Abstract
In Huntington’s disease (HD) atrophy of the caudate nucleus and putamen has been described many years before clinical manifestation. Volume changes of the pallidum, thalamus, brainstem, accumbens nucleus, hippocampus, and amygdala are less well investigated, or reported with contradicting results. The aim of our study is to provide a more precise view of the specific atrophy of the subcortical grey matter structures in different stages of Huntington’s disease, and secondly to investigate how this influences the clinical manifestations. All TRACK-HD subjects underwent standardised T1-weighted 3T MRI scans encompassing 123 manifest HD (stage 1, n = 77; stage 2, n = 46), 120 premanifest HD (close to onset n = 58, far from onset n = 62) and 123 controls. Using FMRIB’s FIRST and SIENAX tools the accumbens nucleus, amygdala, brainstem, caudate nucleus, hippocampus, pallidum, putamen, thalamus and whole brain volume were extracted. Results showed that volumes of the caudate nucleus and putamen were reduced in premanifest HD far from predicted onset (>10.8 years). Atrophy of accumbens nucleus and pallidum was apparent in premanifest HD in the close to onset group (0–10.8 years). All other structures were affected to some degree in the manifest group, although brainstem, thalamus and amygdala were relatively spared. The accumbens nucleus, putamen, pallidum and hippocampus had a strong significant correlation with functional and motor scores. We conclude that volume changes may be a sensitive and reliable measure for early disease detection and in this way serve as a biomarker for Huntington’s disease. Besides the caudate nucleus and putamen, the pallidum and the accumbens nucleus show great potential in this respect.
Electronic supplementary material
The online version of this article (doi:10.1007/s00415-010-5768-0) contains supplementary material, which is available to authorized users.
Keywords: Huntington’s disease, Volumetric MRI, Accumbens nucleus, Pallidum, Biomarker
Introduction
Huntington’s disease (HD) is an autosomal, dominantly inherited, slowly progressive, neurodegenerative disease localised on chromosome four. The pathophysiological mechanism is complex and although impaired energy metabolism and neuronal excitotoxicity are named as major contributors [9, 32], it is still not fully understood, but the main result is neuronal dysfunction and loss in the brain. The disease is characterised by clinical symptoms in the motor, cognitive, and psychiatric domains.
Loss of brain cells and their connections, referred to as atrophy, can be visualised with magnetic resonance imaging (MRI) techniques and has been the focus of many studies in search of objective biomarkers for tracking disease progression. The striatum has been the primary region of interest, due to previous findings in pathological studies that demonstrated the most severe loss of neurons in this region [28]. In manifest HD, the evidence for widespread atrophy is extensive, whereby several MRI studies showed striatal [4, 6, 8, 11, 13, 16, 20, 26, 34] and cortical atrophy [29, 31]. The striatal atrophy occurs in the premanifest stages of HD, with atrophy of the caudate nucleus and putamen present up to a decade or more before clinical manifestation [5, 7, 14, 17, 25, 40].
Other subcortical grey matter structures have been examined to a lesser degree. Thalamic [6, 8, 13, 15] and pallidum [2, 8, 30, 34] atrophy is apparent in the manifest stages of HD; however, findings are controversial in the premanifest stage, as not all studies are in agreement [7, 10, 14, 17, 30, 40]. Atrophy of the hippocampus, amygdala, accumbens nucleus, and brainstem has only been reported in the manifest stage in a very limited number of studies [8, 30]. Whole brain atrophy has been demonstrated in HD [12, 39]. However, the rate of volume reduction of the subcortical structures as compared to whole brain atrophy has not been reported in any of the above mentioned studies and is of importance as this regional atrophy may very well only be a reflection of whole brain atrophy. Correlation studies have been performed for volume reductions and global clinical measures in manifest HD [8, 20, 30]. However, reports on the relationship of atrophy with clinical measures of all these structures in different disease stages in one large cohort has not been previously reported.
With the possibilities of new therapies for HD, there has been increasing interest in the development of robust, reproducible biomarkers to assess the efficacy of potential treatments. The TRACK-HD study is an international multi-centre study investigating candidate biomarkers for HD and encompasses a large, well defined, sample of HD gene carriers [39]. Application of a fully automated MRI-analysis tool to this study sample provides the opportunity to study brain atrophy in detail within a very large HD cohort. The aim of this study is to provide a more precise overview of the specific atrophy of the subcortical grey matter structures in different disease stages, whilst taking into account whole brain atrophy and provide better targets for biomarker research. Secondly, we aim to examine the relationship of all subcortical grey matter structure volumes to clinical measures for HD.
Methods
Subjects
The TRACK-HD study recruited 366 participants from four centres (The National Hospital for Neurology and Neurosurgery in London, The Department of Medical Genetics at University of British Columbia in Vancouver, The Department of Genetics and Cytogenetics at the Hôpital de la Salpetrière-Université Pierre and Marie Curie in Paris and the Department of Neurology at Leiden University Medical Center in Leiden), totalling 123 early manifest HD, 120 premanifest HD and 123 control subjects. Inclusion criteria for the early manifest HD group consisted of genetic confirmation of an expanded CAG repeat of ≥40, presence of motor disturbances on the Unified Huntington’s Disease Rating Scale (UHDRS), defined as a diagnostic motor confidence score of 4 and a total motor score (TMS) of >5, and a total functional capacity (TFC) of ≥7. Inclusion criteria for the premanifest group consisted of genetic confirmation of an expanded CAG repeat of ≥40, absence of motor disturbances on the UHDRS, defined as a TMS of ≤5. Furthermore, premanifest gene carriers required a burden of pathology score >250 [27], based on CAG length and age, to ensure a premanifest HD sample close to onset. Spouses or partners of premanifest and affected subjects or gene-negative siblings were recruited as healthy control subjects. The control group was age- and gender-matched to the combined premanifest and manifest HD group. Further subdivision of the premanifest group was performed on the basis of predicted years to diagnosis into preHD-A (10.8 or more years to disease onset) and preHD-B (closer than 10.8 years to disease onset) based on Langbehn’s et al. survival analysis formula [18, 19]. The early manifest HD subjects were also divided into two groups based on disease stage by means of TFC score, the HD1 group (TFC score 11–13) and the HD2 group (TFC score 7–10) [35]. The study was approved by the local ethical committees and written informed consent was obtained from each subject. For full details on study design see [39].
Clinical measures
From the total TRACK-HD assessment battery, the UHDRS motor scale and TFC were used for this study. The UHDRS TMS is a representation of the severity of motor disturbances, scores potentially range from 0 to 124, with higher scores indicating more severe motor abnormalities. The TFC is a scale used for assessment of global impairments within daily activities, ranging from 0 to 13, with lower scores indicating impaired global functioning.
MRI acquisition
All participants underwent 3 T MRI scanning. T1-weighted image volumes were acquired using a 3D MPRAGE acquisition sequence on a 3 T Siemens or a 3 T Phillips whole body scanner with the following imaging parameters: TR = 2200 ms (Siemens)/7.7 ms (Philips), TE = 2.2 ms (Siemens)/3.5 ms (Philips), FA = 10° (Siemens)/8° (Philips), FOV = 28 cm (Siemens)/24 cm (Philips), matrix size 256 × 256 (Siemens)/224 × 224 (Philips), 208 (Siemens)/164 (Philips), sagittal slices to cover the entire brain with a slice thickness of 1.0 mm with no gap between slices.
Post-processing
All T1-weighed scans were analysed using software provided by FMRIB’s software library (FSL) [37]. Eight subcortical regions were assessed using FMRIB’s integrated registration and segmentation tool (FIRST) [21–23], namely the accumbens nucleus, amygdala, brainstem, caudate nucleus, hippocampus, pallidum, putamen, and the thalamus. Total brain tissue volume was estimated with SIENAX [36, 38]. For details on MRI post-processing, please see the Supplementary data. The whole-brain volume was used to calculate ratios of atrophy of the subcortical structures to whole-brain atrophy. The obtained ratio was divided by the control group value in order to obtain an easily interpretable ratio whereby 1.00 is the control (normal) value ratio. The following formula was used:
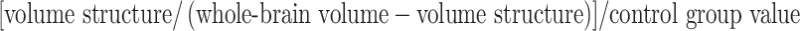 |
A value below 1.00 demonstrates that the structure displays more atrophy than the overall whole-brain atrophy. A value greater than 1.00 demonstrates that the overall whole-brain atrophy is greater than the atrophy of the structure at hand.
Statistics
Analysis of variance for group comparison was performed for volumes and ratios of the eight different subcortical structures, whilst correcting for intracranial volume, study site, age and gender. (Note that each site used a different MRI scanner model, thus, potential scanner effects can not be differentiated from site effects.) Comparisons of each structure were made for each disease group as compared to the control group, and as compared to the previous disease stage group, e.g. the preHD-B group was, besides comparison to the control group, also compared to the preHD-A group. We calculated partial correlations between the various subcortical structures and total motor score and total functional capacity from the UHDRS battery. These correlations used volumes corrected for intracranial volume and were further controlled for age, site, and gender by “partialling” these covariates out during the correlation calculations. The UHDRS TMS and TFC analyses were performed on the basis of a two-way group division, namely the premanifest gene carriers and the manifest HD group in order to keep sufficient power to perform this analysis. The significance level was set at p value <0.05.
Results
Characteristics
The clinical characteristics of the five study groups are shown (Table 1). The progressive nature of HD inherently leads to a higher age of the manifest HD groups. Except for the HD2 group, which displayed lower levels of education, there were no significant differences in education level between the disease groups and the controls. For full details on group characteristics we refer to the recent baseline TRACK-HD paper [39].
Table 1.
Group characteristics
| Control | PreHD-A | PreHD-B | HD1 | HD2 | |
|---|---|---|---|---|---|
| n | 123 | 62 | 58 | 75 | 46 |
| Leiden | 30 | 16 | 14 | 16 | 14 |
| London | 30 | 14 | 16 | 19 | 11 |
| Paris | 30 | 14 | 16 | 25 | 4 |
| Vancouver | 33 | 18 | 12 | 15 | 17 |
| Age (SD and range; years) | 46.1 (10.2, 23.0–65.7) | 41.1 (8.6, 18.6–59.4) | 40.6 (9.2, 22.3–64.1) | 46.9 (10.2, 22.8–64.1) | 51.4 (8.6, 33.3–63.3) |
| Women (%) | 68 (55%) | 33 (53%) | 33 (57%) | 45 (60%) | 21 (46%) |
| Education (SD) levels 1–6 | 4.0 (1.3) | 4.1 (1.1) | 3.8 (1.3) | 3.8 (1.3) | 3.2 (1.4) |
| Disease-burden score (SD) | n.a. | 259.1 (30.1) | 333.1 (30.0) | 364.1 (75.0) | 397.6 (67.5) |
Descriptive statistics of the five different groups. Data are mean (SD, range) or number (%)
Volumes
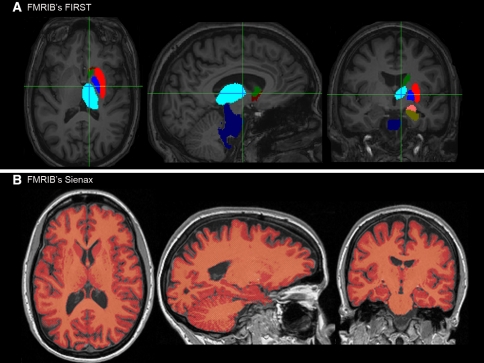
Examples of a typical output from the segmentation by the FIRST and SIENAX tools are depicted (Fig. 1), for the FIRST examples only the left hemisphere segmentations are shown; however, both sides were segmented for the analysis.
Fig. 1.
a Example segmentations by FSL FIRST. Dark blue Brainstem, Blue Pallidum, Light blue Thalamus, Green Caudate nucleus, Dark red Accumbens nucleus, Red Putamen, Copper Hippocampus, Pink Amygdala. b Example output from the FSL SIENAX tool. Red color depicts the final sienax segmentation results
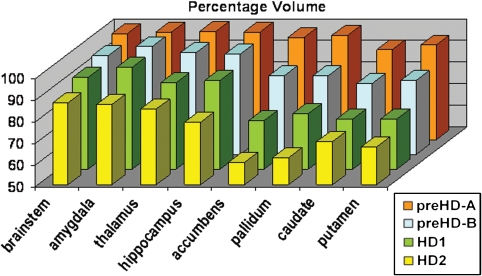
Volume comparisons for eight subcortical structures in five groups are shown (Table 2). Furthermore, Fig. 2 shows the amount of volume reduction in terms of percentage of the control value. The putamen and the caudate nucleus show a similar pattern of a smaller volume in the preHD-A group and in every subsequent disease stage group with a continuous decline in volume. The accumbens nucleus and pallidum show a decreased volume in the preHD-B group and from every following disease stage group as compared to the controls as well as to the previous disease stage group except for the accumbens nucleus between HD1 and HD2.
Table 2.
Volumes
| Control | PreHD-A | PreHD-B | HD1 | HD2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean volume | SD | Mean volume | SD | Mean volume | SD | Mean volume | SD | Mean volume | SD | |
| Accumbens | 1,202 | 251 | 1,170 | 258 | 1,038**†† | 245 | 873**†† | 239 | 752** | 239 |
| Amygdala | 3,345 | 825 | 3,331 | 939 | 3,346 | 869 | 3,246 | 757 | 2,914 | 851 |
| Brainstem | 24,043 | 2,672 | 23,828 | 3,001 | 23,011* | 2,947 | 22,204* | 2,592 | 21,098**†† | 3,083 |
| Caudate | 6,915 | 989 | 6,325** | 1,067 | 5,722**†† | 923 | 5,057**†† | 756 | 4,830** | 816 |
| Hippocampus | 8,417 | 1,271 | 8,391 | 1,319 | 8,115*† | 1,290 | 7,668** | 1,233 | 6,617**†† | 1,351 |
| Pallidum | 3,593 | 505 | 3,526 | 562 | 3,099**†† | 510 | 2,718**†† | 615 | 2,238**†† | 679 |
| Putamen | 10,277 | 1,383 | 9,610** | 1,442 | 8,644**†† | 1,220 | 7,548**†† | 987 | 6,935**†† | 1,203 |
| Thalamus | 16,112 | 1,707 | 16,152 | 1,718 | 15,620** | 1,656 | 14,525**† | 1,524 | 13,681** | 1,448 |
Volumetric analysis of eight structures. Means are the observed unadjusted measured values (mm3). Significance is after correction for ICV, site, gender and age
* significant difference from controls p < 0.05
** significant difference from controls p < 0.001
† significant difference from previous group p < 0.05
†† significant difference from previous group p < 0.01
Fig. 2.
Percentage of volume reduction. Percentage of volume for eight structures as compared to the control value
The brainstem and thalamus show signs of reduced volume in preHD-B, HD1 and HD2 as compared to the control group.
The amygdala showed no reduced volume as compared to the control group. The hippocampus showed volume reduction in stages preHD-B, HD1 and HD2 as compared to controls. In terms of percentages of volume reduction as compared to the control group value it is apparent that the pallidum and accumbens nucleus show marked atrophy with only 62 and 60% of the normal volume remaining in the HD2 group, respectively. These structures are closely followed by the putamen and the caudate nucleus with 67 and 70% remaining volume, respectively. Percentages for all structures in the four disease stages are also available in the supplementary data.
Ratios
Comparisons for volume ratio of eight structures as compared to whole-brain atrophy are shown (Table 3). A smaller ratio indicates that the volume of this specific structure declines faster than the overall brain atrophy (Table 4).
Table 3.
Ratios
| PreHD-A | PreHD-B | HD1 | HD2 | |||||
|---|---|---|---|---|---|---|---|---|
| Ratio | SD | Ratio | SD | Ratio | SD | Ratio | SD | |
| Accumbens nucleus | 1.00 | 0.18 | 0.91**† | 0.18 | 0.82**† | 0.18 | 0.73**† | 0.18 |
| Amygdala | 0.97 | 0.26 | 1.03 | 0.23 | 1.06* | 0.23 | 1.00* | 0.29 |
| Brainstem | 0.99 | 0.07 | 0.99 | 0.08 | 1.04*† | 0.10 | 1.00† | 0.11 |
| Caudate nucleus | 0.92** | 0.13 | 0.87**† | 0.11 | 0.83**† | 0.13 | 0.81** | 0.14 |
| Hippocampus | 1.00 | 0.13 | 1.01 | 0.13 | 1.03 | 0.16 | 0.91**†† | 0.18 |
| Pallidum | 0.97 | 0.12 | 0.91**†† | 0.12 | 0.85**† | 0.21 | 0.70**†† | 0.21 |
| Putamen | 0.93** | 0.11 | 0.87**†† | 0.08 | 0.82**†† | 0.08 | 0.77**† | 0.09 |
| Thalamus | 1.00 | 0.73 | 1.01 | 0.73 | 1.01 | 0.86 | 0.97 | 0.08 |
Ratio analysis of volumes for eight structures. Ratios are the observed unadjusted measured values(mm3). Significance is after correction for ICV, site, gender and age
* p < 0.05 from control group
** p < 0.001 from control group
† p < 0.05 from previous group
†† p < 0.001 from previous group
Table 4.
Volume correlations with motor score and functional capacity
| UDHRS Total motor score | Total functional capacity | |||||||
|---|---|---|---|---|---|---|---|---|
| PreHD | HD | PreHD | HD | |||||
| Partial r | p | Partial r | p | Partial r | p | Partial r | p | |
| Accumbens nucleus | −0.24 | 0.01 | −0.38 | <0.0001 | −0.025 | 0.79 | 0.21 | 0.03 |
| Amygdala | −0.121 | 0.21 | −0.11 | 0.23 | −0.14 | 0.13 | 0.10 | 0.29 |
| Brainstem | −0.12 | 0.23 | −0.22 | 0.02 | 0.00 | >0.99 | 0.20 | 0.03 |
| Caudate nucleus | −0.272 | 0.003 | −0.14 | 0.12 | −0.15 | 0.10 | 0.02 | 0.82 |
| Hippocampus | 0.03 | 0.78 | −0.31 | 0.0008 | 0.03 | 0.73 | 0.24 | 0.01 |
| Pallidum | −0.20 | 0.03 | −0.39 | <0.0001 | 0.03 | 0.75 | 0.23 | 0.01 |
| Putamen | −0.24 | 0.009 | −0.53 | <0.0001 | 0.00 | 0.99 | 0.27 | 0.004 |
| Thalamus | −0.23 | 0.015 | −0.29 | 0.002 | 0.05 | 0.59 | 0.13 | 0.17 |
Statistic is partial correlation of volumes corrected for intracranial volume with statistical (partial) correction for site, gender and age
UHDRS Unified Huntington’s Disease Rating Scale, preHD premanifest Huntington’s disease, HD manifest Huntington’s disease
The putamen atrophy ratio showed a continuous smaller value across subsequent disease stage groups. The caudate nucleus atrophy ratio showed a similar pattern, except within the manifest stage where this ratio did not show a significant difference across HD1 and HD2. The accumbens nucleus and the pallidum atrophy ratio showed a decrease over disease stages from the preHD-B group onwards.
The ratio of whole-brain atrophy to amygdala atrophy is comparable or slightly higher to the control group ratio. The atrophy ratio for the brainstem and thalamus shows no or slightly higher ratios as compared to the control group, stating that atrophy of these structures is comparable or slower to whole brain atrophy. Finally, the hippocampus atrophy ratio shows a drop in value in HD2 as compared to control and HD1.
Clinical measures
The UHDRS TMS in the premanifest stage is most strongly correlated to the atrophy of the caudate nucleus (r = 0.272, p = 0.003) and putamen (r = −0.240, p = 0.009), but also to the atrophy of accumbens nucleus, pallidum and thalamus. The TFC demonstrates a strong ceiling effect in the premanifest stage and no significant correlations are to be found. In the manifest stage of the disease, the UHDRS TMS is most strongly related to the putamen (r = −0.530, p < 0.0001), pallidum (r = −0.390, p < 0.0001) and accumbens nucleus (r < −0.380, p < 0.0001), but also to the hippocampus, thalamus, and brainstem. The TFC is most strongly correlated to atrophy of the putamen (r = 0.270, p = 0.004), hippocampus (r = 0.240, p = 0.01) and pallidum (r = 0.230, p = 0.01) and a weak correlation to accumbens nucleus and brainstem.
Discussion
The aim of this study was to provide a comprehensive overview of the atrophy of subcortical structures, with respect to disease stage while taking into account whole-brain atrophy. The main findings show that all subcortical grey matter structures at some point have a reduced absolute volume. However, the accumbens nucleus, the caudate nucleus, the pallidum and the putamen display a true progressive decline in volume reduction which is disproportionate to overall whole-brain atrophy and this is already visible in the premanifest stages of HD. Conversely, the amygdala, brainstem and thalamus do show reduced volumes, but this merely reflects the overall whole brain atrophy rate. The hippocampus is exceptional in the fact that only in the manifest stage is a true rapid decline in this structure observed. Furthermore, the volume reductions of the accumbens nucleus, putamen, pallidum and hippocampus have a severe impact on clinical measures in manifest HD.
The atrophy of the caudate nucleus and putamen in the premanifest stages confirms earlier studies. The fact that this happens a decade or more before estimated time to onset is in accordance with the findings of Aylward et al. [3], the findings of the PREDICT-HD study [25] and with the results reported in TRACK-HD baseline paper [39].
Atrophy of the pallidum and thalamus in the premanifest stages of HD is controversial. Campodonico et al. and Harris et al. report no significant reduction in volume of the pallidum [7, 10], while others do report this finding [14, 17, 40]. With regard to the thalamus in the premanifest stage, reports of reduced volume are available in favour [10, 40] and in dispute [14, 24] of thalamic involvement. Our findings, using 3 T MRI and in the largest cohort reported thus far, confirms that atrophy in the pallidum is apparent in the premanifest stage closer to predicted disease onset, but not in the further from predicted onset stage. Although thalamic atrophy is also apparent in the premanifest close to onset stage, this merely reflects the whole brain atrophy.
The brainstem, thalamus, and amygdala follow the normal atrophy rate of the whole brain in the manifest stages of the disease. In fact, in HD stage 1 the brainstem and the amygdala show signs of being relatively spared. The hippocampus shows a different pattern of atrophy as it starts to show signs of atrophy in the preHD-B stage, but really becomes significant in terms of volume loss in HD stage 2. To some degree the above described findings have been reported by Rosas et al. and Douaud et al. [8, 30]. The added value of our study lies not only in the large sample size facilitating the further specification of the premanifest and manifest groups, but particularly in the fact that the atrophy described takes into account whole-brain atrophy. It should be noted that some caution is appropriate when interpreting the ratio to whole-brain atrophy, as this method does not necessarily imply that the volume reduction of a certain structure has to be greater than the whole brain atrophy to be of significance in terms of clinical symptoms.
The clinical impact of atrophy of subcortical grey matter is difficult to assess as many brain structures are involved in complex functionality. We have demonstrated that of the subcortical grey matter structures, specifically volume reductions of the accumbens nucleus, putamen and pallidum have the greatest influence on predicting the motor disturbances in manifest HD, and perhaps surprisingly, the impact of the caudate nucleus is minimal. The role of the pallidum in motor function is underestimated and surpasses the caudate nucleus and the thalamus. However, the putamen is the most important structure for this clinical symptom. Our study shows that decreased global functioning of manifest HD was mainly related to volume loss of the hippocampus, putamen and pallidum. Cognitive and motor deficiencies have been shown in a number of separate studies, all examining only part of the deep grey matter structures, to relate to volume reductions of the basal ganglia, thalamus and frontal lobe [1, 4, 6, 7, 10, 14, 26, 33]. Within one small cohort examination of clinical measures with the TFC was carried out by Rosas et al. who provided evidence for an association between each separate structure and the TFC in the manifest stage [30], which, apart from some differences between studies, is largely confirmed by our study. All these studies show that there is an association between volumes and clinical measures; however, we hope that by examining all structures within one large, well defined cohort, we can provide evidence for which structure has the most contribution (or at least the strongest correlation) to global functioning and motor disturbances.
TRACK-HD is a longitudinal observational biomarker study aimed at providing essential methodology for the assessment of therapeutic interventions. With respect to this goal, we can add that the accumbens nucleus and pallidum show similar biomarker potential in addition to the well recognised structures putamen and caudate nucleus. The hippocampus is also of importance as it has a high correlation with clinical symptoms. The important role of the caudate nucleus in the premanifest stage of the disease seems to diminish after manifestation, as was also suggested by the TRACK-HD baseline paper [39]. It is possibly fair to say that at different disease stages different structures play a role. A biomarker would ideally show sensitivity across all disease stages and would reflect the underlying pathologic processes. From this cross-sectional analysis it is not possible to draw any definitive conclusions; however, the putamen possibly shows the greatest potential. Longitudinal evaluation has to be performed to confirm the putamen’s true potential. Furthermore we would like to stress how taking whole brain atrophy into account aids our interpretation of regional atrophy rates and in this way their potential as biomarkers can be assessed more effectively.
A limitation of our study could be the use of a relatively new software package from FSL used for segmentation, which has not specifically been validated in HD. Visual inspection, however, did not reveal any significant mismatches. The method applied gives structure segmentation on an individual basis and can therefore be used to compare groups. In contrast to this limitation several clear reasons exist in favor of using FIRST—first of all, compared to manual segmentation the automated segmentation uses voxel intensity in contrast to the sometimes difficult visual contrast differences, reducing a rater dependent bias. Secondly, this automated technique is suitable for implantation on large datasets, whereas manual segmentation is labour intensive and prone to human error.
In conclusion, we have demonstrated that atrophy of the pallidum and the accumbens nucleus exists in the premanifest stages of the disease and confirmed the well known atrophy of the caudate nucleus and putamen. Furthermore, we have shown the important correlation of the accumbens nucleus, putamen, pallidum and hippocampus with clinical symptoms. The importance of the remaining subcortical structures should be regarded when taking into account that they show similar amounts of atrophy as the brain as a whole. These findings have implications for biomarker research, as several subcortical structures now show great potential for use as a disease progression measure.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgment
The authors wish to thank the TRACK-HD study participants, Cure for Huntington’s Disease Initiative (CHDI)/High Q Foundation, a not-for-profit organisation dedicated to finding treatments for HD, Beth Borowsky, Allan Tobin, Sherry Lifer, Saiqah Munir, Azra Hassanali, Daniel van Kammen, Ethan Signer, Michael Hayden, Susan Creighton, 2mt Software GmbH, Anne Rosser, Andrea Nemeth, Emma Hobson, Huntington’s disease clinic at Guy’s hospital, Centre d’Investigation Clinique Hospital de la Salpêtrière Paris, National Hospital for Neurology London, Leiden University Medical Centre, Katja Vitkin, Felix Mudoh Tita, Irina Vainer, Theresia Kelm, Biorep Technologies, Arthur Toga, Laboratory of Neuro Imaging UCLA (LONI) and IXICO for all their help in enabling all aspects of TRACK-HD to move forward. Some of this work was undertaken at University College London Hospital/University College London, which received funding from the Department of Health NIHR Biomedical Research Centres funding scheme.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
TRACK-HD study group:
Canada—A. Coleman, R. Dar Santos, J. Decolongon, A. Sturrock, B. Leavitt(University of British Columbia, Vancouver). France—E. Bardinet, C. Jauffret, D. Justo, S. Lehericy, C. Marelli, K. Nigaud, R. Valabrègue, A. Durr (APHP, Hôpital Salpêtriere, Paris). Germany—N. Bechtel, R. Reilmann (University of Münster, Munster). A. Hoffman, P. Kraus (University of Bochum). B. Landwehrmeyer (University Ulm, Ulm). The Netherlands—J. van der Grond, EP. t’Hart, C. Jurgens, M–N. Witjes-Ane (Leiden University Medical Centre, Leiden). UK—N. Arran, J. Callaghan, D. Craufurd (St Mary’s Hospital, Manchester), C. Frost, R. Jones (London School of Hygiene and Tropical Medicine, London). N. Hobbs, N. Lahiri, R. Ordidge, G. Owen, T. Pepple, J. Read, M. Say, E. Wild, N. Fox (University College London, London). S. Keenan (Imperial College London, London), DM. Cash (IXICO, London). C. Kennard, S. Hicks (Oxford, UK). USA—E. Axelson, C. Wang (University of Iowa, Iowa City, IA). S. Lee, W. Monaco, D. Rosas (Massachusetts General Hospital, Harvard, MA). C. Campbell, S. Queller, K. Whitlock (Indiana University, IN). Australia—C. Campbell, M. Campbell, E. Frajman, C. Milchman, A. O’Regan, J. Stout (Monash University, Victoria)
Reference
- 1.Aylward EH. Change in MRI striatal volumes as a biomarker in preclinical Huntington’s disease. Brain Res Bull. 2007;72:152–158. doi: 10.1016/j.brainresbull.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Aylward EH, Brandt J, Codori AM, Mangus RS, Barta PE, Harris GJ. Reduced basal ganglia volume associated with the gene for Huntington’s disease in asymptomatic at-risk persons. Neurology. 1994;44(5):823–828. doi: 10.1212/wnl.44.5.823. [DOI] [PubMed] [Google Scholar]
- 3.Aylward EH, Codori AM, Barta PE, Pearlson GD, Harris GJ, Brandt J. Basal ganglia volume and proximity to onset in presymptomatic Huntington disease. Arch Neurol. 1996;53:1293–1296. doi: 10.1001/archneur.1996.00550120105023. [DOI] [PubMed] [Google Scholar]
- 4.Aylward EH, Codori AM, Rosenblatt A, Sherr M, Brandt J, Stine OC, Barta PE, Pearlson GD, Ross CA. Rate of caudate atrophy in presymptomatic and symptomatic stages of Huntington’s disease. Mov Disord. 2000;15:552–560. doi: 10.1002/1531-8257(200005)15:3<552::AID-MDS1020>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 5.Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, Brandt J, Gourley LM, Liang K, Zhou H, Margolis RL, Ross CA. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63:66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 6.Backman L, Robins-Wahlin TB, Lundin A, Ginovart N, Farde L. Cognitive deficits in Huntington’s disease are predicted by dopaminergic PET markers and brain volumes. Brain. 1997;120(Pt 12):2207–2217. doi: 10.1093/brain/120.12.2207. [DOI] [PubMed] [Google Scholar]
- 7.Campodonico JR, Aylward E, Codori AM, Young C, Krafft L, Magdalinski M, Ranen N, Slavney PR, Brandt J. When does Huntington’s disease begin? J Int Neuropsychol Soc. 1998;4:467–473. doi: 10.1017/S1355617798455061. [DOI] [PubMed] [Google Scholar]
- 8.Douaud G, Gaura V, Ribeiro MJ, Lethimonnier F, Maroy R, Verny C, Krystkowiak P, Damier P, Bachoud-Levi AC, Hantraye P, Remy P. Distribution of grey matter atrophy in Huntington’s disease patients: a combined ROI-based and voxel-based morphometric study. Neuroimage. 2006;32:1562–1575. doi: 10.1016/j.neuroimage.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 9.Estrada Sanchez AM, Mejia-Toiber J, Massieu L. Excitotoxic neuronal death and the pathogenesis of Huntington’s disease. Arch Med Res. 2008;39:265–276. doi: 10.1016/j.arcmed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Harris GJ, Codori AM, Lewis RF, Schmidt E, Bedi A, Brandt J. Reduced basal ganglia blood flow and volume in pre-symptomatic, gene-tested persons at-risk for Huntington’s disease. Brain. 1999;122(Pt 9):1667–1678. doi: 10.1093/brain/122.9.1667. [DOI] [PubMed] [Google Scholar]
- 11.Harris GJ, Pearlson GD, Peyser CE, Aylward EH, Roberts J, Barta PE, Chase GA, Folstein SE. Putamen volume reduction on magnetic resonance imaging exceeds caudate changes in mild Huntington’s disease. Ann Neurol. 1992;31:69–75. doi: 10.1002/ana.410310113. [DOI] [PubMed] [Google Scholar]
- 12.Henley SM, Frost C, Macmanus DG, Warner TT, Fox NC, Tabrizi SJ. Increased rate of whole-brain atrophy over 6 months in early Huntington disease. Neurology. 2006;67:694–696. doi: 10.1212/01.wnl.0000230149.36635.c8. [DOI] [PubMed] [Google Scholar]
- 13.Jernigan TL, Salmon DP, Butters N, Hesselink JR. Cerebral structure on MRI, Part II: Specific changes in Alzheimer’s and Huntington’s diseases. Biol Psychiatry. 1991;29:68–81. doi: 10.1016/0006-3223(91)90211-4. [DOI] [PubMed] [Google Scholar]
- 14.Jurgens CK, Van de Wiel WL, van Es AC, Grimbergen YM, Witjes-Ane MN, der van GJ, Middelkoop HA, Roos RA. Basal ganglia volume and clinical correlates in ‘preclinical’ Huntington’s disease. J Neurol. 2008;255:1785–1791. doi: 10.1007/s00415-008-0050-4. [DOI] [PubMed] [Google Scholar]
- 15.Kassubek J, Juengling FD, Ecker D, Landwehrmeyer GB. Thalamic atrophy in Huntington’s disease co-varies with cognitive performance: a morphometric MRI analysis. Cereb Cortex. 2005;15:846–853. doi: 10.1093/cercor/bhh185. [DOI] [PubMed] [Google Scholar]
- 16.Kassubek J, Juengling FD, Kioschies T, Henkel K, Karitzky J, Kramer B, Ecker D, Andrich J, Saft C, Kraus P, Aschoff AJ, Ludolph AC, Landwehrmeyer GB. Topography of cerebral atrophy in early Huntington’s disease: a voxel based morphometric MRI study. J Neurol Neurosurg Psychiatry. 2004;75:213–220. [PMC free article] [PubMed] [Google Scholar]
- 17.Kipps CM, Duggins AJ, Mahant N, Gomes L, Ashburner J, McCusker EA. Progression of structural neuropathology in preclinical Huntington’s disease: a tensor based morphometry study. J Neurol Neurosurg Psychiatry. 2005;76:650–655. doi: 10.1136/jnnp.2004.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65:267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 19.Langbehn DR, Hayden MR, Paulsen JS (2009) CAG-repeat length and the age of onset in Huntington disease (HD): a review and validation study of statistical approaches. Am J Med Genet B Neuropsychiatr Genet [DOI] [PMC free article] [PubMed]
- 20.Muhlau M, Gaser C, Wohlschlager AM, Weindl A, Stadtler M, Valet M, Zimmer C, Kassubek J, Peinemann A. Striatal gray matter loss in Huntington’s disease is leftward biased. Mov Disord. 2007;22:1169–1173. doi: 10.1002/mds.21137. [DOI] [PubMed] [Google Scholar]
- 21.Patenaude B (2007) Bayesian statistical models of shape and appearance for subcortical brain segmentation [DOI] [PMC free article] [PubMed]
- 22.Patenaude B, Smith S, Kennedy D, Jenkinson M (2007) FIRST - FMRIB’s integrated registration and segmentation tool
- 23.Patenaude B, Smith S, Kennedy D, Jenkinson M (2008) Improved Surface Models for FIRST
- 24.Paulsen JS, Hayden M, Stout JC, Langbehn DR, Aylward E, Ross CA, Guttman M, Nance M, Kieburtz K, Oakes D, Shoulson I, Kayson E, Johnson S, Penziner E. Preparing for preventive clinical trials: the Predict-HD study. Arch Neurol. 2006;63:883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- 25.Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peinemann A, Schuller S, Pohl C, Jahn T, Weindl A, Kassubek J. Executive dysfunction in early stages of Huntington’s disease is associated with striatal and insular atrophy: a neuropsychological and voxel-based morphometric study. J Neurol Sci. 2005;239:11–19. doi: 10.1016/j.jns.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Penney JB, Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington’s disease. Ann Neurol. 1997;41:689–692. doi: 10.1002/ana.410410521. [DOI] [PubMed] [Google Scholar]
- 28.Roos RAC. Neuropathology of Huntington’s chorea. In: Vinken PJ, Bruyn GW, Klawans HL, editors. Handbook of Clinical Neurology; Extrapyramidal Disorders. Amsterdam: Elsevier Science Publishers; 1986. [Google Scholar]
- 29.Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65:745–747. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- 30.Rosas HD, Koroshetz WJ, Chen YI, Skeuse C, Vangel M, Cudkowicz ME, Caplan K, Marek K, Seidman LJ, Makris N, Jenkins BG, Goldstein JM. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60:1615–1620. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- 31.Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 32.Roze E, Saudou F, Caboche J. Pathophysiology of Huntington’s disease: from huntingtin functions to potential treatments. Curr Opin Neurol. 2008;21:497–503. doi: 10.1097/WCO.0b013e328304b692. [DOI] [PubMed] [Google Scholar]
- 33.Ruocco HH, Bonilha L, Li LM, Lopes-Cendes I, Cendes F. Longitudinal analysis of regional grey matter loss in Huntington disease: effects of the length of the expanded CAG repeat. J Neurol Neurosurg Psychiatry. 2008;79:130–135. doi: 10.1136/jnnp.2007.116244. [DOI] [PubMed] [Google Scholar]
- 34.Ruocco HH, Lopes-Cendes I, Li LM, Santos-Silva M, Cendes F. Striatal and extrastriatal atrophy in Huntington’s disease and its relationship with length of the CAG repeat. Braz J Med Biol Res. 2006;39:1129–1136. doi: 10.1590/S0100-879X2006000800016. [DOI] [PubMed] [Google Scholar]
- 35.Shoulson I, Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29:1–3. doi: 10.1212/wnl.29.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Smith SM, De Stefano N, Jenkinson M, Matthews PM. Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomogr. 2001;25:466–475. doi: 10.1097/00004728-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 37.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 38.Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 39.Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI, Borowsky B, Tobin AJ, Rosas HD, Johnson H, Reilmann R, Landwehrmeyer B, Stout JC (2009) Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol [DOI] [PMC free article] [PubMed]
- 40.Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, McCusker E, Frackowiak RS. The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain. 2002;125:1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.