
| Model 3 | Older adults | 0.053 (0.030) | 0.035 (0.034) |
| Children | −0.003 (0.007) | −0.005 (0.007) | |
|
| |||
| Pneumonia | |||
| Model 1 | Older adults | 0.026 (0.012)* | 0.019 (0.013) |
|
| |||
| Model 2 | Children | −0.006 (0.003)* | −0.007 (0.003)** |
|
| |||
| Model 3 | Older adults | 0.020 (0.012) | 0.010 (0.013) |
| Children | −0.004 (0.003) | −0.006 (0.003)* | |
|
| |||
| Pneumonia and Influenza | |||
| Model 1 | Older adults | 0.024 (0.012)* | 0.017 (0.013) |
|
| |||
| Model 2 | Children | −0.005 (0.003) | −0.006 (0.003)* |
|
| |||
| Model 3 | Older adults | 0.019 (0.012) | 0.008 (0.013) |
| Children | −0.004 (0.003) | −0.006 (0.003)* | |
p < 0.05
p < 0.01
Adjusted for log of population density, income, and percent of institutionalized population aged 65+
The rate acceleration coefficient reflects the increase in pneumonia and influenza rates (expressed in a log scale) for each single-year increase in age. Exponentiation of the coefficient provides a measure of percent increase in P&I rates with age. Therefore, a negative sign indicates a decline in the acceleration of P&I rates with increased vaccination coverage in children.
DISCUSSION
This study is among the first to directly assess the associations between vaccination of children against influenza and influenza-associated disease burden in the older population. The effect of vaccinating children to impart indirect protection to older adults, versus the prevailing strategy of vaccinating older adults themselves was also directly assessed. Vaccination of older adults against influenza was not associated with a reduction in the influenza burden in the older population, yet vaccination of children against influenza was beneficial in reducing disease in older adults. The modeling approach used a novel measure of population disease burden as the outcome measure, the age acceleration rate of disease in the older population, which quantifies the nearly exponential increase in disease rates with age above age 65.23
A sub-analysis was performed using the outcome of age-specific P&I rates for ten-year age groups (65–74, 75–84, and 85+). The results were similar to those using age-acceleration coefficients as the outcome variable. Vaccination coverage in the older population was actually positively associated with P&I rates, while vaccination coverage in children was either not significantly or negatively associated with age-specific P&I rates in the older population. Age-adjusted rates provide an important summary measure of P&I in older adults. However, this traditional measure does not account for the epidemiological, biological, and demographic processes that give rise to the observed age patterns of P&I in older adults. The age-increase measure used in this analysis, measuring the relative increase in P&I rates with age, takes into account the important relationship between disease rates and age and captures aging and disease dynamics that are masked by age-adjusted P&I rates. This is because P&I, while prevalent in the entire population, are particularly problematic for the oldest of the older population, who, consequently, also experience the highest mortality from P&I. Used together with traditional measurements, alternative measures, such as the P&I rate acceleration, supplement more traditional measures and provide a more complete picture of disease patterns in this vulnerable population.
Increased vaccination coverage in children was associated with reduced P&I for the older population, which could be due to a true biological link between vaccination of children and P&I outcomes in the older population, or because vaccination coverage in children serves as a proxy for some intermediate variable that could mediate or modify the relationship. However, if the results truly reflect the biological relationship between vaccination and P&I outcomes in the older population, these results suggests that one potential means of protecting the older against pneumonia and influenza may be to increase vaccination coverage in children.
The findings of this study should be interpreted with caution, as several limitations of this analysis exist. The data used in this analysis cover pre-school children, aged 19 to 35 months. Previous research has suggested there may be a link between vaccinating school-aged children and P&I morbidity and mortality in the older population13–15; however, influenza vaccination coverage in school-aged children on the state level is not readily available from the National Immunization Survey and other related data sources. Both central exposure measures were based on telephone surveys and are therefore subject to recall bias and sampling error. However, these findings on vaccinating young children are consistent with those of a household-based study that demonstrated a significant reduction in influenza infection in households in which children aged 24 to 60 months who attended day care were immunized.26 Related factors known to have effects, specifically on pneumonia hospitalizations, were not considered in this analysis. These include immunization with polysaccharide pneumococcal vaccine and conjugate vaccine, which have been shown to have potentially beneficial effects for reducing P&I in older adults.17
Another limitation exists with respect to the outcome data. To calculate age-specific P&I rates that were used to estimate age acceleration rates, the denominator used was intercensal population estimates from the US Census Bureau. An alternative denominator that more accurately reflects the Medicare population would be the denominator file of Medicare beneficiaries, which was only available for two of the four years under study. Comparing the two denominators, it was found that there was a consistent and small discrepancy between the two in the estimation of age-specific population counts (3.5–4.8%). Therefore, the P&I rates used in the calculation of age acceleration rates were likely consistent underestimates.
Other limitations involve the modeling strategy used. First, the unit of observation was the state; past research suggests that although states are someone homogeneous, a sizeable amount of heterogeneity exists within states with respect to the distribution of P&I rates and sociodemographic characteristics.27 Second, the dominant circulating influenza virus strain and whether or not the proper vaccine for each influenza season was used were not considered. Effective matching of vaccine strains to the circulating strain could modulate the effectiveness of vaccinating older adults against P&I. A third limitation of the modeling strategy was the assessment of whether vaccination coverage was associated with P&I outcomes in the concurrent influenza and pneumonia season. The strategy employed in this analysis does not taking into account the potential for immune memory to last for consecutive seasons.28 P&I rate acceleration was positively associated with influenza vaccination rates in the older population, which may reflect the possibility for the positive association is a possible lagged effect from the previous year. States with unusually high P&I rates in one season may respond by enhancing vaccination programs and availability for the following year. In the following year, the P&I levels may still be residually high, contributing to the anomalously positive correlation between P&I rate acceleration and vaccination coverage. Despite these limitations, the associations found between influenza vaccination coverage in children and P&I in older adults was generally consistent across models.
Another potential limitation is the ecological nature of the study design, suggesting that individual-level relationships cannot be inferred from these results. The objective of the study, however, was not to examine individual-level relationships between vaccinating children and P&I outcomes in older adults within households or families. This type of research is critical for the understanding of the transmissibility of influenza and pneumonia from child to adult and has been explored on a small scale in the US26 and Europe.29 Instead, the objective of this study was to assess whether vaccination of children against influenza could potentially reduce P&I in older adults in the community, thereby exploring the potential for herd immunity, which cannot be defined on the individual level. If these findings are further validated in future studies, an important issue that must be considered is the coverage of influenza needed to induce herd immunity. Despite the seemingly low levels of vaccination coverage in children, a modest, but detectable level of herd immunity was observed. Also, the time period of study poses unique challenges due to changes to the recommended age groups to get vaccinated. Formal recommendations for universal vaccination of children began in 2004.30 Since, the immunization coverage increased in parallel to the declines in disease, these temporal associations could be, in part, responsible for these findings.
Although the findings are subject to limitations, they have potential implications for future research and policy in the US. Until recently, the prevailing means of controlling influenza transmission in the older population was to vaccinate the older population against influenza. In the past several years, the recommendation has been changed somewhat, including the vaccination of children as a potentially more effective means of controlling influenza for the entire population, particularly for older adults, who tend to experience the most severe influenza-associated health effects. The findings suggest that vaccinating children against influenza may provide an indirect benefit to older adults in reducing exposure to this largely preventable and deadly disease, and induce herd immunity in this vulnerable population of older adults. As the older population continues to grow in the United States and the interactions of children and older adults in households increases, researching and implementing policies to improve population health and reduce disease burden is an increasingly important task. Upon additional validation of these findings, there may still exist numerous barriers to implementing a policy to encourage vaccinating children to protect the population. In the absence of compulsory vaccination, controlling influenza, the eighth leading cause of the death in the United States, may play a substantial role in achieving the goal of a healthy aging population in the coming decades.
Acknowledgments
The following funding agencies are thanked for their continual support: The National Institute of Allergy and Infectious Disease (U19AI062627 and N01 AI50032). We also thank the American Public Health Association (APHA) Gerontological Health Section for their support by selecting this as the winning manuscript of the 2009 James G. Zimmer New Investigator Research Award, along with related financial support at the APHA annual meeting. We also wish to thank the Centers for Medicare and Medicaid Services for supplying the outcome dataset. The use of the dataset was approved by the Tufts Medical Center Institutional Review Board.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions:
Steven Cohen conceived the idea for the manuscript and performed all data analysis and completed all of the writing.
Kenneth Chui managed the data and assisted with the creation of the tables and figures. He also edited the text extensively.
Elena Naumova provided statistical expertise and data for the project. She also edited the manuscript.
Sponsor’s Role: n/a
References
- 1.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Fry AM, Shay DK, Holman RC, Curna AT, et al. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1998–2002. JAMA. 2005;294:2712–2719. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SA, Naumova EN. Population dynamics in the elderly: The need for age-adjustment in national biosurveillance systems. Lec Notes Comp Sci. 2007;4506:47–58. [Google Scholar]
- 4.Kang I, Hong MS, Nolasco H, et al. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–681. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- 5.Fiore AE, Shay DK, Haber P, et al. Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Morb Mortal Wkly Rept. 2007;56(RR-06):1–54. [PubMed] [Google Scholar]
- 6.Gonzalez M, Pirez MC, Ward E, et al. Safety and immunogenicity of a paediatric presentation of an influenza vaccine. Arch Dis Child. 2000;83:488–491. doi: 10.1136/adc.83.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergen R, Black S, Shinefield H, et al. Safety of cold-adapted live attenuated influenza vaccine in a large cohort of children and adolescents. Pediatr Infect Dis J. 2004;23:138–144. doi: 10.1097/01.inf.0000109392.96411.4f. [DOI] [PubMed] [Google Scholar]
- 8.Neuzil KM, Dupont WD, Wright PF, et al. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatr Infect Dis J. 2001;20:733–740. doi: 10.1097/00006454-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Govaert TM, Thijs CT, Masurel N, et al. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272:1661–1665. [PubMed] [Google Scholar]
- 10.Hara M, Sakamoto T, Tanaka K. Effectiveness of influenza vaccination in preventing influenza-like illness among community-dwelling elderly: Population-based cohort study in Japan. Vaccine. 2006;24:5546–5551. doi: 10.1016/j.vaccine.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 12.Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: Reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 13.Reichert TA, Sugaya N, Fedson DS, et al. The Japanese experience with vaccinating schoolchildren against influenza. N Eng J Med. 344:889–896. doi: 10.1056/NEJM200103223441204. [DOI] [PubMed] [Google Scholar]
- 14.Sugaya N, Takeucki Y. Mass vaccination of schoolchildren against influenza and its impact on the influenza-associated mortality rate among children in Japan. Clin Infect Dis. 2005;41:939–947. doi: 10.1086/432938. [DOI] [PubMed] [Google Scholar]
- 15.Ghendon YZ, Kaira AN, Elshina GA. The effect of mass influenza immunization in children on the morbidity of the unvaccinated elderly. Epidemiol Infect. 2006;134:71–78. doi: 10.1017/S0950268805005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glezen WP. Herd protection against influenza. J Clin Virol. 2006;37:237–243. doi: 10.1016/j.jcv.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Cohen SA, Ahmed S, Klassen AC, et al. Childhood Hib vaccination and pneumonia and influenza burden in US seniors. Vaccine. 2010;28:4462–9. doi: 10.1016/j.vaccine.2010.04.035. Epub 2010 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlenker TL, Bain C, Baughman AL, et al. Measles herd immunity: The association of attack rates with immunization rates in preschool children. JAMA. 1992;267:823–826. doi: 10.1001/jama.267.6.823. [DOI] [PubMed] [Google Scholar]
- 19.Emch M, Ali M, Park J, et al. Relationship between neighbourhood-level killed oral cholera vaccine coverage and protective efficacy: Evidence for herd immunity. Int J Epidemiol. 2006;35:1044–1050. doi: 10.1093/ije/dyl100. [DOI] [PubMed] [Google Scholar]
- 20.Monto AS, Davenport FM, Napier AJ, et al. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis. 1970;122:16–25. doi: 10.1093/infdis/122.1-2.16. [DOI] [PubMed] [Google Scholar]
- 21.Talbot HK, Poehling KA, Williams JV, et al. Influenza in older adults: impact of vaccination of school children. Vaccine. 2009;27:1923–1927. doi: 10.1016/j.vaccine.2009.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viboud C, Bjornstad ON, Smith DL, et al. Synchrony, waves, and spatial hierarchies in the spread of influenza. Science. 2006;312:447–451. doi: 10.1126/science.1125237. [DOI] [PubMed] [Google Scholar]
- 23.Cohen SA, Chui KKH, Naumova EN. Measuring disease burden in the older population using the slope-intercept method for population log-linear estimation (SIMPLE) Stat Med. 2010 doi: 10.1002/sim.3886. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong K, Berlin M, Schwartz JS, et al. Barriers to influenza immunization in a low-income urban population. Am J Prev Med. 2001;20:21–25. doi: 10.1016/s0749-3797(00)00263-4. [DOI] [PubMed] [Google Scholar]
- 25.Glezen WP. The changing epidemiology of respiratory syncytial virus and influenza: Impetus for new control measures. Pediatr Infect Dis J. 2004;23:S202–206. doi: 10.1097/01.inf.0000144662.86396.07. [DOI] [PubMed] [Google Scholar]
- 26.Hurwitz ES, Haber M, Chang A, et al. Effectiveness of influenza vaccination of day care children in reducing influenza-related morbidity among household contacts. JAMA. 2000;284:1677–1682. doi: 10.1001/jama.284.13.1677. [DOI] [PubMed] [Google Scholar]
- 27.Naumova EN, Parisi SM, Castronovo D, et al. Pneumonia and influenza hospitalizations in elderly people with dementia. J Am Geriatr Soc. 2009;57:2192–2199. doi: 10.1111/j.1532-5415.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 28.Longini IM, Halloran ME, Nizam A, et al. Estimation of the efficacy of live, attenuated influenza vaccine from a two-year, multi-center vaccine trial: Implications for influenza epidemic control. Vaccine. 2000;18:1902–1909. doi: 10.1016/s0264-410x(99)00419-3. [DOI] [PubMed] [Google Scholar]
- 29.Viboud C, Boëlle P, Cauchemez S, et al. Risk factors of influenza transmission in households. Brit J Gen Pract. 2004;54:684–689. [PMC free article] [PubMed] [Google Scholar]
- 30.American Academy of Pediatrics Committee on Infectious Diseases. Recommendations for influenza immunization of children. Pediatrics. 2004;113:1441–1447. doi: 10.1542/peds.113.5.1441. [DOI] [PubMed] [Google Scholar]


