Abstract
In vitro preparations of whole urinary bladders of neonatal rats exhibit prominent myogenic spontaneous contractions, the amplitude and frequency of which can be increased by muscarinic agonists. The muscarinic receptor subtype responsible for this facilitation was examined in the present experiments. Basal spontaneous contractions in bladders from 1- to 2-wk-old Sprague-Dawley rats were not affected by M2 or M3 receptor antagonists. However, administration of 0.5 μM physostigmine, an anticholinesterase agent that increases the levels of endogenous acetylcholine, or 50–100 nM carbachol, a cholinergic agonist at low concentrations, which did not cause tonic contractions, significantly augmented the frequency and amplitude of spontaneous contractions. Blockade of M2 receptors with 0.1 μM AF-DX 116 or 1 μM methoctramine or blockade of M3 receptors with 50 nM 4-diphenylacetoxy-N-methylpiperidine methiodide or 0.1 μM 4-diphenylacetoxy-N-(2-chloroethyl)piperidine hydrochloride (4-DAMP mustard) reversed the physostigmine and carbachol responses. M2 and M3 receptor blockade did not alter the facilitation of spontaneous contractions induced by 10 nM BAY K 8644, an L-type Ca2+ channel opener, or 0.1 μM iberiotoxin, a large-conductance Ca2+-activated K+ channel blocker. NS-1619 (30 μM), a large-conductance Ca2+-activated K+ channel opener, decreased carbachol-augmented spontaneous contractions. These results suggest that spontaneous contractions in the neonatal rat bladder are enhanced by activation of M2 and M3 receptors by endogenous acetylcholine released in the presence of an anticholinesterase agent or a cholinergic receptor agonist.
Keywords: M2 receptors, M3 receptors, overactive bladder, detrusor overactivity
IN VITRO WHOLE ORGAN or muscle strip preparations of the neonatal rat bladder during postnatal weeks 1 and 2 exhibit high-amplitude low-frequency spontaneous contractions, which, later in postnatal maturation, are converted to low-amplitude high-frequency contractions (32, 34). Although these spontaneous contractions are most likely myogenic in origin because they occur in the absence of neural stimulation (6, 19, 32, 33), they can be modulated by activation of various types of receptors (muscarinic, purinergic, and adrenergic) (9, 10). We previously showed that these contractions are markedly facilitated in the presence of carbachol, a muscarinic agonist (34). Interest in the cholinergic regulation of spontaneous bladder contractions (11) was stimulated by the recent hypothesis that symptoms of overactive bladder may be due to enhancement of spontaneous contractions by leakage of small amounts of acetylcholine from intramural nerves during bladder filling (1).
The present experiments were undertaken to examine the types of receptors involved in the cholinergic modulation of spontaneous bladder activity. On the basis of the ratio of M2 to M3 receptors in the bladder (9:1 in rat and 3:1 in human) (25, 35), one would expect that M2 receptors would be involved in cholinergically evoked bladder contractions. However, it is clear from studies using M2,M3, and M2/M3 receptor-knockout mice (22–24) and pharmacological data (2, 29) that the M3 receptor is primarily responsible for large-amplitude bladder contractions elicited by stimulation of cholinergic nerves (4, 30) and that the M2 receptor works indirectly by potentiating the M3 receptor-mediated contractions or by counteracting β-adrenergic receptor-mediated relaxation (8, 13, 24, 38).
In this study, we provide evidence that activation of M2 as well as M3 receptors by endogenous acetylcholine or exogenous carbachol can enhance spontaneous contractions in whole bladder preparations from normal 1- to 2-wk-old rats. The ability of muscarinic receptor mechanisms to modulate spontaneous bladder contractions raises the possibility that these mechanisms may play a role in the generation of symptoms in patients with detrusor overactivity.
MATERIALS AND METHODS
In vitro whole bladder preparation
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Adult (8 female and 1 male) and 1- to 2-wk-old Sprague-Dawley rats were anesthetized with 4% (vol/vol) isoflurane and killed by cervical dislocation. We used mostly 1- to 2-wk-old rats (n = 96, 48 male and 48 female), because we previously showed that the amplitude of spontaneous contractions at this age is maximal (34, 36); therefore, studies conducted at this age allowed for easier detection of changes elicited by muscarinic receptor activation. We modified our previous technique for whole bladder studies (32) by cannulating the urethra, rather than the dome, and not tying off the ureters, because leakage was not seen from the ureters during bladder filling. The bladder was exposed by a midline abdominal incision and removed from the abdomen by an incision at the bladder neck. A 26-gauge needle was inserted at the bladder neck and tied with 5-0 silk sutures. The needle was connected to an infusion pump and pressure transducer via polyethylene tubing and a three-way stopcock. The needle and tubing were filled with Krebs solution (in mM: 113 NaCl, 19.8 NaHCO3, 11.1 dextrose, 1.2 KH2PO4, 4.7 KCl, 2.5 MgCl2, and 1.7 CaCl2). The bladder was placed between two platinum stimulating electrodes inside an organ bath (Radnoti Glass, Monrovia, CA) filled with 37°C Krebs solution and bubbled with 95% O2-5% CO2. Bladder pressure was recorded by WinDaq Acquisition software (version 2.13 for Windows, Akron, OH). After a 30-min equilibration period, the bladder was filled slowly with Krebs solution in 50-μl increments during intermittent electrical field stimulation (50 V, 1.6-ms pulses at 32 Hz for 15–30 s) to determine the bladder volume necessary to produce maximal bladder contractions. Field stimulation was delivered by a Grass S88 stimulator (Quincy, MA). The distended bladder was washed three times with 15 ml of fresh Krebs solution and equilibrated for another 15 min, and then drug treatment was started. We used the last 5-min interval within the 15-min observation period to calculate the mean amplitude, frequency, and area under the curve (AUC) of the spontaneous contractions. The criteria proposed by Imai et al. (17) were used to define a single spontaneous contraction event: a response with an amplitude ≥30% of the peak spontaneous contraction during the 15-min observation period. Also, when a contraction was superimposed on the previous event before reaching baseline, the two contractions were considered a single contraction event. The peak amplitude of the spontaneous contractions was normalized as a percentage of the maximal K+-evoked contraction amplitude. The K+-evoked contraction was induced at the end of the experiments by a bath solution containing 80 mM KCl. Frequency was determined by counting the number of contractions over a 5-min interval.
Chemicals
All chemicals used for the bath solution in the in vitro whole bladder preparation studies were obtained from Sigma (St. Louis, MO). Recombinant iberiotoxin (IBTX) was obtained from Alomone Lab (Jerusalem, Israel). Carbachol, S-(–)-BAY K 8644, NS-1619, methoctramine tetrahydrochloride, 4-diphenylacetoxy-N-methylpiperidine methiodide (4-Damp), 4-diphenylacetoxy-N-(2-chloroethyl)piperidine hydrochloride (4-DAMP mustard), and physostigmine (eserine) were obtained from Sigma. AF-DX 116 was obtained from Tocris Cookson (Ellisville, MO). The M3 antagonist 4-DAMP was used as a competitive inhibitor and 4-DAMP mustard as a noncompetitive inhibitor in combination with 1 μM AF-DX 116 to protect the M2 receptors during the irreversible alkylation of the M3 receptor caused by 0.1 μM 4-DAMP mustard for 1 h (8). Then 4-DAMP mustard and AF-DX 116 were removed by extensive washes (20 ml) over a 15-min period.
Statistical analysis
Values are means (SD); n is the number of bladders used for each set of experiments. Repeated-measures oneway ANOVA followed by Tukey's multiple posttest was used for multiple group comparison. Paired t-test was used for comparison between two groups. Statistics were calculated using Prism 4 (Windows version 4.02, GraphPad Software, San Diego, CA). Statistical significance was considered when a 95% confidence level (P ≤ 0.05) was reached.
RESULTS
Muscarinic regulation of basal spontaneous contractions
Spontaneous contractions (Fig. 1) appeared in 1- to 2-wk-old bladders after they were filled to 150–200 μl, which was the volume required for maximum field stimulation-evoked contractions. The mean amplitude of the spontaneous contractions was 6.9 cmH2O (SD 4.2), which was 11.5% of K+ evoked contraction (n = 96). The mean frequency was 3.2 contractions/min (SD 1.5) (n = 96).
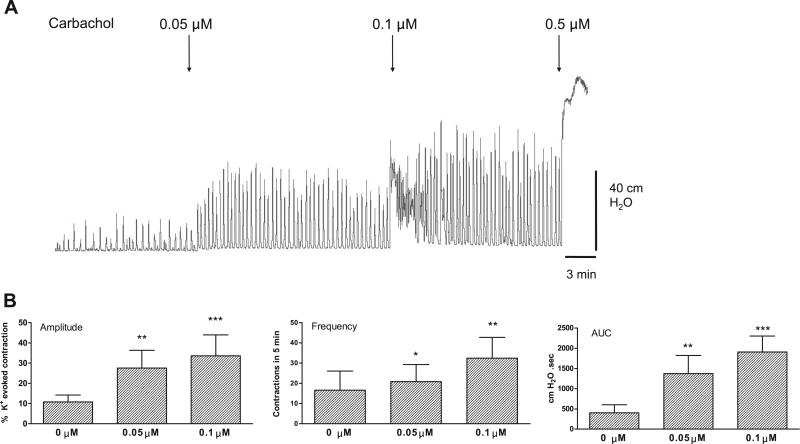
Fig. 1.
Concentration-response study of the effect of carbachol on basal spontaneous contractions. Low concentrations of carbachol enhance spontaneous contractions without producing a tonic contraction; higher concentrations produce tonic contractions. AUC, area under the curve. Values are means (SD); n = 5. *P < 0.05; **P < 0.01; ***P < 0.001 vs. basal (0 μM).
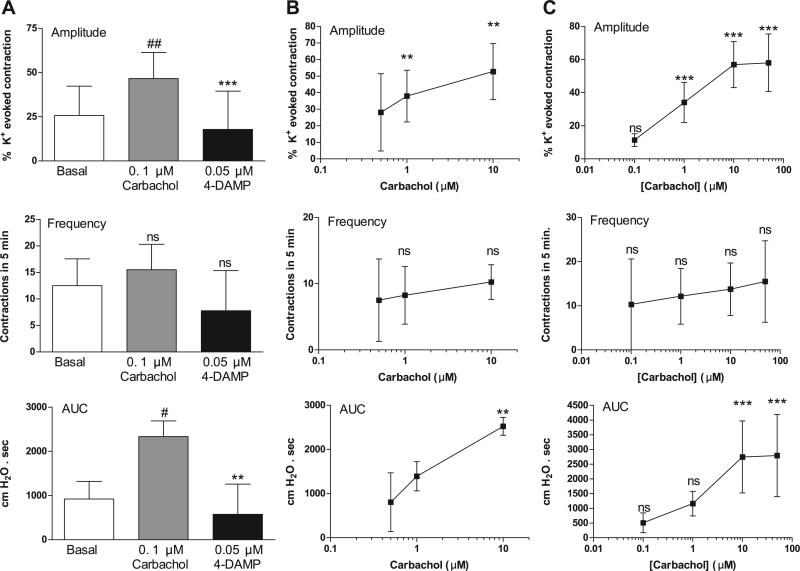
The nonselective muscarinic agonist carbachol enhanced spontaneous bladder contractions in a concentration-dependent manner (Fig. 1). Carbachol (0.05 and 0.1 μM) significantly increased the amplitude, frequency, and AUC of the spontaneous contractions. A low concentration of carbachol (0.05 μM) increased spontaneous contractions without causing a tonic contraction (i.e., an increase in baseline tone), whereas 0.1 μM carbachol caused a 1- to 5-min transient rise in basal tone (Fig. 1A). Higher concentrations of carbachol (0.5–50 μM) elicited large-amplitude tonic contractions (Fig. 1A) followed by high-amplitude, low-frequency spontaneous contractions (40–70 cmH2O) superimposed on an elevated basal tone (n = 4).
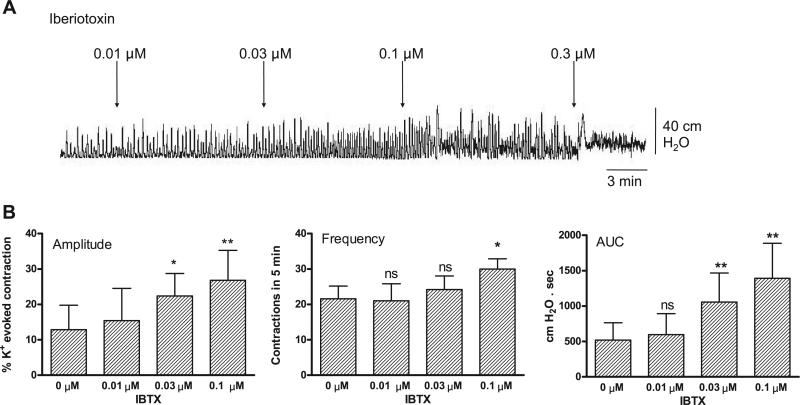
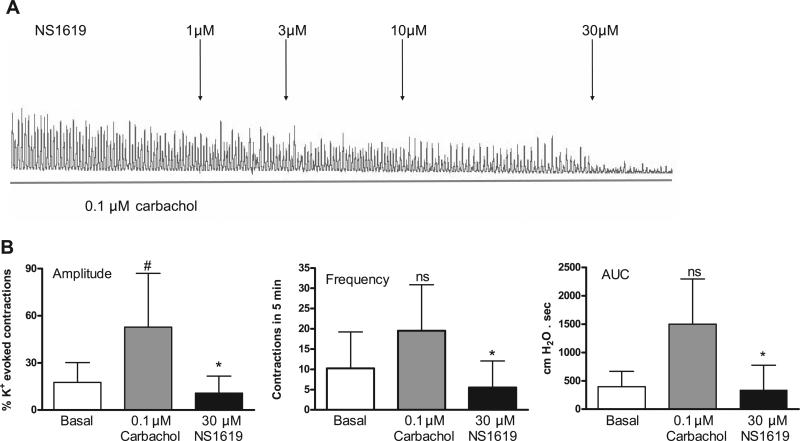
The large conductance Ca2+-activated K+ (BKCa) channel blocker IBTX produced changes in spontaneous activity similar to those induced by carbachol, including a concentration-dependent rise in amplitude, frequency, and AUC of basal spontaneous contractions (Fig. 2). Conversely, the BKCa channel opener NS-1619 elicited a concentration-dependent decrease in amplitude, frequency, and AUC of carbachol-augmented spontaneous contractions (Fig. 3). The L-type Ca2+ channel opener S-(–)-BAY K 8644 also increased the amplitude and AUC of basal spontaneous contractions (Fig. 4A).
Fig. 2.
Concentration-response study of the effect of large-conductance Ca2+-activated K+ (BKCa) channel blocker iberiotoxin (IBTX) on basal spontaneous contractions. ns, Not significant. Values are means (SD); n = 5. *P < 0.05; **P < 0.01 vs. basal.
Fig. 3.
BKCa channel opener NS-1619 reverses carbachol-enhanced spontaneous contractions. Values are means (SD); n = 4. *P < 0.05 vs. carbachol. #P < 0.05 vs. basal.
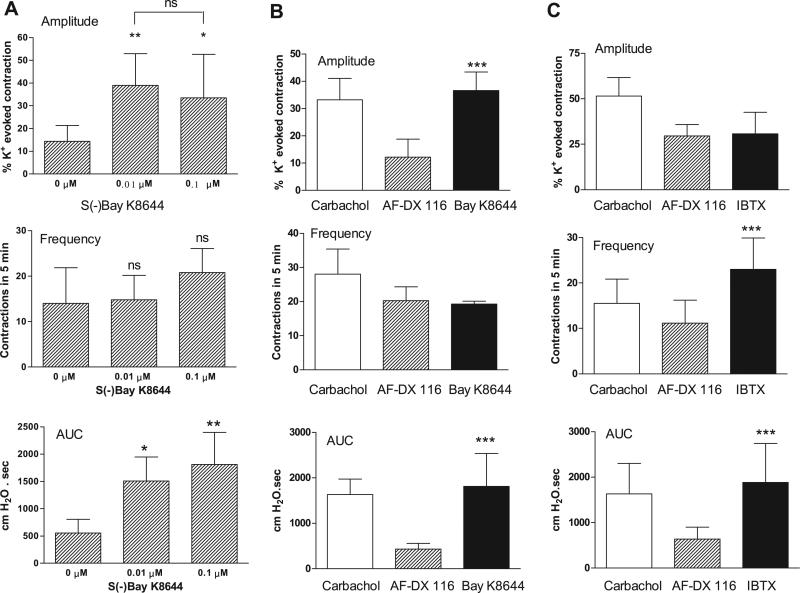
Fig. 4.
A: concentration-response study of effect of L-type Ca2+ channel opener S-(–)-BAY K 8644 on basal spontaneous contractions. Values are means (SD) n = 5. B: BAY K 8644 enhances spontaneous contractions after AF-DX 116 has blocked carbachol facilitation of spontaneous contractions. Values are means (SD); n = 4. C: IBTX enhances spontaneous contractions after AF-DX 116 has blocked carbachol facilitation of spontaneous contractions. Values are means (SD); n = 6. *P < 0.05; **P < 0.01 vs. basal. ***P < 0.001 vs. AF-DX 116.
Effects of muscarinic receptor antagonists
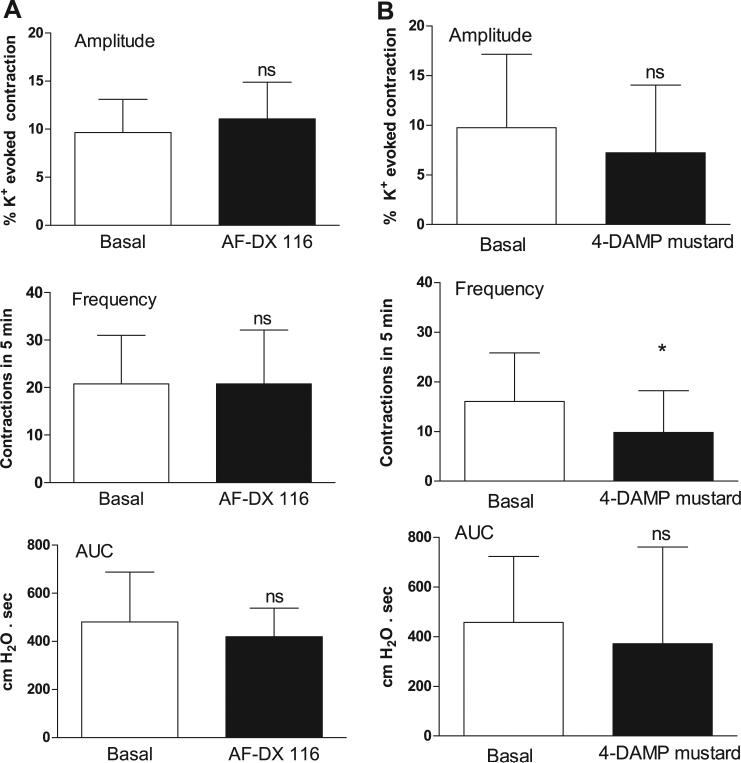
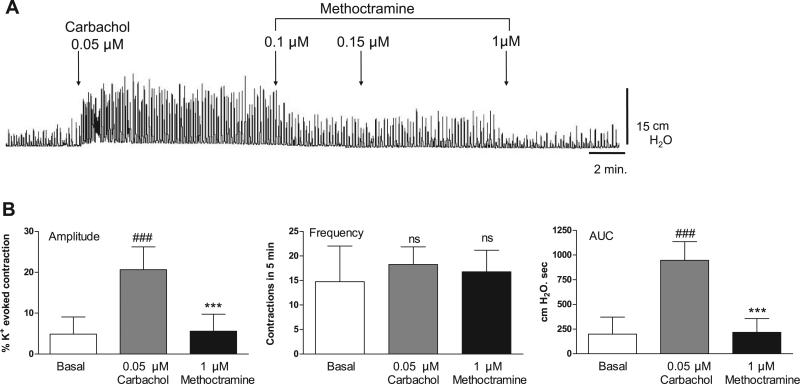
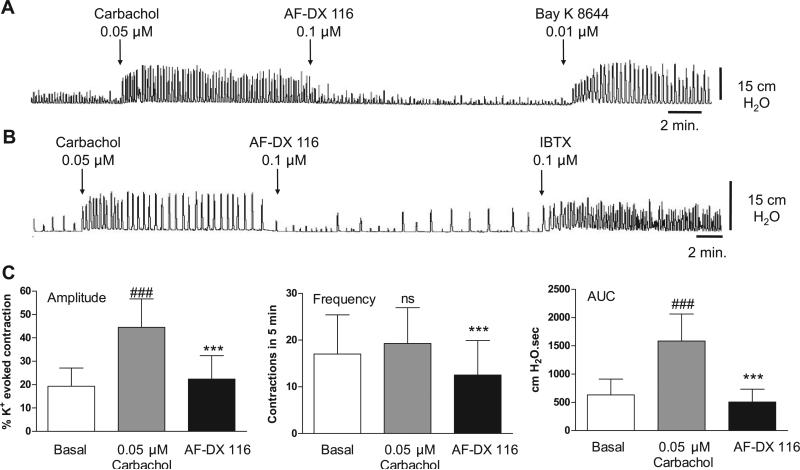
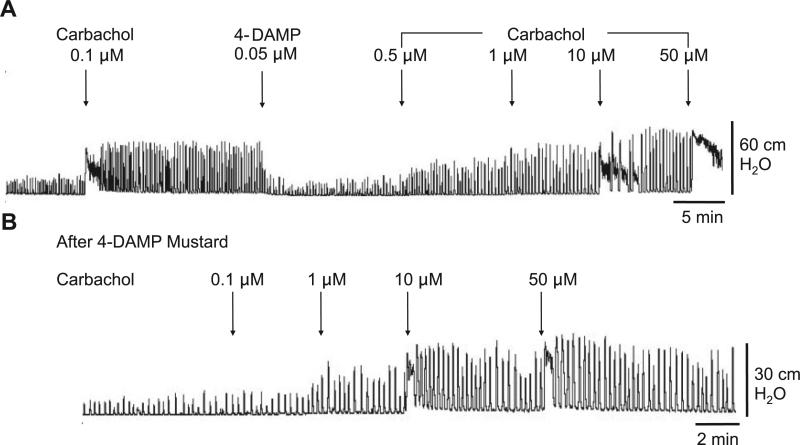
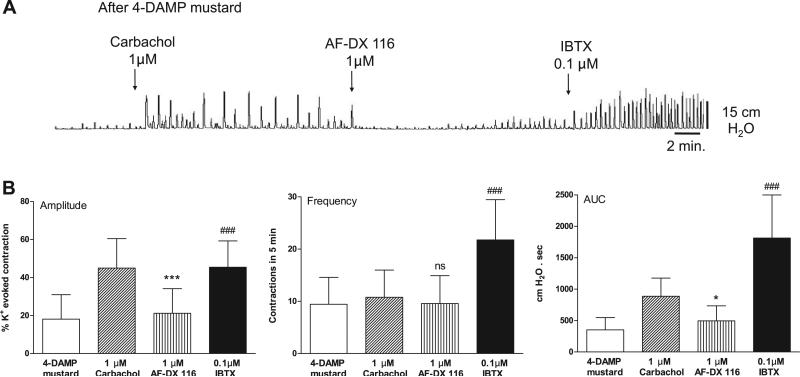
Muscarinic 2 (M2) receptor blockade with 0.1 μM AF-DX 116 or M3 receptor blockade with 0.05 μM 4-DAMP mustard did not affect the amplitude or AUC of basal spontaneous contractions (Fig. 5). 4-DAMP mustard, but not AF-DX 116, decreased the frequency of basal spontaneous contractions (Fig. 5). The antagonists suppressed the effects of carbachol on spontaneous activity. The M2 selective antagonists methoctramine (Fig. 6) and AF-DX 116 (Fig. 7) blocked the carbachol facilitatory effects on amplitude and AUC. AF-DX 116, but not methoctramine, also suppressed the effect of carbachol on the frequency of contractions (Figs. 7C and 6B). After AF-DX 116 blockade of the carbachol response, BAY K 8644 (Figs. 4B and 7A) or IBTX (Figs. 4C and 7B) was still able to enhance spontaneous activity. 4-DAMP (0.05 μM) decreased the carbachol-augmented spontaneous contraction (Figs. 8A and 9A), but this effect could be overcome by a 2-log-unit increase in carbachol concentration (Figs. 8A and 9B). Similarly, the irreversible M3 antagonist 4-DAMP mustard blocked the effect of carbachol, and this blockade could also be reversed by higher concentrations of carbachol (≥10 μM; Figs. 8B and 9C). AF-DX 116 reversed the effects of high concentrations of carbachol in preparations treated with 4-DAMP mustard (Fig. 10A). When M3 and M2 receptors were blocked (Fig. 10B), the BKCa channel blocker IBTX was still able to increase the amplitude, frequency, and AUC of spontaneous contractions.
Fig. 5.
M2 and M3 antagonism does not affect amplitude or AUC of basal spontaneous contractions. A: effect of 0.1 μM AF-DX 116 on basal spontaneous contractions. Values are means (SD); n = 4. B: effect of 0.05 μM 4-diphenylacetoxy-N-(2-chloroethyl)piperidine hydrochloride (4-DAMP mustard) on basal spontaneous contractions. Values are means (SD); n = 12. *P < 0.05 vs. basal. M, muscarinic.
Fig. 6.
Suppression of carbachol-enhanced spontaneous contractions by methoctramine. Values are means (SD); n = 4. ***P < 0.001 vs. carbachol. ###P < 0.001 vs. basal.
Fig. 7.
Inhibition of carbachol-enhanced spontaneous contractions by the M2 antagonist AF-DX 116. S-(–)-BAY K 8644 (n = 4) or IBTX (n = 6) still increases spontaneous contractions in the presence of AF-DX 116. Values are means (SD). ***P < 0.001 vs. carbachol. ###P < 0.001 vs. basal.
Fig. 8.
A: blocking effect of 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP) on carbachol-enhanced spontaneous contractions can be overcome by increasing carbachol concentration. B: concentration response of carbachol after 4-DAMP mustard.
Fig. 9.
A: effect of 4-DAMP on carbachol-enhanced spontaneous contractions. Values are means (SD); n = 4. **P < 0.01; ***P < 0.001 vs. carbachol. #P < 0.05; ##P < 0.01 vs. basal. B: concentration response of carbachol in the presence of 4-DAMP. Values are means (SD); n = 4. **P < 0.01 vs. 0.5 μM carbachol. C: concentration response of carbachol after 4-DAMP mustard. Values are means (SD); n = 6. ***P < 0.001 vs. 0.1 μM carbachol.
Fig. 10.
Effect of AF-DX 116 on carbachol-enhanced spontaneous contractions. A: after combined M2-M3 receptor blockade, IBTX is still effective. B: effect of AF-DX 116 and IBTX on carbachol-enhanced spontaneous contractions after 4-DAMP mustard. Values are means (SD); n = 9. *P < 0.05; ***P < 0.001 vs. carbachol. ###P < 0.001 vs. AF-DX 116.
Physostigmine regulation of spontaneous contractions
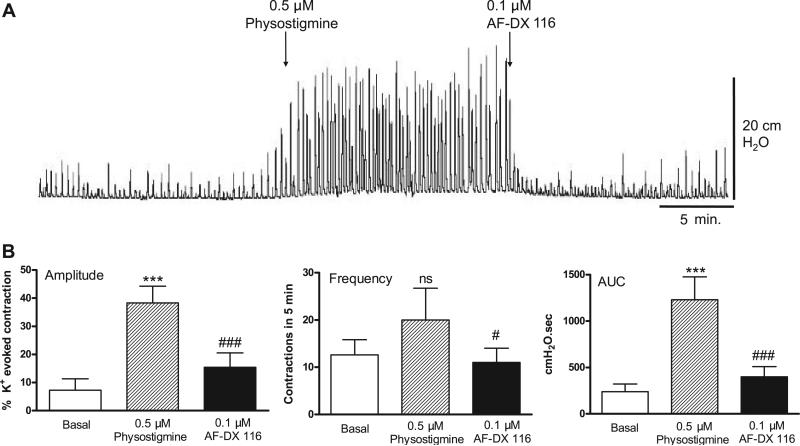
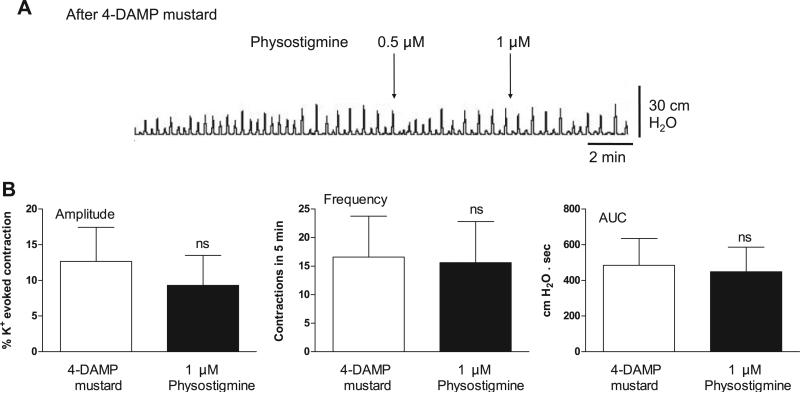
The acetylcholinesterase inhibitor physostigmine (0.5 μM) increased the amplitude and AUC of the spontaneous contractions, producing responses similar to those induced by carbachol (Fig. 11A). This augmentation was reversed by M2 receptor blockade with AF-DX 116 (Fig. 11B). In addition, M3 receptor blockade by pretreatment with 4-DAMP mustard completely blocked the facilitatory effect on spontaneous contractions of a subsequent administration of physostigmine (Fig. 12).
Fig. 11.
Effect of physostigmine on basal spontaneous contractions. Facilitatory effect of physostigmine on spontaneous bladder contractions is reversed by AF-DX 116. Values are means (SD); n = 5. ***P < 0.001 vs. basal. #P < 0.05; ###P < 0.001 vs. physostigmine.
Fig. 12.
Physostigmine does not affect basal spontaneous contractions after irreversible M3 receptor antagonism with 4-DAMP mustard. Values are means (SD); n = 5.
Adult rat bladder spontaneous contractions
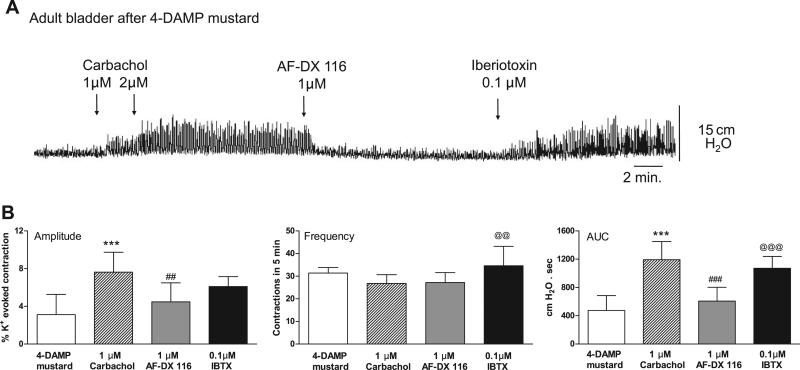
The effects of combined M2 and M3 receptor blockade on muscarinic stimulation of spontaneous bladder contractions were also studied in bladders from adult rats. Consistent with previous results (34), the basal amplitude of spontaneous contractions was lower and the frequency was higher in adult animals than in neonates. M2 receptor antagonism with 1 μM AF-DX 116 after 0.05 μM carbachol decreased amplitude, frequency, and AUC, similar to neonatal bladders (data not shown). After M3 receptor blockade by pretreatment with 4-DAMP mustard, a high concentration of carbachol (2 μM) increased spontaneous contractions (Fig. 13). Addition of AF-DX 116 reduced the contractions to basal levels. As in neonatal bladders, IBTX increased spontaneous contractions after blockade of M3 and M2 receptors (Fig. 13).
Fig. 13.
Effect of carbachol, AF-DX 116, and IBTX on basal spontaneous contractions of adult bladders pretreated with 4-DAMP mustard. After M3 receptors have been inactivated, spontaneous contractions of adult bladders can be enhanced by ≥1 μM carbachol. Effects of AF-DX 116 and IBTX are similar to those in neonatal bladders. Values are means (SD); n = 5. ***P < 0.001 vs. basal. ##P < 0.01; ###P < 0.001 vs. carbachol. @@P < 0.01; @@@P < 0.001 vs. AF-DX 116.
DISCUSSION
The present experiments revealed that spontaneous contractile activity in the in vitro whole bladder preparation of the neonatal rat is not dependent on activation of M3 and M2 receptors by endogenous acetylcholine. However, treatment of the preparations with physostigmine, an anticholinesterase agent that inhibits the metabolism of acetylcholine and increases the extracellular levels of the transmitter, markedly facilitated the spontaneous activity. Administration of carbachol, a cholinergic agonist, mimicked the effect of physostigmine. The effects of both agents were suppressed by M3 and M2 receptor antagonists, indicating that spontaneous contractile activity in the neonatal bladder can be regulated by cholinergic muscarinic mechanisms.
Previous studies in neonatal rat bladder preparations demonstrated large-amplitude rhythmic contractions that persisted after block of nerve action potentials with tetrodotoxin and blockade of muscarinic receptors with atropine (21, 33). These data indicated that the spontaneous contractions were myogenic in origin. The present results confirm that spontaneous contractions in the untreated neonatal bladder are not dependent on activation of M3 and M2 receptors by cholinergic nerves. However, treatment with physostigmine produced a marked enhancement of spontaneous activity that was blocked by muscarinic receptor antagonists. These data indicate that, after acetylcholinesterase inhibition, endogenous acetylcholine can rise to levels sufficient to activate muscarinic receptors in the tissue. The source of the acetylcholine is uncertain, because acetylcholine is known to be released spontaneously from nerves and also from urothelium by bladder distension. However, spontaneous activity in muscle strips from neonatal rat bladders is reduced by atropine (34). This raises the possibility that preparation of muscle strips damages nerves or urothelium, triggering an increase in spontaneous release of acetylcholine.
In contrast to the predominant role of M3 receptors in large-amplitude, neurally evoked bladder contractions, the facilitation of spontaneous contractions in the neonatal bladder seems to depend on the activation of M2 and M3 receptors. The facilitatory effects of physostigmine could be inhibited by pretreatment with 4-DAMP mustard, an irreversible M3 receptor antagonist, and abolished by administration of AF-DX 116, an M2 receptor antagonist. These data indicate that endogenous acetylcholine activation of both types of receptors is required for enhancement of spontaneous activity. The facilitatory effect of carbachol also seems to be mediated by the activation of either type of receptor. However, activation of only one type of receptor by carbachol is sufficient to enhance the contractions. For example, although 4-DAMP or 4-DAMP mustard blocked the effect of low concentrations of carbachol (0.1 μM), increasing the concentration of carbachol to 1–10 μM overcame the block, suggesting that activation of M2 receptors alone can facilitate the spontaneous activity. M2 receptor antagonists (AF-DX 116 or methoctramine) alone also reduced the effect of carbachol.
It is possible that the facilitatory effect of muscarinic receptor activation on spontaneous activity occurs by a mechanism different from that mediating the tonic contractions induced by muscarinic receptor activation (9, 10). As shown in Fig. 1, low concentrations of carbachol (0.05 μM) are sufficient to facilitate spontaneous activity without inducing a tonic contraction. Higher concentrations are needed to induce large-amplitude tonic contractions similar to those induced by nerve stimulation. Similarly, physostigmine facilitated spontaneous contractions without inducing large-amplitude tonic contractions (Fig. 11). The large-amplitude contractions may be mediated by M3 receptors, because they did not return to control levels after pretreatment with 4-DAMP mustard, even when the tissue was exposed to very high concentrations of carbachol (50 μM; Fig. 8B). These findings in neonatal rat bladders confirm previous results in adult guinea pig bladders (9) in which low doses of carbachol (0.1–0.3 μM) induced spontaneous contractions without eliciting tonic contractions. Recent data suggest that M2 receptors are responsible for inhibiting smooth muscle relaxation elicited by increased cytosolic cAMP (13, 38) and potentiating contractions initiated by M3 receptors in gastrointestinal, respiratory, and urinary tract smooth muscles (8). As reviewed by Ehlert (7), the contribution of the M2 receptors to tonic contractions depends on the presence of other direct contractile mechanisms, such as the M3 receptor mechanism.
Although the role of the M2 receptor is recognized in neuropathic bladders, defining its function in normal bladders is more difficult. The spinal cord injury model, in which M2 receptors play a much larger role than M3 receptors, is one in which there is both denervation and hypertrophy (2, 29). It is also possible that changes in tissue perfusion in pathological tissues could affect the local metabolism of acetylcholine and possibly account for some of the shift toward M2, rather than M3, receptor regulation of bladder contraction. The role of muscarinic regulation of bladder contractions appears to decrease with age (28), yet the level of stretch-induced, nonneuronal basal acetylcholine release is higher in the bladders of elderly individuals (39).
M3 receptor antagonism appears to be more effective than M2 receptor antagonism in inhibiting spontaneous contractions. Methoctramine, when used at an M2 receptor-effective concentration of 0.1 μM, decreased the amplitude of spontaneous contractions by only 50%, whereas at an M3 receptor-effective concentration of 1 μM (13), it reduced the amplitude back to baseline, completely reversing the augmentation caused by carbachol (Fig. 6). After treatment with M3 receptor antagonists (4-DAMP and 4-DAMP mustard), the inhibition of spontaneous contractions could be reversed by an increase in the carbachol concentration by 2 log units. One of the problems in interpreting experiments in which muscarinic receptors are blocked is the possibility of incomplete blockade. It has been estimated that ~9% of M3 receptors are still available after 1 h of 4-DAMP mustard treatment (8). Although we would interpret the finding that higher concentrations of carbachol can reverse blockade of M3 receptors as evidence that carbachol is acting via the M2 receptor, it is possible that the effect of carbachol after M3 receptor blockade is due to persistent activation of M3 receptors. The finding that AF-DX 116 administered after 4-DAMP mustard and carbachol results in further blockade of spontaneous contractions in neonatal and adult bladders is additional evidence that carbachol after 4-DAMP mustard acts via the M2 receptors. Similarly, the partial blockade of carbachol effects by low M2 receptor-selective concentrations of methoctramine is evidence that M2 receptors are involved in the control of spontaneous contractions.
Basal spontaneous contractions, which were unaffected by M2 or M3 receptor blockade, were sensitive to BKCa channel and L-type Ca2+ channel regulation. This suggests that although the spontaneous contractions are “myogenic” in origin, the degree to which these contractions are expressed is dependent on properties of the bladder smooth muscle, which may be modulated by muscarinic signaling. After large-amplitude neurally evoked bladder contractions, BKCa channels act as a brake on muscle activity. Elevated intracellular Ca2+ levels after smooth muscle contraction open BKCa channels, repolarize the membrane potential, and inactivate L-type Ca2+ channels. We also found that BKCa channels are important in the postnatal downregulation of spontaneous bladder contractions during maturation (36). Spontaneous contractions in normal adult and diabetic bladders can be augmented by blocking BKCa channels with IBTX (3, 12, 15–17, 26). In this study, blocking BKCa channels with IBTX also enhanced spontaneous activity, whereas opening these channels with NS-1619 reversed the augmentation of spontaneous contractions by carbachol. Nakamura et al. (27) showed that activation of M2 receptors inhibits BKCa channels in rat bladder, but the signaling pathway by which M2 receptors achieve this inhibition is unknown. Our finding that closing large-conductance K+ channels after M2 and M3 receptor blockade (Figs. 7 and 10) results in an increase in spontaneous contractions suggests that cell depolarization, which regulates spontaneous contractions, occurs downstream of the muscarinic receptors.
Control over intracellular Ca2+ levels via the L-type Ca2+ channels is also important in the regulation of spontaneous contractions. Spontaneous contractions are always preceded by spontaneous action potentials (12, 18), the upstroke of which is due to influx of Ca2+ through the L-type channels (14). Blockade of L-type Ca2+ channels with nifidepine abolished spontaneous action potentials and spontaneous contractions in normal bladders (12, 14, 15, 18, 20), whereas activation of L-type Ca2+ channels with the dihydropyridine analog S-(–)-BAY K 8644 enhanced the amplitude and the frequency of spontaneous contractions in normal and diabetic adult rat bladder smooth muscle strips (5, 26). We confirm that enhanced Ca2+ influx by activation of L-type Ca2+ channels alone was sufficient to increase the spontaneous contractions in 1- to 2-wk-old bladders. In addition, the effect of BAY K 8644 after AF-DX 116 on the AUC of spontaneous contractions is similar to that of IBTX (Fig. 11, B and C). However, in contrast to IBTX, BAY K 8644 did not significantly increase the frequency of spontaneous contractions. Thus the frequency of spontaneous contractions appears to be regulated in a more selective manner than amplitude. Although IBTX, NS-1619, and carbachol affected frequency, BAY K 8644, methoctramine, and physostigmine did not.
In conclusion, endogenous acetylcholine or administration of a cholinergic agonist facilitated spontaneous contractions in the neonatal rat bladder by activation of M2 and M3 receptors. Muscarinic mechanisms that modulate spontaneous bladder contractions are of considerable interest, because antimuscarinic drugs are used clinically to treat the sensory and motor symptoms of overactive bladder. The potential clinical relevance of the present findings would be limited if muscarinic modulation of spontaneous activity were only present in neonatal bladders. Thus the finding that muscarinic receptor activation also unmasks spontaneous contractions in adult rat bladders is important, because it indicates that even though spontaneous bladder activity is downregulated during postnatal development, the muscarinic facilitatory mechanisms that regulate spontaneous activity persist in mature bladders.
Acknowledgments
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-65759 (to H.-Y. Wu) and DK-49430 (to W. C. de Groat).
REFERENCES
- 1.Andersson KE, Yoshida M. Antimuscarinics and the overactive detrusor—which is the main mechanism of action? Eur Urol. 2003;43:1–5. doi: 10.1016/s0302-2838(02)00540-7. [DOI] [PubMed] [Google Scholar]
- 2.Braverman AS, Ruggieri MR. Hypertrophy changes the muscarinic receptor subtype-mediated bladder contraction from M3 toward M2. Am J Physiol Regul Integr Comp Physiol. 2003;285:R701–R708. doi: 10.1152/ajpregu.00009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol. 2002;135:639–648. doi: 10.1038/sj.bjp.0704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chess-Williams R, Chapple CR, Yamanishi T, Yasuda K, Sellers DJ. The minor population of M3-receptors mediates contraction of human detrusor muscle in vitro. J Auton Pharmacol. 2001;21:243–248. doi: 10.1046/j.1365-2680.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- 5.Diederichs W, Sroka J, Graff J. Comparison of Bay K 8644, nitrendipine and atropine on spontaneous and pelvic-nerve-induced bladder contractions on rat bladder in vivo. Urol Res. 1992;20:49–53. doi: 10.1007/BF00294335. [DOI] [PubMed] [Google Scholar]
- 6.Drake MJ, Hedlund P, Harvey IJ, Pandita RK, Andersson KE, Gillespie JI. Partial outlet obstruction enhances modular autonomous activity in the isolated rat bladder. J Urol. 2003;170:276–279. doi: 10.1097/01.ju.0000069722.35137.e0. [DOI] [PubMed] [Google Scholar]
- 7.Ehlert FJ. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway, and urinary bladder smooth muscle. Life Sci. 2003;74:355–366. doi: 10.1016/j.lfs.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Ehlert FJ, Griffin MT, Abe DM, Vo TH, Taketo MM, Manabe T, Matsui M. The M2 muscarinic receptor mediates contraction through indirect mechanisms in mouse urinary bladder. J Pharmacol Exp Ther. 2005;313:368–378. doi: 10.1124/jpet.104.077909. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie JI, Harvey IJ, Drake MJ. Agonist- and nerve-induced phasic activity in the isolated whole bladder of the guinea pig: evidence for two types of bladder activity. Exp Physiol. 2003;88:343–357. doi: 10.1113/eph8802536. [DOI] [PubMed] [Google Scholar]
- 10.Gillespie JI. A developing view of the origins of urgency: the importance of animal models. BJU Int. 2005;1(96 Suppl):22–28. doi: 10.1111/j.1464-410X.2005.05652.x. [DOI] [PubMed] [Google Scholar]
- 11.Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol. 2001;530:273–286. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004;141:183–193. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 1997;273:C110–C117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- 15.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol. 2000;279:R60–R68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- 16.Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2001;280:C481–C490. doi: 10.1152/ajpcell.2001.280.3.C481. [DOI] [PubMed] [Google Scholar]
- 17.Imai T, Okamoto T, Yamamoto Y, Tanaka H, Koike K, Shigenobu K, Tanaka Y. Effects of different types of K+ channel modulators on the spontaneous myogenic contraction of guinea-pig urinary bladder smooth muscle. Acta Physiol Scand. 2001;173:323–333. doi: 10.1046/j.1365-201X.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 18.Imai T, Tanaka Y, Okamoto T, Yamamoto Y, Horinouchi T, Tanaka H, Koike K, Shigenobu K. Evidence that action potential generation is not the exclusive determinant to trigger spontaneous myogenic contraction of guinea-pig urinary bladder smooth muscle. Acta Physiol Scand. 2002;176:57–63. doi: 10.1046/j.1365-201X.2002.01009.x. [DOI] [PubMed] [Google Scholar]
- 19.Levin RM, Ruggieri MR, Velagapudi S, Gordon D, Altman B, Wein AJ. Relevance of spontaneous activity to urinary bladder function: an in vitro and in vivo study. J Urol. 1986;136:517–521. doi: 10.1016/s0022-5347(17)44934-2. [DOI] [PubMed] [Google Scholar]
- 20.Maggi CA, Manzini S, Parlani M, Conte B, Giuliani S, Meli A. The effect of nifedipine on spontaneous, drug-induced and reflexly-activated contractions of the rat urinary bladder: evidence for the participation of an intracellular calcium store to micturition contraction. Gen Pharmacol. 1988;19:73–81. doi: 10.1016/0306-3623(88)90008-0. [DOI] [PubMed] [Google Scholar]
- 21.Maggi CA, Santicioli P, Meli A. Postnatal development of myogenic contractile activity and excitatory innervation of rat urinary bladder. Am J Physiol Regul Integr Comp Physiol. 1984;247:R972–R978. doi: 10.1152/ajpregu.1984.247.6.R972. [DOI] [PubMed] [Google Scholar]
- 22.Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi SI, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi SI, Manabe T, Taketo MM. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci. 2002;22:10627–10632. doi: 10.1523/JNEUROSCI.22-24-10627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui M, Griffin MT, Shehnaz D, Taketo MM, Ehlert FJ. Increased relaxant action of forskolin and isoproterenol against muscarinic agonist-induced contractions in smooth muscle from M2 receptor knockout mice. J Pharmacol Exp Ther. 2003;305:106–113. doi: 10.1124/jpet.102.044701. [DOI] [PubMed] [Google Scholar]
- 25.Monferini E, Giraldo E, Ladinsky H. Characterization of the muscarinic receptor subtypes in the rat urinary bladder. Eur J Pharmacol. 1988;147:453–458. doi: 10.1016/0014-2999(88)90180-x. [DOI] [PubMed] [Google Scholar]
- 26.Nakahara T, Mitani A, Kubota Y, Maruko T, Sakamoto K, Tanaka Y, Koike K, Shigenobu K, Ishii K. MaxiK channel-triggered negative feedback system is preserved in the urinary bladder smooth muscle from streptozotocin-induced diabetic rats. J Smooth Muscle Res. 2004;40:97–109. doi: 10.1540/jsmr.40.97. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Kimura J, Yamaguchi O. Muscarinic M2 receptors inhibit Ca2+-activated K+ channels in rat bladder smooth muscle. Int J Urol. 2002;9:689–696. doi: 10.1046/j.1442-2042.2002.00548.x. [DOI] [PubMed] [Google Scholar]
- 28.Oh SJ, Lee KH, Kim SJ, Kim KW, Kim KM, Choi H. Active properties of the urinary bladder: in vitro comparative studies between adult and neonatal rats. BJU Int. 2000;85:1126–1133. doi: 10.1046/j.1464-410x.2000.00621.x. [DOI] [PubMed] [Google Scholar]
- 29.Pontari MA, Braverman AS, Ruggieri MR. The M2 muscarinic receptor mediates in vitro bladder contractions from patients with neurogenic bladder dysfunction. Am J Physiol Regul Integr Comp Physiol. 2004;286:R874–R880. doi: 10.1152/ajpregu.00391.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sellers DJ, Yamanishi T, Chapple CR, Couldwell C, Yasuda K, Chess-Williams R. M3 muscarinic receptors but not M2 mediate contraction of the porcine detrusor muscle in vitro. J Auton Pharmacol. 2000;20:171–176. doi: 10.1046/j.1365-2680.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- 31.Stengel PW, Yamada M, Wess J, Cohen ML. M3-receptor knockout mice: muscarinic receptor function in atria, stomach fundus, urinary bladder, and trachea. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1443–R1449. doi: 10.1152/ajpregu.00486.2001. [DOI] [PubMed] [Google Scholar]
- 32.Sugaya K, de Groat WC. Influence of temperature on activity of the isolated whole bladder preparation of neonatal and adult rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R238–R246. doi: 10.1152/ajpregu.2000.278.1.R238. [DOI] [PubMed] [Google Scholar]
- 33.Sugaya K, de Groat WC. Inhibitory control of the urinary bladder in the neonatal rat in vitro spinal cord-bladder preparation. Brain Res Dev Brain Res. 2002;138:87–95. doi: 10.1016/s0165-3806(02)00468-6. [DOI] [PubMed] [Google Scholar]
- 34.Szell EA, Somogyi GT, de Groat WC, Szigeti GP. Developmental changes in spontaneous smooth muscle activity in the neonatal rat urinary bladder. Am J Physiol Regul Integr Comp Physiol. 2003;285:R809–R816. doi: 10.1152/ajpregu.00641.2002. [DOI] [PubMed] [Google Scholar]
- 35.Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- 36.Wu HY, Ng YK, de Groat WC. Am Urol Assoc Annu Meeting. San Antonio; TX: 2005. Potassium channels regulate spontaneous activity in the neonatal rat bladder. (Abstract 1220) [Google Scholar]
- 37.Yamanishi T, Chapple CR, Yasuda K, Chess-Williams R. The role of M2-muscarinic receptors in mediating contraction of the pig urinary bladder in vitro. Br J Pharmacol. 2000;131:1482–1488. doi: 10.1038/sj.bjp.0703719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanishi T, Chapple CR, Yasuda K, Chess-Williams R. The role of M2 muscarinic receptor subtypes in mediating contraction of the pig bladder base after cyclic adenosine monophosphate elevation and/or selective M3 inactivation. J Urol. 2002;167:397–401. [PubMed] [Google Scholar]
- 39.Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A. Management of detrusor dysfunction in the elderly: changes in acetylcho-line and adenosine triphosphate release during aging. Urology. 2004;3A(63 Suppl):17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]