Abstract
Spontaneous bladder contractions (SBCs) in the neonatal rat urinary bladder change from a high-amplitude, low-frequency pattern to a low-amplitude, high-frequency pattern during the first 6 wk of life. Understanding the mechanism of this developmental change may provide insights into the causes of bladder overactivity in adults. In vitro whole bladder preparations from Sprague-Dawley rats were used to study the modulation of SBCs by calcium-activated potassium channels (KCa) and electrical field stimulation from 3 days to 6 wk of life. SBCs in 3-day-old bladders were unmasked by treatment with iberiotoxin (100 nM), an inhibitor of large conductance KCa (BK) channels, or apamin (100 nM), an inhibitor of small conductance KCa (SK) channels. Iberiotoxin significantly increased the magnitude of SBCs at 2–3 wk, whereas apamin was only effective at 6 wk. In 1–2 wk bladders, exposure to room temperature Krebs solution decreased SBCs. This decrease was reversed by activating intramural nerves with electrical field stimulation. The effect of electrical field stimulation was inhibited by atropine (1 µM), suramin (10 µM), or pretreatment with tetrodotoxin (1 µM) but was not reversed by tetrodotoxin applied after electrical field stimulation. BK-α mRNA increased threefold, and BK-α protein increased fivefold from 3 days to 6 wk. These data suggest that BK channels play an important role in the regulation of SBCs in the neonatal bladder and that both increased BK channel activity, as well as changes in smooth muscle sensitivity to locally released neurotransmitters contribute to the downregulation of SBCs during early postnatal development.
Keywords: large-conductance KCa channel, small-conductance KCa channel, cholinergic, purinergic
The Neural Control and Contractility of the urinary bladder undergo marked changes during postnatal development. Healthy human infants exhibit low bladder capacity, high voiding pressure, and interrupted voiding during the first 18 mo of life (33). Urine storage and elimination become more efficient in older children as voluntary control of the bladder emerges at 3–5 years of age. In animals (rats, dogs, and cats), lower urinary tract function also changes dramatically during the early postnatal period. Voiding is initially controlled by a perigenital-bladder spinal reflex pathway, which is activated when the mother licks the perigenital region. This control is replaced by a supraspinal bladder-to-bladder reflex within the first 3–6 postnatal weeks (1, 9–11, 27, 28, 29, 34).
During the first 6 postnatal weeks, the bladder smooth muscle also exhibits prominent changes in intrinsic activity. In vitro preparations of whole bladders or bladder strips from 1-wk-old rats generate high-amplitude, low-frequency phasic contractions in the absence of neural or electrical stimulation (35, 37). These in vitro spontaneous bladder contractions (SBCs) change to a low-amplitude, high-frequency pattern at 5–6 wk of age (37). The mechanism for this maturational change is unknown but could be related to changes in neural control of smooth muscle contractility, communication between smooth muscle cells via gap junctions, or the activity of pacemaker cells (5).
In this study, we used in vitro whole bladder preparations from neonatal rats to examine the contribution of calcium-activated potassium channels (KCa) and neural mechanisms to the modulation of SBCs from 3 days to 6 wk of life. KCa channels were of particular interest because they have been shown to play a key role in the regulation of SBCs in adult mouse bladders (20, 24, 30, 39). For example, enhanced SBCs and increased sensitivity to electrical field stimulation (EFS) and carbachol occurred in bladder strips from mice with deletion of the α subunit of the large-conductance KCa channel (BK) (30). On the other hand, transgenic mice conditionally overexpressing the small conductance type 3 KCa channel (SK3) have decreased SBCs compared with control mice. When the expression of the SK3 transgene was turned off, the SBCs returned to normal (24, 30). Blocking KCa channels with drugs also markedly increased SBCs in preparations from adult animals (24, 39). Our data suggest that BK, but not SK, channels play an important role in regulating SBCs in the neonatal rat bladder and that during postnatal maturation, the high-amplitude intrinsic contractile activity of the neonatal rat bladder is suppressed by an upregulation of inhibitory control mediated via BK channels and a progressive decrease in sensitivity to locally released excitatory neurotransmitters.
MATERIALS AND METHODS
Chemicals
All chemicals used for the bathing solution of the in vitro whole bladder preparation were obtained from Sigma (St. Louis, MO). Atropine sulfate (muscarinic receptor antagonist), suramin (purinergic antagonist), TTX (Na+ channel blocker), and apamin (SK blocker) were obtained from Sigma. Iberiotoxin (IBTX, BK blocker) was obtained from Alomone Laboratories (Jerusalem, Israel). IBTX and apamin were dissolved in deionized water as a 0.1 mM stock solution.
In vitro whole bladder preparation
All procedures for euthanasia and tissue collection were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Three day to six-wk-old Sprague-Dawley rats were anesthetized with halothane and killed by cervical dislocation. A midline incision was made in the abdomen. The bladder was exposed and removed by cutting at the bladder neck. A 26-gauge syringe needle connected to polyethylene (PE)-50 tubing was filled with Krebs solution (113 mM NaCl, 19.8 mM NaHCO3, 11.1 mM dextrose, 1.2 mM KH2PO4, 4.7 mM KCl, 2.5 mM MgCl2, 1.7 mM CaCl2). The needle was inserted at the neck of the isolated bladder and held in place with 5–0 silk sutures. The needle was connected to an infusion pump and a pressure transducer by polyethylene tubing and a three-way stopcock. The bladder was placed between two platinum stimulating electrodes (Radnoti Glass, Monrovia, CA) in a 15-ml organ bath filled with Krebs solution maintained at 37°C and bubbled with 95% O2-5% CO2. Bladder pressure was recorded by WinDaq Acquisition software (version 2.13 for Windows, Akron, OH). After a 30-min equilibration period, the bladder was filled slowly with Krebs solution in 50-µl increments until the bladder contraction evoked by EFS was maximal. EFS was delivered from a Grass S88 stimulator (Grass Instruments, Quincy, MA) to the platinum electrodes in the organ baths. Our pilot experiments indicated that stimulation at intensities greater than 50 V at 32 Hz in the presence of 1 µM TTX (sodium channel blocker) in 3-day to 6-wk-old bladders could cause direct muscle stimulation. To measure only the neurally evoked contractions using EFS, stimulations of 50 V at 32 Hz (1.6-ms duration) were applied repeatedly for about 10–15 s after each filling to determine the optimal bladder filling volume for maximum EFS-evoked contractions. The use of EFS at 50 V for neurally evoked contractions is consistent with previous studies (6).
After the optimal bladder volume for maximum EFS-evoked contractions was determined, the distended bladder was equilibrated for another 15 min to measure baseline spontaneous activity followed by application of 80 mM KCl. The peak amplitude of the spontaneous contractions was normalized as a percentage of the maximal KCl evoked contraction amplitude. The bladders were then washed with fresh room temperature Krebs solution three times, and then equilibrated at 37°C for another 15 min. Pharmacological agents were sequentially applied to the bath 15 min later. When the combined effect of two agents was being studied, no washes were used between application of the two agents. Apamin (100 nM), atropine (1 µM), IBTX (100 nM), and suramin (10 µM) were used at concentrations reported in previous experiments studying spontaneous contractions to selectively inhibit the target receptor or channel in adult bladders (5, 20, 21, 30, 39). Spontaneous contractions were measured for at least 15 min after each agent. Using the criteria proposed by Imai et al. (25), we identified a single spontaneous contraction as a response with an amplitude of at least 30% of the peak spontaneous contraction occurring during the 15-min observation period. Also, when a contraction was superimposed on the previous event before reaching baseline, the two contractions were considered as a single contraction event. Frequency was determined by counting the number of contractions over a 5-min interval, starting 10 min after a drug was given. The magnitude of spontaneous contractions was estimated as average peak amplitude of a series of contractions or by integrating the area under the curve (AUC) of contractions occurring during a 5-min period.
Semiquantitative RT-PCR assay
Total RNA was isolated from bladders of different ages with Trizol reagent (Invitrogen Carlsbad, CA). The total RNA was further purified by RNAeasy kit (Qiagen, Valencia, CA). One microgram of total RNA was then treated with recombinant bovine RNase-free DNase I (Ambion, Austin, TX) in a 10-µl reaction. The DNase I was inactivated by adding 25 mM EDTA, followed by heating at 65°C for 15 min. Omniscript Reverse Transcriptase (Qiagen, Valencia, CA), ribonuclease inhibitor RNaseOUT (Invitrogen, Carlsbad, CA), 10× RT buffer (Qiagen, Valencia, CA) and oligo-dT primer were added to the reaction mix, and the volume was adjusted to 20 µl with diethyl pyrocarbonate-treated water. The reaction was incubated at 37°C for 1 h and terminated by heating at 95°C for 5 min. Two µl of the first strand cDNA reaction was used in each polymerase chain reaction. Each reaction consisted of 200 µM dNTPs (Genechoice, Frederick, MD), 1.5 mM MgCl2, 0.2 µM gene-specific primer pairs, 2.5 U Taq DNA polymerase (Promega, Madison WI), and 1 × PCR reaction buffer (Promega, Madison WI). GAPDH mRNA in each bladder total RNA sample was used as the loading control among different samples. Reaction without any RNA was used as the negative control for these semiquantitative RT-PCR studies. Thermocycling for the PCR was carried out by a PTC-200 DNA Engine Cycler (MJ Research, Waltham, MA). The reaction mix was first denatured at 92°C for 2 min. The thermocycler program for each gene-specific primer set consisted of 30–40 cycles of a denaturing step of 92°C for 30 s, an annealing step of 55–60°C for 1 min (see Table 1), and an extension step 72°C for 1 min. Afterward, an additional extension at 72°C for 5 min was included. The primer pairs for BK and SK channel subunits were synthesized by Sigma Genosys (The Woodlands, TX). The primer pairs, their corresponding melting temperatures, the annealing temperature, and the numbers of cycles are listed in Table 1. Ten microliters of the PCR products were resolved by 2% (wt/vol) agarose gel electrophoresis along with Multiple-Choice Quantitative DNA ladder II (Genechoice, Frederick, MD). The gel was then stained with 1 × SYBR Gold stain (Molecular Probes, Eugene, OR). The PCR product bands on the stained gel were imaged with a Kodak Image Station 1000 (Eastman Kodak, New Haven, CT). The band intensities were quantified by 1D Capture software (version 3.6 for Windows; Eastman Kodak). To verify the identity of the PCR product, the PCR products from two different time points were first purified by QIA-quick PCR purification kit (Qiagen, Valencia, CA), and the base sequence of the products was determined by the DNA Sequencing Core Facilities of the University of Pittsburgh Molecular Medicine Institute.
Table 1.
Primer sets used in PCR
| Target mRNA | Sequence 5′ → 3′ | Tm, °C | Annealing Temperature, °C | Number of Cycles |
|---|---|---|---|---|
| SK1 | CTGTGGGAAGGGCGTGTGTCT (sense) | 70.8 | 60 | 30 |
| CGAACCCGGCTTTGGTCTG (antisense) | 70.0 | |||
| SK2 | ATGCCCCTTCCACAACCACTGC (sense) | 73.1 | 55 | 30 |
| CACTACGGCTACCACCAAG (antisense) | 60.8 | |||
| SK3 | CAAGAACGCTGCCGCAATGTC (sense) | 74.6 | 55 | 30 |
| CCAGGCTGCCATCTGCTTTTC (antisense) | 70.8 | |||
| BK channel | CGCCATTAAGTCGGCTGAT (sense) | 64.9 | 55 | 40 |
| α-subunit | GACGGCAAATGCTGTCCC (antisense) | 66.8 | ||
| BK channel | TGTGTACCAGCGCCTCTATG (sense) | 63.8 | 58 | 30 |
| β-subunit | CAGAGAGGGACCTGTTGAGC (antisense) | 64.0 | ||
| GAPDH | AAACCCATCACCATCTTCC (sense) | 61.2 | 55–60 | 30–40 |
| GCCTGCTTCACCACCTTCT (antisense) | 64.4 |
Sequences of the small conductance KCa (SK)1–3 primers were published by Tamarina et al. (38), and the large conductance KCa (BK)-α primers are from Gauthier et al. (13). The GAPDH mRNA was amplified as the loading control with the same annealing temperature and the cycle numbers of the target mRNAs.
Western blot analysis
Neonatal bladders (three bladders for 3- to 4-day-old and 1-wk-old animals; one bladder for 2- to 6-wk-old animals) were homogenized in 500-ml ice-cold homogenization buffer (20 mM EDTA, 100 mM Tris · HCl, pH 7.4 with 1 mM PMSF, 1 mM DTT, 3 µg/ml aprotinin, 3 µg/ml pepstatin A, and 1 µg/ml leupeptin) with a hand-held tissue grinder. The homogenate was incubated on ice for 30 min. Cell debris was removed from the homogenate by centrifuging at 3,000 g for 10 min at 4°C. The supernatant was further centrifuged at 10,000 g for 1 h at 4°C. The pellet was then suspended in the homogenization buffer containing 2% (wt/vol) SDS as the crude membrane preparation. The protein content in the membrane preparations was determined by the DC Protein Assay Reagent Kit (Bio-Rad Laboratories, Hercules, CA). The membrane preparations of each time point equivalent to 30-µg protein were resolved in a 7.5% (wt/vol) SDS-PAGE along with Precision Plus Protein Standards (Bio-Rad Laboratories). This amount of protein was determined to be in the linear range for detection. The Western blot analysis conditions were optimized for both the anti-BK antibody and secondary antibody concentrations. The resolved proteins were transferred from the gel to a nitrocellulose membrane (0.45-µm pore size; Bio-Rad Laboratories) for 1 h at room temperature with a constant voltage of 100 V. The membrane was blocked with 1% (wt/vol) milk in Tris-buffered saline-Tween [TBST; 0.05% (vol/vol) Tween-20, 25 mM Tris, 140 mM sodium chloride, 2.7 mM potassium chloride, pH 7.5] at room temperature for 1 h. The membrane was then incubated with 1:500 rabbit anti-BK channel α subunit antibody (Alomone Laboratories, Jerusalem, Israel) at 4°C overnight. After washing with TBST three times, the membrane was then incubated with 1:10,000 horseradish peroxidase-conjugated goat anti rabbit IgG antibody (Zymed, South San Francisco, CA) at room temperature for 1 h. The membrane was washed 3 times with TBST and 2 times with TBS (25 mM Tris, 140 mM sodium chloride, 2.7 mM potassium chloride, pH 7.5). The membrane was then developed by the ECL Plus Western Blotting Detection Reagents (Amersham Bio-sciences, Piscataway, NJ) and a BioMax XAR film (Eastman Kodak) was placed onto the membrane. Because epidermal growth factor receptor (EGFR) expression remains unchanged during postnatal bladder development (2), EGFR was used as an internal control for protein loading. The developed membrane was then stripped with 0.2 M sodium hydroxide, and reblotted with 1:300 sheep anti-EGFR (Upstate, Lake Placid, NY) and 1:8,000 horseradish peroxidase-conjugated goat anti-sheep IgG (Zymed, South San Francisco, CA). The EGFR signal was detected with the ECL Plus Western Blotting Reagents and the XAR films. The images of the BK channel α subunit and the EGFR signals on the developed film were scanned by a conventional desk-top scanner connected to a PC and quantified by the 1D Capture software (version 3.6 for Windows; Eastman Kodak). The specificity of the anti-BK channel α subunit antibody was verified by preincubation of the diluted antibody (1:500) with the blocking antigen peptide (3 µg per microgram antibody; Alomone Laboratories) for 1 h at room temperature. Both the preblocked primary antibody and the antigen peptide were then used for Western blot analysis as described above.
Statistical analysis
Data are expressed as mean (SD). Statistical analysis was performed with Prism 4 for Windows version 4.02 (GraphPad software, San Diego, CA). Comparisons between different time points were made with two-way ANOVA (regular or repeated) with Bonferroni posttests. One-way ANOVA with Tukey’s multiple comparison tests was also used for multiple sample comparison. Unpaired or paired two-tailed t-test was used as appropriate when two samples were compared. Statistical significance was defined as P < 0.05.
RESULTS
SBCs in 1- to 6-wk-old bladders were not observed during initial equilibration in the organ bath but gradually increased in amplitude as the bladders were filled and reached the maximal EFS-evoked contraction. SBCs were not observed during bladder filling in 3-day-old bladders. With increasing age, there was a steady rise in the volume required to evoke maximum EFS contractions during the first 6 postnatal weeks compared with 3- to 4-day-old bladders: 3–4 days: 84.6 µl (SD 8.7, n = 13); 1 wk: 176.9 µl (SD 13.4, n = 13, P < 0.05); 2 wk: 187.5 µl (SD 13.5, n = 16, P < 0.01); 3 wk: 227.3 µl (SD 42.8, n = 11, P < 0.001); and 6 wk: 300 µl (SD 42.8, n = 7, P < 0.001).
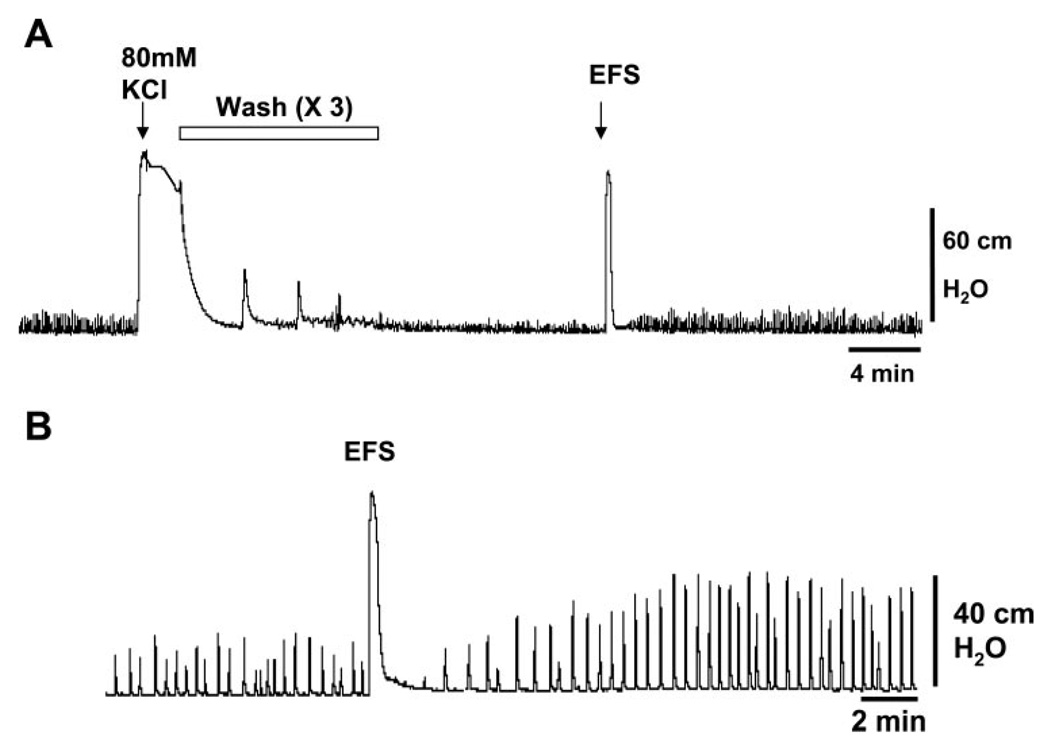
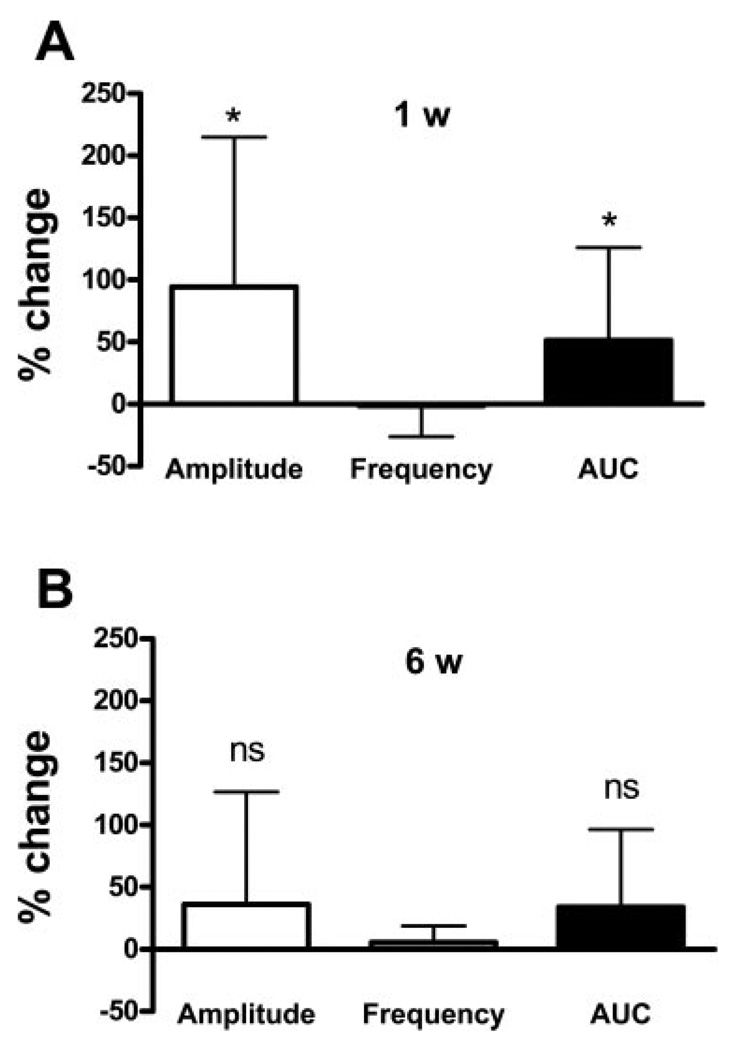
The contractile response to KCl was used to normalize the amplitude of SBCs. However, in the 1- to 3-wk-old bladders, SBCs were often decreased after KCl was washed out with room temperature Krebs (Fig. 1A). For 1- to 2-wk-old bladders, we found that 41% exhibited reduced amplitude SBCs after bath change, and 59% showed very small amplitude or no SBCs. Disappearance of SBCs after bath change was rarely seen in 6-wk-old bladders. We found that a subsequent application of EFS after washing out KCl could restore the level of SBCs back to the pre-KCl state (Fig. 1A). We also noted that EFS increased the amplitude and AUC of SBCs in the 1-wk-old bladders, which had not been exposed to EFS or KCl stimulation (n = 7, Figs. 1B, 2A). EFS did not significantly increase the amplitude and AUC in similarly treated 6-wk-old bladders (Fig. 2B). After EFS, the amplitude of SBCs peaked at 5 min, gradually decreased for 10 min, remained stable for at least 30 min, but then waxed and waned over the subsequent 45 min (n = 3). We did not carry out longer-term studies due to variability in SBCs after 45 min. To standardize our procedures between bladder preparations of different ages, we applied EFS to the bladders after KCl and allowed the SBCs to stabilize for at least 15 min before drug treatment, even if the SBCs persisted after washing out KCl.
Fig. 1.
Enhancement of spontaneous bladder contractions by electrical field stimulation (EFS) in the 1-wk-old bladder. A: spontaneous contractions occur in the absence of electrical or neuronal stimulation. KCl (80 mM) causes a large-amplitude contraction, and changing the bath solution reduces the amplitude of spontaneous contractions. The amplitude was restored by application of EFS (32 Hz, 50 V). B: EFS also increased contraction amplitude in a bladder not previously treated with EFS or KCl.
Fig. 2.
EFS-induced enhancement of spontaneous bladder contractions in 1-wk bladders, but not in 6-wk-old bladders. Basal level of spontaneous contractions was evoked by filling alone (1 wk: 200 µl, 6 wk: 300 µl), without using EFS to optimize the conditions for evoking spontaneous activity. Contractions were evaluated 15 min after EFS. Values are expressed as means (SD). A: EFS significantly increased the amplitude and area under the curve (AUC) of contractions in 1-wk-old bladders but did not significantly change the frequency of contractions, n = 7. *P < 0.05. The basal non-EFS-stimulated amplitude was 11.6 (7.7)% of KCl response, frequency was 15 (8) contractions in 5 min; AUC was 583 (241) cm H2O/s. B: EFS did not significantly change the amplitude, frequency, or AUC, of the spontaneous contractions in 6-wk-old bladders (n = 5). ns, not significant. The basal non-EFS-stimulated amplitude was 5.3 (4.2)% of KCl response; frequency was 35 (3.5) contractions in 5 min; AUC was 809 (632) cm H2O/s.
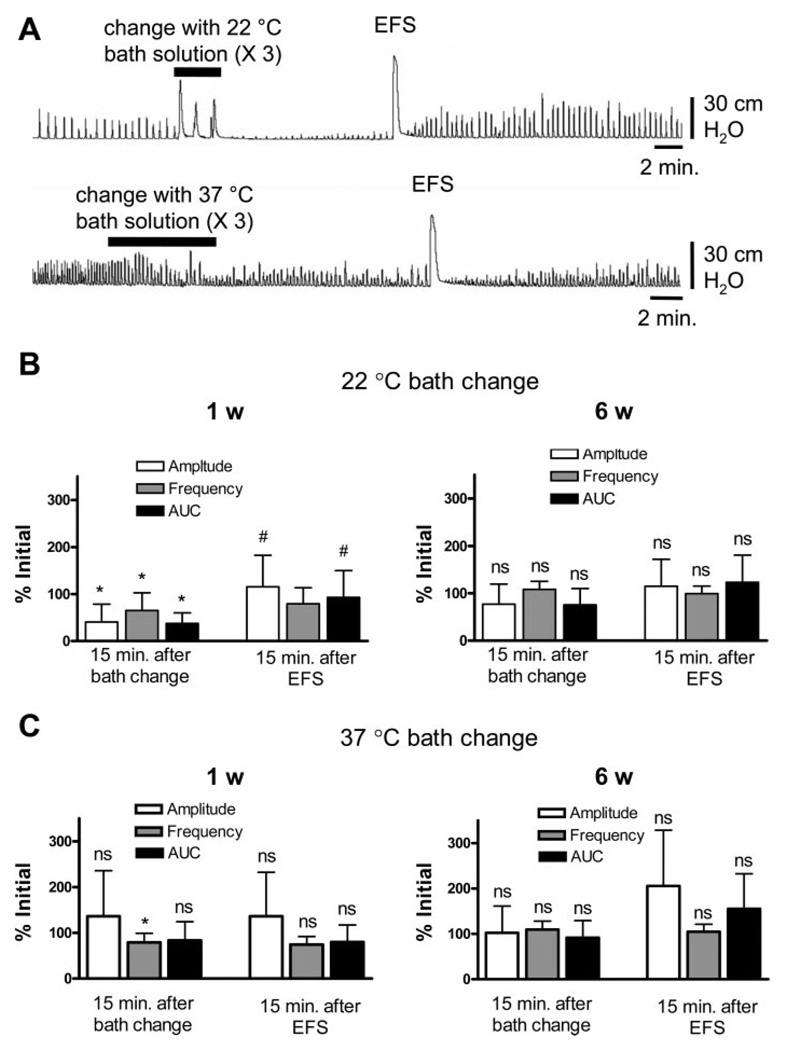
Because the washout after KCl-evoked contraction was performed with room temperature Krebs solution (22°C), washing with room-temperature solution was also evaluated. The addition of 22°C Krebs solution decreased the amplitude, frequency, and AUC of SBCs in the 1-wk-old bladders (Fig. 3A). Although the room temperature Krebs reached 37°C in the organ bath within 5 min, the SBCs did not return to previous levels, even when the temperature reached 37°C. Application of EFS after 22°C Krebs restored the amplitude and AUC of SBCs close to the initial values (Fig. 3B). Replacement of the bath solution with 37°C Krebs transiently reduced the frequency but not the amplitude of contractions. EFS after the 37°C bath change did not change the SBCs (Fig. 3C). Similar experiments carried out in the 6-wk-old bladders showed no significant drop in amplitude and AUC after 22°C Krebs (Fig. 3B) and no change after 37°C Krebs (Fig. 3C). Replacement of the bath solution with 37°C KCl (80 mM), followed by 37°C Krebs in the 1-wk-old bladders also did not change the amplitude or frequency of SBCs (n = 4, data not shown).
Fig. 3.
The temperature of the bath solution affects contractions in 1-wk-old bladders, but not in 6-wk-old bladders. A: changes in spontaneous contractions in a 1-wk-old bladder after changing the bath solution three times with fresh Krebs solution, and subsequent application of EFS after a 15-min equilibration period. Upper trace: bath change with 22°C Krebs solution. Lower trace: bath change with 37°C Krebs solution. B: flushing the bath with 22°C solution significantly reduced the amplitude, frequency, and AUC of contractions in the 1-wk-old bladder (n = 7) but not in the 6-wk-old bladders (n = 5) Values are expressed as means (SD). *P < 0.05 compared with the initial value. EFS restored the amplitude and the AUC in the 1-wk-old bladders but not in 6-wk-old bladders. #P < 0.05 compared with the value 15 min after the bath change. C: flushing the bath with 37°C solution decreased the frequency but not the amplitude or AUC of contractions in the 1-wk-old bladders (n = 7) but did not significantly change these parameters in the 6-wk-old bladder (n = 5). Values are mean (SD). *P < 0.05 compared with the initial value. Subsequent EFS did not significantly change the spontaneous contractions in 1-wk-old or 6-wk-old bladders.
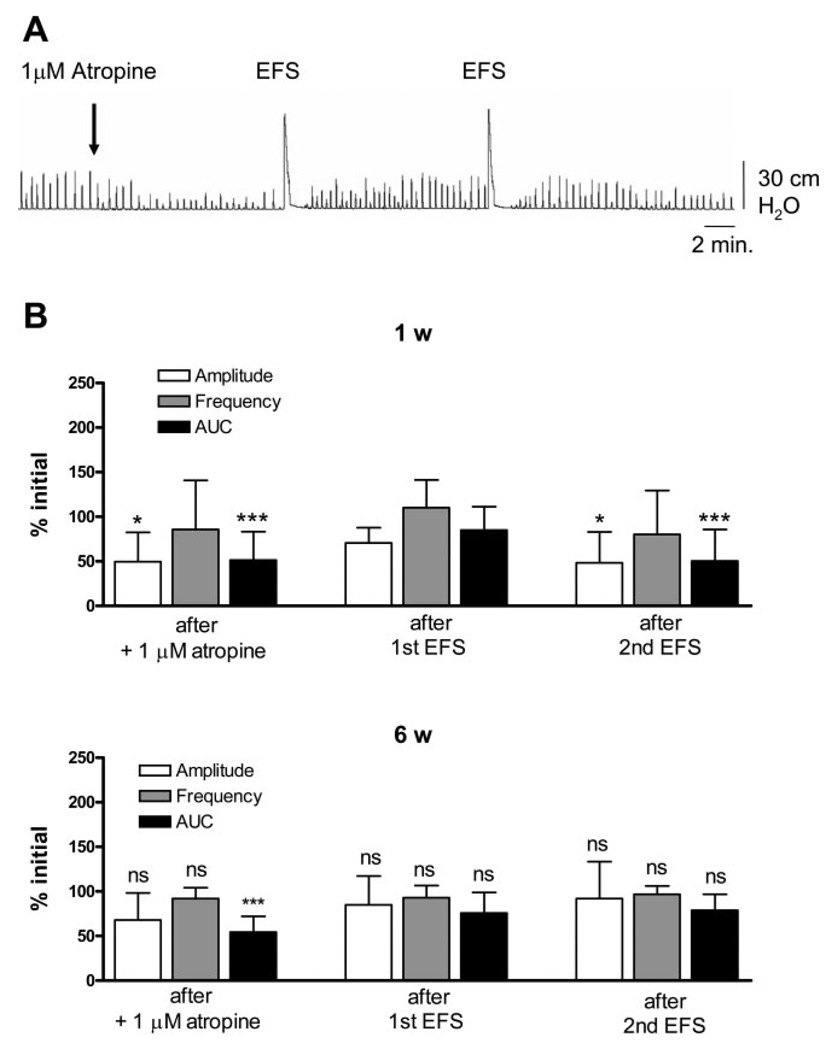
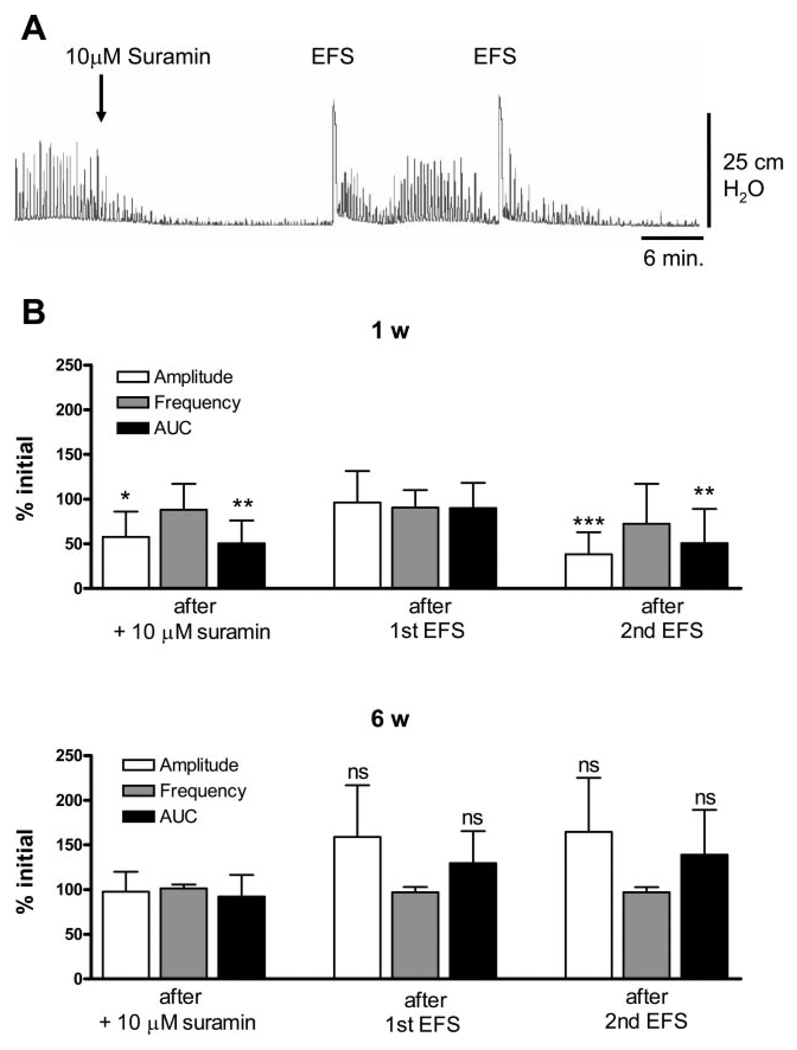
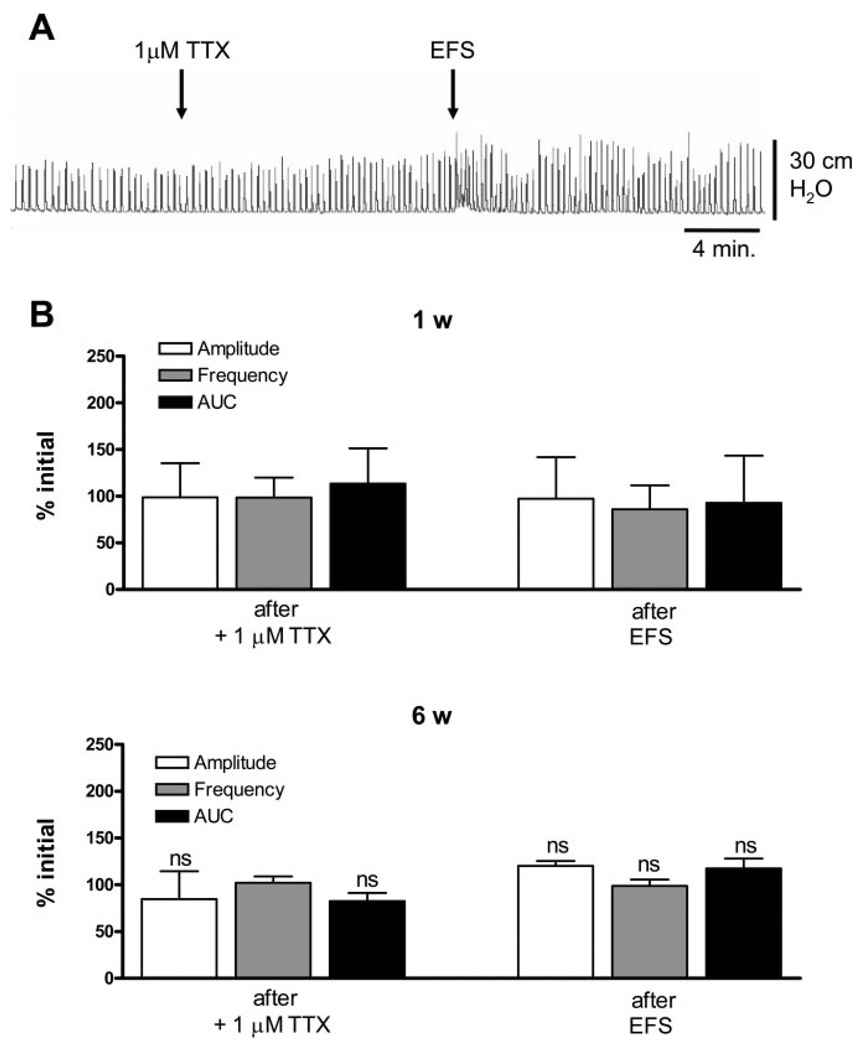
To evaluate the mechanisms underlying the facilitatory effects of EFS, several blocking agents were tested. Block of muscarinic or purinergic receptors significantly decreased the amplitude and AUC of EFS-enhanced SBCs in 1- to 2-wk-old bladders (Figs. 4A and 5A), but not in 6-wk-old bladders (Figs. 4B and 5B). Basal values for EFS-enhanced SBCs in 1- and 6-wk-old bladders are shown in Fig. 7. Atropine (1 µM, a muscarinic receptor antagonist) and suramin (10 µM, a purinergic P2 antagonist) reduced the amplitude and AUC by 50% in 1-to 2-wk-old bladders. The SBCs were partially and temporarily restored by a subsequent application of EFS but eventually decreased after a second EFS (Figs. 4B and 5B). In 6-wk-old bladders, there was a significant decrease in AUC of SBCs after atropine, but after one EFS application, the spontaneous contractions returned to basal levels (Fig. 4B). Suramin did not significantly affect the SBCs in 6-wk-old bladders. Furthermore, subsequent EFS after suramin did not significantly increase the amplitude and AUC of the SBCs (Fig. 5B). Inhibition of sodium channels by 1 µM TTX after EFS did not affect the basal level of EFS-enhanced SBCs in the 1- to 2-wk-old (Fig. 6, A and B) or the 6-wk-old bladders (Fig. 6B). However, pretreatment with TTX before EFS blocked the enhancement or unmasking of SBCs (Fig. 6, A and B).
Fig. 4.
Effects of atropine on EFS-enhanced spontaneous contractions. A: effect of 1 µM atropine on spontaneous contractions and subsequent EFS, on EFS-enhanced spontaneous contractions in the 1-wk-old bladder. B: amplitude and AUC are decreased by 1 µM atropine in the 1-wk-old bladder. The first EFS was able to restore the amplitude and AUC without affecting the frequency. However, the second EFS caused a decrease in amplitude and AUC (n = 11). Values are expressed as means (SD). *P < 0.05; ***P < 0.001. All statistics are for comparison with the basal level prior to atropine. The spontaneous contractions in the 6-wk-old bladders were only affected by a drop in AUC after 1 µM atropine, which was restored by one EFS (n = 4). ***P < 0.001 compared with basal level before atropine.
Fig. 5.
Effects of suramin on EFS-enhanced spontaneous contractions. A: effect of 10 µM suramin and subsequent EFS, on EFS-enhanced spontaneous contractions in the 1-wk-old bladder. B: amplitude and AUC are decreased by 10 µM suramin in the 1-wk-old bladder. The first EFS was able to restore amplitude and AUC without affecting the frequency. However, the second EFS caused a decrease in amplitude and AUC (n = 9). Values are expressed as means (SD). *P < 0.05; **P < 0.01, ***P < 0.001; All statistics are for comparison to basal level before suramin. C: spontaneous contractions in the 6-wk-old bladders were not affected by 10 µM suramin, (n = 4). Values are expressed as means (SD). ns, compared with basal level before suramin.
Fig. 7.
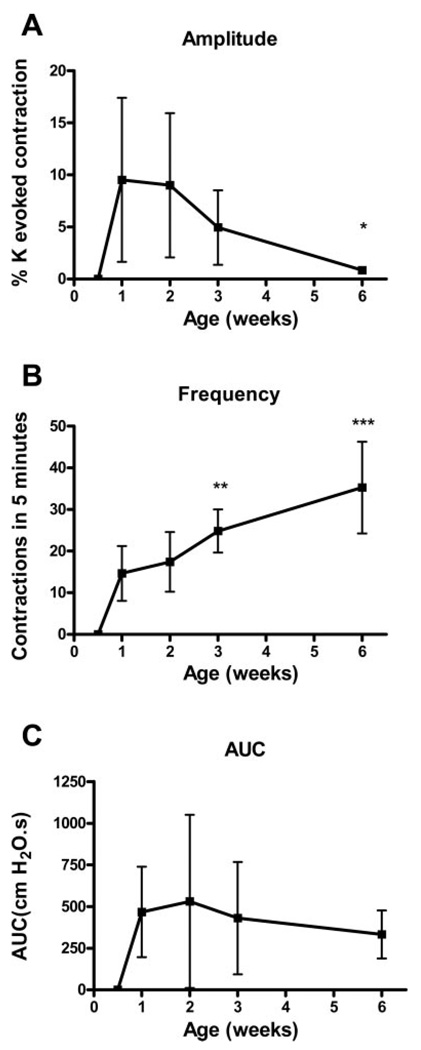
Changes in basal bladder spontaneous contractions from 3 days to 6 wk. Parallel decrease in amplitude (A) and increase in frequency (B) result in no net change in AUC (C) from 1 to 6 wk. n = 13 for 3 days, 12 for 1 wk, 13 for 2 wk, 11 for 3 wk, 8 for 6 wk. Values are mean (SD). *P < 0.05; **P < 0.01, ***P < 0.001. Comparisons are to 1-wk values.
Fig. 6.
Effects of TTX on EFS-enhanced spontaneous contractions. A: Effect of 1 µM TTX and subsequent EFS on EFS-enhanced spontaneous contractions in the 1-wk-old bladder. B: EFS-enhanced spontaneous contractions were not affected by 1 µM TTX in both the 1-wk-old (n = 6) and 6-wk-old bladder (n = 3). Subsequent EFS was not able to further enhance spontaneous contractions. Values are expressed as means (SD). ns, compared with basal level before TTX.
Spontaneous contractions during postnatal development
As reported in previous experiments (36), the amplitude of SBCs decreased from 1 wk to 6 wk of age (Fig. 7A), while the frequency increased over the same time period (Fig. 7B). The amplitude of the SBCs, even with EFS-induced enhancement, was much smaller than the EFS- and KCl-evoked contractions. The average amplitude was highest (9%) at 1 and 2 wk, then declined to 1% by 6 wk (P < 0.05, n ≥ 7). SBCs were not detected after EFS in 3-day-old bladders but did occur after application of KCa antagonists (Fig. 9A and see below).
Fig. 9.
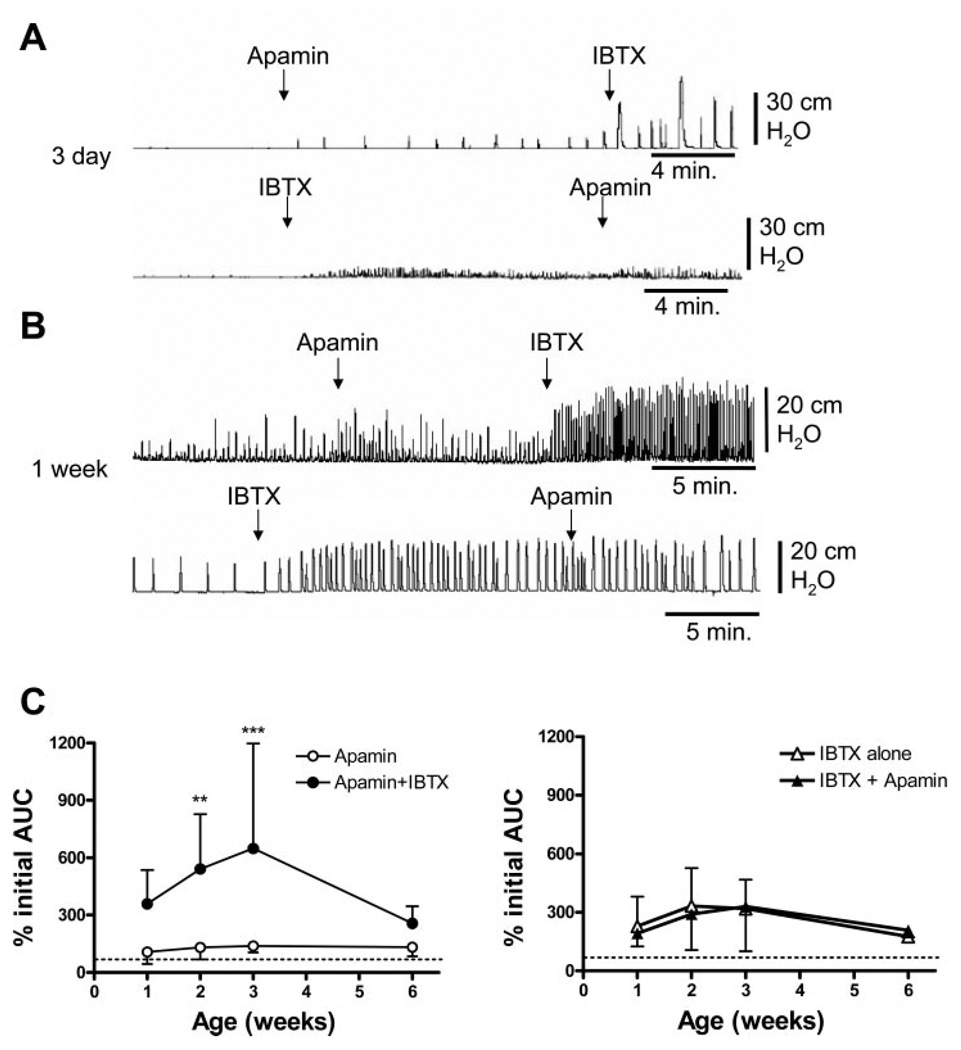
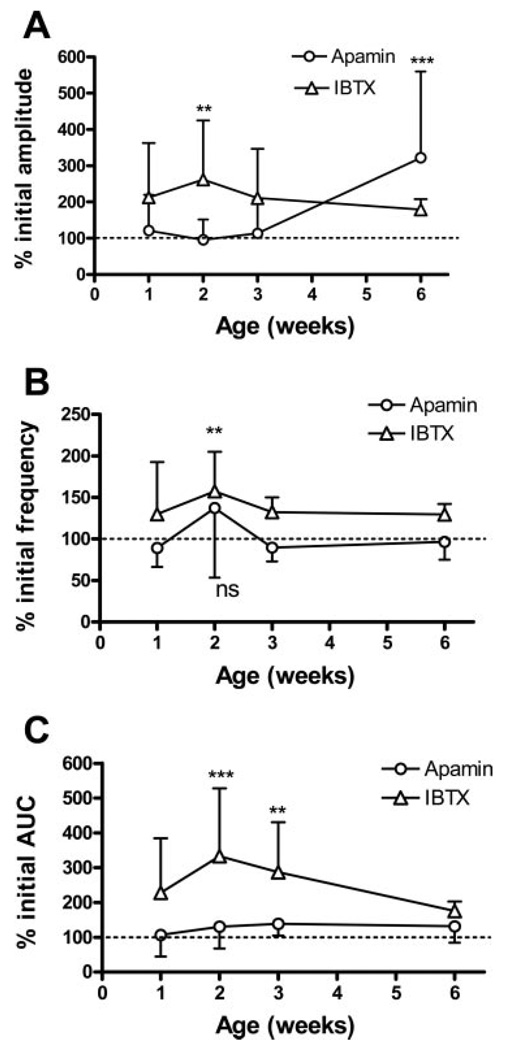
Combined small-conductance KCa (SK) and large-conductance KCa (BK) blockade. Both IBTX and apamin were used at 100 nM concentrations. A: effect of apamin followed by IBTX on spontaneous contractions in the 3-day-old bladder, then the effects of IBTX followed by apamin. B: effect of apamin followed by IBTX on spontaneous contractions in the 1-wk-old bladder, then the effects of IBTX followed by apamin. C: apamin followed by iberiotoxin results in a significantly larger AUC at 2 and 3 wk of life compared with apamin alone. **P < 0.01; ***P < 0.001. Dotted line indicates 100%. Iberiotoxin followed by apamin results in a similar increase in AUC compared with iberiotoxin alone. Dotted line indicates 100%. n = 8–12 animals at each time point. Values are expressed as means (SD). Apamin/IBTX is not significantly greater than IBTX/apamin.
SK and BK antagonism of spontaneous contractions
In 3- to 4-day-old bladders, SBCs were unmasked by the SK channel blocker apamin in a concentration (100 nM) shown to facilitate SBCs in adult bladders (5, 21) (Fig. 9A, n = 3). However, apamin did not change the amplitude or frequency of spontaneous contractions in 1- to 3-wk-old bladders (Fig. 8, A and B). Apamin increased the amplitude but not the AUC of contractions in 6-wk-old bladders [222.1% (SD 240) of control, n = 7; Fig. 8, A and C]. The BK channel blocker IBTX (100 nM) also unmasked spontaneous contractions in 3-day-old bladders (Fig. 9A, n = 5). IBTX increased both the amplitude and frequency in bladders from 1 to 6 wk of age (Fig. 8, A and B), with a statistically significant increase at 2 wk. IBTX alone increased the AUC of SBCs by threefold in 2- to 3-wk-old bladders (Fig. 9C), and the subsequent addition of apamin did not elicit a further increase. In preparations pretreated with apamin, which did not significantly affect the SBCs (Fig. 9C), the subsequent application of IBTX in 2- to 3-wk-old bladders increased the AUC by five- to sixfold (Fig. 9C). However, the effect of apamin followed by IBTX was not significantly greater than that of IBTX followed by apamin (P > 0.05).
Fig. 8.
Effects of KCa channel blockers on spontaneous contractions. Both iberiotoxin (IBTX) and apamin were used at 100 nM concentrations. Values are expressed as means (SD). A: IBTX increased the amplitude in 2 wk-old bladders, whereas apamin increased the amplitude in 6-wk-old bladders. B: IBTX increased the frequency in 2-wk-old bladders, whereas apamin did not affect frequency at any time. C: IBTX increased AUC in the 2- to 3-wk-old bladders, whereas apamin did not affect AUC at any time. Values are expressed as means (SD). **P < 0.01, ***P < 0.001, compared with basal level before either IBTX or apamin was given.
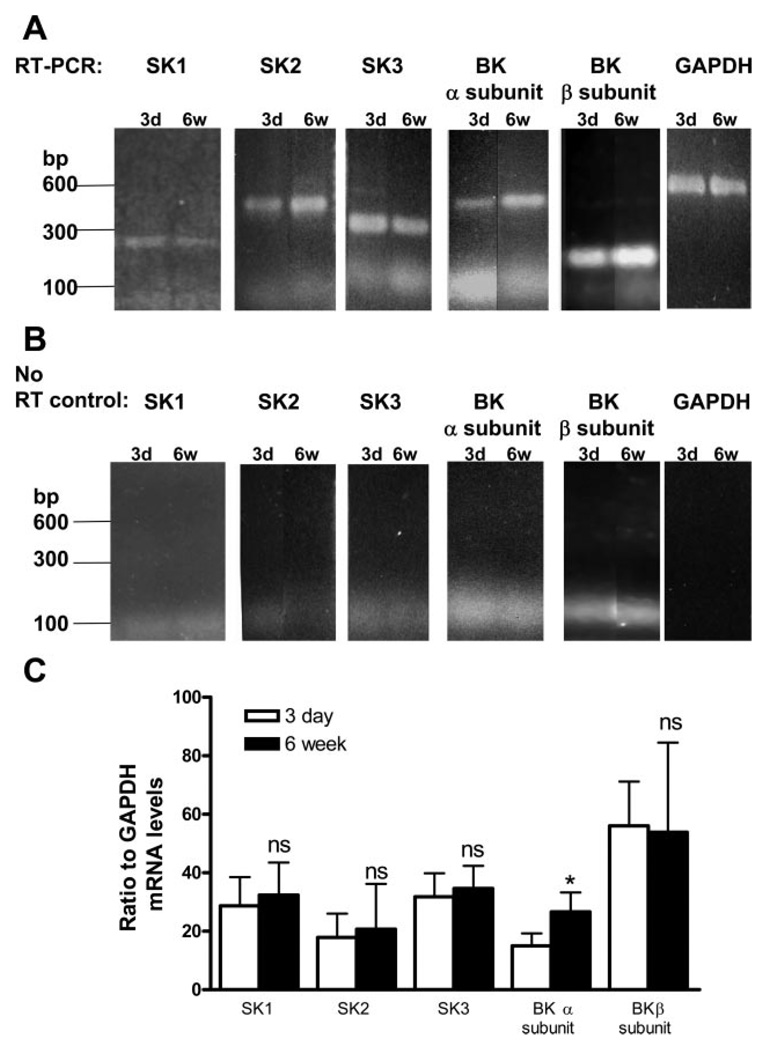
Expression of KCa channels during postnatal development
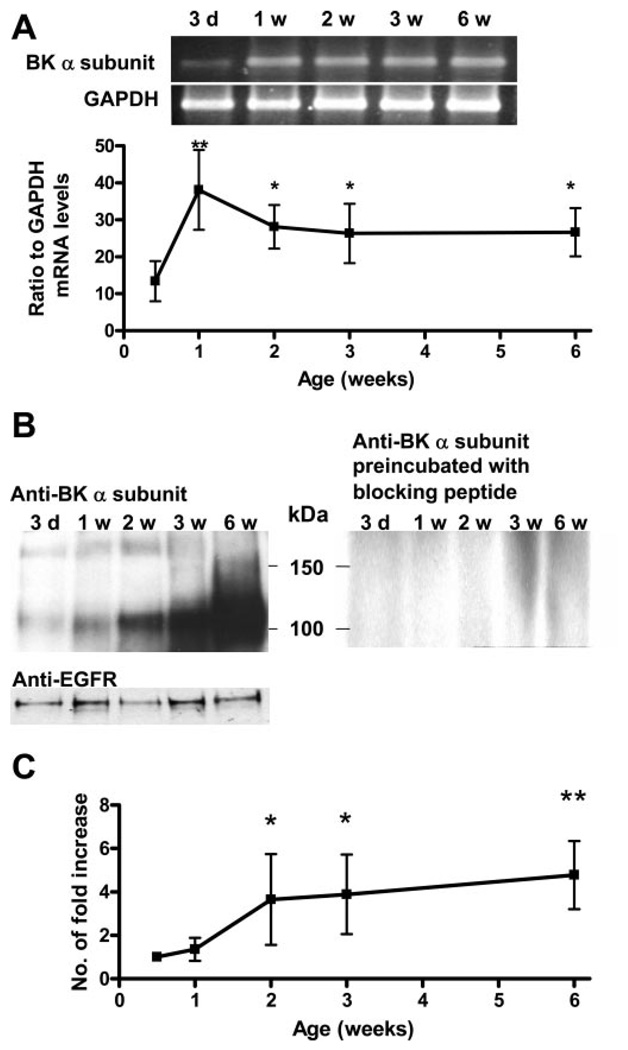
The steady-state mRNA levels of SK1, 2, and 3 did not change significantly between 3-day-old and 6-wk-old bladders (Fig. 10, A and C). For the BK channels, the mRNA levels of both the pore-forming α subunit and the regulatory α subunit were measured. The BK-α but not BK-α subunit transcript showed a significant increase between 3 days and 6 wk (Fig. 10C). The expression of the BK-α subunit transcript greatly increased within the first week and then was maintained at a steady level from 2 to 6 wk (Fig. 11A). A 120-kDa protein identified with an antibody for the BK-α subunit was detected starting on day 3 (Fig. 11B). When the membrane protein loading between samples was normalized with the EGFR protein level, the level of the 120-kDa protein progressively increased between the 1st and 6th wk (Fig. 11, B and C). The 120-kDa protein detection could be blocked by preincubation of the BK-α subunit antibody with the specific blocking peptide (Fig. 11B).
Fig. 10.
Expression of SK and BK in 3-day-old and 6-wk-old bladders. A: RT-PCR products detected from mRNA of SK1, 2, 3, and BK-α and -β subunits. The RT-PCR of GAPDH was used as an internal loading control among samples. B: negative controls of PCR using mRNA samples of SK1, 2, 3, and BK-α and -β subunits in the absence of RT (No-RT). C: quantitation of steady-state mRNA levels (expressed as ratio to GAPDH) of SK1, 2, 3 channels and BK channel α and β subunits between 3-day and 6-wk bladders. (n = 4 or 5 bladders per reaction). Values are expressed as means (SD). *P < 0.05, compared with day 3.
Fig. 11.
Upregulation of BK-α subunit during the first 6 postnatal weeks. A, top: RT-PCR of BK-α subunit in bladders of different ages. The RT-PCR product of GAPDH is shown as a loading control. Bottom: mRNA levels of the α subunit between 3 days and 6 wk (n = 5). Values are expressed as means (SD). *P < 0.05; **P < 0.01, compared with day 3. B: A 120-kDa protein equivalent to the BK-α subunit was detected from 3 days to 6 wk in the Western blot of the bladder membrane protein using anti-BK-α subunit primary antibody. The 120-kDa protein was not detected when the primary antibody was preincubated with the blocking peptide. The EGFR level was used as a loading control for the membrane protein at each time point. C: increase in the α subunit protein levels between 3 days and 6 wk (n = 4). Values are expressed as means (SD). *P < 0.05; **P < 0.01, compared with day 3.
DISCUSSION
The present experiments revealed that the SBCs in the in vitro whole bladder preparation, which are very prominent in 1- to 2-wk-old bladders and markedly decline in 6-wk-old bladders (35, 37) are modulated by electrical stimulation of intramural nerves and that this effect, which is mediated by cholinergic and purinergic mechanisms declines with age. The change in spontaneous contractile activity occurred in concert with an increased expression of BK, but not SK channels. Block of BK channels with IBTX enhanced SBCs in 1- to 6-wk-old bladders, most significantly at 2–3 wk, suggesting that tonic activation of BK channels modulates SBCs, and increased expression of BK channels might contribute to the postnatal maturation of bladder activity. It is concluded that changes in both neural mechanisms and expression of KCa channels in bladder smooth muscle might be involved in the downregulation of SBCs during postnatal maturation.
Neural modulation of spontaneous contractions in the neonatal bladder
Although SBCs in the neonatal rat bladder are myogenic and occur in the absence of neural stimulation, it is clear that they can be modulated by activation of muscarinic, purinergic, and other currently unidentified neurotransmitter receptors (14). SBCs in the in vitro brain stem-spinal cord-bladder preparation are tonically inhibited by a parasympathetic preganglionic efferent pathway arising in the lumbosacral spinal cord (34). The transmitter responsible for this inhibition is unknown. Carbachol, a muscarinic agonist, enhances SBCs in neonatal rat bladder strips. Atropine blocks the effect of carbachol and also reduces the amplitude of SBCs in the absence of carbachol, suggesting that there is a spontaneous release of ACh from bladder strips (36). Physostigmine, an anticholinesterase agent, also markedly enhances SBCs in neonatal rat whole bladders (32), suggesting that when metabolism of endogenous ACh in the bladder is blocked, ACh increases sufficiently to activate postjunctional muscarinic receptors and facilitate SBCs.
In the present experiments in 1- to 3-wk-old neonatal rat bladders, EFS elicited a temporary decrease in amplitude (Fig. 3A), followed by a subsequent increase in amplitude and AUC of SBCs, which developed over a period of several minutes. In untreated preparations, the amplitude of SBCs 15 min after EFS increased 100% over control in 1-wk-old bladders (Figs. 1B and 2A) but not in 6-wk-old bladders (Fig. 2B). These effects were blocked by TTX, indicating that they were mediated by activation of intramural nerves (Fig. 6, A and B). The long-lasting EFS enhancement of SBCs was reversed by atropine or suramin, indicating that EFS facilitates SBCs by releasing ACh and ATP, which then activate postjunctional muscarinic and purinergic receptors. It seems unlikely that the long-lasting facilitation of SBCs by EFS is only due to a brief release of ACh and ATP from postganglionic nerves because these neurotransmitters are rapidly metabolized by endogenous cholinesterases and nucleotidases. Thus a different mechanism, such as EFS-induced enhancement of spontaneous release of ACh or ATP from intramural nerves might be involved. TTX did not affect the amplitude or frequency of spontaneous contractions after they had been enhanced by EFS, suggesting that ongoing neural activity is not necessary for maintenance of established SBCs. However, ACh and ATP must be released continuously in the distended bladder after EFS, because application of atropine or suramin markedly decreased the amplitude and AUC of EFS-enhanced SBCs. Subsequent application of EFS in the presence of either antagonist elicited only a transient reversal of the blocking effect of the drugs (Figs. 4 and 5).
EFS also reversed a suppression of SBCs that occurred after washing the preparations with room temperature (22°C) Krebs solution. This effect of temperature change on SBCs was noted in a previous study, in which bath temperature was altered in 1°C steps between 19°C and 38°C (35). In 1- and 2-wk-old bladders, the amplitude of SBCs, as well as neurally evoked contractions was decreased at low temperatures, unlike 5- 6-wk-old and 6-mo-old bladders, where low temperatures unmasked or increased the amplitude of SBCs and increased neurally evoked contractions. Bladders from adult rats with chronic urethral outlet obstruction exhibited prominent SBCs and exhibited temperature responses similar to neonatal bladders. It was suggested that pathology can reverse the developmental changes in SBCs (4, 35).
The inhibitory effect of low temperature was first noted in the present experiments after washing out KCl, which was used to evoke maximal contractions in each preparation and to normalize the amplitudes of SBCs between different experiments. We initially hypothesized that this effect was due to the elimination of a facilitatory factor that had accumulated in the bath. However, subsequent experiments showed that the inhibition only occurred with 22°C solutions, not with 37°C solutions. In addition, even after the bath temperature recovered to 37°C, the SBCs did not recover to control levels in 1-wk-old bladders. These effects were not observed in 6-wk-old bladders. Thus a brief exposure to low temperatures has a prolonged suppressant effect on SBCs in 1-wk-old bladders, which can be reversed by the cholinergic and purinergic mechanisms activated by EFS (Fig. 3, A and B). Although the mechanism of low-temperature inhibition is unknown, it is possible that it is due to reduced metabolism of an inhibitory factor, which produces a long-lasting suppression of SBCs. Alternatively, low temperatures could suppress the spontaneous release of transmitters from intramural nerves, and high-frequency EFS could produce a long-lasting posttetanic potentiation of spontaneous transmitter release, as has been reported in peripheral and central synapses (43).
SK and BK antagonism
SBCs are initiated by spontaneous action potentials in the smooth muscle (5) that are thought to propagate throughout the bladder via intercellular connections (gap junctions) between muscle cells or via interstitial cells that provide a cellular link between the muscle cells. The upstroke of muscle action potentials is due to Ca2+ influx through voltage-dependent, nifedipine-sensitive l-type Ca2+ channels (17). This Ca2+ influx induces Ca2+ release from ryanodine-sensitive internal stores (Ca2+-induced Ca2+ release) and activates the actin-myosin contractile apparatus. Increased intracellular calcium also activates KCa channels, causing K+ efflux, repolarization of the membrane potential (mediated by BK channels) (19), and an after-hyperpolarization following the action potential (mediated by SK channels) (3, 8). The activity of KCa channels thus modulates contractility by regulating membrane potential and excitability. Both BK and SK channels are expressed in normal adult bladders and control electrical and contractile activities (5, 7, 15–18, 21–25, 30). The BK channel blocker IBTX enhances spontaneous contractions in adult bladder smooth muscle by increasing the amplitude and duration of spontaneous action potentials (15, 16). Blocking SK channels with apamin increases the amplitude while decreasing the frequency of spontaneous contractions by altering the pattern of the spontaneous action potentials (17, 21, 25). BK channels are both Ca2+ and voltage sensitive, whereas SK channels are Ca2+ sensitive but voltage insensitive (20). Compared with adult bladders, the contractions in neonatal bladders are more dependent on Ca2+ entry through l-type Ca2+ channels than Ca2+-induced Ca2+ release from the sarcoplasmic reticulum (40–42). How this difference in calcium-mediated contractions in neonatal bladders affects the function of KCa channels is unknown.
BK channels appear to be the major KCa channel regulating SBCs during the early postnatal period between 1 and 3 wk, when the bladder undergoes a major developmental change in its pattern of spontaneous activity. Block of BK channels with IBTX increased the amplitude and AUC of SBCs two- to threefold in 1- to 3-wk-old bladders and unmasked SBCs in 3-day-old bladders. Block of SK channels with apamin also unmasked spontaneous contractions in 3-day-old bladders and enhanced contractions in 6-wk-old bladders but did not change SBCs in bladders from 1–3 wk. The lack of an effect of apamin on SBCs between 1 and 3 wk was unexpected because RTPCR indicated stable expression of the SK gene from 3 days to 6 wk. In adult bladders, apamin enhances spontaneous contractions by converting single spontaneous action potentials into bursts of action potentials without changing the amplitude or time course of the potentials (15–17). Each burst of action potentials in apamin-treated bladder muscle was found to be coupled with a phasic contraction of higher amplitude and lower frequency than in untreated muscle (17). Apamin also increased the amplitude of SBCs in adult bladders (12, 21). Thus the failure of apamin to enhance SBCs in neonatal bladders raises the possibility that the burst pattern of action potentials is already present in the neonatal bladders and that blocking SK channels with apamin does not change the action potential pattern. On the basis of the differences between our findings in 1- and 3-wk-old bladders and other studies in adult bladders, it appears that SK channels become more important in controlling the activity of the bladder after 6 wk of age.
IBTX significantly enhanced the AUC of SBCs at 2–3 wk, the same time when levels of the BK channel α (pore-forming) subunit reached a maximum (Fig. 11C). The increase in BK-α protein occurred 1 wk later than the increase in BK-α mRNA, which peaked at 1 wk. This difference may be due to a time lag between the accumulation of mRNA and subsequent increased expression and membrane targeting of proteins. The BK-α regulatory subunit mRNA level remained stable during this period, suggesting that the α and β subunits are regulated differently in the bladder. This is not surprising, as other studies have shown that the expression of α and β subunits can be regulated independently in smooth muscle in response to hormonal stimulation (26, 31). BK channels contribute to the repolarization phase of the action potentials. Blocking BK channels increases action potential duration (5) and should increase Ca2+ influx, thereby increasing the amplitude of SBCs. While BK channel expression is maximal at 6 wk, the effect of IBTX blockade at 6 wk is not different compared with the effect at 1 wk (Fig. 8C), when BK-α protein expression is lowest (Fig. 11C). The larger effects of IBTX at 2- to 3-wk than at 6 wk may be partially due to the greater ability of the sarcoplasmic reticulum to buffer Ca2+ influx in older bladders (40–42).
In adult bladders, both apamin and IBTX decreased the frequency of SBCs, while increasing their amplitude (5, 21, 39). In this study, apamin did not change the frequency in 1- to 3-wk-old bladders, and IBTX significantly increased the frequency in 2-wk-old bladders. For adult bladders, it has been proposed that the decrease in contraction frequency after KCa channel blockade is due to release of Ca2+ from the sarcoplasmic reticulum via activation of ryanodine receptors (21). The released Ca2+ then either inactivates voltage-dependent Ca2+ channels or activates KCa channels to change membrane excitability. The failure of KCa channel blockers to decrease frequency of SBCs in neonatal bladders is consistent with the view that intracellular Ca2+ release mechanisms are not fully developed in the neonatal bladder.
In adult rat bladders, combined administration of apamin and IBTX produced large chaotic SBCs that were difficult to evaluate (21). In neonatal bladders, the minor role played by SK channels allowed for evaluation of synergistic interactions between SK and BK channels in 2- and 3-wk-old bladders. Apamin followed by IBTX produced a slightly larger increase (sixfold) (Fig. 9C) in AUC than IBTX followed by apamin (threefold), but this difference was not statistically significant (Fig. 9C). In adult mouse urinary bladders, simultaneous application of apamin and IBTX also produced additive effects on neurally evoked contractions (20). This was attributed to the drugs acting on two different mechanisms regulating cell membrane excitability and bladder contractility. However, because apamin and IBTX were added simultaneously to the organ bath, the effect of sequential blockade was not examined. It has been suggested that intracellular Ca2+ release limits contractility in the presence of apamin by the activation of BK (21). Thus blocking both channels produces a greater effect than blocking either one alone. The reason for the slightly greater effect of the IBTX-apamin combination treatment on AUC of SBCs in the 2- and 3-wk-old bladders when apamin is administered first is unknown.
Conclusions and clinical translation
Although the decrease in smooth muscle sensitivity to neurotransmitters and the increased expression of BK channel activity seem to contribute to the developmental downregulation of SBCs, other factors that inhibit cell-cell interaction via gap junctions or reduce the efficiency of pacemaker cells in the bladder may also play an important role in transforming the bladder from an overactive neonatal organ to an adult organ that stores urine efficiently. Understanding the mechanisms involved in the postnatal downregulation of spontaneous bladder contractions has clinical implications for both children and adults who exhibit overactive bladder dysfunction. Furthermore, although it is believed that the development of inhibitory mechanisms in the brain and spinal cord is essential for creating a stable adult bladder (36), it is also important to determine how these changes in the central nervous system are coordinated with the maturation of the bladder smooth muscle and peripheral neural pathways. If signals from the spinal cord and brain regulate the emergence of mature bladder smooth muscle function, then further study of the postnatal changes in urinary bladder activity might provide new insights into the mechanisms of urinary tract dysfunction in people with neuropathic bladders due to spina bifida and spinal cord injury.
Acknowledgments
GRANTS
This work was supported by National Institute of Health Grants DK-65759 (to H.-Y. Wu) and DK-49430 (to W. C. de Groat).
REFERENCES
- 1.Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci. 1997;17:8402–8407. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baskin LS, Sutherland RS, Thomson AA, Hayward SW, Cunha GR. Growth factors and receptors in bladder development and obstruction. Lab Invest. 1996;75:157–166. [PubMed] [Google Scholar]
- 3.Brading AF. Ion channels and control of contractile activity in urinary bladder smooth muscle. Jpn J Pharmacol. 1992;58 Suppl 2:120P–127P. [PubMed] [Google Scholar]
- 4.Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57–67. doi: 10.1016/s0090-4295(97)00591-8. discussion 68–73. [DOI] [PubMed] [Google Scholar]
- 5.Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol. 2006;570:12–22. doi: 10.1113/jphysiol.2005.097311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brading AF, Williams JH. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and α,β-methylene ATP. Br J Pharmacol. 1990;99:493–498. doi: 10.1111/j.1476-5381.1990.tb12956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol. 2002;135:639–648. doi: 10.1038/sj.bjp.0704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creed KE, Ishikawa S, Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol. 1983;338:149–164. doi: 10.1113/jphysiol.1983.sp014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groat WC. Plasticity of bladder reflex pathways during postnatal development. Physiol Behav. 2002;77:689–692. doi: 10.1016/s0031-9384(02)00919-8. [DOI] [PubMed] [Google Scholar]
- 10.de Groat WC, Araki I. Maturation of bladder reflex pathways during postnatal development. In: Baskin LS, Hayward SW, editors. Advances in Bladder Research. New York: Kluwer Academic/Plenum; 1999. pp. 253–263. [DOI] [PubMed] [Google Scholar]
- 11.de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 12.Fujii K, Foster CD, Brading AF, Parekh AB. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol. 1990;99:779–785. doi: 10.1111/j.1476-5381.1990.tb13006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauthier KM, Liu C, Popovic A, Albarwani S, Rusch NJ. Freshly isolated bovine coronary endothelial cells do not express the BKCa channel gene. J Physiol. 2002;545:829–836. doi: 10.1113/jphysiol.2002.029843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillespie JI, Harvey IJ, Drake MJ. Agonist- and nerve-induced phasic activity in the isolated whole bladder of the guinea pig: evidence for two types of bladder activity. Exp Physiol. 2003;88:343–357. doi: 10.1113/eph8802536. [DOI] [PubMed] [Google Scholar]
- 15.Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol. 2003;140:146–158. doi: 10.1038/sj.bjp.0705319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004;141:183–193. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol. 2001;530:273–286. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 1997;273:C110–C117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- 20.Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R402–R409. doi: 10.1152/ajpregu.00488.2004. [DOI] [PubMed] [Google Scholar]
- 21.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol. 2000;279:R60–R68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- 22.Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2001;280:C481–C490. doi: 10.1152/ajpcell.2001.280.3.C481. [DOI] [PubMed] [Google Scholar]
- 23.Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol. 2003;551:893–903. doi: 10.1113/jphysiol.2003.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai T, Okamoto T, Yamamoto Y, Tanaka H, Koike K, Shigenobu K, Tanaka Y. Effects of different types of K+ channel modulators on the spontaneous myogenic contraction of guinea-pig urinary bladder smooth muscle. Acta Physiol Scand. 2001;173:323–333. doi: 10.1046/j.1365-201X.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 26.Jamali K, Naylor BR, Kelly MJ, Ronnekleiv OK. Effect of 17beta-estradiol on mRNA expression of large-conductance, voltage-dependent, and calcium-activated potassium alpha and beta subunits in guinea pig. Endocrine. 2003;20:227–237. doi: 10.1385/ENDO:20:3:227. [DOI] [PubMed] [Google Scholar]
- 27.Kruse MN, de Groat WC. Micturition reflexes in decerebrate and spinalized neonatal rats. Am J Physiol Regul Integr Comp Physiol. 1990;258:R1508–R1511. doi: 10.1152/ajpregu.1990.258.6.R1508. [DOI] [PubMed] [Google Scholar]
- 28.Kruse MN, Noto H, Roppolo JR, de Groat WC. Pontine control of the urinary bladder and external urethral sphincter in the rat. Brain Res. 1990;532:182–190. doi: 10.1016/0006-8993(90)91758-9. [DOI] [PubMed] [Google Scholar]
- 29.Maggi CA, Santicioli P, Meli A. Postnatal development of micturition reflex in rats. Am J Physiol Regul Integr Comp Physiol. 1986;250:R926–R931. doi: 10.1152/ajpregu.1986.250.5.R926. [DOI] [PubMed] [Google Scholar]
- 30.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- 31.Nagar D, Liu XT, Rosenfeld CR. Estrogen regulates α 1-subunit expression in Ca2+-activated K+ channels in arteries from reproductive tissues. Am J Physiol Heart Circ Physiol. 2005;289:H1417–H1427. doi: 10.1152/ajpheart.01174.2004. [DOI] [PubMed] [Google Scholar]
- 32.Ng YK, de Groat WC, Wu HY. Muscarinic regulation of neonatal rat bladder spontaneous contractions. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1049–R1059. doi: 10.1152/ajpregu.00236.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sillen U. Bladder function in healthy neonates and its development during infancy. J Urol. 2001;166:2376–2381. doi: 10.1016/s0022-5347(05)65594-2. [DOI] [PubMed] [Google Scholar]
- 34.Sugaya K, de Groat WC. Micturition reflexes in the in vitro neonatal rat brain stem-spinal cord-bladder preparation. Am J Physiol Regul Integr Comp Physiol. 1994;266:R658–R667. doi: 10.1152/ajpregu.1994.266.3.R658. [DOI] [PubMed] [Google Scholar]
- 35.Sugaya K, de Groat WC. Influence of temperature on activity of the isolated whole bladder preparation of neonatal and adult rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R238–R246. doi: 10.1152/ajpregu.2000.278.1.R238. [DOI] [PubMed] [Google Scholar]
- 36.Sugaya K, de Groat WC. Inhibitory control of the urinary bladder in the neonatal rat in vitro spinal cord-bladder preparation. Brain Res Dev Brain Res. 2002;138:87–95. doi: 10.1016/s0165-3806(02)00468-6. [DOI] [PubMed] [Google Scholar]
- 37.Szell EA, Somogyi GT, de Groat WC, Szigeti GP. Developmental changes in spontaneous smooth muscle activity in the neonatal rat urinary bladder. Am J Physiol Regul Integr Comp Physiol. 2003;285:R809–R816. doi: 10.1152/ajpregu.00641.2002. [DOI] [PubMed] [Google Scholar]
- 38.Tamarina NA, Wang Y, Mariotto L, Kuznetsov A, Bond C, Adelman J, Philipson LH. Small-conductance calcium-activated K+ channels are expressed in pancreatic islets and regulate glucose responses. Diabetes. 2003;52:2000–2006. doi: 10.2337/diabetes.52.8.2000. [DOI] [PubMed] [Google Scholar]
- 39.Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol. 2005;289:F604–F610. doi: 10.1152/ajprenal.00060.2005. [DOI] [PubMed] [Google Scholar]
- 40.Zderic SA, Hypolite J, Duckett JW, Snyder HM, 3rd, Wein AJ, Levin RM. Developmental aspects of bladder contractile function: sensitivity to extracellular calcium. Pharmacology. 1991;43:61–68. doi: 10.1159/000138829. [DOI] [PubMed] [Google Scholar]
- 41.Zderic SA, Sillen U, Liu GH, Snyder H, 3rd, Duckett JW, Wein AJ, Levin RM. Developmental aspects of bladder contractile function: evidence for an intracellular calcium pool. J Urol. 1993;150:623–625. doi: 10.1016/s0022-5347(17)35564-7. [DOI] [PubMed] [Google Scholar]
- 42.Zderic SA, Sillen U, Liu GH, Snyder MC, 3rd, Duckett JW, Gong C, Levin RM. Developmental aspects of excitation contraction coupling of rabbit bladder smooth muscle. J Urol. 1994;152:679–681. doi: 10.1016/s0022-5347(17)32679-4. [DOI] [PubMed] [Google Scholar]
- 43.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]