Abstract
Objective
To compare objective polysomnographic parameters between three cohorts: children with autism, with typical development, and with developmental delay without autism.
Design
Overnight polysomnographic recordings were scored for sleep architecture according to American Academy of Sleep Medicine criteria by a board certified sleep medicine specialist blind to diagnosis for studies collected between July 2006, September 2009.
Setting
Subjects evaluated pediatric ward in Clinical Center of the National Institutes of Health.
Participants
First 60 consecutive children with autism, 15 with typical development, and 13 with developmental delay matched for non-verbal IQ to the autism group. Ranging in age from 2 to 13, selected without regard to the presence or absence of sleep problem behavior.
Main Outcome Measure
Total Sleep Time (TST), latencies to non-REM sleep and REM and percentages of total sleep time for stages 1, 2, slow wave sleep (SWS) and REM.
Results
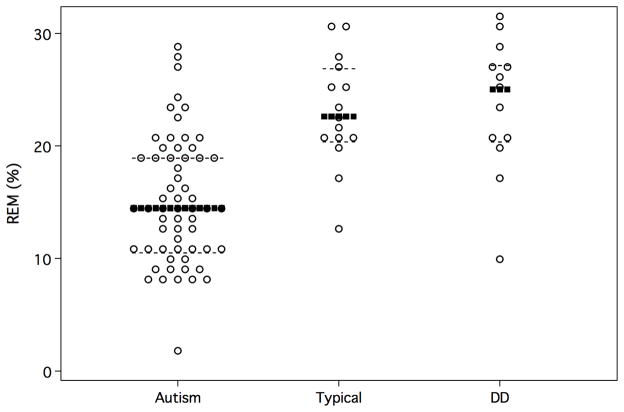
There were no differences between typical v developmental delay groups. Autism v typical revealed shorter TST (p = .004), greater SWS percentage (p=.001), and much smaller REM percentage, 14.5 v 22.6 (p<.001). Autism v developmental delay revealed shorter TST (p=.001), greater stage 1 percentage (p= <.001), greater SWS percentage (p = <.001) and much less REM percentage, 14.5 v 25 (p= <.001).
Conclusions
A relative deficiency of REM may indicate an abnormality in neural organization in young children with autism that is not directly associated or related to inherent intellectual disability but may serve as a window into understanding core neurotransmitter abnormalities unique to this disorder.
INTRODUCTION
Autism is a neurodevelopmental disorder characterized by abnormalities in social interaction and communication and by the presence of repetitive behaviors.1 Abnormalities in gross brain structure, neuronal growth patterns and neurotransmitter profiles have all been preliminarily explored with little consensus on findings as they relate to pathophysiology.2 Consistent behavioral disturbances may offer insights into core physiologic or anatomic abnormalities and may help to elucidate biological signatures or endophenotypes. Disturbances in sleep are well known correlates of autism3 and sleep disturbances in children with autism are a major clinical concern for caretakers and clinicians. The prevalence has been estimated to be between 44–83% for this population.4 Irregular sleep, frequent night awakenings, prolonged awakenings, and hypersomnia alternating with severely reduced sleep times are the most often reported abnormalities.5 Most of the previous studies addressing sleep in autism have relied on parent report measures alone 6, 7, 8, 9 or in conjunction with actigraphic recordings, 8,10 which may allow for the tracking of overall sleep wake cycles more objectively, but does not inform on specific sleep architecture such as relative ratios of REM and non-REM sleep. Although there is clearly a great need for therapeutic interventions that will alleviate these sleep disturbances, progress towards developing such therapies has been stymied by a lack of useful information regarding the underlying neurobiological abnormality.11
Markers of sleep architecture and sleep state can be objectively and non-invasively measured and referenced to developmental norms. Polysomnography is a reliable, non-invasive tool used to study the basic mechanisms of sleep and has proven useful to neuropsychiatric medicine by serving to identify trait markers for diseases such as narcolepsy and depression. 12 Previous studies in people with autism have identified various abnormalities in REM sleep including: immature organization, decreased quantity, abnormal twitches, undifferentiated sleep and REM Sleep Behavior Disorder, which is characterized by the absence of the muscle atonia that is normal during REM sleep. 13,14,15,16 There have been relatively few, exclusively pediatric, polysomnographic studies of autism to date. Existing studies have consistently confirmed various abnormalities of sleep but are often difficult to compare due to dissimilar exclusion and inclusion criteria, different ages groups and small number of patients. 17–18
Given the recent progress in delineating the neurobiology of sleep, investigating putatively abnormal sleep architecture (as it may reflect abnormal neurotransmission) in a large, well defined, young cohort of children with autism offers the opportunity of identifying trait markers in autism that may be rapidly linkable to emerging genetic findings. Even if such an ambitious goal is not realized, the information gained could have immediate benefits in terms of developing more targeted therapies for the sleep disturbances of autism.
METHODS
Subjects
Our institutional review board approved this study and the cohort presented is a subset from two ongoing National Institute of Mental Health investigations-a small clinical trial of minocycline and a larger phenomenologic investigation of the medical and clinical subtypes of autism. Children were recruited to participate in the studies without regard to the presence or absence of sleep problem behavior. This report includes the first 60 children between the ages of 2 and 13 to be given a research diagnosis of autism (see below), to complete an overnight polysomnogram at the National Institute of Health’s Clinical Research Center and to have a technically complete study (studies where eye leads were lost were not included). Autism symptoms were assessed using the Autism Diagnostic Observation Schedule (ADOS),19 a clinician-administered structured play interview designed to elicit behaviors relevant to a diagnosis of autism, and the Autism Diagnostic Interview-Revised (ADI-R),20 a semi-structured parent interview concerning all domains of impairment in autism spectrum disorders. The developmentally delayed group was ascertained for matching on developmental and adaptive capacity to the autism group. Criteria for this group included a non-ASD clinical judgment based on administration of the ADI-R and ADOS. Of the first 15 children with developmental delay to complete an overnight polysomnogram, 13 (ages 2–7) had technically complete studies and are included in this dataset. The typical control group was recruited at a younger age to better match maturational and neurodevelopment levels with the delays seen in the autism group and consists of the first 15 children (ages 1 through 5) to complete an overnight study. Each parent or guardian also completed the Children’s Sleep Habits Questionnaire (CSHQ).21
Medication histories were obtained from all participants in order to evaluate potential effects of medication on sleep architecture.
Polysomnogram
Children were admitted for a continuous overnight recording that included a referential, 21 lead electroencephalogram montage, electro-oculogram, electrocardiogram, and surface electromyogram (chin, anterior tibialis). Respiratory parameters were not measured. Lights out approximated child’s actual bedtime. All recordings were videotaped and ended at a median wake up time of 0700 for all groups. The clinical readings were provided by the same neurophysiologist (SS) using National Institute of Neurological Disorders and Stroke EEG lab standards for sleep architecture; these early reports of decreased REM percentage among the children with autism were the impetus for this systemic investigation. A second, blinded reading for sleep architecture and leg movements was done using GRASS telefactor software R by a different neurologist, board certified in neurology, neurophysiology, and sleep medicine (AR). Wake/sleep was subdivided into 30-s epochs and scored according to the guidelines contained in the American Academy of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events.22 The following sleep variables were calculated: total sleep time (TST = the entire sleep period time minus the time spent in wakefulness after sleep onset), sleep efficiency index (SE = TST/Time in Bed x 100), the minutes spent in each stage (stage 1, stage 2, stage 3 or slow wave sleep and REM), the percentage of each stage relative to TST, the latency to sleep onset (measured from lights out to the first epoch of stage 1) and the latency to REM sleep (measured from the first epoch of stage 1 to the first epoch of REM sleep).
All participants were allowed to continue any prescribed medications during the hospitalization. One child in the typical group was taking diphenhydramine and cetirizine on the night of the study. Four children in the DD group reported taking a medication on the night of the study that could potentially affect sleep architecture: clobazam (1), somatostatin (1), cetirizine (1) and montelukast (1). In the autism group, 11 children took the following medications on the night of the study: clonidine alone (2), risperdal alone (2), melatonin alone (1), tegretol and fluoxetine (1), melatonin and seroquel (1), melatonin, fluvoxamine, trileptal and trazodone (1), topiramate and clonazepam (1) citirizine (1) and valproate (1).
Statistical Analysis
Descriptive statistics, including medians and interquartile ranges, were computed for all sleep parameters and additional measures (age, non-verbal ratio intelligence quotient). The normality of distributions of the sleep parameters was assessed using the Shapiro-Francia W’ test, revealing significant non-normality in four of the eight measures (WASO, sleep efficiency, stage 1 percentage and latency to REM, p <.0001). Sample variances for all parameters were compared between groups using Brown and Forsythe’s robust test using the sample median as the measure of location; no variances were found to be statistically significantly different at the p= .01 level. Pairwise differences between groups (autism, typical, and developmentally delayed) on all measures were thus evaluated by comparing medians to account for non-normality, and statistical significance was assessed via the Mann-Whitney U-test. Tests were two-sided, and a p-value of .01 was considered to indicate statistical significance. In addition, 99% confidence intervals for median differences were computed based on the Hodges-Lehmann statistic.23 All calculations were generated using the Stata statistical analysis software, version 10.1.
RESULTS
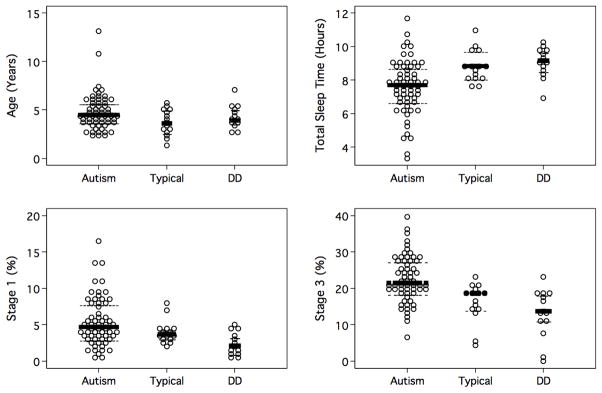
Means, standard deviations, and ranges of the observed data are presented in Table 1. Table 2 presents medians, interquartile ranges, and the results of Mann-Whitney tests and Hodges-Lehmann confidence intervals. As Table 2 shows, compared to the typically developing group, the subjects with autism showed a significantly shorter total sleep time (p = .004), a significantly higher percentage of stage 3 sleep (p = .001) and a significantly lower percentage of REM sleep (p<.001).
Table 1.
Descriptive statistics
| Autism | Typical | DD | |
|---|---|---|---|
| Number of observations | 60 | 15 | 13 |
| Proportion male | 0.82 | 0.73 | 0.54 |
| Age (years) | 4.81 (1.88) 2.24–13.11 |
3.69 (1.33) 1.35–5.84 |
4.29 (1.19) 2.69–7.11 |
| Non-verbal ratio IQ | 56.92 (17.96) 25.07–114.20 |
107.97 (15.86) 80.57–142.81 |
58.06 (18.84) 9.61–76.63 |
| Total sleep time (hr) | 7.59 (1.66) 3.23–11.59 |
8.81 (0.93) 7.61–10.85 |
9.01 (0.88) 6.91–10.13 |
| Latency to sleep (min) | 39.4 (41.2) 0.0–144.0 |
44.8 (32.8) 6.5–114.0 |
33.2 (22.7) 0.0–93.5 |
| Sleep Efficiency (%) | 80.2 (12.7) 43.5–97.3 |
84.7 (7.7) 69.3–95.0 |
84.6 (8.1) 63.2–92.5 |
| Wake after sleep onset (min) | 73.5 (72.2) 0.5–358.9 |
51.7 (52.1) 10.0–178.7 |
66.2 (59.5) 14.0–203.9 |
| Stage 1 (%) | 5.4 (3.4) 0.4–16.6 |
4.0 (1.6) 2.0–7.9 |
2.3 (1.5) 0.6–4.8 |
| Stage 2 (%) | 56.9 (7.7) 37.8–75.3 |
55.9 (5.7) 47.6–69.5 |
60.9 (8.2) 50.6–74.4 |
| Stage 3 (%) or SWS (%) | 22.6 (6.6) 6.3–39.6 |
16.3 (5.4) 4.4–23.5 |
13.1 (6.8) 0.0–22.6 |
| REM (%) | 15.1 (5.6) 1.4–28.8 |
23.0 (4.9) 12.9–30.6 |
23.7 (6.2) 9.6–31.8 |
| REM latency (min) | 126.3 (86.8) 36.5–556.0 |
79.8 (34.2) 36.0–144.5 |
76.0 (28.6) 32.5–149.0 |
Note: Table shows means (first line), standard deviations (second line, in parentheses), and ranges (third line) by group (autism, typical, developmentally delayed).
Table 2.
Children with autism have shorter total sleep time, increased SWS and relative REM deficiency.
| Autism | Typical | DD | Autism vs. Typical | Autism vs. DD | Typical vs. DD | |
|---|---|---|---|---|---|---|
| Age (years) | 4.49 (1.95) | 3.60 (2.42) | 3.91 (1.44) | .038 [−0.22, 2.07] | .37 [−0.75, 1.54] | .26 [−1.95, 0.86] |
| Non-verbal ratio IQ | 56.06 (24.11) | 105.90 (26.96) | 67.25 (22.55) | <.001 [−64.71, −38.54] | .41 [−17.97, 12.50] | <.001 [29.80, 66.34] |
| Total sleep time (hr) | 7.70 (2.04) | 8.83 (1.66) | 9.15 (1.14) | .004 [−2.13, −0.15] | .001 [−2.39, −0.33] | .34 [−1.23, 0.72] |
| Latency to sleep (min) | 28.5 (65.5) | 37.5 (34.5) | 33.0 (11.5) | .29 [−34.0, 20.0] | .84 [−27.0, 31.0] | .38 [−16.5, 37.5] |
| Sleep Efficiency (%) | 83.7 (16.5) | 86.2 (11.8) | 87.8 (8.9) | .29 [−11.3, 4.4] | .36 [−12.0, 4.2] | .89 [−8.7, 7.4] |
| Wake after sleep onset (min) | 50.1 (82.7) | 37.0 (40.0) | 45.1 (43.8) | .31 [−16.3, 52.0] | .97 [−34.1, 46.5] | .42 [−54.5, 23.0] |
| Stage 1 (%) | 4.7 (4.9) | 3.7 (1.2) | 2.1 (2.3) | .14 [−0.8, 2.8] | <.001 [0.6, 4.7] | .020 [−0.1, 3.1] |
| Stage 2 (%) | 56.4 (12.3) | 55.8 (8.5) | 57.3 (10.7) | .62 [−4.4, 7.0] | .18 [−10.9, 3.1] | .10 [−13.8, 2.7] |
| Stage 3 (%) or SWS (%) | 21.5 (9.0) | 18.6 (5.4) | 13.7 (7.1) | .001 [1.1, 10.4] | <.001 [3.1, 15.1] | .08 [−3.3, 9.7] |
| REM (%) | 14.5 (8.4) | 22.6 (6.5) | 25.0 (6.8) | <.001 [−12.1, −3.7] | <.001 [−13.9, −4.1] | .61 [6.7, 4.8] |
| REM latency (min) | 108.5 (80.3) | 64.0 (59.0) | 69.0 (27.5) | .016 [−2.5, 71.0] | .012 [−0.5, 74.0] | .89 [−28.0, 42.0] |
Note: First three columns present medians and interquartile ranges (in parentheses) for each measure by group (autism, typical, developmentally delayed). Last three columns show p-values from pairwise, two-tailed Mann-Whitney U-tests and 99% confidence intervals for the Hodges-Lehmann median differences (in brackets). Number of observations = 60 (autism), 15 (typical), and 13 (DD).
Compared to the DD group, the subjects with autism showed a significantly shorter total sleep time (p = .001), a significantly longer stage 1 (p<.001), a significantly higher percentage of stage 3 (p< .001) and a significantly lower percentage of REM sleep (p <.001).
There were no statistically significant differences between the typical group and the DD group on any sleep measurements. Notably, sleep efficiencies and minutes awake after sleep onset did not differ between groups.
The median wake time in the clinical setting was 0617 for the autism group (range = 0310–0834), 0645 for the developmental delay group (range = 0546–0751), and 0646 for the typical group (range = 0603–0836). The median habitual wake time as reported on the CSHQ was 0700 for the autism group, 0630 for the group with developmental delay and 0700 for the typical group (n = 80 after listwise deletion of CSHQ non-respondents).
Figure 1 presents differences among the three cohorts on age in years, total sleep time, stage 1 percentage, and stage 3 percentage graphically. Figure 2 shows the group differences in REM percentage.
Figure 1.
Age in Years, Total sleep time, Stage 1 percent, and Stage 3 (SWS) percent by group.
Note: Dark lines denote group medians; dashed lines denote interquartile range.
Figure 2.
REM percentage by group
Note: Dark lines denote group medians; dashed lines denote interquartile range.
DISCUSSION
Our data reveal autism specific differences in sleep architecture with the largest median differences occurring in the percentage of REM sleep attained in the autism group in comparison to both a typically developing cohort and a cohort of children with nonspecific developmental delay. This relative deficiency of REM sleep warrants further investigation. The function of REM sleep and its relationship to cognition and overall neurological health is unknown and a subject of ongoing research. We know from animal studies that REM sleep increases after intensive learning sessions 24, 25 which may imply that this stage is important for particular cognitive processes and that REM sleep may be useful as an indicator of brain plasticity. Current studies continue to add support for this postulate. 26, 27, 28, 29 REM sleep has most recently been implicated in the process of human memory consolidation and several studies suggest that it is crucial to normal cognitive function and in the processing of emotion in memory systems. 30,31
Additionally, there is converging evidence that REM sleep serves a specific developmental role. The amount of time spent in REM sleep decreases over the lifespan. About 50% of a neonate’s sleep is devoted to REM versus about 20% to 25% of adult sleep. 32. The decline is the most rapid over the first few years of life and adult values are achieved before puberty. 33 One hypothesis, first proposed in 1966 by Roffwarg and associates and continuing to be explored today, is that the increased ponto-thalamo-cortical activity evident during REM sleep provides the endogenous stimulation needed to form and stabilize durable synaptic connections in the developing brain. 34,35,36 In support of the theory that REM sleep abnormalities early in life may have long term consequences, Vogel et al. have shown that REM sleep deprivation in the neonatal rat induces social deficiencies in the adult animal.37–38 Furthermore, in recent animal models of learning and memory exploring this question, REM deficiency has been found to have differential effects on markers of neuronal plasticity (as measured by changes in molecular components of glutamatergic synapses) that are age dependent.39 For example, in work published by Lopez and colleagues in 2008, the authors suggest that REM sleep deprivation early in life may interfere with the correct formation of neural circuits in the hippocampus by “preventing expression of synaptic proteins that are important for the maintenance and stability of neural connections.” 38
Our study has several limitations. The recordings were conducted on a regular pediatric ward and not in a specialized sleep lab. While the total sleep times for the typical controls are comparable to other pediatric lab-based studies40 the sleep efficiency is not as great for any of the three groups as might have been obtained in a more controlled environment. Another limitation is that respiratory parameters were not measured, rendering it impossible to know the potential contribution of obstructive sleep apnea (OSA) to sleep architecture in any of the cohorts. The prevalence of OSA in the general pediatric population is estimated at 2%. This is an important consideration, but it may not have a direct impact on the interpretation of these results. While it is true that OSA is more likely to occur in REM than NREM sleep in children, sleep architecture appears to be preserved in children with OSA without the associated EEG arousal and measurable sleep fragmentation that often follows obstructive events in adults.41 A third limitation is the potential effects of various medications on sleep stages. Being medication-free was not an inclusion criterion for either of the parent studies. In the autism group, there was no statistically significant difference in the median REM% among those on any medication (11.1) and those not (14.7; p= 0.17).
Investigation of sleep state transitions in autism offers an opportunity to speculate on the underlying neurotransmission. The control of wake/Non-REM/REM rhythms is complex, involving GABA, glutamate and three major neurotransmitter systems: serotonin, produced by cells in the dorsal raphe nucleus; norepinephrine, from the locus coeruleus; and acetylcholine, with neurons in the basal forebrain and the brainstem and interneurons in the striatum. Acetylcholine is the main driver of REM sleep and in concert with serotonin and norepinephrine, it acts on brainstem structures in the pons and adjacent areas of the midbrain via ‘REM/On’ and ‘REM/Off’ cells to coordinate REM and non-REM (NREM) sleep. 42 It is this reciprocal model of inhibitory aminergic neurons and excitatory cholinergic neurons that modulates brain activity between the, NREM and REM states. An imbalance in either arm may result in state instability. It is our conjecture that in the developing brain the proper stabilization of synaptic pathways is at least partly dependent upon the rich periods of disinhibition that occur during the rapid eye movement state. Neuropathologic investigations by Bauman and Kemper in 1994 43, Perry et al in 200144, Lee et al in 2002 45, and Martin-Ruiz et al in 2004 46, implicate the cholinergic system in the abnormal development of the autistic brain. When considered in conjunction with the knowledge that cholinergic afferents innervate the cerebral cortex during a period of intense neuronal differentiation and synapse formation, 47 the findings highlight the relevance of further investigation of the cholinergic system in autism, particularly early in development.
The differences in slow wave sleep (SWS) % between the autism group and both the typically developing cohort and the developmental delay group without autism, may offer further clues as to whether or not the underlying differences in sleep architecture between cohorts are mediated by cholinergic abnormalities. During SWS, a different neurotransmission milieu is evident than is present during REM sleep. Slow wave appearance is greatly influenced by the presence of acetylcholine. Unlike in REM sleep where cholinergic input is high, SWS is characterized by a relative diminution of this neurotransmitter 48–49. A more global (not just in the pontine brainstem where REM is orchestrated) cholinergic deficiency would predict a more permissive environment for slow wave generation and could conceivably be represented by a higher percentage of this state, which was, in fact, what we found in the autism group.
In summary, the extant literature supports a far-reaching hypothesis that a primary cholinergic deficiency may simultaneously produce deficits in REM sleep in autism and contribute to the social-emotional deficits that are at the core of the autism phenotype both directly and indirectly (through the lack of appropriate developmental support provided by REM sleep early in development). Future studies should attempt to disentangle these possibilities as a means of linking the profound social and emotional processing deficits that characterize autism to a fundamental deficit in physiological regulation. Should such a link be discovered, it would provide an immediate target for pharmacologic therapies and could also lead to the development of an objective biomarker for identification of infants at-risk for autism.
Acknowledgments
The authors report no potential conflicts of interest. All research funded by NIH intramural research program. The first author acknowledges full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank Allan Rechtschaffen, PhD, for critical insights and helpful discussions.
Contributor Information
Ashura Williams Buckley, National Institute of Mental Health, New York University
Alcibiades J. Rodriguez, New York University
Kaitlin Jennison, National Institute of Mental Health.
Jack Buckley, New York University
Audrey Thurm, National Institute of Mental Health
Susumu Sato, National Institute of Neurological Disorders and Stroke
Susan Swedo, National Institute of Mental Health
References
- 1.Rapin I. Autism. N Engl J Med. 1997;337:97–104. doi: 10.1056/NEJM199707103370206. [DOI] [PubMed] [Google Scholar]
- 2.Penn HE. Neurobiological correlates of autism: A review of recent research. Child Neuropsych. 2006;12:57–79. doi: 10.1080/09297040500253546. [DOI] [PubMed] [Google Scholar]
- 3.Johnson K, Malow BA. Assessment and pharmacologic treatment of sleep disturbance in autism. Child Adolesc Psychiatric Clin N AM. 2008;17:773–785. doi: 10.1016/j.chc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Richdale AL. Sleep problems in autism: prevalence, cause, and intervention. Develop Med Child Neurol. 1999;41:60–6. doi: 10.1017/s0012162299000122. [DOI] [PubMed] [Google Scholar]
- 5.Malow BA. Sleep disorders, epilepsy, and autism. Mental Retardation and Developmental Disabilities, Research Reviews. 2004;10:122–125. doi: 10.1002/mrdd.20023. [DOI] [PubMed] [Google Scholar]
- 6.Patzold LM, Richdale AL, Tonge BJ. An investigation into sleep characteristics of children with autism and asperger’s disorders. Journal of Paediatrics and Child Health. 1998;34(6):528–533. doi: 10.1046/j.1440-1754.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- 7.Stores G, Wiggs L. Abnormal sleep patterns associated with autism: A brief review of research findings, assessment methods and treatment strategies. Autism. 1998;2:157–169. [Google Scholar]
- 8.Gail Williams PG, Sears LL, Allard A. Sleep problems in children with autism. Journal of Sleep Research. 2004;13:265–268. doi: 10.1111/j.1365-2869.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- 9.Segawa M, Katoh M, Katoh J, Nomura Y. Early modulation of sleep parameters and its importance in later behavior. Brain Dysfunction. 1992;5:211–223. [Google Scholar]
- 10.Goodlin-Jones BL, Tang K, Liu J, Anders TF. Sleep patterns in preschool-age children with autism, developmental delay, and typical development. J Am Acad Child Adolesc Psychiatry. 2008;47(8):930–8. doi: 10.1097/CHI.ObO13e3181799f7c. [DOI] [PubMed] [Google Scholar]
- 11.Meyers SM, Johnson CP. Management of children with autism spectrum disorders. Pediatrics. 2007;120:5. doi: 10.1542/peds.2007-2362. [DOI] [PubMed] [Google Scholar]
- 12.Kryer MH, Roth T, Dement W. Principles and Practice of sleep medicine. 4. Philadelphia: Elsevier Saunders; 2005. [Google Scholar]
- 13.Tanguay PE, Ornitz EM, Forsythe AB, Ritvo ER. Rapid eye movement (REM) activity in normal and autistic children during REM sleep. J Autism Child Schizophr. 1976;6(3):275–288. doi: 10.1007/BF01543468. [DOI] [PubMed] [Google Scholar]
- 14.Diomedi M, Curatolo P, Scalise A, Placidid F, Caretto F, Gigli GL. Sleep abnormalities in mentally retarded autistic subjects: Down’s syndrome with mental retardation and normal Subjects. Brain Dev. 1999;21:548–553. doi: 10.1016/s0387-7604(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 15.Elia M, Ferri R, Musumeci SA, Del Gracco S, Bottitta M, Scuderi C, Miano G, Panerai S, Bertrand T, Grubar JC. Sleep in subjects with autistic disorder: A neuropsychological and psychological study. Brain and Development. 2000;22: 2(14):88–92. doi: 10.1016/s0387-7604(99)00119-9. [DOI] [PubMed] [Google Scholar]
- 16.Thirumalai SS, Shubin RA, Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurology. 2002;17(3):173–178. doi: 10.1177/088307380201700304. [DOI] [PubMed] [Google Scholar]
- 17.Johnson KP, Gianotti F, Cortesi F. Sleep paterns in autism spectrum disorders. Child adolesc psychiatric clin N Am. 2009;18:917–928. doi: 10.1016/j.chc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Medicine Reviews. 2009;13:403–411. doi: 10.1016/j.smrv.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- 20.Lord C, Rutter M, le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 21.Owens J, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM Manual Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. 1. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 23.Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann Math Sta. 1963;34:598–611. [Google Scholar]
- 24.McGrath MJ, Cohen DB. REM sleep facilitation of adaptive waking behavior: a review of the literature. Psychol Bull. 1978;85:24–57. [PubMed] [Google Scholar]
- 25.Mirmiran M, van den Dungenn H, Uylings H. Sleep patterns during rearing under different environmental conditions in juvenile rats. Brain Research. 1982;233:287–298. doi: 10.1016/0006-8993(82)91203-3. [DOI] [PubMed] [Google Scholar]
- 26.Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Suppression of hippocampus-related gene expression by sleep deprivation in rats. J Physiol. 2006;575(3):807–19. doi: 10.1113/jphysiol.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25(17):4365–9. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Datta S, Mavanji V, Ulloor J, Patterson EH. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2004;24(6):1416–27. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaffery JP, Sinton CM, Bissette G, Roffwarg HP, Marks GA. Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience. 2002;110(3):4331–43. doi: 10.1016/s0306-4522(01)00589-9. [DOI] [PubMed] [Google Scholar]
- 30.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437(27) doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 31.Maquet P. The role of sleep in learning and memory. Science. 2001;294(2) doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 32.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nature Reviews/Neuroscience. 2002;3:679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 33.Hobson JA. Sleep. New York, NY: Scientific American Library; 1989. [Google Scholar]
- 34.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:602–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 35.Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg Howard. A functional role for REM sleep in brain maturation. Behavioral Brain Research. 1995;69(1995):1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- 36.Hobson A. REM sleep and dreaming: towards a theory of protoconsciousness. Nature Reviews/Neuroscience. 2009;10:803–813. doi: 10.1038/nrn2716. [DOI] [PubMed] [Google Scholar]
- 37.Vogel G, Neill D, Kors D, Hagler M. REM sleep abnormalities in a new animal model of endogenous depression. Neurosci Biobehav Rev. 1990;14(1):77–83. doi: 10.1016/s0149-7634(05)80163-0. [DOI] [PubMed] [Google Scholar]
- 38.Vogel G, Hagler M. Effects of neonatally administered iprindole on adult behaviors of rats. Pharmacol Biochem Behav. 1996;55(1):157–61. doi: 10.1016/0091-3057(95)02286-4. [DOI] [PubMed] [Google Scholar]
- 39.Lopez J, Roffwarg H, Dreher A, Bissette G, Karolwicz, Shaffert J. Rapid eye movement sleep deprivation decreases long-term potentiation stability and affects some glutamatergic signaling proteins during hippocampal development. Neuroscience. 2008;153:44–5. doi: 10.1016/j.neuroscience.2008.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 41.Marcus CL. Sleep-disordered breathing in children. AmJ Respir Crit Care Med. 2001;164:16–30. doi: 10.1164/ajrccm.164.1.2008171. [DOI] [PubMed] [Google Scholar]
- 42.McMarley RW. Neurobiology of REM and NREM sleep. Sleep medicine. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Bauman ML, Kemper TL. The Neurobiology of Autism. Baltimore, MD: Johns Hopkins University Press; 1994. Neuroanatomic observations of the brain in autism. [Google Scholar]
- 44.Perry E, Mandy LW, Martin-Ruiz CM, Court JA, Volsen SG, Folly E, Iversen P, Bauman ML, Perry RH, Wenk GL. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry. 2001;158:1058–1066. doi: 10.1176/appi.ajp.158.7.1058. [DOI] [PubMed] [Google Scholar]
- 45.Lee M, Martin-Ruiz A, Graham A, Court J, Jaros E, Perry R, Iversen P, Bauman M, Perry E. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain. 2002;125:1483–1495. doi: 10.1093/brain/awf160. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Ruiz CM, Lee M, Perry RH, Bauman M, Court JA, Perry EK. Molecular analysis of nicotinic receptor expression in autism. Molecular Brain Research. 2004;123:81–90. doi: 10.1016/j.molbrainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Hohmann CF, Berger-Sweeney J. Cholinergic regulation of cortical development and plasticity: new twists to an old story. Perspect Dev Neurobiol. 1998;5:401–425. [PubMed] [Google Scholar]
- 48.Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10(8):2541–59. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rainnie DG, Heinz CR, Grunze, McCarley RW, Greene RW. Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science New Series. 1994;263(5147):689–692. doi: 10.1126/science.8303279. [DOI] [PMC free article] [PubMed] [Google Scholar]