Abstract
Deinococcus maricopensis (Rainey and da Costa 2005) is a member of the genus Deinococcus, which is comprised of 44 validly named species and is located within the deeply branching bacterial phylum Deinococcus–Thermus. Strain LB-34T was isolated from a soil sample from the Sonoran Desert in Arizona. Various species of the genus Deinococcus are characterized by extreme radiation resistance, with D. maricopensis being resistant in excess of 10 kGy. Even though the genomes of three Deinococcus species, D. radiodurans, D. geothermalis and D. deserti, have already been published, no special physiological characteristic is currently known that is unique to this group. It is therefore of special interest to analyze the genomes of additional species of the genus Deinococcus to better understand how these species adapted to gamma- or UV ionizing-radiation. The 3,498,530 bp long genome of D. maricopensis with its 3,301 protein-coding and 66 RNA genes consists of one circular chromosome and is a part of the Genomic Encyclopedia of Bacteria and Archaea project.
Keywords: aerobic, non-motile, Gram-positive, radiation-resistant, mesophilic, chemoorganotrophic, Deinococcaceae, GEBA
Introduction
Strain LB-34T (= DSM 21211 = NRRL B-23946 = LMG 22137) is the type strain of Deinococcus maricopensis [1]. In addition to the type strain LB-34T, two more strains of this species, KR 1 and KR 23, were characterized by Rainey et al. [1]. The generic name derives from the Greek words ‘deinos’ meaning ‘strange or unusual’ and ‘coccus’ meaning ‘a grain or berry’ [2]. The species epithet is derived from the Neo-Latin word ‘maricopensis’ referring to the Maricopa Nation, a native tribe in Arizona [1]. Strain LB 34T was isolated from desert soil in Arizona and described by Rainey et al. in 2005 [1]. The genus Deinococcus was proposed in 1981 by Brooks and Murray [2] to separate the distinct radiation-resistant species from the genus Micrococcus in which those species were originally classified. With the description of Deinobacter grandis by Oyaizu et al. [3], a second genus was placed to the family Deinococcaceae, and in 1997 Rainey et al. proposed to transfer Deinobacter to the genus Deinococcus, based on investigations of the phylogenetic diversity of the Deinococci as determined by 16S rRNA gene sequence analysis. In conclusion, an emended description of the genus Deinococcus was published, showing that the cells can be spherical or rod-shaped [4]. Members of the genus Deinococcus were isolated from various environmental habitats including air [5-7], arid soil [1,8-12], water and activated sludge [13-15], alpine environments [16], rhizosphere [17], Antarctica [18], hot springs [19], aquifer [20], marine fish [21] and radioactive sites [22]. Here we present a summary classification and a set of features for D. maricopensis LB-34T, together with the description of the complete genomic sequencing and annotation.
Classification and features
A representative genomic 16S rRNA sequence of strain LB-34T was compared using NCBI BLAST under default settings (e.g., considering only the high-scoring segment pairs (HSPs) from the best 250 hits) with the most recent release of the Greengenes database [23] and the relative frequencies, weighted by BLAST scores, of taxa and keywords (reduced to their stem [24]) were determined. The single most frequent genus was Deinococcus (100.0%) (114 hits in total). Regarding the three hits to sequences from members of the species, the average identity within HSPs was 99.9%, whereas the average coverage by HSPs was 97.6%. Regarding the 77 hits to sequences from other members of the genus, the average identity within HSPs was 91.5%, whereas the average coverage by HSPs was 60.5%. Among all other species, the one yielding the highest score was D. radiodurans, which corresponded to an identity of 91.2% and an HSP coverage of 88.0%. The highest-scoring environmental sequence was AY905380 ('Extensive ionizing-radiation-resistant recovered sonoran and description nine new species genus Deinococcus obtained single mixed agricultural/open desert soil clone L14-471'), which showed an identity of 98.1% and a HSP coverage of 70.2%. The five most frequent keywords within the labels of environmental samples which yielded hits were 'skin' (7.7%), 'litholog/stream' (2.8%), 'fossa' (2.4%), 'microbi' (2.4%) and 'forearm' (2.1%) (136 hits in total). Environmental samples which yielded hits of a higher score than the highest scoring species were not found.
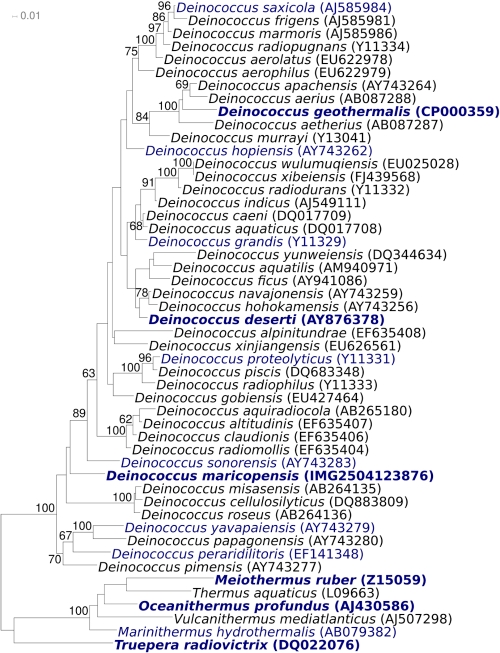
Figure 1 shows the phylogenetic neighborhood of D. maricopensis LB-34T in a 16S rRNA based tree. The sequences of the four identical 16S rRNA gene copies in the genome differ by one nucleotide from the previously published 16S rRNA sequence (AY743274).
Figure 1.
Phylogenetic tree highlighting the position of D. maricopensis relative to the other type strains within the family Deinococcaceae. The tree was inferred from 1,382 aligned characters [25,26] of the 16S rRNA gene sequence under the maximum likelihood criterion [27] and rooted in accordance with the current taxonomy. The branches are scaled in terms of the expected number of substitutions per site. Numbers above branches are support values from 1,000 bootstrap replicates [28] if larger than 60%. Lineages with type strain genome sequencing projects registered in GOLD [29] are shown in blue, and published genomes in bold [30-34]. The genome of D. radiodurans published by White at al. in 1999 [35] later turned out not to be from the type strain [36].
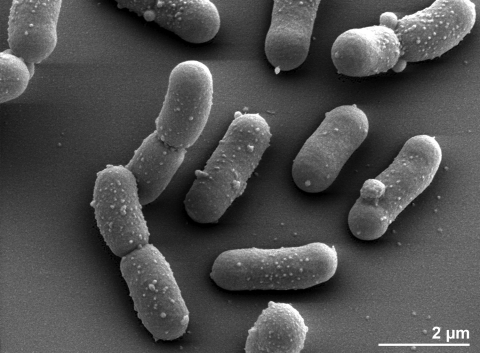
The cells of D. maricopensis are rod-shaped, up to 6 µm in length and 2.0 µm wide (Figure 2). D. maricopensis is a Gram-positive, non-spore-forming bacterium (Table 1). Colonies on Rich medium are orange to pink. The cells are non-motile. The organism is chemoorganotrophic [1]. The temperature range for growth is 10° to 45°C, with an optimum at 40°C [1]. Cytochrome oxidase and catalase activity have been observed [1]. Strains may utilize L-arabinose, cellobiose, galactose, glucose, mannose, maltose, sucrose, trehalose, glucosamine, glycerol, malate, asparagine, aspartate, glutamate, L-glutamine, ornithine and proline. Fructose can be used by strain KR23, but not by strain LB-34T [1]. Strain LB-34T showed similar levels of desiccation tolerance of up to four weeks as compared to D. radiodurans strain R1T. Strain LB-34T is resistant to > 10kGy, but more sensitive to ionizing radiation than strain D. radiodurans R1T [1].
Figure 2.
Scanning electron micrograph of D. maricopensis LB-34T
Table 1. Classification and general features of D. maricopensis LB-34Taccording to the MIGS recommendations [37].
| MIGS ID | Property | Term | Evidence code |
|---|---|---|---|
| Current classification | Domain Bacteria | TAS [38] | |
| Phylum Deinococcus-Thermus | TAS [39] | ||
| Class Deinococci | TAS [40,41] | ||
| Order Deinococcales | TAS [4] | ||
| Family Deinococcaceae | TAS [2,4] | ||
| Genus Deinococcus | TAS [2,4] | ||
| Species Deinococcus maricopensis | TAS [1,42] | ||
| Type strain LB-34 | TAS [1] | ||
| Gram stain | positive | TAS [1] | |
| Cell shape | rods | TAS [1] | |
| Motility | non-motile | TAS [1] | |
| Sporulation | none | TAS [1] | |
| Temperature range | mesophile, 10°C–45°C | TAS [1] | |
| Optimum temperature | 40°C | TAS [1] | |
| Salinity | not reported | ||
| MIGS-22 | Oxygen requirement | aerobic | TAS [1] |
| Carbon source | carbohydrates | TAS [1] | |
| Energy metabolism | chemoorganotroph | TAS [1,2] | |
| MIGS-6 | Habitat | soil | TAS [1] |
| MIGS-15 | Biotic relationship | free-living | NAS |
| MIGS-14 | Pathogenicity | none | NAS |
| Biosafety level | 1 | TAS [43] | |
| Isolation | soil | TAS [1] | |
| MIGS-4 | Geographic location | Sonoran Desert, Arizona, USA | TAS [1] |
| MIGS-5 | Sample collection time | 1999 | NAS |
| MIGS-4.1 | Latitude | 32.93 | NAS |
| MIGS-4.2 | Longitude | -112.30 | NAS |
| MIGS-4.3 | Depth | not reported | |
| MIGS-4.4 | Altitude | not reported |
Evidence codes - IDA: Inferred from Direct Assay (first time in publication); TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from of the Gene Ontology project [44]. If the evidence code is IDA, then the property was directly observed by one of the authors or an expert mentioned in the acknowledgements.
Chemotaxonomy
The major cellular fatty acids of the strain LB-34T were identified as iso-C15:0, iso-C17:0 and C16:0. Menaquinone 8 (MK-8) was determined as the major respiratory quinone of the strain. Phosphoglycolipid and glycolipid pattern are similar to those of other Deinococcus species [1]. No data are available for strain LB-34T showing the peptidoglycan type of the cell wall.
Genome sequencing and annotation
Genome project history
This organism was selected for sequencing on the basis of its phylogenetic position [45], and is part of the Genomic Encyclopedia of Bacteria and Archaea project [46]. The genome project is deposited in the Genomes On Line Database [29] and the complete genome sequence is deposited in GenBank. Sequencing, finishing and annotation were performed by the DOE Joint Genome Institute (JGI). A summary of the project information is shown in Table 2.
Table 2. Genome sequencing project information.
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | Finished |
| MIGS-28 | Libraries used | Three genomic libraries: one 454 pyrosequence standard library, one 454 PE library (7 kb insert size), one Illumina library |
| MIGS-29 | Sequencing platforms | Illumina GAii, 454 GS FLX Titanium |
| MIGS-31.2 | Sequencing coverage | 170.9 × Illumina; 75.4 × pyrosequence |
| MIGS-30 | Assemblers | Newbler version 2.3-PreRelease-10-21-2009-gcc-4.1.2-threads, Velvet version 0.7.63, phrap |
| MIGS-32 | Gene calling method | Prodigal 1.4, GenePRIMP |
| INSDC ID | CP002454 | |
| Genbank Date of Release | January 20, 2011 | |
| GOLD ID | Gc01597 | |
| NCBI project ID | 43461 | |
| Database: IMG-GEBA | 2503982045 | |
| MIGS-13 | Source material identifier | DSM 21211 |
| Project relevance | Tree of Life, GEBA |
Growth conditions and DNA isolation
D. maricopensis LB-34T, DSM 21211, was grown in DSMZ medium 736 (Rich Medium) [47] at 28°C. DNA was isolated from 0.5-1 g of cell paste using MasterPure Gram-positive DNA purification kit (Epicentre MGP04100) following the standard protocol as recommended by the manufacturer, with a modification in cell lysis by adding 20 μl lysozyme (100 mg/μl), and 10 μl mutanolysine, achromopeptidase and lysostphine, each, for 40 min at 37°C, followed by one hour incubation on ice after the MPC step. DNA is available through the DNA Bank Network [48,49].
Genome sequencing and assembly
The genome was sequenced using a combination of Illumina and 454 sequencing platforms. All general aspects of library construction and sequencing can be found at the JGI website [50]. Pyrosequencing reads were assembled using the Newbler assembler version 2.3 (Roche). The initial Newbler assembly consisting of 58 contigs in two scaffolds was converted into a phrap assembly by [51] making fake reads from the consensus, to collect the read pairs in the 454 paired end library. Illumina GAii sequencing data (957.8 Mb) were assembled with Velvet version 0.7.63 [52] and the consensus sequences were shredded into 1.5 kb overlapped fake reads and assembled together with the 454 data. The 454 draft assembly was based on 234.5 Mb 454 draft data and all of the 454 paired end data. Newbler parameters are -consed -a 50 -l 350 -g -m -ml 20. The Phred/Phrap/Consed software package [51] was used for sequence assembly and quality assessment in the subsequent finishing process. After the shotgun stage, reads were assembled with parallel phrap (High Performance Software, LLC). Possible mis-assemblies were corrected with gapResolution [50], Dupfinisher [53], or sequencing cloned bridging PCR fragments with subcloning or transposon bombing (Epicentre Biotechnologies, Madison, WI). Gaps between contigs were closed by editing in Consed, by PCR and by Bubble PCR primer walks (J.-F.Chang, unpublished). A total of 255 additional reactions were necessary to close gaps and to raise the quality of the finished sequence. Illumina reads were also used to correct potential base errors and increase consensus quality using a software Polisher developed at JGI [54]. The error rate of the completed genome sequence is less than 1 in 100,000. Together, the combination of the Illumina and 454 sequencing platforms provided 246.3 × coverage of the genome. The final assembly contained 872,337 pyrosequence and 16,604,657 Illumina reads.
Genome annotation
Genes were identified using Prodigal [55] as part of the Oak Ridge National Laboratory genome annotation pipeline, followed by a round of manual curation using the JGI GenePRIMP pipeline [56]. The predicted CDSs were translated and used to search the National Center for Biotechnology Information (NCBI) nonredundant database, UniProt, TIGR-Fam, Pfam, PRIAM, KEGG, COG, and InterPro databases. Additional gene prediction analysis and functional annotation was performed within the Integrated Microbial Genomes - Expert Review (IMG-ER) platform [57].
Genome properties
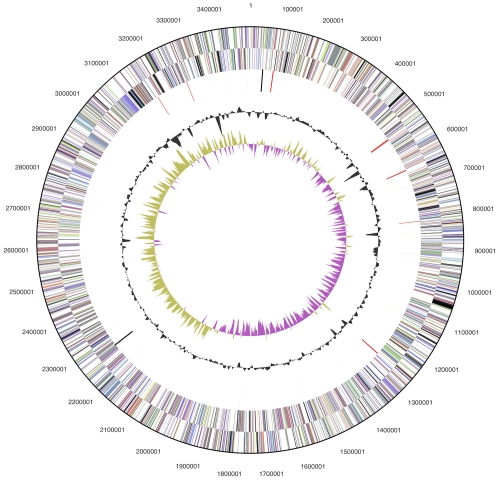
The genome consists of a 3,498,530 bp long chromosome with a G+C content of 69.8% (Table 3 and Figure 3). Of the 3,367 genes predicted, 3,301 were protein-coding genes, and 66 RNAs; 37 pseudogenes were also identified. The majority of the protein-coding genes (70.3%) were assigned with a putative function while the remaining ones were annotated as hypothetical proteins. The distribution of genes into COGs functional categories is presented in Table 4.
Table 3. Genome Statistics.
| Attribute | Value | % of Total |
|---|---|---|
| Genome size (bp) | 3,498,530 | 100.00% |
| DNA coding region (bp) | 3,127,041 | 89.38% |
| DNA G+C content (bp) | 2,442,849 | 69.83% |
| Number of replicons | 1 | |
| Extrachromosomal elements | 0 | |
| Total genes | 3,367 | 100.00% |
| RNA genes | 66 | 1.96% |
| rRNA operons | 4 | |
| Protein-coding genes | 3,301 | 98.04% |
| Pseudo genes | 37 | 1.10% |
| Genes with function prediction | 2,366 | 70.27% |
| Genes in paralog clusters | 368 | 10.93% |
| Genes assigned to COGs | 2,412 | 71.64% |
| Genes assigned Pfam domains | 2,495 | 74.10% |
| Genes with signal peptides | 1,005 | 29.85% |
| Genes with transmembrane helices | 662 | 19.66% |
| CRISPR repeats | 0 |
Figure 3.
Graphical circular map of the chromosome. From outside to the center: Genes on forward strand (color by COG categories), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, rRNAs red, other RNAs black), GC content, GC skew.
Table 4. Number of genes associated with the general COG functional categories.
| Code | value | %age | Description |
|---|---|---|---|
| J | 160 | 6.0 | Translation, ribosomal structure and biogenesis |
| A | 0 | 0.0 | RNA processing and modification |
| K | 188 | 7.1 | Transcription |
| L | 109 | 4.1 | Replication, recombination and repair |
| B | 2 | 0.1 | Chromatin structure and dynamics |
| D | 29 | 1.1 | Cell cycle control, cell division, chromosome partitioning |
| Y | 0 | 0.0 | Nuclear structure |
| V | 45 | 1.7 | Defense mechanisms |
| T | 195 | 7.3 | Signal transduction mechanisms |
| M | 137 | 5.2 | Cell wall/membrane/envelope biogenesis |
| N | 15 | 0.6 | Cell motility |
| Z | 1 | 0.0 | Cytoskeleton |
| W | 0 | 0.0 | Extracellular structures |
| U | 43 | 1.6 | Intracellular trafficking, secretion, and vesicular transport |
| O | 113 | 4.3 | Posttranslational modification, protein turnover, chaperones |
| C | 125 | 4.7 | Energy production and conversion |
| G | 205 | 7.7 | Carbohydrate transport and metabolism |
| E | 237 | 8.9 | Amino acid transport and metabolism |
| F | 77 | 2.9 | Nucleotide transport and metabolism |
| H | 119 | 4.5 | Coenzyme transport and metabolism |
| I | 105 | 4.0 | Lipid transport and metabolism |
| P | 121 | 4.6 | Inorganic ion transport and metabolism |
| Q | 60 | 2.3 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 334 | 12.6 | General function prediction only |
| S | 238 | 9.0 | Function unknown |
| - | 955 | 28.4 | Not in COGs |
Acknowledgements
We would like to gratefully acknowledge the help of Gabriele Gehrich-Schröter (DSMZ) for growing D. maricopensis cultures. This work was performed under the auspices of the US Department of Energy Office of Science, Biological and Environmental Research Program, and by the University of California, Lawrence Berkeley National Laboratory under contract No. DE-AC02-05CH11231, Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344, and Los Alamos National Laboratory under contract No. DE-AC02-06NA25396, UT-Battelle and Oak Ridge National Laboratory under contract DE-AC05-00OR22725, as well as German Research Foundation (DFG) INST 599/1-2.
References
- 1.Rainey FA, Ray K, Ferreira M, Gatz BZ, Nobre MF, Bagaley D, Rash BA, Park NJ, Earl AM, Shank NC, et al. Extensive diversity of ionizing-radiation-resistant bacteria recovered from Sonoran Desert soil and description of nine new species of the genus Deinococcus obtained from a single soil sample. Appl Environ Microbiol 2005; 71:5225-5235 10.1128/AEM.71.9.5225-5235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks BW, Murray RGE. Nomenclature for "Micrococcus radiodurans" and other radiation-resistant cocci: Deinococcaceae fam. nov. and Deinococcus gen. nov., including five species. Int J Syst Bacteriol 1981; 31:353-360 10.1099/00207713-31-3-353 [DOI] [Google Scholar]
- 3.Oyaizu H, Stackebrandt E, Schleifer KH, Ludwig W, Pohla H, Ito H, Hirata A, Oyaizu Y, Komagata K. A radiation-resistant rod-shaped bacterium, Deinobacter grandis gen. nov., sp. nov., with peptidoglycan containing ornithine. Int J Syst Bacteriol 1987; 37:62-67 10.1099/00207713-37-1-62 [DOI] [Google Scholar]
- 4.Rainey FA, Nobre MF, Schumann P, Stackebrandt E, da Costa MS. Phylogenetic diversity of the Deinococci as determined by 16S ribosomal DNA sequence comparison. Int J Syst Bacteriol 1997; 47:510-514 10.1099/00207713-47-2-510 [DOI] [PubMed] [Google Scholar]
- 5.Weon HY, Kim BY, Schumann P, Son JA, Jang J, Go SJ, Kwon SW. Deinococcus cellulosilyticus sp. nov., isolated from air. Int J Syst Evol Microbiol 2007; 57:1685-1688 10.1099/ijs.0.64951-0 [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Itoh T, Yokobori S, Itahashi S, Shimada H, Satoh K, Ohba H, Narumi I, Yamagishi A. Deinococcus aerius sp. nov., isolated from the high atmosphere. Int J Syst Evol Microbiol 2009; 59:1862-1866 10.1099/ijs.0.007963-0 [DOI] [PubMed] [Google Scholar]
- 7.Yoo SH, Weon HY, Kim SJ, Kim YS, Kim BY, Kwon SW. Deinococcus aerolatus sp. nov. and Deinococcus aerophilus sp. nov., isolated from air samples. Int J Syst Evol Microbiol 2010; 60:1191-1195 10.1099/ijs.0.016030-0 [DOI] [PubMed] [Google Scholar]
- 8.Rainey FA, Ferreira M, Nobre MF, Ray K, Bagaley D, Earl AM, Battista JR, Gómez-Silva B, McKay CP, da Costa MS. Deinococcus peraridilitoris sp. nov., isolated from a coastal desert. Int J Syst Evol Microbiol 2007; 57:1408-1412 10.1099/ijs.0.64956-0 [DOI] [PubMed] [Google Scholar]
- 9.de Groot A, Chapon V, Servant P, Christen R, Saux MF, Sommer S, Heulin T. Deinococcus deserti sp. nov., a gamma-radiation-tolerant bacterium isolated from the Sahara Desert. Int J Syst Evol Microbiol 2005; 55:2441-2446 10.1099/ijs.0.63717-0 [DOI] [PubMed] [Google Scholar]
- 10.Peng F, Zhang L, Luo X, Dai J, An H, Tang Y, Fang C. Deinococcus xinjiangensis sp. nov., isolated from desert soil. Int J Syst Evol Microbiol 2009; 59:709-713 10.1099/ijs.0.004564-0 [DOI] [PubMed] [Google Scholar]
- 11.Yuan M, Zhang W, Dai S, Wu J, Wang Y, Tao T, Chen M, Lin M. Deinococcus gobiensis sp. nov., an extremely radiation-resistant bacterium. Int J Syst Evol Microbiol 2009; 59:1513-1517 10.1099/ijs.0.004523-0 [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Mao J, Zhang Z, Tang Q, Xie Y, Zhu J, Zhang L, Liu Z, Shi Y, Goodfellow M. Deinococcus wulumuqiensis sp. nov., and Deinococcus xibeiensis sp. nov., isolated from radiation-polluted soil. Int J Syst Evol Microbiol 2010; 60:2006-2010 10.1099/ijs.0.015917-0 [DOI] [PubMed] [Google Scholar]
- 13.Im WT, Jung HM, Ten LN, Kim MK, Bora N, Goodfellow M, Lim S, Jung J, Lee ST. Deinococcus aquaticus sp. nov., isolated from fresh water, and Deinococcus caeni sp. nov., isolated from activated sludge. Int J Syst Evol Microbiol 2008; 58:2348-2353 10.1099/ijs.0.64082-0 [DOI] [PubMed] [Google Scholar]
- 14.Kämpfer P, Lodders N, Huber B, Falsen E, Busse HJ. Deinococcus aquatilis sp. nov., isolated from water. Int J Syst Evol Microbiol 2008; 58:2803-2806 10.1099/ijs.0.2008/001206-0 [DOI] [PubMed] [Google Scholar]
- 15.Asker D, Awad TS, Beppu T, Ueda K. Deinococcus aquiradiocola sp. nov., isolated from a radioactive site in Japan. Int J Syst Evol Microbiol 2009; 59:144-149 10.1099/ijs.0.65762-0 [DOI] [PubMed] [Google Scholar]
- 16.Callegan RP, Nobre MF, McTernan PM, Battista JR, Navarro-González R, McKay CP, da Costa MS, Rainey FA. Description of four novel psychrophilic, ionizing radiation-sensitive Deinococcus species from alpine environments. Int J Syst Evol Microbiol 2008; 58:1252-1258 10.1099/ijs.0.65405-0 [DOI] [PubMed] [Google Scholar]
- 17.Lai WA, Kämpfer P, Arun AB, Shen FT, Huber B, Rekha PD, Young CC. Deinococcus ficus sp. nov., isolated from the rhizosphere of Ficus religiosa L. Int J Syst Evol Microbiol 2006; 56:787-791 10.1099/ijs.0.64007-0 [DOI] [PubMed] [Google Scholar]
- 18.Hirsch P, Gallikowski CA, Siebert J, Peissl K, Kroppenstedt R, Schumann P, Stackebrandt E, Anderson R. Deinococcus frigens sp. nov., Deinococcus saxicola sp. nov., and Deinococcus marmoris sp. nov., low temperature and draught-tolerating, UV-resistant bacteria from continental Antarctica. Syst Appl Microbiol 2004; 27:636-645 10.1078/0723202042370008 [DOI] [PubMed] [Google Scholar]
- 19.Ferreira AC, Nobre MF, Rainey FA, Silva MT, Wait R, Burghardt J, Chung AP, da Costa MS. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov., two extremely radiation-resistant and slightly thermophilic species from hot springs. Int J Syst Bacteriol 1997; 47:939-947 10.1099/00207713-47-4-939 [DOI] [PubMed] [Google Scholar]
- 20.Suresh K, Reddy GS, Sengupta S, Shivaji S. Deinococcus indicus sp. nov., an arsenic-resistant bacterium from an aquifer in West Bengal, India. Int J Syst Evol Microbiol 2004; 54:457-461 10.1099/ijs.0.02758-0 [DOI] [PubMed] [Google Scholar]
- 21.Shashidhar R, Bandekar JR. Deinococcus piscis sp. nov., a radiation-resistant bacterium isolated from a marine fish. Int J Syst Evol Microbiol 2009; 59:2714-2717 10.1099/ijs.0.003046-0 [DOI] [PubMed] [Google Scholar]
- 22.Asker D, Awad TS, Beppu T, Ueda K. Deinococcus misasensis and Deinococcus roseus, novel members of the genus Deinococcus, isolated from a radioactive site in Japan. Syst Appl Microbiol 2008; 31:43-49 10.1016/j.syapm.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 23.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 2006; 72:5069-5072 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter MF. An algorithm for suffix stripping. Program: electronic library and information systems 1980; 14:130-137.
- 25.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 2000; 17:540-552 [DOI] [PubMed] [Google Scholar]
- 26.Lee C, Grasso C, Sharlow MF. Multiple sequence alignment using partial order graphs. Bioinformatics 2002; 18:452-464 10.1093/bioinformatics/18.3.452 [DOI] [PubMed] [Google Scholar]
- 27.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol 2008; 57:758-771 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- 28.Pattengale ND, Alipour M, Bininda-Emonds ORP, Moret BME, Stamatakis A. How many bootstrap replicates are necessary? Lect Notes Comput Sci 2009; 5541:184-200 10.1007/978-3-642-02008-7_13 [DOI] [PubMed] [Google Scholar]
- 29.Liolios K, Chen IM, Mavromatis K, Tavernarakis N, Hugenholtz P, Markowitz VM, Kyrpides NC. The Genomes On Line Database (GOLD) in 2009: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res 2009; 38:D346-D354 10.1093/nar/gkp848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Groot A, Dulermo R, Ortet P, Blanchard L, Guerin P, Fernandez B, Vacherie B, Dossat C, Jolivet E, Siguier P, et al. Alliance of proteomics and genomics to unravel the specificities of Sahara bacterium Deinococcus deserti. PLoS Genet 2009; 5:e1000434 10.1371/journal.pgen.1000434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makarova KS, Omelchenko MV, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Lapidus A, Copeland A, Kim E, Land M, et al. Deinococcus geothermalis: the pool of extreme radiation resistance genes shrinks. PLoS ONE 2007; 2:e955 10.1371/journal.pone.0000955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanova N, Rhode C, Munk C, Nolan M, Lucas S, Glavina Del Rio T, Tice H, Deshpande S, Cheng JF, Tapia R, et al. Complete genome sequence of Truepera radiovictrix type strain (RQ-24T). Stand Genomic Sci 2011; 4:91-99 10.4056/sigs.1563919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tindall BJ, Sikorski J, Lucas S, Goltsman E, Copeland A, Glavina Del Rio T, Nolan M, Tice H, Cheng JF, Han C, et al. Complete genome sequence of Meiothermus ruber type strain (21T). Stand Genomic Sci 2010; 3:26-36 10.4056/sigs.1032748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pati A, Zhang X, Lapidus A, Nolan M, Lucas S, Glavina Del Rio T, Tice H, Cheng JF, Tapia R, Han C, et al. Complete genome sequence of Oceanithermus profundus type strain (506T). Stand Genomic Sci 2011; 4:210-220 10.4056/sigs.1513795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White O, Eisen JA, Heidelberg JF, Hickey EK, Peterson JD, Dodson RJ, Haft DH, Gwinn ML, Nelson WC, Richardson DL, et al. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 1999; 286:1571-1577 10.1126/science.286.5444.1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corrections and Clarifications [Erratum: Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1.]. Science 2004; 303:766b 10.1126/science.303.5659.766b [DOI] [PubMed] [Google Scholar]
- 37.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen MJ, Angiuoli SV, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol 2008; 26:541-547 10.1038/nbt1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 1990; 87:4576-4579 10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weisburg WG, Giovannoni SJ, Woese CR. The Deinococcus-Thermus phylum and the effect of rRNA composition on phylogenetic tree construction. Syst Appl Microbiol 1989; 11:128-134 [DOI] [PubMed] [Google Scholar]
- 40.List Editor Validation List no. 85. Validation of publication of new names and new combinations previously effectively published outside the IJSEM. Int J Syst Evol Microbiol 2002; 52:685-690 10.1099/ijs.0.02358-0 [DOI] [PubMed] [Google Scholar]
- 41.Garrity GM, Holt JG. Class I. Deinococci class. nov. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 395. [Google Scholar]
- 42.List Editor Validation of publication of new names and new combinations previously effectively published outside the IJSEM. List no. 106. Int J Syst Evol Microbiol 2005; 55:2235-2238 10.1099/ijs.0.64108-0 [DOI] [PubMed] [Google Scholar]
- 43.Classification of bacteria and archaea in risk groups. TRBA 466, www.baua.de
- 44.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene Ontology: tool for the unification of biology. Nat Genet 2000; 25:25-29 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klenk HP, Göker M. En route to a genome-based classification of Archaea and Bacteria? Syst Appl Microbiol 2010; 33:175-182 10.1016/j.syapm.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 46.Wu D, Hugenholtz P, Mavromatis K, Pukall R, Dalin E, Ivanova NN, Kunin V, Goodwin L, Wu M, Tindall BJ, et al. A phylogeny-driven genomic encyclopaedia of Bacteria and Archaea. Nature 2009; 462:1056-1060 10.1038/nature08656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.List of growth media used at DSMZ: http//www.dsmz.de/microorganisms/media_list.php
- 48.Gemeinholzer B, Dröge G, Zetzsche H, Haszprunar G, Klenk HP, Güntsch A, Berendsohn WG, Wägele JW. The DNA Bank Network: the start from a German initiative. Biopreservation and Biobanking (In press). [DOI] [PubMed] [Google Scholar]
- 49.DNA Bank Network http://www.dnabank-network.org
- 50.DOE Joint Genome Institute http://www.jgi.doe.gov/
- 51.Phrap and Phred for Windows. MacOS, Linux, and Unix. http://www.phrap.com
- 52.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 2008; 18:821-829 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han C, Chain P. 2006. Finishing repeat regions automatically with Dupfinisher. in Proceeding of the 2006 international conference on bioinformatics & computational biology. Edited by Hamid R. Arabnia & Homayoun Valafar, CSREA Press. June 26-29, 2006: 141-146. [Google Scholar]
- 54.Lapidus A, LaButti K, Foster B, Lowry S, Trong S, Goltsman E. POLISHER: An effective tool for using ultra short reads in microbial genome assembly and finishing. AGBT, Marco Island, FL, 2008. [Google Scholar]
- 55.Hyatt D, Chen GL, LoCascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 2010; 11:119 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pati A, Ivanova NN, Mikhailova N, Ovchinnikova G, Hooper SD, Lykidis A, Kyrpides NC. GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods 2010; 7:455-457 10.1038/nmeth.1457 [DOI] [PubMed] [Google Scholar]
- 57.Markowitz VM, Ivanova NN, Chen IMA, Chu K, Kyrpides NC. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 2009; 25:2271-2278 10.1093/bioinformatics/btp393 [DOI] [PubMed] [Google Scholar]