Abstract
Vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) are expressed in the neural pathways regulating the lower urinary tract. VIP-immunoreactivity (IR) is present in afferent and autonomic efferent neurons innervating the bladder and urethra, whereas PACAP-IR is present primarily in afferent neurons. Exogenously applied VIP relaxes bladder and urethral smooth muscle and excites parasympathetic neurons in bladder ganglia. PACAP relaxes bladder and urethral smooth muscle in some species (pig) but excites the smooth muscle in other species (mouse). Intrathecal administration of VIP in cats with an intact spinal cord suppresses reflex bladder activity, but intrathecal administration of VIP or PACAP in rats enhances bladder activity and suppresses urethral sphincter activity. PACAP has presynaptic facilitatory effects and direct excitatory effects on lumbosacral parasympathetic preganglionic neurons. Chronic spinal cord transection produces an expansion of VIP-IR (cats) and PACAP-IR (rats) in primary afferent axons in the lumbosacral spinal cord and unmasks spinal excitatory effects of VIP on bladder reflexes in cats. Intrathecal administration of PACAP6–38, a PAC1 receptor antagonist, reduces bladder hyperactivity in chronic spinal-cord-injured rats. These observations raise the possibility that VIP or PACAP have a role in the control of normal or abnormal voiding.
Keywords: Urinary bladder, Urethra, Primary afferent neurons, Autonomic neurons, Spinal cord injury
Introduction
Vasoactive intestinal polypeptide (VIP), a 28-amino acid peptide, and pituitary adenylate cyclase-activating polypeptide (PACAP), which exists as two alternatively spliced forms (PACAP-27 and PACAP-38), are members of the secretin/glucagon/vasoactive intestinal polypeptide super family of neuropeptides (Arimura 1992; Pantaloni et al. 1996). PACAP and VIP bind to three types of G-protein-coupled receptors (PAC1, VPAC1, and VPAC2). PACAP has a high affinity for all three types of receptors, whereas VIP has a high affinity for VPAC1 and VPAC2 (Arimura 1998; Vaudry et al. 2000). Activation of all three subtypes of receptors stimulates adenylate cyclase and increases the production of cAMP, while activation of PAC1 can also stimulate phospholipase C and associated downstream signaling pathways (Sherwood et al. 2000; Laburthe et al. 2002).
PACAP and VIP are widely distributed in the peripheral nervous system in primary afferent and autonomic efferent neurons innervating various peripheral organs such as the pancreas, gastrointestinal tract, the eye, and the urogenital tract (Arimura and Shioda 1995; Fahrenkrug and Hannibal 1998a, b; Reubi 2000; Laburthe et al. 2002; Hernández et al. 2006a, b). The peptides are also contained in neurons in the central nervous system. This paper will review the expression and functions of VIP and PACAP in neural pathways controlling the lower urinary tract.
Anatomy and Innervation of the Lower Urinary Tract
The storage and periodic elimination of urine are dependent upon the activity of two functional units in the lower urinary tract: (1) a reservoir (the urinary bladder) and (2) an outlet consisting of the urethra and striated muscles of the external urethral sphincter (EUS, Fig. 1). The urinary bladder is divided into two parts (body and base), each composed of several layers: serosal, muscle, lamina propria, and urothelium.
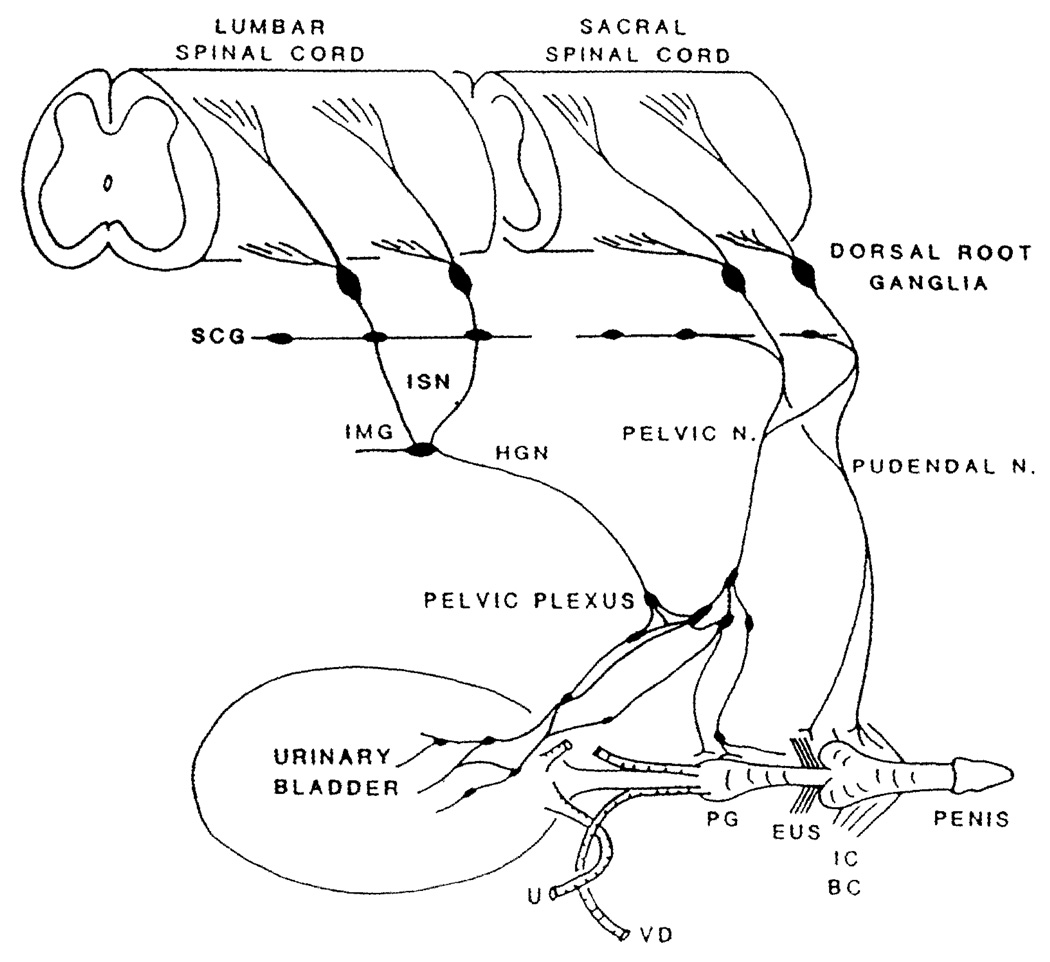
Fig. 1.
Diagram showing the sympathetic, parasympathetic, and somatic innervation of the urogenital tract of the male cat. Sympathetic preganglionic pathways emerge from the lumbar spinal cord and pass to the sympathetic chain ganglia (SCG) and then via the inferior splanchnic nerves (ISN) to the inferior mesenteric ganglia (IMG). Preganglionic and postganglionic sympathetic axons then travel in the hypogastric nerve (HGN) to the pelvic plexus and the urogenital organs. Parasympathetic preganglionic axons which originate in the sacral spinal cord pass in the pelvic nerve to ganglion cells in the pelvic plexus and to distal ganglia in the organs. Sacral somatic pathways are contained in the pudendal nerve, which provides an innervation to the penis, the ischiocavernosus (IC), bulbocavernosus (BC), and external urethral sphincter (EUS) muscles. The pudendal and pelvic nerves also receive postganglionic axons from the caudal sympathetic chain ganglia. These three sets of nerves contain afferent axons from the lumbosacral dorsal root ganglia. U ureter, PG prostate gland, VD vas deferens
The lower urinary tract is regulated by three sets of peripheral nerves: sacral parasympathetic (pelvic nerves), thoracolumbar sympathetic (hypogastric nerves and sympathetic chain), and somatic nerves (pudendal nerves) distributed bilaterally (Fig. 1). These nerves consist of efferent and afferent axons originating at thoracolumbar and sacral spinal levels (de Groat 1986; Jänig and Morrison 1986; Häbler et al.1990). Sacral parasympathetic efferent nerves provide an excitatory cholinergic input to the bladder smooth muscle and a nitric-oxide-mediated inhibitory input to the urethral smooth muscle. Lumbar sympathetic nerves provide a noradrenergic inhibitory input to the bladder and excitatory input to the urethra. Somatic cholinergic nerves excite the EUS.
Afferent axons in the pelvic and pudendal nerves arise from neurons located in the dorsal root ganglia (DRG) at caudal lumbosacral levels (L6–S3), whereas those in the hypogastric nerves originate in the caudal thoracic-rostral lumbar (T11-L2) segmental levels (de Groat 1986; Jänig and Morrison 1986; Yoshimura and de Groat 1997a; Fig. 1). The bladder is innervated by two types of afferent axons (small myelinated Aδ and unmyelinated C-fiber axons) that are distributed throughout the bladder wall (Uemura et al. 1973; Gabella and Davis 1998) from the serosal layer to the urothelium. The majority of the afferents (70%) innervating the rat bladder are of the C-fiber type (Nadelhaft and Vera 1991; Yoshimura et al. 2003). The density of afferent innervation is greater in the bladder base than in the bladder body.
Distribution of VIP and PACAP Containing Neurons and Nerves in the Lower Urinary Tract
VIP and PACAP immunoreactivity (IR) has been identified at various sites in the neural pathways to the lower urinary tract in many species. Although there are some differences between species, peptide-IR has been detected in: (1) axons and varicosities in the bladder wall (Gu et al. 1984; Mattiasson et al. 1985b; Lasanen et al. 1992), (2) neurons and axons in bladder autonomic ganglia (Kawatani et al. 1985b), (3) lumbosacral dorsal root ganglion cells (Kawatani et al. 1985c, 1986, 1990; de Groat 1986; Keast and de Groat 1992; Vizzard 2000a; Zvarova et al. 2005), and (4) primary afferent projections to the dorsal horn and the region of the parasympathetic nucleus in the lumbosacral spinal cord (Fig. 2; Kawatani et al. 1985c; Zvarova et al. 2005)
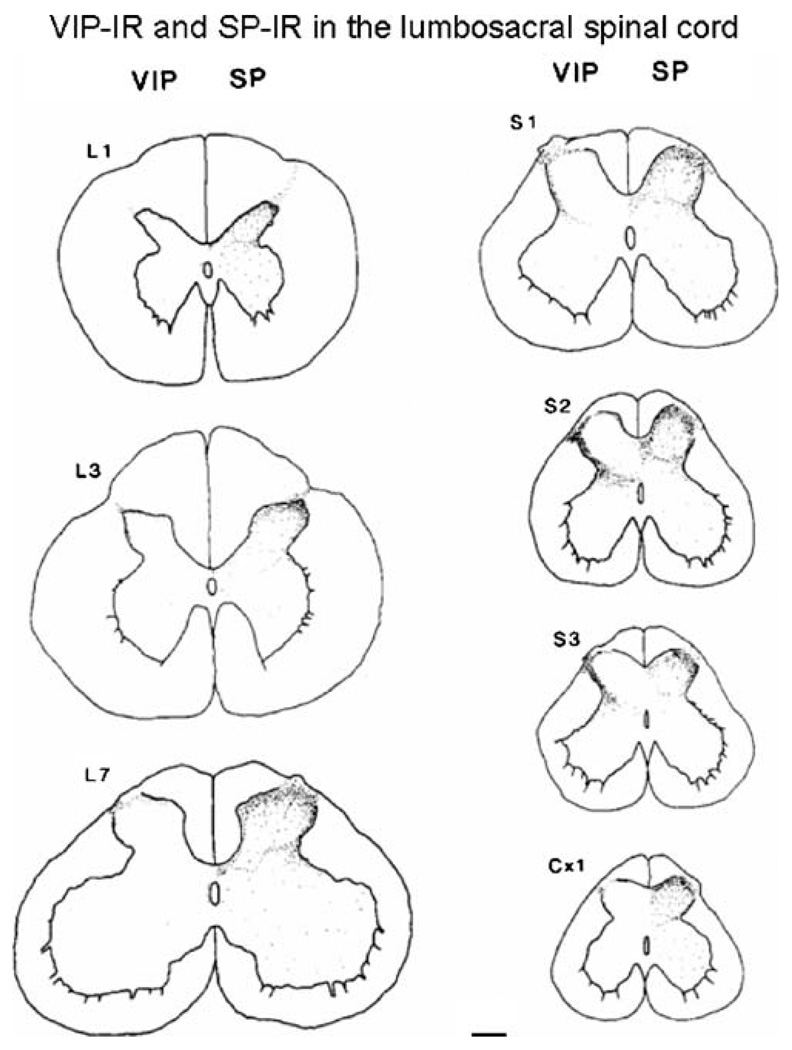
Fig. 2.
Camera lucida drawings of VIP- and substance P (SP)-IR at various levels of the lumbosacral and coccygeal spinal cord. Note that VIP-IR is present at all levels in Lissauer’s tract and lamina I of the dorsal horn, but was most prominent in the sacral segments on the lateral side of the dorsal horn. SP-IR is distributed throughout laminae I–III at all segmental levels. Calibration bar=200 µm. Reproduced with permission from Kawatani et al. (1985a)
In the bladder, VIP/PACAP-IR nerves have been detected in all regions and layers of the bladder, but are most dense in the bladder base and in a sub-epithelial plexus (Gu et al. 1984; Mattiasson et al. 1985b; Crowe et al. 1996; Fahrenkrug and Hannibal 1998a, b; Mohammed et al. 2002; Zvarova et al. 2005). PACAP-38-IR is more prominent than PACAP-27-IR. VIP/PACAP-IR nerves have also been identified in the smooth and striated muscle regions of the urethra in several species (Crowe et al. 1986b; Milner et al. 1987; Persson et al. 1995; Fahrenkrug and Hannibal 1998a; Radziszewski et al. 1996; Werkström et al.1998; Busacchi et al. 2004; Sienkiewicz et al. 2004). In infants and young children, VIP is present in 40% of the intramural ganglion cells in the bladder neck (Dixon et al. 1997) where it is co-localized with tyrosine hydroxylase in 40% of the cells (Jen et al. 1996) and with neuropeptide Y in 90% of the cells (Dixon et al. 1997). The intramural ganglion cells in the human bladder are surrounded by VIP-IR varicosities. In the pig, VIP-IR is co-localized with nitric oxide synthase (NOS)-IR in afferent and efferent nerves in the bladder base and urethra (Persson et al. 1995).
In bladder ganglia of the cat, VIP-IR is present in 10–13% of the ganglion cells where it is co-localized with acetylcholinesterase, indicating that it is present in cholinergic neurons (Kawatani et al. 1985b). The cat bladder ganglion cells are also surrounded by VIP-IR pericellular varicosities (Alm et al. 1980; Kawatani et al. 1985b). These varicosities are not eliminated after transecting the pelvic and hypogastric nerves, indicating that they originate from neurons in the peripheral nervous system (Kawatani et al. 1985b). VIP-IR is also expressed in a subpopulation of intramural bladder ganglion cells in the guinea pig (Crowe et al. 1986a; Werkström et al.1998) and in bladder ganglion cells in the major pelvic ganglia in the rat (Mattiasson et al. 1985b; Keast and de Groat 1989; Keast 2006) and mouse (Wanigasekara et al. 2003) where it is co-localized with choline acetyltransferase or NOS-IR (Zhou and Ling 1998, 1999).
The afferent innervation to the lower urinary tract exhibits prominent VIP/PACAP-IR. A large percentage (42%) of pelvic afferent neurons in the cat exhibit VIP-IR, whereas only 10% of pudendal afferent neurons exhibit VIP-IR, indicating that VIP expression is greater in visceral than in somatic afferent pathways (Kawatani et al. 1986; de Groat 1986, 1989). A similar conclusion emerged from an analysis of the segmental distribution of VIP-IR in the spinal cord of the cat (Basbaum and Glazer 1983; Kawatani et al. 1983, 1986; Anand et al. 1983; Honda et al. 1983), monkey (Gibson et al. 1984), and human (Anand et al. 1983). These studies revealed that the sacral spinal cord has a markedly higher density of VIP-IR afferent nerves than other levels of the cord. This is consistent with the large population of VIP-IR afferent neurons that innervate the pelvic visceral organs. For example, in the cat, 30% and 70% of the bladder and uterine cervix afferent neurons, respectively, exhibit VIP-IR (de Groat 1989; Kawatani et al. 1990). In the cat, VIP is expressed in small-diameter sacral DRG cells and in the lumbosacral spinal cord (Fig. 2) is contained exclusively in unmyelinated axons (Morgan et al. 1999). Thus, VIP-IR is considered a marker for unmyelinated afferent nerves. In the spinal cord, the VIP-IR afferent axons have a distribution similar to that of bladder afferent projections labeled by anterograde tracing techniques (Morgan et al. 1981). In the rat, VIP-IR is present in a smaller number of bladder afferent neurons (Keast and de Groat 1989), and VIP-IR in the rat bladder is not reduced by treatment with capsaicin, the C-fiber afferent neurotoxin, indicating that it is present either in efferent nerves or in capsaicin-resistant afferent nerves (Fahrenkrug and Hannibal 1998a; Avelino and Cruz 2000).
PACAP-IR in the rat lower urinary tract is mainly located in afferent nerves (Vizzard 2000a; Zvarova et al. 2005). It is present in approximately 40% of small-diameter bladder dorsal root ganglion cells (Moller et al. 1993; Zvarova et al. 2005; Papka et al. 2006) and is co-localized with immunochemical staining for the capsaicin receptor (i.e., the transient receptor potential vanilloid receptor 1, TRPV1; Zvarova et al. 2005) or calcitonin-gene-regulated peptide (CGRP; Fahrenkrug and Hannibal 1998a, b) in axons in the bladder and with CGRP-IR in the urethra (Fahrenkrug and Hannibal 1998a). Treatment of neonatal rats with capsaicin caused a marked reduction in PACAP-IR in all regions of the urinary tract (Fahrenkrug and Hannibal 1998a), indicating that the peptide is contained in capsaicin-sensitive C-fiber afferent nerves. PACAP-IR nerves are in low density in the muscle layers of the rat bladder but are more prominent in the sub-epithelial plexus (Fahrenkrug and Hannibal 1998a; Mohammed et al. 2002). PACAP-IR in the cat bladder has not been reported. Thus, in the cat, more attention has been focused on the role of VIP as a possible transmitter in bladder afferents, whereas in the rat, PACAP-27 or PACAP-38 have attracted the most attention as putative afferent transmitters.
Distribution of PACAP Receptors
In the rat bladder, PAC1 receptors are present in the smooth muscle and urothelium, whereas VPAC2 receptors are present only in the smooth muscle (Braas et al. 2006). VPAC1 receptors are present in the epithelium of the mouse urethra (Harmar et al. 2004). PAC1 and VPAC2 receptors are expressed in rat DRG and in the dorsal horn of the spinal cord (Braas et al. 2006). Alternative splicing of the PAC1 receptor gives rise to two splice variants (HIP and HOP). The HOP splice variant has been detected in bladder smooth muscle, urothelium, the sacral DRG, and the sacral spinal cord (Braas et al. 2006).
Effect of VIP/PACAP on Smooth Muscle in the Lower Urinary Tract
The effects of VIP and PACAP on lower urinary tract smooth muscle vary in different species. In some species (cat, rabbit, pig, human, dog), VIP reduces basal tension as well as neurally and agonist evoked contractions in the bladder body (Larsen et al. 1981; Klarskov et al. 1984, 1987; Kawatani et al. 1985b; Sjögren et al.1985; Callahan and Creed 1986; Hosokawa and Kaseda 1993; Ückert et al.2002), bladder base (Hills et al. 1984; Klarskov et al. 1984, 1987; Mattiasson et al. 1990; Hosokawa and Kaseda 1993), and urethra (Andersson et al. 1983; Mattiasson et al. 1985a; Blank et al. 1986; Hashimoto et al. 1992, 1993; Hosokawa and Kaseda 1993; Ohnishi et al. 1997; Werkström et al.1997). On the other hand, in rat and guinea pig, VIP had no effect or a weak contractile effect on the detrusor (Andersson et al. 1988, 1992; Igawa et al. 1993; Saito et al. 1993; Braas et al. 2006) and a relaxant effect on the urethral smooth muscle (Igawa et al. 1993; Werkström et al.1998). In the rat, PACAP-27 had weak or no relaxant effects on isolated bladder or urethral smooth muscle preparations in some studies (Ishizuka et al. 1995), but in other studies (Braas et al. 2006), PACAP-27 or PACAP-38 increased detrusor smooth muscle tone and enhanced the amplitude of nerve-evoked contractions. In the isolated mouse bladder, PACAP-27 and PACAP-38 increased basal tone, enhanced spontaneous contractions, and enhanced neurally evoked contractions (Herrera et al. 2006).
In the pig, PACAP-38 and VIP relax the bladder neck by activating muscle VPAC2 receptors linked to the cyclic adenosine monophosphate-protein kinase A (cAMP-PKA) pathway and involve activation of voltage-gated K channels. Two additional mechanisms that contribute to the smooth muscle relaxation include: (1) activation of facilitatory PAC1 receptors located on capsaicin-sensitive afferent terminals which induces the release of nitric oxide, an inhibitory agent that relaxes smooth muscle and (2) activation of presynaptic inhibitory VPAC receptors at autonomic nerve terminals (Hernández et al. 2006a, b). PACAP-27 or PACAP-38 also relax urethral smooth muscle in the pig (Werkström et al. 1997).
Effects of VIP/PACAP on Autonomic Ganglia
The presence of VIP-IR in ganglion cells in the bladder wall and in the pelvic plexus or in pericellular varicosities around ganglion neurons in the bladder stimulated interest in the effects of VIP on ganglionic transmission. In vivo experiments revealed that intra-arterial administration of VIP to bladder ganglia of the cat enhanced muscarinic ganglionic transmission and postganglionic firing induced by a muscarinic agonist (Kawatani et al. 1985b). VIP also suppressed muscarinic inhibition in bladder ganglia, but did not alter nicotinic transmission or the inhibitory effects of norepinephrine, leucine enkephalin, or gamma-aminobutyric acid (GABA). The facilitatory effects of VIP persisted in chronically decentralized ganglia and were not blocked by nicotinic receptor antagonists, indicating that they were due to a direct effect on the ganglion cells. The studies raised the possibility that intra-ganglionic pathways containing VIP may exert a selective modulatory influence on muscarinic transmission in bladder ganglia (Kawatani et al. 1985b).
Subsequent in vitro experiments revealed that VIP elicited a depolarization of neurons in cat sympathetic ganglia (Kawatani et al. 1985a) and cat bladder ganglia (Akasu et al. 1986) as well as in hamster submandibular parasympathetic ganglia (Suzuki 1992). In bladder and submandibular ganglia, the depolarizing effect of VIP was associated with the generation of spontaneous action potentials in quiescent neurons, was resistant to tetrodotoxin, and was mediated by a block of a novel type of voltage-insensitive K channel.
PACAP-27 also elicited a depolarization and increased firing in guinea pig cardiac and hamster submandibular parasympathetic ganglia (Braas et al. 1998; Suzuki et al. 2003; Tomkins et al. 2007). The effects in cardiac ganglia were mimicked by electrical stimulation of preganglionic nerves and were reduced by PACAP6–38, a PAC1 receptor antagonist. These findings raised the possibility that endogenous PACAP-27 is a modulator of transmission in cardiac ganglia (Tomkins et al. 2007).
Effect of VIP/PACAP on Reflex Pathways in the Spinal Cord
The projections of VIP-IR and PACAP-IR afferent axons to the regions of the lumbosacral spinal cord containing interneurons and preganglionic neurons involved in the control of the lower urinary tract (de Groat et al. 1993) raised the possibility that these peptides might be transmitters in spinal pathways controlling micturition. Intrathecal administration of VIP at the sacral level of the spinal cord in the cat in doses ranging from 1 to 10 µg suppressed reflex bladder contractions (Fig. 3) and reflex firing on bladder postganglionic nerves. The effects appeared 2–5 min after injection and persisted for 5–30 min (de Groat et al. 1990). On the other hand, in rats, intrathecal administration of 10 µg ofVIP during continuous infusion cystometry elicited excitatory effects that were evident as a decrease in bladder capacity and micturition volume and an increase in spontaneous bladder contractions (Igawa et al. 1993). However, in other studies (Tiseo and Yaksh 1990), the intrathecal injection of a VIP antagonist ([4-Cl-D-Phe6,Leu17]VIP) dose-dependently produced a similar increase in spontaneous bladder contractions and a decrease in the bladder volume to evoke micturition.
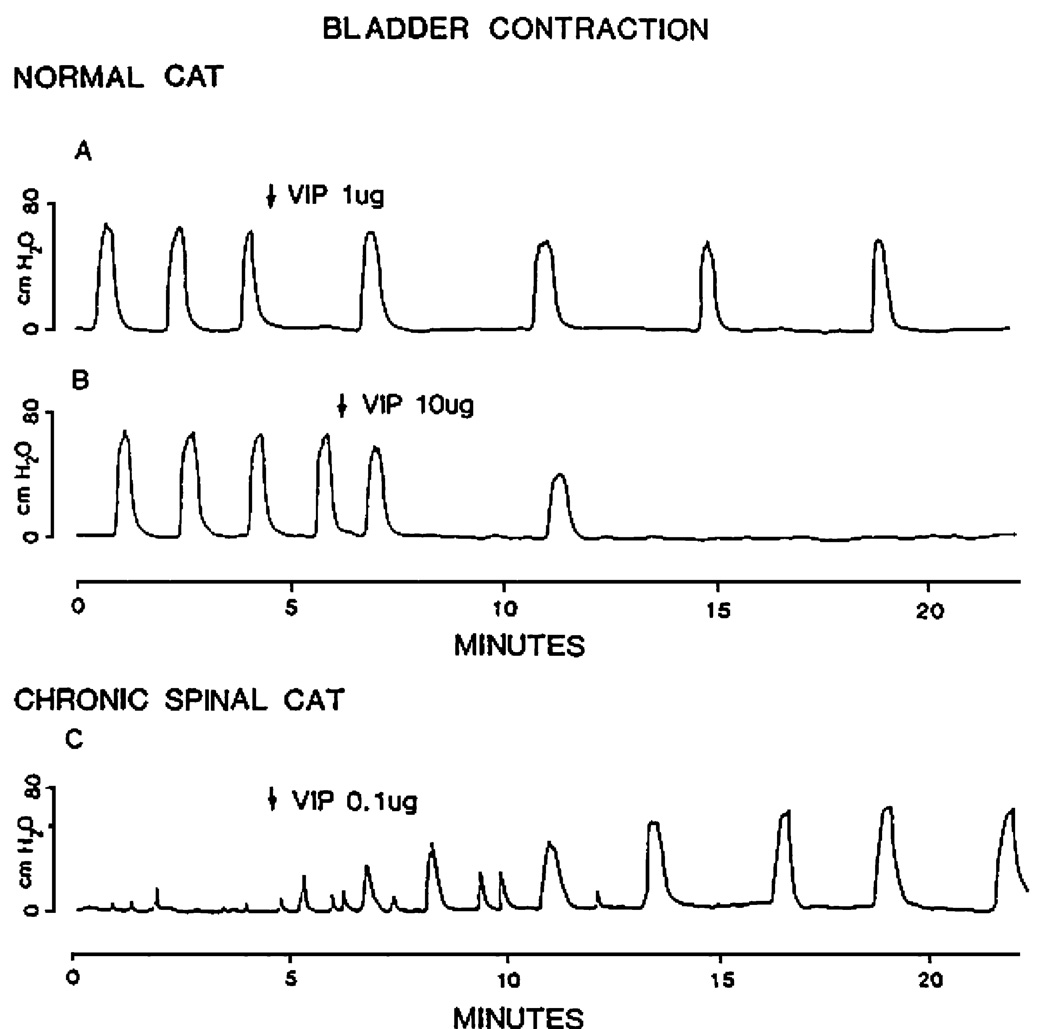
Fig. 3.
Effects of intrathecal administration of VIP on reflex bladder contractions recorded under isovolumetric conditions in chloralose-anesthetized cats. A, B In a cat with an intact spinal cord, large doses of VIP (1 and 10 µg) inhibited bladder activity. C In a chronic spinal cord transected cat, a small dose of VIP (0.1 µg) facilitated bladder activity
Intrathecal administration of PACAP-27 dose-dependently (0.1–1 nmol/rat), in normal unanesthetized rats, decreased bladder capacity, decreased micturition volume, and increased micturition pressure. The effects appeared within 10 min, disappeared after 40 min, and were repeatable in the same rat (Ishizuka et al. 1995).
Intrathecal administration of PACAP-38 was tested on reflex bladder activity in unanesthetized decerebrate rats under isovolumetric conditions and during continuous infusion cystometry (Fig. 4; Yoshiyama and de Groat 2008). Under isovolumetric conditions, 30 µg of PACAP-38, but not lower doses (0.1–10 µg), significantly increased the amplitude of bladder contractions but reduced the frequency of the contractions. Because PACAP can also stimulate thoraco-lumbar sympathetic preganglionic neurons which could potentially inhibit bladder activity, PACAP was also tested after bilateral transection of the hypogastric nerves which contain the sympathetic inhibitory pathway to the bladder. Hypogastric nerve transection unmasked a bladder facilitatory effect of a lower dose (10 µg) of PACAP but did not significantly change the response to the higher dose.
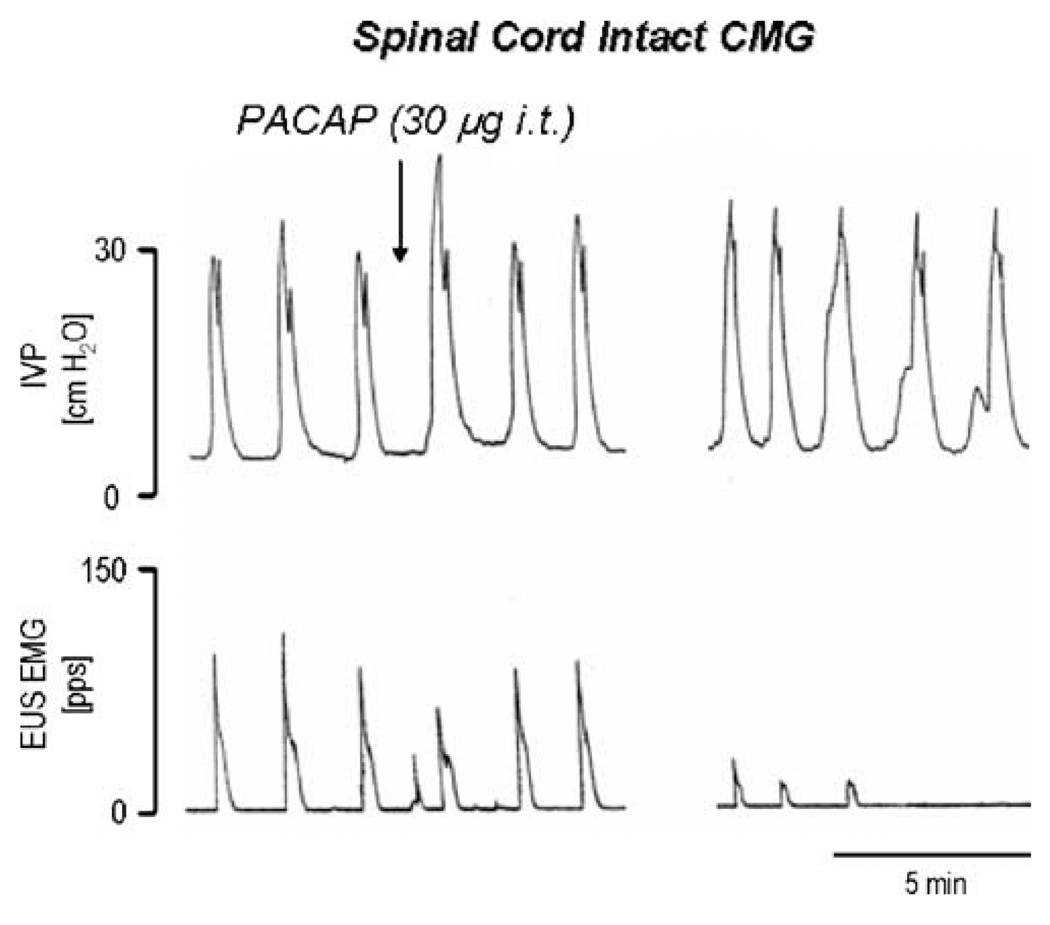
Fig. 4.
The effects of intrathecal administration of PACAP-38 (30 µg i.t.) on reflex bladder activity (top trace) and EUS EMG activity (bottom trace) during continuous filling (0.21 ml/min) CMGs in a spinal cord intact decerebrate unanesthetized female rat with intact hypogastric nerves. Recordings on the left and right sides of the figure are separated by a time gap of 6 min. Each bladder contraction represents voiding. Note that PACAP-38 (injected at the arrow) produced a transient increase in peak micturition pressure and then produced a more prolonged 20–25% increase in pressure which was accompanied by a marked decrease in the EUS EMG activity. Vertical calibrations are intravesical pressure (IVP) in cm H2O and EUS EMG activity in pulses per second. EMG activity was recorded with a pulse height discriminator connected to a ratemeter. Horizontal calibration represents 5 min
During continuous infusion cystometrograms (CMG), PACAP was tested on bladder and external urethral sphincter (EUS) electromyographic (EMG) activity in animals with intact or sectioned hypogastric nerves (Yoshiyama and de Groat 2008). PACAP (10–30 µg) increased the amplitude of bladder contractions (18–43%) but reduced EUS EMG activity (68–100%; Fig. 4). It was concluded that PACAP facilitated micturition by enhancing the parasympathetic excitatory pathway to the bladder and inhibiting the somatic excitatory pathway to the EUS (Fig. 5). The excitatory effects on micturition appear to be countered somewhat by simultaneous stimulation of the sympathetic inhibitory pathway to the bladder.
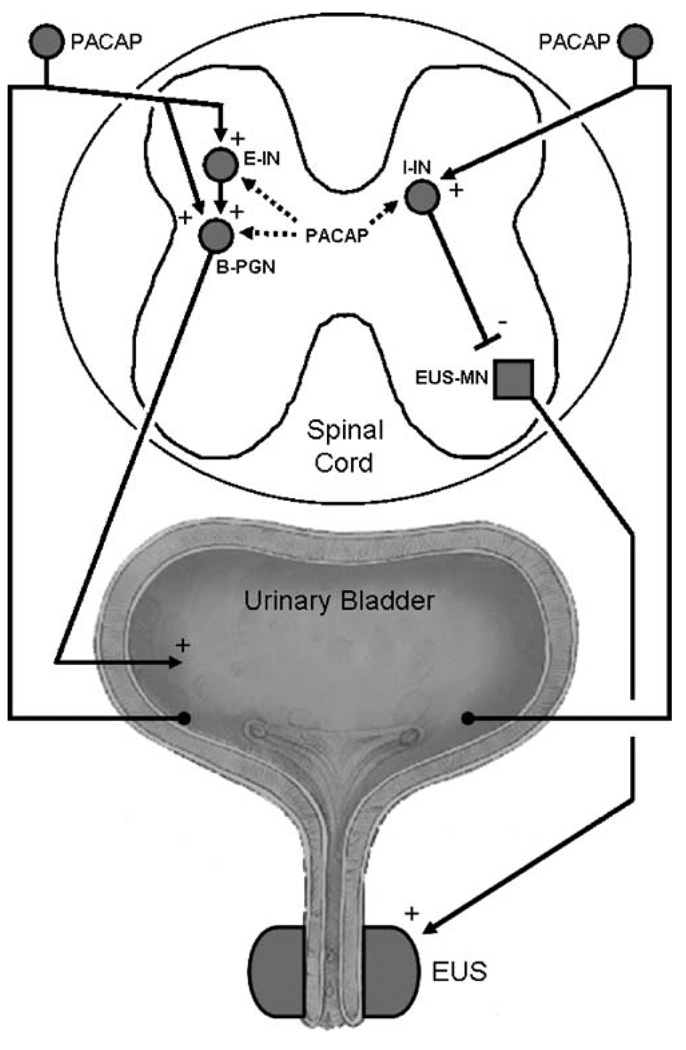
Fig. 5.
Diagram showing the possible sites in the spinal cord where PACAP acts to modulate reflex bladder and external urethral sphincter (EUS) activity in the chronic spinal cord injured rat. Left side shows the micturition reflex pathway consisting of afferent limb passing from the bladder through the dorsal roots, making excitatory synaptic connections with an excitatory interneuron (E-IN) or with a bladder preganglionic neuron (B-PGN). PACAP may function as an excitatory transmitter in bladder afferent neurons. The right side shows a putative inhibitory pathway that controls the activity of the external urethral sphincter (EUS). This pathway consists of an afferent limb arising in the bladder which synapses on an inhibitory interneuron (I-IN) which in turn makes inhibitory synaptic connections with an external sphincter motoneuron (EUS-MN) that provides the excitatory input to the EUS. The I-IN is thought to be located in the dorsal commissure region of the spinal cord. PACAP administered intrathecally could excite the bladder by activating E-INs or B-PGNs and could inhibit the EUS by activating I-INs. Excitatory pathways from the bladder to the EUS which mediate detrusor-sphincter dyssynergia and which are also prominent after spinal cord injury are not shown in the diagram. Plus and minus signs indicate excitatory and inhibitory synapses, respectively
The site and mechanism of action of PACAP on spinal micturition reflex pathways was explored using patch clamp recording in lumbosacral parasympathetic preganglionic neurons in spinal slice preparations from neonatal rats (Miura et al. 2001). The experiments revealed that PACAP-38 had direct excitatory effects on the preganglionic neurons and enhanced excitatory input to the neurons, suggesting that it might act at several sites in the spinal micturition reflex pathway (Fig. 5). In parasympathetic preganglionic neurons, PACAP-38 decreased the electrical threshold for triggering action potentials, increased the number of action potentials induced by depolarizing current pulses, increased input resistance, and suppressed a 4-aminopyridine-sensitive outward current. PACAP-38 also induced spontaneous firing and increased the frequency of spontaneous excitatory postsynaptic potentials in the presence of tetrodotoxin. Because excitatory synaptic inputs are mediated primarily by alpha-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid and N-methyl-d-aspartic acid (NMDA) glutamatergic synapses (Araki and de Groat 1996), it is proposed that PACAP-38 facilitates glutamatergic excitatory synaptic input to the parasympathetic neurons in addition to directly enhancing the excitability of the neurons by blocking K channels. PACAP-38 could act presynaptically to enhance the firing of excitatory interneurons, enhance glutamate release from interneuronal terminals, or act postsynaptically directly on parasympathetic neurons (Fig. 5) to enhance glutamatergic currents as noted in cortical neurons (Liu and Madsen 1997) where PACAP enhances NMDA-induced currents.
It is interesting that the VIP has an excitatory effect on peripheral bladder ganglion cells by suppressing K currents (Akasu et al. 1986), and PACAP also has an excitatory effect on spinal preganglionic neurons by suppressing K currents (Miura et al. 2001), although it is likely that different types of K channels are involved in the actions at the two sites. However, the facilitatory effects of PACAP on parasympathetic preganglionic neurons and cardiac ganglion neurons (Tomkins et al. 2007) to induce repetitive firing during depolarizing current pulses may be mediated by the same mechanism, i.e., by blockade of A-type K channels.
The effect of PACAP to block reflex activity of the EUS is most reasonably attributed to an action on interneuronal mechanisms (Fig. 5) because PACAP-IR axons do not project directly into the EUS motor nucleus in the lumbosacral spinal cord. This inhibitory effect could be due to stimulation of interneurons located in the region of the dorsal commissure which provide an inhibitory input to EUS motoneurons (Fig. 5; Blok et al. 1998; de Groat et al. 2001; Sie et al. 2001). Immunohistochemical studies have revealed that this region exhibits low levels of PACAP-IR in rats (Zvarova et al. 2005) and VIP-IR in cats (Kawatani et al. 1985c) (Fig. 2). Thus, endogenous PACAP could suppress EUS EMG activity by activating inhibitory interneurons which in turn release GABA or glycine at inhibitory synapses on the EUS motoneurons (Sie et al. 2001).
Role of VIP/PACAP Pathways in Lower Urinary Tract Dysfunction after Spinal Cord Injury
Micturition is mediated by activation of the sacral parasympathetic efferent pathway to the bladder and the urethra as well as reciprocal inhibition of the somatic pathway to the urethral sphincter. Studies in animals using brain lesioning techniques revealed that neurons in the brainstem at the level of the inferior colliculus (i.e., the pontine micturition center) have an essential role in the regulation of the bladder and sphincter (Barrington 1925; Kuru 1965; de Groat and Ryall 1969; Mallory et al. 1991; de Groat et al. 1993). Bilateral lesions in the rostral pons in the region of the pontine micturition center or transections of the neuraxis at any point below the colliculi abolish micturition (Barrington 1925), whereas electrical or chemical stimulation in the pontine micturition center triggers bladder contractions, sphincter relaxation, and micturition (Kuru 1965; Sugaya et al. 1987; Kruse et al. 1990; Mallory et al. 1991; Noto et al. 1991). These observations led to the concept of a spinobulbospinal micturition reflex pathway that passes through the pontine micturition center which functions as “on–off” switch (Fig. 6; de Groat 1975) that is activated by a critical level of Aδ afferent activity arising from tension receptors in the bladder and is in turn modulated by inhibitory and excitatory influences from areas of the brain rostral to the pons (Torrens and Morrison 1987; de Groat et al. 1993). Spinal cord injury (SCI) rostral to the lumbosacral level eliminates voluntary and supraspinal control of voiding, leading initially to an areflexic bladder and complete urinary retention followed by a slow development of automatic micturition and bladder hyperactivity (de Groat and Yoshimura 2006). However, voiding after SCI is commonly inefficient due to simultaneous contractions of the bladder and urethral sphincter (i.e., detrusor-sphincter dyssynergia, DSD).
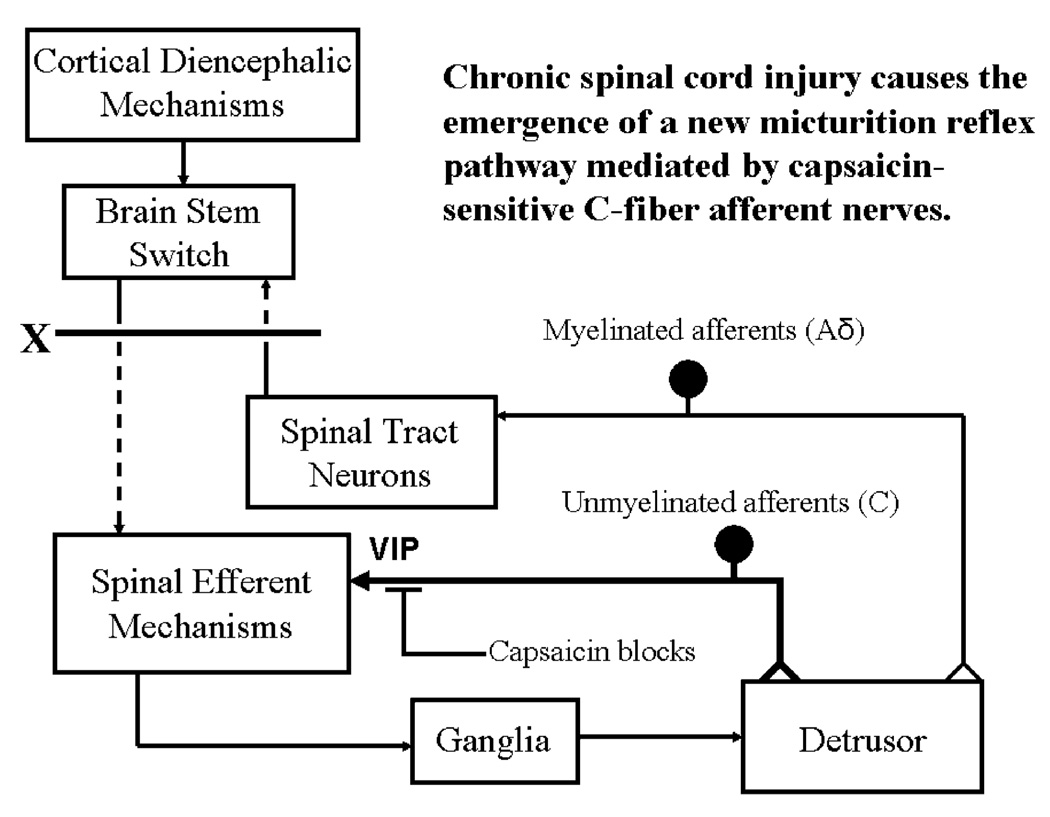
Fig. 6.
Diagram showing the organization of the parasympathetic excitatory reflex pathway to the detrusor muscle. Scheme is based on electrophysiologic studies in cats. In animals with an intact spinal cord, micturition is initiated by a supraspinal reflex pathway passing through a center in the brain stem. The pathway is triggered by myelinated afferents (Aδ fibers) which are connected to the tension receptors in the bladder wall. Injury to the spinal cord above the sacral segments (X) interrupts the connections between the brain and spinal autonomic centers and initially blocks micturition. However, over a period of several weeks after cord injury, a spinal reflex mechanism emerges, which is triggered by unmyelinated vesical afferents (C-fibers); the A-fiber afferent inputs are ineffective. The C-fiber reflex pathway is usually weak or undetectable in animals with an intact nervous system. Capsaicin (20–30 mg, subcutaneously) blocks the C-fiber reflex in chronic spinal cats, but does not block micturition reflexes in intact cats. Intravesical capsaicin also suppresses detrusor hyperreflexia in patients with neurogenic bladder dysfunction
Electrophysiological studies in cats have shown that the recovery of bladder function after SCI is mediated by a change in the afferent limb of the micturition reflex pathway (Fig. 6) and remodeling of synaptic connections in the spinal cord. In chronic spinal cats, unmyelinated C-fiber afferents rather than Aδ afferents initiate voiding, and the spinal micturition reflex occurs with a short central delay (15 ms) in contrast to the long central delay (60 ms) of the reflex in cats with an intact spinal cord (de Groat and Ryall 1969; de Groat et al. 1981). This conclusion is supported by the finding that in chronic spinal cats, subcutaneous administration of capsaicin, a C-fiber neurotoxin, completely blocked reflex bladder contractions induced by bladder distention (Fig. 6), whereas capsaicin had no inhibitory effects on reflex bladder contractions in spinal intact cats (de Groat et al. 1990; Cheng et al. 1999). Thus, it is plausible that C-fiber bladder afferents which usually do not respond to bladder distention (i.e., silent C-fibers; Häbler et al.1990) become mechano-sensitive and initiate automatic micturition after spinal cord injury.
The effect of VIP which is contained in bladder C-fiber afferents in the cat (Morgan et al. 1999) is also changed after SCI. Intrathecal administration of VIP, which suppressed reflex bladder activity in cats with an intact spinal cord (Fig. 3A and B), enhanced or unmasked reflex bladder activity in chronic SCI cats (Fig. 3C). In addition, C-fiber afferent projections to the sacral spinal cord exhibiting VIP-IR expanded and reorganized after SCI (Thor et al. 1986). This was evident as: (1) a wider distribution of VIP-IR axons in lateral lamina I of the dorsal horn forming an almost continuous band of axons in the rostrocaudal direction in comparison to a discontinuous distribution in normal cats, (2) the appearance of rostrocaudal axons in this region where they were not normally present, (3) more extensive contralateral projections to lamina I, and (4) a more extensive ipsilateral projection to lateral lamina VII which contains bladder preganglionic neurons. These observations raise the possibility that C-fiber afferents sprout and contribute to the synaptic remodeling in the spinal micturition reflex pathway that occurs after SCI.
Although changes in VIP-IR in the human spinal cord have not been examined after SCI, changes in peripheral VIP-IR nerves in the urinary tract have been reported. In patients with lower motor neuron lesions which damaged the sacral spinal cord or the cauda equina, a decrease in the density of VIP-IR nerves was detected in the bladder muscle (Crowe et al. 1991; Drake et al. 2000), suggesting that urinary retention, bladder areflexia, and deficient sensation may be directly linked to neuropeptide neuropathy. On the other hand, in patients with lower motor neuron lesions, there was a marked increase in the density of VIP-IR nerves in the striated muscle of the external urethral sphincter (Milner et al. 1987). In patients with cord injuries at the cervical or thoracic levels, VIP-IR in the urinary tract was not changed (Crowe et al. 1988).
Plasticity in C-fiber bladder afferents has also been detected in the rat after SCI. The changes include (1) somal hypertrophy of bladder afferent neurons (45% to 50% increase in cross-sectional area) in the L6-S1 DRG (Kruse et al. 1995; Yoshimura and de Groat 1997b; Yoshimura et al. 1998), (2) increased expression of PACAP-IR in bladder DRG neurons and expansion of PACAP-IR afferent axons in the lumbosacral spinal cord (Zvarova et al. 2005), and (3) increased excitability of dissociated L6–S1 bladder afferent neurons due to changes in the properties of Na and K channels (Yoshimura and de Groat 1997b; de Groat and Yoshimura 2005).
Detailed studies of Zvarova et al. (2005) revealed that 6 weeks after spinal cord injury in the rat, PACAP-IR dramatically increased in spinal segments and DRG (L1, L2, L6, S1) involved in micturition reflexes, but no changes occurred in adjacent spinal segments (L4–L5). The density of PACAP-IR was increased in the superficial laminae (I–II) of the L1, L2, L6, and S1 spinal segments and in a fiber bundle extending ventrally from Lissauer’s tract in lamina I along the lateral edge of the dorsal horn to the sacral parasympathetic nucleus in the L6–S1 spinal segments. This is the same region in which VIP-IR increased in the cat after SCI (Thor et al. 1986). After SCI, PACAP-IR in cells in the L1, L2, L6, and S1 DRG significantly increased, and the percentage of bladder afferent cells expressing PACAP-IR also significantly increased. No changes were observed in the L4–L5 DRG. PACAP-IR was reduced throughout the urothelium and detrusor smooth muscle after SCI (Zvarova et al. 2005). These studies demonstrate changes in PACAP expression in micturition reflex pathways after SCI that may contribute to urinary bladder dysfunction or reemergence of primitive voiding reflexes after SCI.
Nerve growth factor (NGF) has been implicated as a chemical mediator of pathology-induced changes in C-fiber afferent nerve excitability and reflex bladder activity (Yoshimura 1999; Vizzard 2000b). The levels of neurotrophic factors including NGF increase in the bladder after SCI (Vizzard 2000b, 2006), and increased levels of NGF have been detected in the lumbosacral spinal cord and DRG of rats after SCI (Seki et al. 2002). It is known that NGF upregulates PACAP expression in DRG neurons (Jongsma Wallin et al. 2003), and it has been demonstrated that chronic administration of NGF into the spinal cord of rats induces bladder hyperactivity and increases in the firing frequency of dissociated bladder afferent neurons (Yoshimura et al. 2006). NGF seems to contribute to the lower urinary tract dysfunction after SCI because intrathecal application of NGF antibodies, which neutralized NGF in the spinal cord, suppressed detrusor hyperreflexia and DSD in SCI rats (Seki et al. 2002; Seki et al., 2004). Intrathecal administration of NGF antibodies also blocked autonomic dysreflexia induced by bladder distension in SCI rats (Krenz et al. 1999).
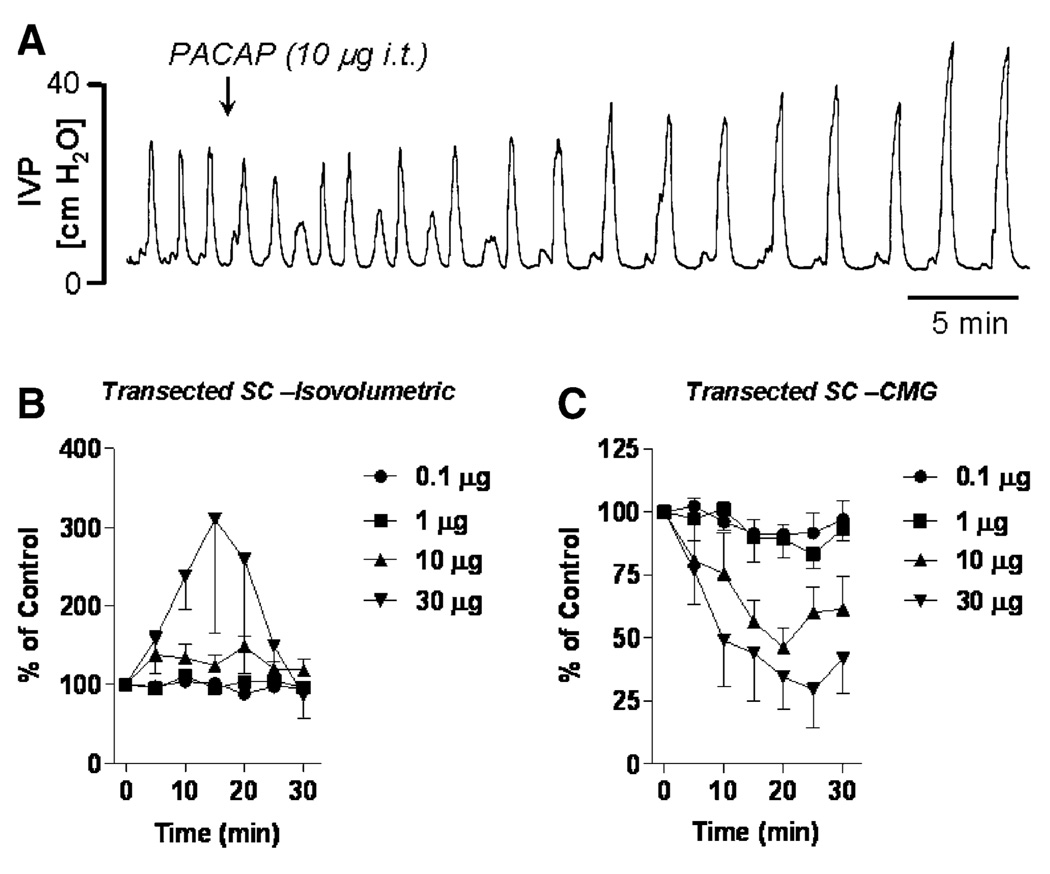
The increased expression of PACAP in bladder afferent pathways after SCI prompted an investigation of the effects of PACAP in SCI rats (Yoshiyama and de Groat 2008). In SCI rats, the effects of PACAP-38 were different under different experimental conditions (Fig. 7). When recording isovolumetric bladder contractions, PACAP-38 (10 and 30 µg intrathecally) produced larger amplitude and longer duration bladder contractions (Fig. 7A and B). These excitatory effects were enhanced after transection of the hypogastric nerves (HGNs), indicating that PACAP may also activate the sympathetic inhibitory pathway as well as the parasympathetic excitatory pathway to the bladder.
Fig. 7.
Effect of intrathecal administration of PACAP-38 on reflex bladder activity recorded under isovolumetric conditions (A, B) or during continuous infusion cystometrograms (C) in chronic spinal cord transected, decerebrate, unanesthetized rats. A PACAP 10 µg intrathecally enhances the amplitude of isovolumetric bladder contractions but reduces the frequency of the contractions. B Dose–response curves showing the time-dependent effect of PACAP on the amplitude of bladder contractions recorded under isovolumetric conditions. Graph represents the average of five to seven experiments. C Dose–response curves showing the time-dependent effect of PACAP on the amplitude of micturition contractions during continuous filling cystometrograms. Graph represents the average of five experiments. Vertical calibrations represent intravesical pressure in cm H2O (A) and percentage change in amplitude of bladder contractions from control contractions before the administration of PACAP (B, C)
During continuous infusion CMGs in SCI rats with the urethra open to allow voiding, PACAP-38 had a markedly different effect. In doses of 10–30 µg administered intrathecally, the peptide decreased the amplitude of bladder contractions (Fig. 7C) and suppressed EUS EMG activity. This unexpected result was attributed to the combined effect of PACAP-38 on bladder and sphincter where the excitatory effect of PACAP-38 on bladder activity was masked by a simultaneous inhibitory effect on the EUS (Fig. 5) which in turn blocks DSD and reduces urethral outlet resistance. This would indirectly lower intravesical pressure during voiding. A similar effect in SCI rats has been noted after the administration of α-bungarotoxin, a potent neuromuscular blocking agent that selectively suppresses striated muscle activity without altering bladder activity. Alpha-bungarotoxin reduced maximal voiding pressure and increased voiding efficiency by blocking DSD in SCI rats (Yoshiyama et al. 2000). Because the high dose of PACAP-38 induced overflow incontinence in a majority of SCI animals with only a small increase in intravesical pressure, it is likely that this is due to a combined action to facilitate the spinal parasympathetic reflex to the bladder and inhibit the motor outflow to the EUS.
The pathophysiological role of PACAP in the control of bladder function in chronic SCI rats was studied by evaluating the effect of PACAP6–38, a PAC1 receptor antagonist on bladder activity during continuous infusion CMGs in awake rats (Zvara et al. 2006). Intrathecal administration of the antagonist reduced non-voiding contractions during bladder filling and reduced maximal voiding pressure, suggesting that activation of PAC1 receptors by endogenous PACAP was contributing to the micturition reflex and bladder hyperreflexia. Because PACAP is contained in C-fiber afferents, it is noteworthy that systemic administration of capsaicin, a C-fiber afferent neurotoxin, produced a similar reduction in non-voiding contractions in SCI rats (Cheng et al. 1995; Cheng and de Groat 2004).
However, paradoxically, the antagonist also reduced bladder capacity and increased voiding frequency (Zvara et al. 2006). This raises the possibility that after SCI, different functions of the bladder may be regulated independently by separate spinal mechanisms and that endogenous PACAP may modulate these different functions by acting on multiple receptors that have opposing effects on bladder function. It is known that PAC1 and VPAC2 but not VPAC1 receptors are present in the sacral spinal cord of the rat (Braas et al. 2006). If PAC1 receptors are involved in triggering non-voiding contractions but are also involved in inhibiting the mechanisms that control bladder capacity, then blocking PAC1 receptors with PACAP6–38 could elicit the combination of effects noted in the experiments. Furthermore, if both PAC1 and VPAC2 receptors are activated by endogenous PACAP and mediate opposite effects on bladder capacity, then blocking PAC1 inhibitory receptors would be expected to reduce bladder capacity and enhance voiding frequency. The effect of capsaicin treatment in chronic SCI rats to selectively suppress non-voiding contractions without changing bladder capacity supports the idea that these two functions are regulated independently (Cheng et al. 1995). This hypothesis should be examined further in future experiments in SCI rats by evaluating the effects of selective PACAP receptor antagonists on bladder and urethral sphincter dysfunction and on the responses of both organs to PACAP-38 and PACAP-27.
Conclusions
The expression of VIP and PACAP in peripheral and central neural pathways regulating the lower urinary tract coupled with the prominent excitatory and inhibitory effects of the peptides on voiding reflexes and the changes in expression and actions of the peptides in pathological conditions raise the possibility that VIP or PACAP may have a role in the regulation of normal or abnormal voiding. However, more detailed in vivo studies are needed using receptor antagonists and receptor knockout animals to clearly establish the contribution of VIP and PACAP to the neural control of the lower urinary tract.
Acknowledgments
This work was supported by NIH grants DK 49430 and DK 77783 to WD.
Contributor Information
Mitsuharu Yoshiyama, Yamanashi Rehabilitation Hospital, Fuefuki, Yamanashi 406-0004, Japan; Department of Urology, University of Yamanashi Interdisciplinary, Graduate School of Medicine and Engineering, Chuo, Yamanashi 409-3898, Japan; Department of Pharmacology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA.
William C. de Groat, Email: deGroat@server.pharm.pitt.edu, Department of Pharmacology, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA.
References
- Akasu T, Gallagher JP, Hirai K, Shinnick-Gallagher P. Vasoactive intestinal polypeptide depolarizations in cat bladder parasympathetic ganglia. Journal of Physiology (London) 1986;374:457–473. doi: 10.1113/jphysiol.1986.sp016091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm P, Alumets J, Hakanson R, Owman O, Sjoberg NO, Sundler F, et al. Origin and distribution of VIP (vasoactive intestinal polypeptide)-nerves in the genito-urinary tract. Cell and Tissue Research. 1980;205:337–347. doi: 10.1007/BF00232276. [DOI] [PubMed] [Google Scholar]
- Anand P, Gibson SJ, McGregor GP, Blank MA, Ghatei MA, Bacarese-Hamilton AJ, et al. AVIP-containing system concentrated in the lumbosacral region of human spinal cord. Nature. 1983;305:143–145. doi: 10.1038/305143a0. [DOI] [PubMed] [Google Scholar]
- Andersson PO, Andersson KE, Fahrenkrug J, Mattiasson A, Sjogren C, Uvelius B. Contents and effects of substance P and vasoactive intestinal polypeptide in the bladder of rats with and without infravesical outflow obstruction. Journal of Urology. 1988;140:168–172. doi: 10.1016/s0022-5347(17)41520-5. [DOI] [PubMed] [Google Scholar]
- Andersson PO, Fahrenkrug J, Malmgren A, Uvelius B. Effects of age and streptozotocin-induced diabetes on contents and effects of substance P and vasoactive intestinal polypeptide in the lower urinary tract of the rat. Acta Physiologica Scandanavia. 1992;144:361–368. doi: 10.1111/j.1748-1716.1992.tb09306.x. [DOI] [PubMed] [Google Scholar]
- Andersson KE, Mattiasson A, Sjögren C. Electrically induced relaxation of the noradrenaline contracted isolated urethra from rabbit and man. Journal of Urology. 1983;129:210–214. doi: 10.1016/s0022-5347(17)51986-2. [DOI] [PubMed] [Google Scholar]
- Araki I, de Groat WC. Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. Journal of Neurophysiology. 1996;76:215–226. doi: 10.1152/jn.1996.76.1.215. [DOI] [PubMed] [Google Scholar]
- Arimura A. Pituitary adenylate cyclase activating polypeptide (PACAP): Discovery and current status of research. Regulatory Peptides. 1992;37:287–303. [PubMed] [Google Scholar]
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Japanese Journal of Physiology. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: Neuroendocrine and endocrine interaction. Frontiers in Neuroendocrinology. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- Avelino A, Cruz F. Peptide immunoreactivity and ultrastructure of rat urinary bladder nerve fibers after topical desensitization by capsaicin or resiniferatoxin. Autonomic Neuroscience. 2000;86:37–46. doi: 10.1016/S1566-0702(00)00204-6. [DOI] [PubMed] [Google Scholar]
- Barrington FJF. The effect of lesion of the hind- and mid brain on micturition in the cat. Quarterly Journal of Experimental Physiology. 1925;15:81–102. [Google Scholar]
- Basbaum AI, Glazer EJ. Immunoreactive vasoactive intestinal polypeptide is concentrated in the sacral spinal cord: A possible marker for pelvic visceral afferent fibers. Somatosensory Research. 1983;1:69–82. doi: 10.3109/07367228309144541. [DOI] [PubMed] [Google Scholar]
- Blank MA, Brown JR, Hunter JC, Bloom SR, Tyers MB. Effects of VIP and related peptides and Gila monster venom on genitourinary smooth muscle. European Journal of Pharmacology. 1986;132:155–161. doi: 10.1016/0014-2999(86)90600-x. [DOI] [PubMed] [Google Scholar]
- Blok BF, van Maarseveen JT, Holstege G. Electrical stimulation of the sacral dorsal gray commissure evokes relaxation of the external urethral sphincter in the cat. Neuroscience Letters. 1998;249:68–70. doi: 10.1016/s0304-3940(98)00382-6. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. Journal of Neuroscience. 1998;18:9766–9779. doi: 10.1523/JNEUROSCI.18-23-09766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, et al. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. American Journal of Physiology. 2006;290:R951–R962. doi: 10.1152/ajpregu.00734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busacchi P, Perri T, Paradisi R, Oliverio C, Santini D, Guerrini, et al. Abnormalities of somatic peptide-containing nerves supplying the pelvic floor of women with genitourinary prolapse and stress urinary incontinence. Urology. 2004;63:591–595. doi: 10.1016/j.urology.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Callahan SM, Creed KE. Non-cholinergic neurotransmission and the effects of peptides on the urinary bladder of guinea-pigs and rabbits. The Journal of Physiology. 1986;374:103–115. doi: 10.1113/jphysiol.1986.sp016068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Experimental Neurology. 2004;187:445–454. doi: 10.1016/j.expneurol.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. American Journal of Physiology. 1999;277:R786–R794. doi: 10.1152/ajpregu.1999.277.3.R786. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Ma CP, Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Research. 1995;678:40–48. doi: 10.1016/0006-8993(95)00212-9. [DOI] [PubMed] [Google Scholar]
- Crowe R, Burnstock G, Light JK. Spinal cord lesions at different levels affect either the adrenergic or vasoactive intestinal polypeptide-immunoreactive nerves in the human urethra. Journal of Urology. 1988;140:1412–1414. doi: 10.1016/s0022-5347(17)42058-1. [DOI] [PubMed] [Google Scholar]
- Crowe R, Haven AJ, Burnstock G. Intramural neurons of the guinea-pig urinary bladder: Histochemical localization of putative neurotransmitters in cultures and newborn animals. Journal of the Autonomic Nervous System. 1986a;15:319–339. doi: 10.1016/0165-1838(86)90018-4. [DOI] [PubMed] [Google Scholar]
- Crowe R, Light K, Chilton CP, Burnstock G. Vasoactive intestinal polypeptide-, somatostatin- and substance P-immunoreactive nerves in the smooth and striated muscle of the intrinsic external urethral sphincter of patients with spinal cord injury. Journal of Urology. 1986b;136:487–491. doi: 10.1016/s0022-5347(17)44927-5. [DOI] [PubMed] [Google Scholar]
- Crowe R, Moss HE, Chapple CR, Light JK, Burnstock G. Patients with lower motor spinal cord lesion: A decrease of vasoactive intestinal polypeptide, calcitonin gene-related peptide and substance P, but not neuropeptide Y and somatostatin-immunoreactive nerves in the detrusor muscle of the bladder. Journal of Urology. 1991;145:600–604. doi: 10.1016/s0022-5347(17)38403-3. [DOI] [PubMed] [Google Scholar]
- Crowe R, Vale J, Trott KR, Soediono P, Robson T, Burnstock G. Radiation-induced changes in neuropeptides in the rat urinary bladder. Journal of Urology. 1996;156:2062–2066. [PubMed] [Google Scholar]
- de Groat WC. Nervous control of the urinary bladder of the cat. Brain Research. 1975;87:201–211. doi: 10.1016/0006-8993(75)90417-5. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Spinal cord projections and neuropeptides in visceral afferent neurons. Progress in Brain Research. 1986;67:165–187. doi: 10.1016/s0079-6123(08)62762-4. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Neuropeptides in pelvic afferent pathways. In: Polak JM, editor. Regulatory peptides. Basel: Birkhauser; 1989. pp. 334–361. [Google Scholar]
- de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The autonomic nervous system, vol. 3, nervous control of the urogenital system. London: Harwood Academic Publishers; 1993. pp. 227–290. [Google Scholar]
- de Groat WC, Fraser MO, Yoshiyama M, Smerin S, Tai C, Chancellor, et al. Neural control of the urethra. Scandinavian Journal of Urology and Nephrology. 2001;35:35–43. doi: 10.1080/003655901750174872. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Kawatani M, Hisamitsu T, Cheng C-L, Ma C-P, Thor K, et al. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. Journal of the Autonomic Nervous System. 1990;30:S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. Journal of the Autonomic Nervous System. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Ryall RW. Reflexes to sacral preganglionic parasympathetic neurons concerned with micturition in the cat. Journal of Physiology (London) 1969;200:87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Progress in Brain Research. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- Dixon JS, Jen PY, Gosling JA. A double-label immunohistochemical study of intramural ganglia from the human male urinary bladder neck. Journal of Anatomy. 1997;190(Pt 1):125–134. doi: 10.1046/j.1469-7580.1997.19010125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake MJ, Hedlund P, Mills IW, McCoy R, McMurray G, Gardner BP, et al. Structural and functional denervation of human detrusor after spinal cord injury. Laboratory Investigations. 2000;80:1491–1499. doi: 10.1038/labinvest.3780158. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J. PACAP in visceral afferent nerves supplying the rat digestive and urinary tracts. Annals of the New York Academy of Sciences. 1998a;865:542–546. doi: 10.1111/j.1749-6632.1998.tb11233.x. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J. Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience. 1998b;83:1261–1272. doi: 10.1016/s0306-4522(97)00474-0. [DOI] [PubMed] [Google Scholar]
- Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. Journal of Neurocytology. 1998;27:141–155. doi: 10.1023/a:1006903507321. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Polak JM, Anand P, Blank MA, Morrison JF, Kelly JS, et al. The distribution and origin of VIP in the spinal cord of six mammalian species. Peptides. 1984;5:201–207. doi: 10.1016/0196-9781(84)90207-9. [DOI] [PubMed] [Google Scholar]
- Gu J, Blank MA, Huang WM, Islam KN, McGregor GP, Christofides N, et al. Peptide-containing nerves in human urinary bladder. Urology. 1984;24:353–357. doi: 10.1016/0090-4295(84)90209-7. [DOI] [PubMed] [Google Scholar]
- Häbler HJ, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. Journal of Physiology (London) 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Sheward WJ, Morrison CF, Waser B, Gugger M, Reubi JC. Distribution of the VPAC2 receptor in peripheral tissues of the mouse. Endocrinology. 2004;145:1203–1210. doi: 10.1210/en.2003-1058. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Kigoshi S, Muramatsu I. Neurogenic responses of urethra isolated from the dog. European Journal of Pharmacology. 1992;213:117–123. doi: 10.1016/0014-2999(92)90240-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Kigoshi S, Muramatsu I. Nitric oxide-dependent and -independent neurogenic relaxation of isolated dog urethra. European Journal of Pharmacology. 1993;231:209–214. doi: 10.1016/0014-2999(93)90451-m. [DOI] [PubMed] [Google Scholar]
- Hernández M, Barahona MV, Recio P, Benedito S, Martinez AC, Rivera L, et al. Neuronal and smooth muscle receptors involved in the PACAP- and VIP-induced relaxations of the pig urinary bladder neck. British Journal of Pharmacology. 2006a;149:100–109. doi: 10.1038/sj.bjp.0706832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández M, Barahona MV, Recio P, Bustamante S, Benedito S, Rivera L, et al. PACAP 38 is involved in the non-adrenergic non-cholinergic inhibitory neurotransmission in the pig urinary bladder neck. Neurourology and Urodynamics. 2006b;25:490–497. doi: 10.1002/nau.20287. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Braas KM, May V, Vizzard MA. PACAP enhances mouse urinary bladder contractility and is upregulated in micturition reflex pathways after cystitis. Annals of the New York Academy of Sciences. 2006;1070:330–336. doi: 10.1196/annals.1317.040. [DOI] [PubMed] [Google Scholar]
- Hills J, Meldrum LA, Klarskov P, Burnstock G. A novel non-adrenergic, non-cholinergic nerve-mediated relaxation of the pig bladder neck: An examination of possible neurotransmitter candidates. European Journal of Pharmacology. 1984;99:287–293. doi: 10.1016/0014-2999(84)90135-3. [DOI] [PubMed] [Google Scholar]
- Honda CN, Rethelyi M, Petrusz P. Preferential immunohistochemical localization of vasoactive intestinal polypeptide (VIP) in the sacral spinal cord of the cat: light and electron microscope observations. Journal of Neuroscience. 1983;3:2183–2196. doi: 10.1523/JNEUROSCI.03-11-02183.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa H, Kaseda M. Experimental studies on VIP as non-cholinergic and non-adrenergic neurotransmitter in bladder neck and posterior urethra. Nippon Hinyokika Gakkai Zasshi. 1993;84:440–449. doi: 10.5980/jpnjurol1989.84.440. [DOI] [PubMed] [Google Scholar]
- Igawa Y, Persson K, Andersson K-E, Uvelius B, Mattiasson A. Facilitatory effect of vasoactive intestinal polypeptide on spinal and peripheral micturition reflex pathways in conscious rats with and without detrusor instability. Journal of Urology. 1993;149:884–889. doi: 10.1016/s0022-5347(17)36252-3. [DOI] [PubMed] [Google Scholar]
- Ishizuka O, Alm P, Larsson B, Mattiasson A, Andersson KE. Facilitatory effect of pituitary adenylate cyclase activating polypeptide on micturition in normal, conscious rats. Neuroscience. 1995;66:1009–1014. doi: 10.1016/0306-4522(95)00038-k. [DOI] [PubMed] [Google Scholar]
- Jänig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Progress in Brain Research. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- Jen PY, Dixon JS, Gosling JA. Co-localisation of tyrosine hydroxylase, nitric oxide synthase and neuropeptides in neurons of the human postnatal male pelvic ganglia. Journal of the Autonomic Nervous System. 1996;59:41–50. doi: 10.1016/0165-1838(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Jongsma Wallin H, Petterson LM, Verge VM, Danielsen N. Effect of anti-nerve growth factor treatment on pituitary adenylate cyclase activating polypeptide expression in adult sensory neurons exposed to adjuvant induced inflammation. Neuroscience. 2003;120:325–331. doi: 10.1016/s0306-4522(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Kawatani M, Erdman SL, de Groat WC. VIP and substance P in primary afferent pathways to the sacral spinal cord of the cat. Journal of Comparative Neurology. 1985a;241:327–347. doi: 10.1002/cne.902410307. [DOI] [PubMed] [Google Scholar]
- Kawatani M, Lowe IP, Nadelhaft I, Morgan C, De Groat WC. Vasoactive intestinal polypeptide in visceral afferent pathways to the sacral spinal cord of the cat. Neuroscience Letters. 1983;42:311–316. doi: 10.1016/0304-3940(83)90280-x. [DOI] [PubMed] [Google Scholar]
- Kawatani M, Nagel J, de Groat WC. Identification of neuropeptides in pelvic and pudendal nerve afferent pathways to the sacral spinal cord of the cat. Journal of Comparative Neurology. 1986;249:117–132. doi: 10.1002/cne.902490109. [DOI] [PubMed] [Google Scholar]
- Kawatani M, Rutigliano M, de Groat WC. Depolarization and muscarinic excitation induced in a sympathetic ganglion by vasoactive intestinal polypeptide. Science. 1985b;229:879–881. doi: 10.1126/science.3895438. [DOI] [PubMed] [Google Scholar]
- Kawatani M, Rutigliano M, de Groat WC. Selective facilitatory effect of vasoactive intestinal polypeptide (VIP) on muscarinic firing in vesical ganglia of the cat. Brain Research. 1985c;336:223–234. doi: 10.1016/0006-8993(85)90649-3. [DOI] [PubMed] [Google Scholar]
- Kawatani M, Takeshige C, de Groat WC. Central distribution of afferent pathways from the uterus of the cat. Journal of Comparative Neurology. 1990;302:294–304. doi: 10.1002/cne.903020208. [DOI] [PubMed] [Google Scholar]
- Keast JR. Plasticity of pelvic autonomic ganglia and urogenital innervation. International Review of Cytology. 2006;248:141–208. doi: 10.1016/S0074-7696(06)48003-7. [DOI] [PubMed] [Google Scholar]
- Keast JR, de Groat WC. Immunohistochemical characterization of pelvic neurons which project to the bladder, colon, or penis in rats. Journal of Comparative Neurology. 1989;288:387–400. doi: 10.1002/cne.902880303. [DOI] [PubMed] [Google Scholar]
- Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. Journal of Comparative Neurology. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Klarskov P, Gerstenberg T, Hald T. Vasoactive intestinal polypeptide influence on lower urinary tract smooth muscle from human and pig. Journal of Urology. 1984;131:1000–1004. doi: 10.1016/s0022-5347(17)50748-x. [DOI] [PubMed] [Google Scholar]
- Klarskov P, Holm-Bentzen M, Norgaard T, Ottesen B, Walter S, Hald T. Vasoactive intestinal polypeptide concentration in human bladder neck smooth muscle and its influence on urodynamic parameters. British Journal of Urology. 1987;60:113–118. doi: 10.1111/j.1464-410x.1987.tb04944.x. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Meakin SO, Krassioukov AV, Weaver LC. Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. Journal of Neuroscience. 1999;19:7405–7414. doi: 10.1523/JNEUROSCI.19-17-07405.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse MN, Bray LA, de Groat WC. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. Journal of the Autonomic Nervous system. 1995;54:215–224. doi: 10.1016/0165-1838(95)00011-l. [DOI] [PubMed] [Google Scholar]
- Kruse MN, Noto H, Roppolo JR, de Groat WC. Pontine control of the urinary bladder and external urethral sphincter in the rat. Brain Research. 1990;532:182–190. doi: 10.1016/0006-8993(90)91758-9. [DOI] [PubMed] [Google Scholar]
- Kuru M. Nervous control of micturition. Physiological Reviews. 1965;45:425–494. doi: 10.1152/physrev.1965.45.3.425. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Marie JC. VPAC receptors for VIP and PACAP. Receptors & Channels. 2002;8:137–153. [PubMed] [Google Scholar]
- Larsen JJ, Ottesen B, Fahrenkrug J, Fahrenkrug L. Vasoactive intestinal polypeptide (VIP) in the male genitourinary tract: concentration and motor effect. Investigative Urology. 1981;19:211–213. [PubMed] [Google Scholar]
- Lasanen LT, Tammela TL, Liesi P, Waris T, Polak JM. The effect of acute distension on vasoactive intestinal polypeptide (VIP), neuropeptide Y (NPY) and substance P (SP) immunoreactive nerves in the female rat urinary bladder. Urological Research. 1992;20:259–263. doi: 10.1007/BF00300255. [DOI] [PubMed] [Google Scholar]
- Liu GJ, Madsen BW. PACAP38 modulates activity of NMDA receptors in cultured chick cortical neurons. Journal of Neurophysiology. 1997;78:2231–2234. doi: 10.1152/jn.1997.78.4.2231. [DOI] [PubMed] [Google Scholar]
- Mallory BS, Roppolo JR, de Groat WC. Pharmacological modulation of the pontine micturition center. Brain Research. 1991;546:310–320. doi: 10.1016/0006-8993(91)91495-m. [DOI] [PubMed] [Google Scholar]
- Mattiasson A, Andersson KE, Andersson PO, Larsson B, Sjogren C, Uvelius B. Nerve-mediated functions in the circular and longitudinal muscle layers of the proximal female rabbit urethra. Journal of Urology. 1990;143:155–160. doi: 10.1016/s0022-5347(17)39901-9. [DOI] [PubMed] [Google Scholar]
- Mattiason A, Andersson KE, Sjögren C. Adrenergic and non-adrenergic contraction of isolated urethral muscle from rabbit and man. Journal of Urology. 1985a;133:298–303. doi: 10.1016/s0022-5347(17)48927-0. [DOI] [PubMed] [Google Scholar]
- Mattiasson A, Ekblad E, Sundler F, Uvelius B. Origin and distribution of neuropeptide Y-, vasoactive intestinal polypeptide-and substance P-containing nerve fibers in the urinary bladder of the rat. Cell and Tissue Research. 1985b;239:141–146. doi: 10.1007/BF00214914. [DOI] [PubMed] [Google Scholar]
- Milner P, Crowe R, Burnstock G, Light JK. Neuropeptide Y- and vasoactive intestinal polypeptide-containing nerves in the intrinsic external urethral sphincter in the areflexic bladder compared to detrusor-sphincter dyssynergia in patients with spinal cord injury. Journal of Urology. 1987;138:888–892. doi: 10.1016/s0022-5347(17)43409-4. [DOI] [PubMed] [Google Scholar]
- Miura A, Kawatani M, de Groat WC. Effects of pituitary adenylate cyclase activating polypeptide on lumbosacral preganglionic neurons in the neonatal rat spinal cord. Brain Research. 2001;895:223–232. doi: 10.1016/s0006-8993(01)02112-6. [DOI] [PubMed] [Google Scholar]
- Mohammed H, Hannibal J, Fahrenkrug J, Santer R. Distribution and regional variation of pituitary adenylate cyclase activating polypeptide and other neuropeptides in the rat urinary bladder and ureter: Effects of age. Urological Research. 2002;30:248–255. doi: 10.1007/s00240-002-0261-6. [DOI] [PubMed] [Google Scholar]
- Moller K, Zhang YZ, Hakanson R, Luts A, Sjolund B, Uddman R, et al. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: Immunocytochemical and immunochemical evidence. Neuroscience. 1993;57:725–732. doi: 10.1016/0306-4522(93)90018-b. [DOI] [PubMed] [Google Scholar]
- Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. Journal of Comparative Neurology. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- Morgan CW, Ohara PT, Scott DE. Vasoactive intestinal polypeptide in sacral primary sensory pathways in the cat. Journal of Comparative Neurology. 1999;407:381–394. doi: 10.1002/(sici)1096-9861(19990510)407:3<381::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Conduction velocity distribution of afferent fibers in the female rat hypogastric nerve. Brain Research. 1991;539:228–232. doi: 10.1016/0006-8993(91)91625-b. [DOI] [PubMed] [Google Scholar]
- Noto H, Roppolo JR, Steers WD, de Groat WC. Electrophysiological analysis of the ascending and descending components of the micturition reflex pathway in the rat. Brain Research. 1991;549:95–105. doi: 10.1016/0006-8993(91)90604-t. [DOI] [PubMed] [Google Scholar]
- Ohnishi N, Park YC, Kurita T, Kajimoto N. Role of ATP and related purine compounds on urethral relaxation in male rabbits. International Journal of Urology. 1997;4:191–197. doi: 10.1111/j.1442-2042.1997.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Pantaloni C, Brabet P, Bilanges B, Dumuis A, Houssami S, Spengler D, et al. Alternative splicing in the N-terminal extracellular domain of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor modulates receptor selectivity and relative potencies of PACAP-27 and PACAP-38 in phospholipase C activation. Journal of Biological Chemistry. 1996;271:22146–22151. doi: 10.1074/jbc.271.36.22146. [DOI] [PubMed] [Google Scholar]
- Papka RE, Workley M, Usip S, Mowa CN, Fahrenkrug J. Expression of pituitary adenylate cyclase activating peptide in the uterine cervix, lumbosacral dorsal root ganglia and spinal cord of rats during pregnancy. Peptides. 2006;27:743–752. doi: 10.1016/j.peptides.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Persson K, Alm P, Johansson K, Larsson B, Andersson KE. Co-existence of nitrergic, peptidergic and acetylcholine esterase-positive nerves in the pig lower urinary tract. Journal of the Autonomic Nervous System. 1995;52:225–236. doi: 10.1016/0165-1838(94)00160-l. [DOI] [PubMed] [Google Scholar]
- Radziszewski P, Ekblad E, Sundler F, Mattiasson A. Distribution of neuropeptide-, tyrosine hydroxylase- and nitric oxide synthase containing nerve fibers in the external urethral sphincter of the rat. Scandinavian Journal of Urology and Nephrology. 1996;179:81–85. [PubMed] [Google Scholar]
- Reubi JC. In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues. Clinical implications. Annals of the New York Academy of Sciences. 2000;921:1–25. doi: 10.1111/j.1749-6632.2000.tb06946.x. [DOI] [PubMed] [Google Scholar]
- Saito M, Kondo A, Gotoh M, Kato K, Levin RM. Age-related changes in the response of the rat urinary bladder to neurotransmitters. Neurourology and Urodynamics. 1993;12:191–200. doi: 10.1002/nau.1930120214. [DOI] [PubMed] [Google Scholar]
- Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, et al. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. Journal of Urology. 2002;168:2269–2274. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, De Groat WC, et al. Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. Journal of Urology. 2004;171:478–482. doi: 10.1097/01.ju.0000088340.26588.74. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocrine Reviews. 2000;21:619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- Sie JA, Blok BF, de Weerd H, Holstege G. Ultrastructural evidence for direct projections from the pontine micturition center to glycine-immunoreactive neurons in the sacral dorsal gray commissure in the cat. Journal of Comparative Neurology. 2001;429:631–637. doi: 10.1002/1096-9861(20010122)429:4<631::aid-cne9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Sienkiewicz W, Kaleczyc J, Czaja K, Lakomy M. Adrenergic, nitrergic and peptidergic innervation of the urethral muscle in the boar. Folia histochemica et cytobiologica/Polish Academy of Sciences, Polish Histochemical and Cytochemical Society. 2004;42:89–94. [PubMed] [Google Scholar]
- Sjögren C, Andersson KE, Mattiasson A. Effects of vasoactive intestinal polypeptide on isolated urethral and urinary bladder smooth muscle from rabbit and man. Journal of Urology. 1985;133:136–140. doi: 10.1016/s0022-5347(17)48822-7. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Matsuyama K, Takakusaki K, Mori S. Electrical and chemical stimulations of the pontine micturition center. Neuroscience Letters. 1987;80:197–201. doi: 10.1016/0304-3940(87)90653-7. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Vasoactive intestinal polypeptide-depolarizations in hamster submandibular ganglion. Bulletin of Tokyo Dental College. 1992;33:71–74. [Google Scholar]
- Suzuki T, Ono H, Ikegami H. PACAP-induced depolarizations in hamster submandibular ganglion neurons. Bulletin of Tokyo Dental College. 2003;44:21–24. doi: 10.2209/tdcpublication.44.21. [DOI] [PubMed] [Google Scholar]
- Thor K, Kawatani M, de Groat WC. Plasticity in the reflex pathways to the lower urinary tract of the cat during postnatal development and following spinal cord injury. In: Goldberger ME, Gorio A, Murray M, editors. Development and plasticity of the mammalian spinal cord. vol. 1. Padova: Liviana Press; 1986. pp. 65–80. [Google Scholar]
- Tiseo PJ, Yaksh TL. The spinal pharmacology of urinary function: Studies on urinary continence in the unanaesthetized rat. Ciba Foundation Symposium. 1990;151:91–104. doi: 10.1002/9780470513941.ch6. [DOI] [PubMed] [Google Scholar]
- Tomkins JD, Ardell JL, Hoover DB, Parsons RL. Neurally released pituitary adenylate cyclase-activating polypeptide enhances guinea pig intrinsic cardiac neurone excitability. Journal of Physiology (London) 2007;582:87–93. doi: 10.1113/jphysiol.2007.134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens M, Morrison JFB. In: The physiology of the lower urinary tract. Torrens M, Morrison JFB, editors. Heidelberg: Springer; 1987. pp. 3–350. [Google Scholar]
- Ückert S, Stief CG, Lietz B, Burmester M, Jonas U, Machtens SA. Possible role of bioactive peptides in the regulation of human detrusor smooth muscle—Functional effects in vitro and immunohistochemical presence. World Journal of Urology. 2002;20:244–249. doi: 10.1007/s00345-002-0287-y. [DOI] [PubMed] [Google Scholar]
- Uemura E, Fletcher TF, Dirks VA, Bradley WE. Distribution of sacral afferent axons in cat urinary bladder. American Journal of Anatomy. 1973;136:305–313. doi: 10.1002/aja.1001360305. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: From structure to functions. Pharmacological Reviews. 2000;52:269–324. [PubMed] [Google Scholar]
- Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. Journal of Comparative Neurology. 2000a;420:335–348. [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Experimental Neurology. 2000b;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Progress in Brain Research. 2006;152:97–115. doi: 10.1016/S0079-6123(05)52007-7. [DOI] [PubMed] [Google Scholar]
- Wanigasekara Y, Kepper ME, Keast JR. Immunohistochemical characterisation of pelvic autonomic ganglia in male mice. Cell and Tissue Research. 2003;311:175–185. doi: 10.1007/s00441-002-0673-1. [DOI] [PubMed] [Google Scholar]
- Werkström V, Alm P, Persson K, Andersson KE. Inhibitory innervation of the guinea-pig urethra: Roles of CO, NO and VIP. Journal of the Autonomic Nervous System. 1998;74:33–42. doi: 10.1016/s0165-1838(98)00135-0. [DOI] [PubMed] [Google Scholar]
- Werkström V, Persson K, Andersson KE. NANC transmitters in the female pig urethra—Localization and modulation of release via alpha 2-adrenoceptors and potassium channels. British Journal of Pharmacology. 1997;121:1605–1612. doi: 10.1038/sj.bjp.0701308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N. Bladder afferent pathway and spinal cord injury: Possible mechanisms inducing hyperreflexia of the urinary bladder. Progress in Neurobiology. 1999;57:583–606. doi: 10.1016/s0301-0082(98)00070-7. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. Journal of Neuroscience. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Neural control of the lower urinary tract. International Journal of Urology. 1997a;4:111–125. doi: 10.1111/j.1442-2042.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Plasticity of Na channels in afferent neurons innervating rat urinary bladder following spinal cord injury. Journal of Physiology (London) 1997b;503:269–276. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Erdman SL, Snider MW, de Groat WC. Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience. 1998;83:633–643. doi: 10.1016/s0306-4522(97)00376-x. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, Erickson KA, Erickson VL, Chancellor MB, de Groat WC. Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. Journal of Neuroscience. 2003;23:4355–4361. doi: 10.1523/JNEUROSCI.23-10-04355.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama M, de Groat WC. Effects of intrathecal administration of pituitary adenylate cyclase activating polypeptide on lower urinary tract functions in rats with intact or transected spinal cords. Experimental Neurology. 2008;211:449–455. doi: 10.1016/j.expneurol.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama M, de Groat WC, Fraser MO. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urology. 2000;55:956–960. doi: 10.1016/s0090-4295(00)00474-x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ling EA. Colocalization of nitric oxide synthase and some neurotransmitters in the intramural ganglia of the guinea pig urinary bladder. Journal of Comparative Neurology. 1998;394:496–505. [PubMed] [Google Scholar]
- Zhou Y, Ling EA. Nitric oxide synthase—Its distribution and alteration in the intramural ganglia of the urinary bladder in normal and urethra-obstructed guinea pigs. Annals of the Academy of Medicine, Singapore. 1999;28:49–61. [PubMed] [Google Scholar]
- Zvara P, Braas KM, May V, Vizzard MA. A role for pituitary adenylate cyclase activating polypeptide (PACAP) in detrusor hyperreflexia after spinal cord injury (SCI) Annals of the New York Academy of Sciences. 2006;1070:622–628. doi: 10.1196/annals.1317.092. [DOI] [PubMed] [Google Scholar]
- Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Experimental Neurology. 2005;192:46–59. doi: 10.1016/j.expneurol.2004.10.017. [DOI] [PubMed] [Google Scholar]