Abstract
Objective
Heart failure (HF) disproportionately affects older adults and previous studies have suggested that the demographic as well as clinical profile of older patients with HF is different from that of younger patients. However, population-based data on the clinical, treatment, and prognostic profile of older as compared to middle aged and younger patients with HF are lacking.
Design/Setting/Participants
A total of 4,534 residents of the Worcester (MA) metropolitan area hospitalized for decompensated HF at 11 greater Worcester medical centers during 1995 and 2000 comprised the study sample.
Measurements
Medical records were reviewed for demographic, clinical, and treatment characteristics and hospital survival status. Long-term follow-up of discharged hospital patients was carried out through 2005. Patients were compared according to 4 age groups (<65, 65–74, 75–84, and ≥85 years).
Results
The mean age was 76 years and 24% were ≥85 years. Older patients (≥75) were more likely to be female, to have multiple comorbidities, a lower body mass index at the time of hospitalization, and higher ejection fraction findings. Older patients were significantly more likely to receive symptom modifying medications, and less likely to receive disease modifying medications, than younger patients. Advanced age was directly associated with increased in-hospital, 30-day, and 1-year death rates in both crude and multivariable adjusted analyses.
Conclusions
The results of this community-wide study suggest that clinical, treatment, and prognostic factors differ by age in patients hospitalized for decompensated HF. These high-risk patients warrant special attention in future studies in order to improve their management and long-term survival.
Keywords: Heart failure, elderly, epidemiology
Introduction
Heart failure (HF) disproportionately affects older adults with the incidence rates of this clinical syndrome increasing with advancing age.1 Previous studies have suggested that the demographic as well as clinical profile of older patients with HF is different from that of younger patients, with elderly patients more often being female, more likely to present with multiple comorbidities and preserved systolic function, and to have a poorer short and long-term prognosis.2–4 The management of HF is more complex in elderly patients because older patients with HF are more likely to have other comorbid conditions present that may interact with prescribed medications and contribute to problems with adherence to treatment regimens and recommended lifestyle changes.2 Despite their high risk status, or because of it, treatment of older patients with HF may be less aggressive than that of their younger counterparts.3, 5–7
Despite the growing epidemic of HF, particularly in the elderly, contemporary data describing differences in the clinical, treatment, and prognostic profile of older as compared to middle aged and younger patients with HF are limited, particularly from the broader perspective of a population-based investigation. The purpose of the present study was to examine age-specific differences in clinical presentation, receipt of therapeutic practices and lifestyle recommendations, and hospital and long-term survival in patients hospitalized for acute HF in a large New England community.
Methods
The study sample was comprised of residents of the Worcester, MA, metropolitan area hospitalized for possible HF at all 11 greater Worcester medical centers during 1995 and 2000. These 2 periods were originally selected to coincide with an intercensal and a decennial census period and so that there would be considerable long-term follow-up data available when we initiated the study in 2003. The details of the Worcester Heart Failure Study have been previously described.4, 8 In brief, the medical records of patients with primary and/or secondary International Classification of Disease (ICD)-9 discharge diagnoses consistent with the possible presence of acute HF were reviewed in a standardized manner using the Framingham criteria to confirm the presence of HF.4, 8 Patients with a discharge diagnosis of HF (ICD-9 code 428) were the principal diagnostic category reviewed. In addition, the medical records of patients with discharge diagnoses of hypertensive heart and renal disease, acute cor pulmonale, cardiomyopathy, pulmonary congestion, acute lung edema, and respiratory abnormalities were reviewed to identify patients who may also have had new onset HF. Patients who developed HF secondary to admission for another acute illness (e.g., acute myocardial infarction), or after an interventional procedure (e.g., coronary artery bypass surgery), were not included in this sample.
Data Collection
Information was collected about patient’s demographic (e.g., age, sex, race), medical history (e.g., coronary heart disease, diabetes, renal failure, stroke), and clinical characteristics (e.g., laboratory test results, physiologic findings) through the review of hospital medical records by trained nurse and physician reviewers in whom regular quality control checks were performed. Quality control checks, which were conducted by the PI (Dr. Goldberg) and a physician study coordinator, included a re-review of data abstraction in a 10% random sample of all charts. Intra-rater quality control checks were also performed in a 5% random sample of all charts by having reviewers repeat data abstraction a few months after the initial abstraction.
Emergency department physician’s and nurse’s notes were reviewed to identify patient’s presenting symptoms. Hospital medical records were reviewed to ascertain the prescribing of effective cardiac therapies (e.g., angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and beta blockers), therapies designed to provide symptomatic relief (e.g., diuretics and digoxin), and documentation of health care provider recommendations to modify various lifestyle and/or dietary practices (e.g., low-fat or low-salt diet, exercise).4 Hospital survival status was ascertained through the review of hospital medical records by trained physician and nurse data abstractors. Information about patients’ long-term survival status (including date of death where applicable) was obtained through the review of hospital medical records at all participating greater Worcester medical centers for subsequent hospitalizations or medical care contacts, as well as through the review of the Social Security Death Index and death certificates at the Massachusetts State Health Department.9
Data Analysis
Differences in the demographic, clinical characteristics, hospital treatment practices, and hospital death rates of patients with decompensated HF were examined according to 4 age groups (<65, 65–74, 75–84, and ≥85 years) through the use of chi-square tests and analysis of variance for discrete and continuous variables respectively. Logistic regression analysis was utilized to examine the independent association of age with the receipt of disease modifying (ACE inhibitors, ARBs and beta (β) blockers and symptom modifying (diuretics and digoxin) medications and with short-term prognosis after decompensated HF, controlling for a number of potentially confounding demographic (age, sex, race) and clinical factors (medical history of: coronary heart disease, chronic obstructive pulmonary disease, diabetes, hypertension, peripheral vascular disease, cancer, renal disease, stroke, heart failure, estimated GFR, serum urea nitrogen, hematocrit, diastolic and systolic blood pressure, and heart rate) as well as length of stay and year of hospitalization. These pre-defined variables (Tables 3 and 4) were controlled for either because they have been shown to be of prognostic importance in previous studies and/or because they differed between our respective comparison groups. Data from 1995 and 2000 were combined in the regression models; the results did not differ significantly when we examined the two study years separately. The main study findings are reported for all patients, however, for selected analyses, several sub-group analyses are also performed. These subgroups analyses include: incident HF cases, the presence of a DNR order, and patients with EF data findings.
Table 3.
Association of age with not being treated with selected cardiac medications in patients hospitalized with acute heart failure (Worcester Heart Failure Study)
| Not Receiving Symptom Modifying Medications* | Not Receiving Disease Modifying Medications* | |||
|---|---|---|---|---|
| Age Group† | Crude OR | Adjusted OR† | Crude OR | Adjusted OR† |
| <65 | 1.0 | 1.0 | 1.0 | 1.0 |
| 65–74 | 0.56 (0.30, 1.05) | 0.71 (0.35, 1.45) | 1.04 (0.86, 1.27) | 1.03 (0.82, 1.27) |
| 75–84 | 0.28 (0.14, 0.54) | 0.29 (0.13, 1.45) | 1.28 (1.07, 1.53) | 1.29 (1.05, 1.58) |
| ≥85 | 0.45 (0.24, 0.86) | 0.47 (0.21, 1.09) | 1.51 (1.24, 1.83) | 1.43 (1.13, 1.80) |
Note.
< 65 is reference group.
Symptom modifying medications = diuretics and digoxin; Disease modifying medications = ACE inhibitors, angiotensin receptor blockers, and beta blockers.
Adjusted for sex, race, length of stay, medical history of: coronary heart disease, chronic obstructive pulmonary disease, diabetes, hypertension, peripheral vascular disease, cancer, renal disease, stroke, heart failure, estimated GFR, serum urea nitrogen, hematocrit, diastolic and systolic blood pressure, heart rate, and year of hospitalization.
Table 4.
Association of age with mortality at selected time points in patients hospitalized with acute heart failure (Worcester Heart Failure Study)
| Outcome | Age (years)
|
|||
|---|---|---|---|---|
| <65 | 65–74 | 75–84 | ≥85 | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Hospital Mortality Crude rate (%) | 3.0 | 5.5 | 8.2 | 8.2 |
| Adjusted OR* | reference (1.0) | 2.14 (1.22, 3.76) | 3.06 (1.79, 5.23) | 2.90 (1.64, 5.12) |
| 30-Day† Mortality Crude rate (%) | 5.5 | 11.2 | 14.0 | 17.6 |
| Adjusted OR* | reference (1.0) | 2.05 (1.35, 3.09) | 2.57 (1.73, 3.81) | 2.99 (1.97, 4.52) |
| 1-Year‡ Mortality Crude rate (%) | 26.9 | 34.1 | 40.6 | 47.7 |
| Adjusted OR* | reference (1.0) | 1.13 (0.89, 1.45) | 1.54 (1.22, 1.94) | 2.02 (1.56, 2.60) |
Note.
30-days post admission.
1-year post discharge.
Adjusted for sex, race, length of hospital stay, medical history of: coronary heart disease, chronic obstructive pulmonary disease, diabetes, hypertension, peripheral vascular disease, cancer, renal disease, stroke, heart failure, estimated GFR, serum urea nitrogen, hematocrit, diastolic and systolic blood pressure, heart rate, and year of hospitalization.
A life table analysis was utilized to examine differences in long-term survival according to age, while including patients with varying duration of follow-up. A Cox proportional hazards regression approach was used to examine differences in long-term survival according to age while controlling for duration of follow-up and a variety of potentially confounding prognostic characteristics.
Results
A total of 4,534 men and women from the Worcester metropolitan area with independently confirmed decompensated HF requiring hospital admission comprised the study population. The mean age of our study sample was 76 years and the majority (56.9%) were women; among all patients studied, 1,153 (25.4%) experienced a first (incident) episode of HF.
Ejection fraction (EF) data were available in only 37% of hospitalized study patients. Compared to patients without EF data, those with available EF data were younger, had a lower body mass index (BMI), were less likely to have a medical history of coronary heart disease, chronic obstructive pulmonary disease (COPD), diabetes, cancer, renal disease, stroke and congestive heart failure (all p’s <0.05). Patients with EF data were more likely to report chest pain, edema, orthopnea and weight gain, were more likely to be treated with ACE inhibitors, ARBs, digoxin, β blockers, lipid lowering agents and nitrates, and were more likely to survive the index hospitalization (all p’s <.05). In a multivariable regression analysis, patients with EF assessments were younger and had a lower prevalence of several comorbidities, including coronary heart disease, COPD, diabetes or stroke (all p’s <0.05), compared to patients without EF measurements. Patients in the 1995 cohort were more likely to have EF data compared to patients in the 2000 cohort.
Baseline Characteristics According to Age
Older patients hospitalized with decompensated HF were more likely to be female, Caucasian and to have a lower BMI compared to patients who were less than 65 years (Table 1). Older patients had lower serum creatinine and higher blood urea nitrogen levels, higher systolic and lower diastolic blood pressure findings, a lower heart rate, and were less likely to smoke and to have preserved renal function, as represented by an estimated glomerular filtration fate (eGFR) ≥60. Compared to younger patients, older patients were more likely to have a medical history of coronary heart disease, stroke, cancer, and heart failure, and less likely to have a history of chronic obstructive pulmonary disease, diabetes, peripheral vascular disease, and renal disease. Older patients were also more likely to have had multiple comorbidities previously diagnosed than younger patients (Table 1). Older patients with decompensated HF were more likely to have a do not resuscitate order (DNR) in their clinical charts, have a shorter average hospital stay, and were more likely to die during the index hospitalization as compared to younger patients hospitalized with acute HF.
Table 1.
Characteristics of patients with acute heart failure according to age (Worcester Heart Failure Study)
| Characteristic | Age (years)
|
p-value | |||
|---|---|---|---|---|---|
| <65 (n = 675) | 65–74 (n = 1040) | 75–84 (n = 1731) | ≥85 (n = 1088) | ||
| Age, Mean (SD) | 54.7 (8.3) | 70.2 (2.7) | 79.7 (2.8) | 89.0 (3.4) | |
| Female, % | 47.7 | 49.7 | 56.3 | 70.3 | <0.001 |
| White race/ethnicity, % | 82.1 | 93.6 | 96.7 | 98.1 | <0.001 |
| Body mass index, % | |||||
| <25 | 24.5 | 34.6 | 49.9 | 61.4 | |
| 25–29.9 | 24.3 | 31.8 | 26.7 | 24.7 | |
| ≥30 | 51.2 | 33.6 | 23.4 | 13.8 | <0.001 |
| Laboratory values, means | |||||
| Serum urea nitrogen, mg/dL | 32.8 (29.0) | 33.2 (23.4) | 34.9 (23.8) | 35.7 (24.3) | 0.03 |
| Creatinine, md/dL | 1.8 (1.9) | 1.6 (1.2) | 1.6 (1.1) | 1.5 (0.8) | <0.001 |
| Sodium, mEq/L | 137.1 (5.1) | 137.0 (4.9) | 137.3 (6.0) | 137.5 (5.3) | 0.23 |
| Hematocrit, % | 37.0 (7.5) | 36.3 (6.7) | 36.3 (6.6) | 36.2 (6.3) | 0.11 |
| Cholesterol, mean (SD) | 174.4 (52.9) | 175.7 (51.7) | 173.7 (57.4) | 171.2 (50.7) | 0.92 |
| Estimated Glomerular Filtration Rate (GFR) (ml/min per 1.73 m2) | |||||
| <30 | 17.6 | 15.2 | 15.4 | 13.7 | |
| 30–59 | 18.3 | 34.3 | 36.9 | 36.14 | |
| ≥60 | 64.2 | 50.5 | 47.7 | 50.1 | <0.001 |
| Blood pressure (mmHg) mean (SD) | |||||
| Systolic | 142.9 (33.3) | 146.0 (33.2) | 144.8 (32.6) | 144.0 (31.7) | <0.001 |
| Diastolic | 80.5 (19.7) | 78.0 (18.8) | 75.8 (19.6) | 74.7 (19.0) | <0.001 |
| Heart rate (beats/min) | 95.3 (23.4) | 90.0 (22.0) | 89.8 (22.3) | 89.9 (22.6) | <0.001 |
| Left ventricular ejection fraction (mean %) | 40.6 (17.0) | 45.0 (37.9) | 44.4 (20.9) | 51.2 (44.7) | <0.001 |
| Left ventricular ejection fraction | |||||
| <40% | 50.2 | 45.3 | 39.3 | 29.5 | |
| 40–49% | 13.0 | 14.4 | 17.8 | 17.8 | |
| ≥50% | 36.8 | 40.3 | 42.9 | 52.6 | <0.001 |
| Medical History | |||||
| Coronary heart disease | 48.3 | 61.5 | 58.5 | 54.9 | <0.001 |
| Chronic obstructive pulmonary disease | 34.9 | 41.5 | 34.5 | 28.8 | <0.001 |
| Diabetes mellitus | 50.3 | 46.1 | 41.1 | 25.4 | <0.001 |
| Hypertension | 63.9 | 63.0 | 66.6 | 65.7 | 0.21 |
| Peripheral vascular disease | 16.9 | 24.1 | 19.6 | 11.0 | <0.001 |
| Renal disease | 25.8 | 25.6 | 22.8 | 20.2 | 0.01 |
| Stroke | 7.6 | 15.2 | 17.4 | 13.9 | <0.001 |
| Heart failure | 69.8 | 75.7 | 74.4 | 76.7 | 0.009 |
| Cancer | 6.4 | 19.7 | 21.2 | 23.0 | <0.001 |
| Number of Comorbid conditions | |||||
| ≤2 | 34.9 | 22.0 | 25.8 | 33.3 | |
| 3 | 20.3 | 24.4 | 21.5 | 24.4 | |
| 4 | 18.5 | 20.8 | 25.5 | 23.5 | |
| ≥5 | 26.2 | 32.8 | 27.2 | 18.8 | <0.001 |
| Signs or symptoms at hospital presentation | |||||
| Chest pain | 37.2 | 30.9 | 29.3 | 28.2 | <0.001 |
| Dyspnea | 95.6 | 95.9 | 94.6 | 92.6 | 0.006 |
| Edema | 70.1 | 69.5 | 66.1 | 66.2 | 0.09 |
| Nausea or vomiting | 17.6 | 14.1 | 14.4 | 13.5 | 0.10 |
| Orthopnea | 42.7 | 36.5 | 32.1 | 26.9 | <0.001 |
| Generalized weakness | 20.9 | 24.9 | 28.3 | 30.4 | <0.001 |
| Weight gain | 14.9 | 9.1 | 6.4 | 5.2 | <0.001 |
| Smoker | 26.1 | 11.6 | 5.7 | 3.1 | <0.001 |
| Do not resuscitate order | 10.2 | 18.3 | 30.7 | 50.6 | <0.001 |
| Length of hospital stay, mean, days | 7.2 (9.7) | 6.7 (7.5) | 6.1 (7.2) | 5.6 (5.3) | <0.001 |
Compared to patients <65 years, older patients were less likely to exhibit all signs and symptoms of acute HF with the exception of generalized weakness. Because symptoms may differ by level of EF, we examined association of age and acute symptomatology in patients with data on EF and according to preserved (EF≥50%) vs. impaired EF and found that age related differences in the reporting of chest pain, nausea, orthopnea, and weight gain were observed only in patients with impaired EF.
Treatment Practices According to Age
Older patients were less likely to have been prescribed the majority of effective cardiac therapies examined during hospitalization for decompensated HF including ACE inhibitors, β blockers, and ARBs (Table 2). Older patients were more likely than younger patients to have received diuretics during their index hospitalization. Older adults were also significantly less likely to have been recommended to adopt various non-pharmacologic interventions, such as low fat or low salt diets, fluid restriction, and cardiac rehabilitation by their physicians as compared to younger patients.
Table 2.
Treatment practices in patients with acute heart failure according to age (Worcester Heart Failure Study)
| Characteristic | Age (years)
|
p-value | |||
|---|---|---|---|---|---|
| <65 (n = 675) | 65–74 (n = 1040) | 75–84 (n = 1731) | ≥85 (n = 1088) | ||
| Cardiac medications(%) | |||||
| Lipid lowering agents | 22.7 | 18.9 | 14.2 | 5.6 | <0.001 |
| Nitrates | 59.6 | 65.8 | 66.8 | 63.9 | <0.01 |
| Disease modifying medications(%) | |||||
| Angiotensin-converting enzyme inhibitors | 57.0 | 54.6 | 50.4 | 46.0 | <0.001 |
| Angiotensin receptor blockers | 3.7 | 3.6 | 2.5 | 2.2 | 0.11 |
| B-blockers Symptom modifying medications(%) | 40.4 | 41.7 | 43.1 | 36.5 | <0.01 |
| Digoxin | 49.0 | 47.6 | 53.2 | 49.8 | 0.03 |
| Diuretics | 95.8 | 97.7 | 98.4 | 98.0 | <0.01 |
| Non pharmacologic interventions(%) | |||||
| Low fat diet | 61.3 | 62.7 | 57.4 | 51.6 | <0.001 |
| Fluid restriction | 26.1 | 22.9 | 20.4 | 18.2 | <0.001 |
| Increased physical activity | 45.3 | 41.2 | 41.3 | 39.9 | 0.16 |
| Reduced alcohol intake | 3.3 | 1.0 | 0.0 | 0.0 | <0.001 |
| Cardiac rehabilitation | 9.8 | 10.4 | 6.9 | 5.0 | <0.001 |
| Low salt diet | 82.7 | 82.9 | 79.9 | 76.7 | <0.01 |
| Smoking cessation | 4.2 | 1.6 | 0.5 | 0.0 | <0.001 |
Because treatment practices evolved considerably over the period under study, we examined age differences in treatment practices separately by study year and found that disease modifying medications were prescribed significantly more often in 2000 compared to 1995, particularly in patients <65 years and in those 75–84 years old (p=<.01).
Factors associated with pharmacologic interventions according to age
In a series of regression analyses, we examined the association between age and the failure to receive symptom modifying medications (diuretics and digoxin) and disease modifying medications (ACE inhibitors, ARBs and β blockers) while controlling for a variety of demographic and clinical factors that may affect the prescribing of these medications (Table 3). The crude odds of receiving symptom modifying medications was significantly higher in older, compared to younger, patients. While older patients were still more likely than younger patients to receive symptom modifying medications, the association between age and receipt of symptom modifying medications was somewhat attenuated, however, in multivariable adjusted models. In contrast, older patients were significantly less likely than younger patients to receive disease modifying medications in both crude and multivariable adjusted models. When regression analyses were stratified by study year (1995 and 2000) findings did not vary significantly.
When we carried out a series of regression analyses among patients in whom EF data were available (n=1,509), the failure to receive symptom modifying medications according to patient’s age was similar to those found in the total study sample. The crude and multivariable adjusted odds of receiving symptom modifying medications was higher in older, as compared to younger, participants, although the adjusted odds ratios were not statistically significant, possibly due to the smaller sample size (adjusted ORs compared to those <65 years: OR 65–74 years = 0.91, 95% CI = 0.19, 4.21; OR 75–84 years = 0.29, 95% CI = 0.05, 1.45; OR ≥85 years = 0.54 95% CI = 0.09, 3.27). In contrast to findings in the total sample, receipt of disease modifying medications did not vary according to age in patients with EF data (adjusted ORs of failure to receive disease modifying medications compared to those <65 years: OR 65–74 years = 0.76, 95% CI = 0.52, 1.10; OR 75–84 years = 1.11, 95% CI = 0.78, 1.57; OR ≥85 years = 0.92 95% CI = 0.61, 1.38).
Age-specific differences in hospital and long-term mortality
We examined the association between age and in-hospital death adjusting for several demographic, medical history and clinical characteristics as well as time period of hospitalization (Table 4). In-hospital death rates increased markedly with advancing age from 3% in patients <65 years to 8.2% in those ≥75 years. In multivariable adjusted analyses, the odds of dying during hospitalization remained considerably higher in older, compared to younger, patients. When we restricted this analysis to patients with EF data, results were similar with the odds of dying during hospitalization significantly higher in older, as compared to younger, patients (adjusted ORs compared to those <65 years: OR 65–74 years = 1.58, 95% CI = 0.55, 4.56; OR 75–84 years = 3.03, 95% CI = 1.13, 8.11; OR ≥85 years = 3.36 95% CI = 1.15, 9.88).
In a similar manner, we examined the relation between age and the risk of dying at 30-days post hospital admission, and 1-year after hospital discharge, adjusting for several demographic, medical history, and clinical characteristics (Table 4). Thirty-day crude mortality rates increased with age from 5.5% in patients <65 years to 17.6% in patients ≥85 years. In the multivariable adjusted analyses, the odds of dying during the first 30-days after hospital admission increased with advancing age. Results were similar when the analysis was restricted to patients with available EF data (adjusted ORs compared to those <65 years: OR 65–74 years = 1.45, 95% CI = 0.68, 3.10; OR 75–84 years = 2.68, 95% CI = 1.33, 5.42; OR ≥85 years = 3.58 95% CI = 1.68, 7.00).
Because DNR orders may impact that association between age and mortality, we also stratified this analysis according to the presence of DNR orders during hospitalization and found that the odds of 30-day mortality increased with advancing age in those without a DNR (OR 65–74 years = 1.79; 95% CI = 0.96, 3.34; OR ≥85 years = 2.46; 95% CI = 1.27, 4.77) but were nonsignificantly associated with age among those with a DNR order present in their hospital charts (OR 65–74 years = 1.50; 95% CI = 0.78, 2.87; OR ≥85 years = 0.86; 95% CI = 0.46, 1.61).
One-year crude death rates increased with advancing age from 26.9% in patients <65 years to 47.7% in patients ≥85 years (Table 4). In the multivariable adjusted models, the odds of dying during the first year after hospital discharge increased with advancing age. The results were similar when we carried out this analysis only in patients with EF data available (adjusted ORs compared to those <65 years: OR 65–74 years = 1.08, 95% CI = 0.69, 1.69; OR 75–84 years = 1.83, 95% CI = 1.21, 2.79; OR ≥85 years = 2.84 95% CI = 1.78, 4.54). Similar results were also obtained with regards to the association between advancing age and the risk of dying during the first year after hospital discharge, as were observed with regards to the 30 day death rates after hospital admission, when we carried out separate multivariable adjusted analyses in those who did or did not have a DNR order during their index hospitalization for HF.
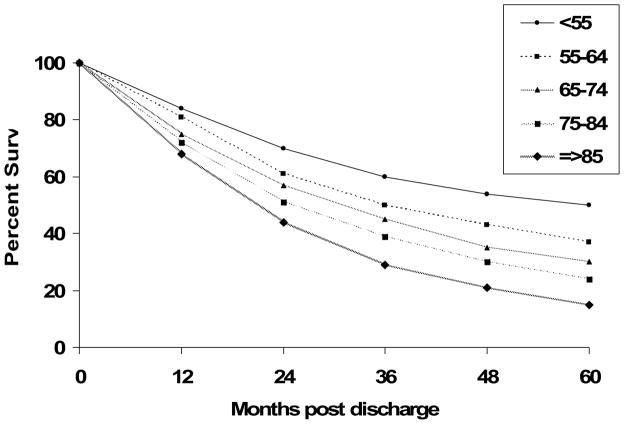
Advanced patient age was also negatively associated with more extended long-term survival. The five year post hospital discharge survival rates were approximately 50% in patients <55 years, 35% in those 55–65 years, 27% in those 65–74 years, 21% in those 75–84 years, and 12% in patients ≥85 years (Figure 1). A proportional hazards regression analysis was carried out to control for previously described potentially confounding prognostic factors in examining the association of age with long-term survival. Consistent with our univariate findings, post-discharge mortality was directly related to advancing age (adjusted OR compared to patients <65 years: 65–74 years = 1.48, 95% CI 1.32, 1.65; 75–84 years = 1.84, 95% CI 1.67, 2.04; ≥85 years = 2.23, 95% CI 2.00, 2.49).
Figure 1.
Long-term Survival According to Age (Worcester Heart Failure Study)
Among patients with EF data, we stratified the proportional hazards regression analysis according to EF findings [preserved left ventricular function (EF≥50%; n=652) compared with impaired ventricular function (EF<50%; n=857)] and controlled for the same potentially confounding factors described above. In both groups, post-discharge mortality was directly associated with advancing age (adjusted ORs compared to those <65 years: preserved function: OR 65–74 years = 1.23, 95% CI = 0.87, 1.74; OR 75–84 years = 1.75, 95% CI = 1.26, 2.43; OR ≥85 years = 2.75, 95% CI = 1.92, 3.94; impaired function: OR 65–74 years = 1.37, 95% CI = 1.06, 1.77; OR 75–84 years = 1.74, 95% CI = 1.37, 2.23; OR ≥85 years = 2.34, 95% CI = 1.74, 3.14).
Discussion
The results of this community-wide study in residents from a large New England metropolitan area hospitalized with decompensated HF in 1995 and 2000 provide insights into the clinical features, treatment practices, and hospital and more long-term outcomes of older as compared to younger patients. Despite the growing epidemic of HF, particularly among older adults, there are limited contemporary data available, particularly from a “real world” population-based perspective, with which to systematically compare characteristics of the acute HF episode and outcomes in younger and older adults.
Clinical characteristics and advancing age
Similar to the results of other studies,3, 7 we found that older patients were more likely to be female and to have preserved left ventricular function; among those with EF data available (approximately 40% of the sample), more than one half of older hospitalized patients had an ejection fraction >50%. We found that older patients were more likely to have a medical history of some (e.g., stroke and HF), but not all (diabetes), co-morbidities compared to younger patients with these differences being most pronounced in our most elderly patients (≥85 years). While unknown, the lower frequency of certain comorbidities, such as diabetes, in younger as compared to older patients may reflect survival bias, real or artifactual differences in the likelihood of hospitalization for acute HF, diagnosis of these comorbidities, medical record documentation, or in the occurrence of out-of hospital deaths due to HF in our study sample.
The majority of previously published studies comparing the clinical features of HF in older as compared to younger patients have reported on either younger patient samples and/or compared only patients <75 years to those ≥75 years.3, 7 Our results suggest that patients ≥85 years are different from younger patients, even those aged 75–84 years, with respect to comorbidities and receipt of different treatment practices and lifestyle recommendations. Older patients appear to be less likely to have some of the usual comorbidities associated with HF present and are also less likely to experience typical symptoms of HF, presenting more often with only generalized weakness. These factors may impact older patients’ ability to recognize the signs and symptoms of HF, resulting in a longer delay in seeking medical care, and, in turn, poorer clinical outcomes. These high-risk patients may warrant special attention in future studies.
Age-specific differences in the receipt of effective treatment practices
While there have been significant advances in the treatment of patients with acute HF during the past decade, many clinical trials have excluded older patients, due partially to their age and clinical profile (e.g., preserved left ventricular systolic function, presence of comorbidities); thus there are fewer proven effective therapies for the treatment of HF in older patients.
Few population-based studies have compared differences in treatment practices in younger and older patients with acute HF. A study of 818 patients discharged with a diagnosis of HF from 81 acute care hospitals in Italy in 1998 found that prescriptions for ACE inhibitors were inversely associated with advancing age.6 Similarly, in a study of 799 patients hospitalized for an incident episode of HF in Somme, France in 2000, older patients were significantly less likely than younger patients to have been treated with ACE inhibitors and β blockers.5 In the present study, we found that hospital treatment practices varied by age. Older patients were more likely to have been prescribed symptom modifying medications and were less likely to have been prescribed disease modifying medications than younger patients. This is particularly concerning because β blockers and ACE inhibitors have been shown to improve survival and reduce hospitalizations in patients with HF.10–12 The reluctance to use these medications in older patients is not well understood but may be related to the higher prevalence of comorbid conditions in elderly patients. However, it has been suggested that the prescribing of these as well as other therapies may still be warranted in these high risk patients.13 In addition, our results suggest that, independent of age, effective disease modifying medications are underutilized in patients with acute HF; less than one half of all patients hospitalized with acute HF were treated with β blockers at all greater Worcester medical centers during our most recent study year of 2000.
In the subsample of patients with assessment of EF, receipt of disease modifying medications did not vary by age. This lack of association may be due to the reduced sample size in this subgroup analysis, particularly among the oldest age group. Alternatively, since patients with EF assessments were younger and had fewer comorbidities, the prevalence of selected comorbidities (such as CHD, COPD and diabetes) may confound the association between age and disease modifying medications observed in the overall sample.
The prevalence of preserved left ventricular function in older patients may have contributed to observed differences in treatment practices with regards to the use of disease modifying medications. Findings from the Cardiovascular Health Study showed that, compared to those with preserved ventricular function, patients with a low ejection fraction were twice as likely to be treated with ACE inhibitors between 1989 and 1995 and that the use of β blockers increased by 10% between 1995 and 2000 in the subgroup of patients with impaired systolic function.14, 15 Given the high proportion of older patients with HF with preserved ventricular function, the association between age, treatment practices, and type of HF may be confounded and needs to be further explored in other ongoing observational studies and HF registries.
Irrespective of age, and with the exception of low salt diets, non-pharmacologic treatments were infrequently prescribed in the present study cohort. We found, however, that provider recommendations for the use of several non-pharmacologic treatment regimens differed according to age. It is unclear whether or not several of these treatments, such as fluid restriction, are beneficial in patients with preserved ejection fraction, which is more commonly noted in older patients. Therefore, age differences in the prescribing of this or other treatment modalities may be confounded and should be interpreted with appropriate caution.
Age related differences in Prognosis after hospitalization for HF
Age has been consistently associated with higher mortality after hospitalization for HF with older patients faring worse than younger patients.2, 16, 17 While the few population-based studies of patients with acute HF have shown that the short-term survival in patients with HF has improved over the past few decades, these improvements have been most pronounced in younger patients.1, 18 A national sample of nearly 4 million Medicare beneficiaries, with an average age of 79 years, found no improvements in 30-day and 1-year mortality in patients with HF during the 1990’s.19 Mortality rates were not, however, reported according to age. Examination of trends in cardiovascular disease (CVD) mortality in residents of Olmsted County, MN, showed that while overall CVD mortality declined over the 25-year period under study (1979–2003), there were significant age differences in the rate of decline.20 Although mortality trends were not reported separately for HF, adults <75 years had more than a two-fold greater rate of decline in overall CVD mortality than those 85 years and older. In the present study we found that the odds of in-hospital, 30-day, and 1-year mortality increased with advancing age in both crude and multivariable adjusted analyses, with no differences noted during the 2 years under study. It is possible that with more contemporary data, age gaps in survival following hospitalization for HF may narrow.
Study strengths and limitations
The strengths of this study include its large sample of patients hospitalized with independently validated HF from a well-characterized New England metropolitan area. We were also able to control for a number of factors that could affect hospital treatment practices as well as hospital and long-term prognosis after hospitalization for HF. This study has a number of limitations, however, that must be kept in mind when interpreting the study results. We had ejection fraction data available on only a subsample of hospitalized patients (<40%) and had limited numbers with which to examine findings in patients with diastolic as compared to systolic HF. We did not have data available with regards to medication use following hospital discharge. Our data were derived from a single, primarily Caucasian, New England population, and our findings may not reflect national patterns. Lastly, we examined medication use in patients hospitalized for HF in 1995 and 2000. These data predate the 2001 announcement by The Joint Commission of Hospital Accreditation regarding the HF core measurement guidelines for hospitals21 which likely had an influence on the management of HF in older patients and also predate the publication of several major beta blocker trials.22–24
Conclusions
The results of our study in residents of a large, central New England metropolitan area suggest that clinical factors, treatment practices, and long-term survival differ according to age in patients hospitalized for decompensated HF. Our findings highlight the importance of examining age- specific differences in the treatment and natural history of HF and suggest that patients 85 years and older may represent a distinct group with respect to clinical factors, treatment factors, and mortality. This high risk subgroup warrants special attention in future studies in order to improve their management and long-term outcomes.
Acknowledgments
This research was made possible by the cooperation of the medical records, administration, and cardiology departments of participating hospitals in the Worcester Metropolitan area and through funding support provided by the National Institutes of Health (R37 HL69874).
Footnotes
Conflict of Interests: The authors have no conflicts of interest to report.
Author contributions: Jane S. Saczynski: Analysis and interpretation of data, drafting of article and critical review, final approval; Chad E. Darling: Analysis and interpretation of data, drafting and critical review of article for important intellectual content, final approval ; Frederick A. Spencer: Conception and design, critical review of article for important intellectual content, final approval; Darleen Lessard: Analysis and interpretation of data, critical review of article for important intellectual content, final approval; Joel M. Gore: Conception and design, acquisition of data, critical review of article for important intellectual content, final approval; Robert J. Goldberg: Conception and design, acquisition of data, analysis and interpretation of data, critical review of article for important intellectual content, final approval.
Sponsor’s Role: None
References
- 1.Roger VL, Weston SA, Redfield MM, et al. Trends in Heart Failure Incidence and Survival in a Community-Based Population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 2.Huynh BC, Rovner A, Rich MW. Long-term Survival in Elderly Patients Hospitalized for Heart Failure: 14-Year Follow-up From a Prospective Randomized Trial. Arch Intern Med. 2006;166(17):1892–1898. doi: 10.1001/archinte.166.17.1892. [DOI] [PubMed] [Google Scholar]
- 3.Pulignano G, Del Sindaco D, Tavazzi L, et al. Clinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: Data from a large nationwide cardiology database (IN-CHF Registry) American Heart Journal. 2002;143(1):45–55. doi: 10.1067/mhj.2002.119608. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RJ, Spencer FA, Farmer C, Lessard D, Pezzella SM, Meyer TE. Use of Disease-Modifying Therapies in Patients Hospitalized with Heart Failure: A Population-Based Perspective. The American Journal of Medicine. 2007;120(1):98. doi: 10.1016/j.amjmed.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 5.Mahjoub H, Rusinaru D, Souliere V, Durier C, Peltier M, Tribouilloy C. Long-term survival in patients older than 80 years hospitalised for heart failure. A 5-year prospective study. European Journal of Heart Failure. 2008;10(1):78–84. doi: 10.1016/j.ejheart.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Pedone C, Pahor M, Carosella L, Bernabei R, Carbonin P for the GI. Use of Angiotensin-Converting Enzyme Inhibitors in Elderly People With Heart Failure: Prevalence and Outcomes. J Gerontol A Biol Sci Med Sci. 2004;59(7):M716–721. doi: 10.1093/gerona/59.7.m716. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Selles M, Garcia Robles JA, Prieto L, Dominguez Munoa M, Frades E. Heart failure in the elderly: age-related differences in clinical profile and mortality. International Journal of Cardiology. 2005;102(1):55–60. doi: 10.1016/j.ijcard.2004.03.072. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RJ, Spencer FA, Farmer C, Meyer TE, Pezzella S. Incidence and hospital death rates associated with heart failure: A community-wide perspective. The American Journal of Medicine. 2005;118(7):728–734. doi: 10.1016/j.amjmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RJ, Ciampa J, Lessard D, Meyer TE, Spencer FA. Long-term Survival After Heart Failure: A Contemporary Population-Based Perspective. Arch Intern Med. 2007;167(5):490–496. doi: 10.1001/archinte.167.5.490. [DOI] [PubMed] [Google Scholar]
- 10.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 11.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. The Lancet. 1999;353(9146):9–13. [PubMed] [Google Scholar]
- 12.Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of Controlled-Release Metoprolol on Total Mortality, Hospitalizations, and Well-being in Patients With Heart Failure: The Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF) JAMA. 2000;283(10):1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation. 2003;107(12):1570–1575. doi: 10.1161/01.CIR.0000065187.80707.18. [DOI] [PubMed] [Google Scholar]
- 14.Smith NL, Psaty BM, Pitt B, Garg R, Gottdiener JS, Heckbert SR. Temporal Patterns in the Medical Treatment of Congestive Heart Failure With Angiotensin-Converting Enzyme Inhibitors in Older Adults, 1989 Through 1995. Arch Intern Med. 1998;158(10):1074–1080. doi: 10.1001/archinte.158.10.1074. [DOI] [PubMed] [Google Scholar]
- 15.Smith NL, Chan JD, Rea TD, et al. Time trends in the use of [beta]-blockers and other pharmacotherapies in older adults with congestive heart failure. American Heart Journal. 2004;148(4):710–717. doi: 10.1016/j.ahj.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting Mortality Among Patients Hospitalized for Heart Failure: Derivation and Validation of a Clinical Model. JAMA. 2003;290(19):2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 17.MacIntyre K, Capewell S, Stewart S, et al. Evidence of Improving Prognosis in Heart Failure : Trends in Case Fatality in 66 547 Patients Hospitalized Between 1986 and 1995. Circulation. 2000;102(10):1126–1131. doi: 10.1161/01.cir.102.10.1126. [DOI] [PubMed] [Google Scholar]
- 18.Levy D, Kenchaiah S, Larson MG, et al. Long-Term Trends in the Incidence of and Survival with Heart Failure. N Engl J Med. 2002;347(18):1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 19.Kosiborod M, Lichtman JH, Heidenreich PA, et al. National Trends in Outcomes Among Elderly Patients with Heart Failure. The American Journal of Medicine. 2006;119(7):616.e611–616. doi: 10.1016/j.amjmed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Gerber Y, Jacobsen SJ, Frye RL, Weston SA, Killian JM, Roger VL. Secular Trends in Deaths From Cardiovascular Diseases: A 25-Year Community Study. Circulation. 2006;113(19):2285–2292. doi: 10.1161/CIRCULATIONAHA.105.590463. [DOI] [PubMed] [Google Scholar]
- 21.Joint Commission on Accreditation of Healthcare Organizations. Heart Failure Core Measurement Set. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Heart+Failure+Core+Measure+Set.htm.
- 22.Gottlieb SS, Fisher ML, Kjekshus J, et al. Tolerability of {beta}-Blocker Initiation and Titration in the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF) Circulation. 2002;105(10):1182–1188. doi: 10.1161/hc1002.105180. [DOI] [PubMed] [Google Scholar]
- 23.Packer M, Coats AJS, Fowler MB, et al. Effect of Carvedilol on Survival in Severe Chronic Heart Failure. N Engl J Med. 2001;344(22):1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 24.Packer M, Fowler MB, Roecker EB, et al. Effect of Carvedilol on the Morbidity of Patients With Severe Chronic Heart Failure: Results of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study. Circulation. 2002;106(17):2194–2199. doi: 10.1161/01.cir.0000035653.72855.bf. [DOI] [PubMed] [Google Scholar]