Abstract
Aims
Recent studies show that ischaemic postconditioning (PostC), similar to the well-established ischaemic preconditioning (IPC), confers cardioprotection against ischaemia/reperfusion (IR) injury, and both IPC and PostC can activate the reperfusion injury salvage kinase (RISK) pathway and the survivor activating factor enhancement (SAFE) pathway. PostC is clinically more attractive because of its therapeutic application at the predictable onset of reperfusion. Our previous studies have demonstrated that MG53 is a primary component of the IPC machinery. Here, we investigated the potential role of MG53 in PostC-mediated myocardial protection and explored the underlying mechanism.
Methods and results
Using Langendorff perfusion, we investigated IR injury in wild-type (wt) and MG53-deficient (mg53−/−) mouse hearts with or without PostC. IR-induced myocardial damage was markedly exacerbated in mg53−/− hearts compared with wt controls. PostC protected wt hearts against IR-induced myocardial infarction, myocyte necrosis, and apoptosis, but failed to protect mg53−/− hearts. The loss of PostC protection in mg53−/− hearts was attributed to selectively impaired PostC-activated RISK signalling. Mechanistically, MG53 is required for the interaction between caveolin 3 (CaV3) and the p85 subunit of phosphoinositide 3-kinase (p85-PI3K) and PostC-mediated activation of the RISK pathway. Importantly, a structure–function study revealed that the MG53 tripartite motif (TRIM) domain (aa1–284) physically interacted with CaV3 but not p85-PI3K, whereas the MG53 SPRY domain (aa285–477) interacted with p85-PI3K but not CaV3, indicating that MG53 binds to CaV3 and p85 at its N- and C-terminus, respectively.
Conclusions
We conclude that MG53 participates in PostC-mediated cardioprotection largely through tethering CaV3 and PI3K and subsequent activation of the RISK pathway.
Keywords: MG53, Ischaemic postconditioning, Ischaemic preconditioning, Ischaemia/reperfusion injury, Myocardial infarction
1. Introduction
Ischaemic preconditioning (IPC), discovered by Murry et al. in 1986,1 is a well-established endogenous mechanism of cardioprotection against ischaemia/reperfusion (IR) injury. Recently, it has been shown that ‘postconditioning' (PostC), similar to IPC, is another form of endogenous cardioprotection.2 PostC is induced by a series of short repetitive cycles of reperfusion and re-occlusion of coronary blood flow applied immediately at the onset of reperfusion. This has been independently confirmed by several research groups in several mammalian species, including mouse,3 rat,4 rabbit,5 dog,6 and human.7 Many studies have shown that the cardioprotective effect of PostC, as is the case for IPC, is associated with activation of cell survival signalling pathways, including reperfusion injury salvage kinases (RISKs)4 and survivor activating factor enhancement (SAFE) pathways.3 Because it is temporally well controlled, PostC may represent a potentially more important and practical clinical intervention to treat ischaemic heart disease.
MG53 [also named (tripartite motif) TRIM72], a newly identified TRIM-containing family protein primarily expressed in cardiac and skeletal muscles, has been implicated in intracellular vesicle trafficking, myogenesis, and acute membrane repair in skeletal and cardiac muscles.8–10 Our recent studies have demonstrated that MG53 is indispensable for IPC-mediated cardioprotection through activating prosurvival kinases such as phosphoinositide 3-kinase (PI3K), Akt, GSK3β, and ERK1/2.11 Although IPC and PostC can activate overlapping signalling events, it remains elusive as to whether and how MG53 contributes to the cardiac PostC response.
In this study, we determined whether MG53 is involved in the cardioprotective effect of PostC treatment and, if so, what is the underlying mechanism. Our study demonstrated that PostC-mediated cardioprotection is missing in the MG53-deficient heart because of impairments of the RISK pathway.
2. Methods
2.1. Animals
Animals were maintained in the Center for Experimental Animals (an AAALAC accredited experimental animal facility) at Peking University, Beijing, China. All procedures involving experimental animals were performed in accordance with protocols approved by the Committee for Animal Research of Peking University, and conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, revised 1996).
2.2. Isolated mouse heart Langendorff perfusion
Adult mg53−/− mice and wt littermates (20–30 g) were used for mouse heart ex vivo Langendorff perfusion. Details of the experiments were as previously described.11 Mice were anaesthetized by ip injection of pentobarbital (70 mg/kg). The heart was excised and perfused on a Langendorff apparatus at a constant pressure of 55 mmHg. The buffer was continuously gassed with 95% O2/ 5% CO2 (pH 7.4) and warmed by a heating bath/circulator. The heart temperature was continuously monitored and maintained at 37 ± 0.5°C. Global ischaemia was induced by cessation of perfusion for 20 or 30 min followed by reperfusion. PostC was achieved by 6 episodes of 10 s ischaemia and 10 s reperfusion which followed the 20 or 30 min ischaemia.
2.3. Measurement of myocardial infarct size
To measure the infarct size, isolated perfused mouse hearts subjected to global ischaemia were frozen at −80°C for 10 min and cut into slices (5–6 slices/heart), which were then incubated in a sodium phosphate buffer containing 1% 2,3,5-triphenyl-tetrazolium chloride for 15 min to visualize the unstained infarct region. The infarct and ventricle areas were determined by planimetry with Image/J software from NIH. The infarct size was calculated as infarct area divided by ventricle area.
2.4. Histology and TUNEL staining of myocardial sections
Hearts were fixed in 4% paraformaldehyde (pH 7.4) overnight, embedded in paraffin, and serially sectioned at 5 µm. A CardioTACSTM in situ apoptosis detection kit (#TA5353; R&D Systems Inc.,) was used for TUNEL staining of these sections following the manual.
2.5. Determination of myocardial injury by lactate dehydrogenase release
The effluent from the perfused heart was accumulated at 10 min of reperfusion. Lactate dehyrogenase (LDH) was spectrophotometrically assayed using a kit from Sigma Chemical Co.
2.6. Plasmid construction
2.6.1. Flag-MG53 and MG53-NT (non-tagged MG53)
p3X-flag-CMV-MG53 expresses flag-tagged MG53. The coding sequence of MG53 was amplified from a mouse skeletal muscle cDNA library by using forward primer MG53-FP bearing a KpnI site and reverse primer MG53-RP bearing an XbaI site and cloned into the expression vector p3X-flag-CMV-9 (Sigma Chemical Co) using these two sites: MG53-FP, 5′-AATGGTACCGCCACCATGTCGGCTGCACCCGGCCTTC-3′; and MG53-RP, 5′-AATCTCGAGCGGGCCTGTTCCTGCTCCGGCC-3′. For MG53-NT, the MG53 coding sequence was amplified by using forward primer MG53-NT-FP bearing a KpnI site and reverse primer MG53-NT-RF bearing an XhoI site followed the stop codon and cloned into the vector pcDNA4/TO/myc-HisB (invitrogen) using these two sites: MG53-NT-FP, 5′-AATGGTACCGCCACCATGTCGGCTGCACCCGGCCTTC-3′; and MG53-NT-RF, 5′-AATCTCGAGTTAGGCCTGTTCCTGCTCCGGCC-3′.
2.6.2. Flag-MG53-TRIM and Flag-MG53-splA and ryanodine receptors (SPRY)
p3X-flag-CMV-TRIM and p3X-flag-CMV-SPRY express the flag-tagged MG53 TRIM domain and SPRY domain, respectively. The TRIM domain (1–284 amino acids region of MG53) and the SPRY domain (285–477 amino acids region of MG53) were amplified by PCR. Both of the products beard a NotI site at the 5′ terminus and a BglII site at the 3′ terminus. They were cloned into the p3X-flag-CMV using these two sites: pTRIM-FP, 5′-AAGCGGCCGCCACCATGTCGGCTGCACCCGGCCTT-3′ and pTRIM-RP, 5′-AAAGATCTTCACAGAGCCCGGAACATCTT-3′; pSPRY-FP, 5′-AAGCGGCCGCCACCATGCCAGCGCTGGAGGAACTG-3′; and pSPRY-RP, 5′-AAAGATCTTCAGGCCTGTTCCTGCTCCGG-3′.
2.6.3. P85 subunit (p85)-PI3K-myc and flag-p85
pcDNA4/TO/myc-p85 expresses myc-tagged p85. The coding sequence of p85 was amplified from a mouse foetal liver cDNA library by using forward primer p85-FP bearing a BamHI site and reverse primer p85-RP bearing an XbaI site and cloned into the expression vector pcDNA4/TO/myc-HisB by using these two sites: p85-FP, 5′-ATCGGATCCGCCACCATGAGTGCAGAGGGCTACCAG-3′; and p85-RP, 5′-AGTTCTAGACCTCGCCTCTGTTGTGCATATAC-3′. P3X-flag-p85 expresses Flag-tagged p85. The coding sequence of p85 was amplified by flag-p85-FP bearing an EcoRI site and Flag-p85-RF bearing a BamHI site and cloned into the expression vector p3X-flag-CMV-9 by using these two sites: flag-P85-FP, 5′-AGAGAATTCAAGTGCAGAGGGCTACC-3′ and flag-p85-RF, 5′-CCCGGGATCCTCATCGCCTCTGTTGTGCA-3′.
2.6.4. Caveolin (CaV) 3-myc
pcDNA3.1 (+)-myc-CaV3 (caveolin) expresses myc-tagged CaV3. The coding sequence of CaV3 was amplified from a mouse foetal liver cDNA library by using forward primer pCaV3-FP bearing an EcoRI site and reverse primer pCaV3-RP bearing an XbaI site and cloned into the expression vector pcDNA3.1(+)-myc-HisA by using these two sites: pCaV3-FP, 5′-CGGAATTCCCCCAGCCTCACAATGATGAC-3′ and pCaV3-RP, 5′-CTAGTCTAGAGCCTTCCCTTCGCAGCACCAC-3′.
2.7. Cell transfection
Human embryonic kidney 293 (HEK293) cells were maintained in DMEM supplemented with 10% FBS. Transfection was performed using lipofectamine 2000TM (Invitrogen) following the manufacturer's instructions. When performing co-immunoprecipitation (Co-IP), two plasmids were transfected at the ratio 2 µg:2 µg per 60 mm plate.
2.8. Western blot and Co-IP
Western blot and Co-IP, together with subcellular fractionation, were performed as previously described.11,12 Cells were lysed in lysis buffer A (30 mM HEPES (pH 7.6), 100 mM NaCl, 0.5% Nonidet P-40, and protease inhibitor mixture) on ice for 30 min, and the lysates were clarified by centrifugation at 4°C for 10 min at 12 000 g. The supernatant was mixed with protein A plus agarose (Santa Cruz Biotechnology) and the antibody and incubated at 4°C for 3 h. The resins were then washed five times with lysis buffer A, and the bound proteins were detected by western blot.
2.9. Production of recombinant protein and pull-down assay
HEK293 Cells expressing MG53-myc, CaV3-myc, or p85-myc were lysed in lysis buffer A on ice for 10 min, and the lysates were clarified by centrifugation at 4°C for 10 min at 13 000 g in a microcentrifuge. The supernatant was mixed with EZviewTM Red Anti-myc Affinity Gel (Sigma Chemical Co.,) to precipitate myc-tagged proteins. After incubation at 4°C for 2 h, the resins were washed three times with lysis buffer A and eluted proteins with 0.1 mM Glycine (pH, 2.8). The antibody to MG53 was coupled onto CNBr-activated SepharoseTM 4B (Amersham Biosciences) which we named anti-MG53-beads. HEK293 cells expressing MG53-NT were lysed in lysis buffer A. MG53-NT proteins were purified by using anti-MG53-beads. HEK293 cells expressing flag-p85 were purified by the same strategy using anti-flag M2 Affinity Gel (Sigma Chemical Co., Cat# A2220). To test the interaction between MG53 and CaV3 or p85, the MG53-myc proteins were immobilized onto anti-MG53 beads and incubated with purified p85-myc or CaV3-myc for 4 h at 4°C. The anti-MG53 beads were incubated with only p85-myc or CaV3-myc served as negative controls. The resins were washed and boiled in the sample loading buffer. The proteins were resolved by SDS/PAGE and detected by western blotting with the anti-myc antibody. To determine the role of MG53 in the interaction of p85 and CaV3, CaV3-myc proteins were first immobilized onto Anti-myc Affinity Gel and then incubated with purified flag-p85 with or without purified MG53-NT for 4 h at 4°C. The Anti-myc Affinity Gel was incubated with only flag-p85, MG53-NT, or CaV3-myc served as negative controls. The resins were washed and boiled in the sample loading buffer. The proteins were resolved by SDS/PAGE and detected by western blotting with respective specific antibody.
2.10. Immunofluorescence and confocal imaging
For immunostaining, cells were incubated with anti-myc and anti-flag antibody (1:100) at 4°C overnight after fixation and blocking, and then stained with TRITC-conjugated and FITC-conjugated secondary antibody (1:500, Santa Cruz Biotechnology). DAPI staining was performed before mounting the cover slip. Images were detected with a laser scanning confocal microscope (LSM-700, Zeiss) with a 40× water immersion objective (numeric aperture 1.0), with excitation wavelengths of 405, 488, and 555 nm, and emission wavelengths of 350–490, 490–539, and 550–600 nm for DAPI, FITC, and TRITC, respectively.
2.11. Materials
Antibodies to p-ERK1/2, ERK1/2, p-Akt, Akt, p-GSK3β, GSK3, p-STAT3, signal transducer and activator of transcription-3 (STAT3), and GAPDH were from Cell Signaling Technology. Antibodies to flag and myc were from Sigma Chemical Co. MG53 antibody was described previously.8 SB216763 was from Sigma Chemical Co. (Cat# S3442).
2.12. Statistical analysis
Data are expressed as the mean ± s.e.m. Statistical comparisons used one-way analysis of variance, followed by Bonferroni's procedure for multiple-group comparisons. P < 0.05 was considered statistically significant unless otherwise noted for a specific experiment.
3. Results
3.1. PostC protection is abolished in MG53-deficient hearts
In ex vivo perfused mouse hearts, we found that 15 min reperfusion caused a significant downregulation of MG53 in wt mouse hearts, and that PostC treatment fully blocked IR-induced MG53 reduction (Supplementary material online, Figure S1). This result suggests a potential role of MG53 in PostC-mediated cardioprotection or alternatively an epiphenomenon.
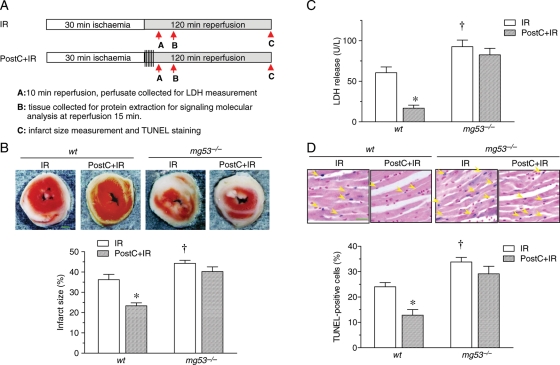
To define whether MG53 is involved in PostC-mediated cardiac protection, we investigated the response to PostC in wt and mg53−/− mouse hearts in the presence of IR insult following the protocol in Figure 1A. IR-induced myocardial damage during Langendorff perfusion was markedly worsened in the mg53−/− heart (Figure 1B–D). PostC was able to profoundly attenuate IR-induced myocardial infarct size (23.37 ± 1.45% vs. 36.24 ± 2.63%, P < 0.05), LDH release (16.80 ± 3.74 vs. 60.56 ± 7.15 U/L, P < 0.05), and apoptotic cell death (12.86 ± 2.20% vs. 24.04 ± 1.67%, P < 0.05) in wt mouse hearts (Figure 1B–D). Importantly, in MG53-defiecient mouse hearts, PostC was unable to reduce IR-induced myocardial infarction (40.24 ± 2.34% vs. 44.26 ± 1.51%, P > 0.05), LDH release (82.56 ± 8.08 vs. 92.66 ± 8.10 U/L, P > 0.05), or apoptosis (29.15 ± 2.98% vs. 33.39 ± 1.64%, P > 0.05) (Figure 1B–D), highlighting an essential role of MG53 in PostC-mediated cardiac protection (Figure 1B–D).
Figure 1.
MG53 knockout hearts are vulnerable to IR injury and resistant to ischaemic PostC protection. (A) Schematic illustration of the protocol used for mouse global ex vivo IR (30 min ischaemia followed by reperfusion) with or without PostC (6 episodes of 10 s ischaemia followed by 10 s reperfusion). (B) Representative photographs and statistical data of infarct size in perfused wt and mg53−/− mouse hearts subjected to IR with or without PostC (n = 8, scale bar denotes 1 mm). (C) Change of LDH concentration in the efflux of perfused hearts from wt and mg53−/− mice subjected to 30 min ischaemia and 10 min of reperfusion with or without PostC (n = 8). (D) Representative photographs and statistical data of TUNEL staining of myocardial sections from perfused hearts of wt and mg53−/− mice subjected to IR with or without PostC (n = 8, scale bar denotes 30 µm). For (B–D), data are presented as mean ± s.e.m. (*P < 0.05 vs. all three other groups; †P < 0.05 vs. wt IR).
To determine whether the failure of PostC to protect mg53−/− mouse hearts is because of PostC starting with a larger infarct size, we tested the potency of PostC in 20 min ischaemia followed by 120 min reperfusion, which caused a smaller infarct size compared with that induced by 30 min ischaemia in wt mice (Supplementary material online, Figure S2). We found that for 20 min ischaemia, the infarct size in the hearts from mg53−/− mice was still larger than that of wt mice, and the protection of PostC was absent in mg53−/− mice, similar to the situation of 30 min ischaemia (Supplementary material online, Figure S2). Together, our present and previous studies have demonstrated that MG53 is essential not only for IPC-11 but also for PostC-mediated cardioprotection (Figure 1), and that the failure of PostC to protect MG53-deficient hearts is not because of starting with a greater infarct size.
3.2. Activation of RISK pathway by PostC is blunted in MG53-deficient hearts
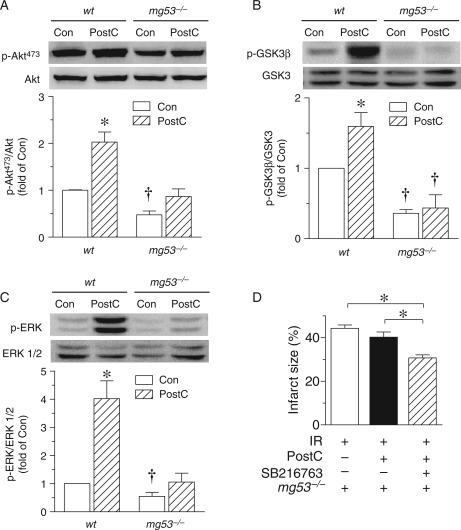
Next, we sought to delineate the mechanism underlying the MG53-dependent PostC response. In this regard, previous studies have shown that multiple protective pathways, including RISK4,13,14 and SAFE,3 are implicated in cardioprotection by PostC. Consistent with previous studies,4 PostC significantly elevated the phosphorylation levels of several important components of the RISK pathway, including Akt, GSK3β, and ERK1/2 in wt mouse hearts (Figure 2A–C). But in the mg53−/− hearts, while the expression levels of Akt, GSK3β, and ERK1/2 were not altered, PostC failed to increase the phosphorylation levels of the survival kinases (Figure 2A–C), suggesting that MG53 is essential for the PostC-induced activation of the RISK pathway.
Figure 2.
MG53 is essential for PostC-induced activation of RISK pathway. (A–C): Representative immunoblots and statistical data of phosphorylated and total Akt (A), GSK3β (B), and ERK 1/2 (C) in lysates from perfused wt and mg53−/− mouse hearts with or without PostC (n = 8, *P < 0.05 vs. all three other groups; †P < 0.05 vs. both wt groups). Note that MG53 ablation impaired PostC-induced phosphorylation of Akt, GSK3β, and ERK 1/2. (D) Average data of the infarct size in perfused mg53−/− hearts subjected to 30 min ischaemia followed by 120 min reperfusion without or with PostC or SB216763 pretreatment (3 µM for 10 min before 30 min ischaemia. n = 8, *P < 0.05 as indicated).
Furthermore, prior to the 30 min ischaemia, pretreatment of the mg53−/− hearts with a GSK inhibitor, SB216763 (3 µM) for 10 min significantly decreased the infarct size induced by IR (Figure 2D). This indicates that RISK pathway is still capable of being activated by signalling events downstream of PI3K such as GSK3β inhibition in mg53−/− mouse hearts.
3.3. Activation of SAFE pathway by PostC is unaltered in MG53-deficient hearts
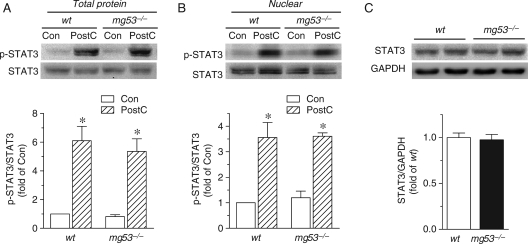
Equally important, the SAFE pathway, namely a ‘RISK-free' pathway, which involves activation of tumour necrosis factor α (TNFα) and the transcription factor STAT3, is important for PostC- as well as IPC-mediated cardiac protection.3,15 Indeed, PostC led to activation of STAT3 in both total cellular and nuclear fractions (Figure 3A and B), consistent with previous studies.3,16 MG53 ablation did not alter STAT3 expression or PostC-induced STAT3 phosphorylation (Figure 3A–C). Thus, MG53 is essentially involved in the signalling of the PostC-evoked RISK pathway but not the SAFE pathway in mammalian heart.
Figure 3.
SAFE pathway is independent of MG53. (A and B) Representative immunoblots and average data of phosphorylated and total STAT3 protein from total (A) and nuclear fraction (B) in hearts from wt and mg53−/− mice with or without PostC (n = 6 for each group, *P < 0.05 vs. control). (C) Representative immunoblots and average data of STAT3 protein levels in myocardial tissue from wt and mg53−/− mice (n = 8).
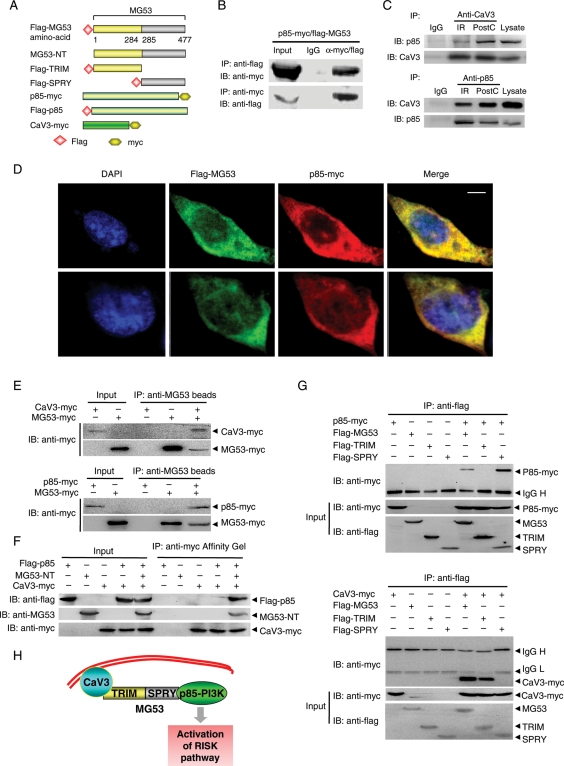
3.4. MG53 tethers p85-PI3K and CaV3
Our previous studies have demonstrated that MG53 and CaV3 form a physical complex which is essential for the interaction of CaV3 and the p85 subunit of PI3K and subsequent activation of the IPC-evoked RISK pathway.11 However, it remains unknown as to how MG53 mediates the interaction between CaV3 and p85-PI3K. To address this question, we constructed vectors expressing Flag-MG53 and p85-PI3K-myc (Figure 4A). Co-IP and immunostaining revealed a physical interaction between MG53 and p85-PI3K (Figure 4B and D). To explore the physiological and pathological relevance of the signalling complex in PostC-mediated cardioprotection, we performed Co-IP assay with proteins from perfused mouse hearts subjected to IR with or without PostC treatment. Importantly, the formation of p85-PI3K and CaV3 complex was clearly increased by PostC (Figure 4C). Using recombinant MG53, CaV3, or p85-PI3K proteins and pull-down assay, we confirmed the direct interaction between MG53 protein with CaV3 as well as p85-PI3K, and the failure of CaV3 to interact with p85-PI3K in the absence of recombinant MG53 protein (Figure 4E and F). Furthermore, structure–function studies showed that MG53-TRIM (aa1–284) interacted with CaV3, but not p85-PI3K, while MG53-SPRY (aa285–477) interacted with p85-PI3K but not CaV3 (Figure 4G), indicating that MG53 acts as bridge tethering CaV3 and p85, contributing to the PostC-activated RISK pathway (Figure 4H).
Figure 4.
MG53 interacts with p85 subunit of PI3K as well as CaV3. (A) Schematic diagram of structure of the plasmids expressing flag-MG53, MG53-NT, flag-MG53-TRIM and flag-MG53-SPRY, p85-myc, flag-p85, or CaV3-myc. (B) Co-IP of flag-MG53 and p85-myc in lysates of HEK293 cells (n = 4). (C) Co-IP of p85-PI3K and CaV3 in the lysates of the perfused mouse hearts subjected to IR with or without PostC treatment (n = 4). (D) Confocal immunofluorescence co-staining to visualize the colocalization of flag-MG53 (green) and p85-myc (red) in HEK293 cells (Scale bar denotes 5 µm). (E and F) Representative blots of pull-downed MG53-myc recombinant protein with CaV3-myc or p85-myc protein, and CaV3-myc with flag-p85 in the presence or absence of MG53-NT recombinant protein, respectively. (G) Co-IP of MG53-TRIM or MG53-SPRY with p85-PI3K-myc or CaV3-myc in lysates of HEK293 cells (n = 4). (H) Schematic presentation to show the role of MG53 in tethering CaV3 and p85-PI3K and subsequent activation of the RISK pathway.
4. Discussion
There are three main findings in the current study, including (i) MG53 is an essential component of cardiac PostC machinery in addition to its known role in the IPC response,11 (ii) MG53 specifically participates in PostC-induced activation of the RISK pathway but not the SAFE pathway, and (iii) MG53 acts as a bridge with its N-terminus interacting with CaV3 and its C-terminus interacting with p85-PI3K, contributing to PostC-induced activation of the RISK pathway.
It is well accepted that myocardial infarction caused by prolonged ischaemia results in irreversible myocardial damage and permanent loss of contractile mass. Although timely reperfusion of the ischaemic heart is the best way to preserve cardiomyocyte viability, reperfusion of the ischaemic heart leads to further damage to the myocardium, i.e. IR injury.17–19 Both IPC1 and the newly discovered PostC2 are the most powerful intrinsic cellular mechanism to protect the heart from IR injury. IPC protection can be beneficial only when it is applied before the occurrence of a severe ischaemic episode.1 But in clinical settings, the index ischaemic episode is often unpredictable. Fortunately, PostC, where brief repetitive IR episodes are applied after a prolonged period of ischaemia, can also reduce myocardial injury to an extent comparable with that induced by IPC, but is more temporally well controlled than IPC. Thus, PostC offers a more promising approach to alleviate cardiac IR injury.
MG53 is a novel TRIM family protein, which is primarily expressed in cardiac and skeletal muscle. The structure of MG53 includes an N-terminal TRIM domain and a C-terminal SPRY domain.8 MG53 participates in multiple physiological and pathological processes, including acute membrane repair, intracellular vesicle trafficking and myogenesis in skeletal muscle and cardiac IPC.8–11 In the current study, we have shown that PostC can restore MG53 protein abundance in mouse hearts subjected to IR injury, and that MG53 is indeed required for PostC-induced activation of the RISK pathway, thus contributing to PostC-mediated cardiac protection. Interestingly, the defect in the RISK pathway occurs in early signalling steps of the phosphorylation cascade of the kinases, while the distal pathway is still functional. Indeed, inhibition of GSK with SB216763 is able to elicit a significant cardiac protective effect, as evidenced by the reduction in IR-induced infarct size in mg53−/− mouse hearts.
Caveolae, a fraction of the lipid membrane, are cholesterol- and sphingolipid-enriched invaginations of the plasma membrane, and play an important role in cellular signal transduction. The scaffold protein CaV, which is essential for caveolae formation, interacts with the p85 subunit of PI3K, facilitating the activation of its downstream signalling.20 CaV3, a CaV family member which is specifically expressed in striated muscles, forms a complex with p85, and is involved in IPC protection.11,21
In this study, we have demonstrated that MG53 can form a complex with both CaV3 and p85-PI3K in the absence of any other bridging molecule, and that the signalling complex can be enhanced by PostC treatment. It is noteworthy that the MG53 N-terminal TRIM domain interacts with CaV3, whereas its C-terminal SPRY domain binds to p85-PI3K. Furthermore, we have shown that MG53-mediated interaction of Cav3 and p85 is indispensable for PostC-induced activation of RISK signalling, including PI3K/Akt/GSK3β cascade and ERK1/2 pathway. Thus, MG53 bridges CaV3 and p85-PI3K forming a functional complex of CaV3–MG53–PI3K (Figure 4H), which, in turn, activates the RISK pathway. MG53 deficiency leads to dissociation of CaV3 and p85-PI3K, disrupting PostC-induced RISK signalling and cardioprotection.
In our previous studies, it has been demonstrated that MG53 plays an essential role in membrane repair in cardiac myocytes as well as skeletal muscle.8,22 It merits future investigation to determine whether the role of MG53 in membrane repair contributes to PostC-mediated cardioprotection and whether there is cross-talk between RISK signalling and membrane repair machinery.
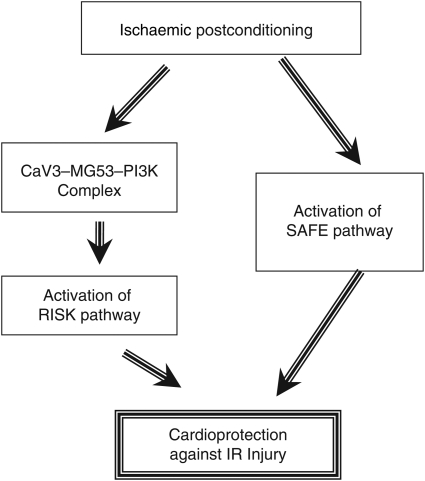
In summary, we have demonstrated, for the first time, that MG53 is an essential component of the cardiac PostC machinery in addition to its known function in the IPC response, and that MG53 specifically participates in PostC-induced activation of the RISK pathway but not the SAFE pathway (Figure 5). The present findings not only unveil an important physiological and pathological function of the newly identified muscle-specific TRIM family protein but also provide a promising therapeutic target for the treatment of ischaemic heart disease.
Figure 5.
Schematic shows that MG53 forms a complex with CaV3 and p85-PI3K, which is involved in PostC-induced activation of the RISK but not the SAFE pathway.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the National Basic Research Program of China (2007CB512100), the Beijing Municipal Scientific and Technology Commission (2007B005) and National Natural Science Foundation of China (81000054 and 81000051) and in part, by the extramural National Institutes of Health (NIH) grant support to J.M.
Supplementary Material
Acknowledgements
We thank I. C. Bruce for language editing. We thank N. Hou, L. Huang, and W. Q. Zhang for their excellent technical assistance. Special thanks to H. Takeshima for his generous support in providing mg53−/− mice.
Conflict of interest: none declared.
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 3.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 4.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 5.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 6.Couvreur N, Lucats L, Tissier R, Bize A, Berdeaux A, Ghaleh B. Differential effects of postconditioning on myocardial stunning and infarction: a study in conscious dogs and anesthetized rabbits. Am J Physiol Heart Circ Physiol. 2006;291:H1345–1350. doi: 10.1152/ajpheart.00124.2006. [DOI] [PubMed] [Google Scholar]
- 7.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, et al. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 8.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11:56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai C, Masumiya H, Weisleder N, Pan Z, Nishi M, Komazaki S, et al. MG53 regulates membrane budding and exocytosis in muscle cells. J Biol Chem. 2009;284:3314–3322. doi: 10.1074/jbc.M808866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3 and dysferlin. J Biol Chem. 2009;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao C, Zhang Y, Weisleder N, Ferrante C, Wang X, Lv F, et al. MG53 Constitutes a primary determinant of cardiac ischemic preconditioning. Circulation. 2010;121:2565–2574. doi: 10.1161/CIRCULATIONAHA.110.954628. [DOI] [PubMed] [Google Scholar]
- 12.Peng W, Zhang Y, Zheng M, Cheng H, Zhu W, Cao CM, et al. Cardioprotection by CaMKII-deltaB is mediated by phosphorylation of heat shock factor 1 and subsequent expression of inducible heat shock protein 70. Circ Res. 2010;106:102–110. doi: 10.1161/CIRCRESAHA.109.210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 14.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 15.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci U S A. 2001;98:9050–9055. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman MD, Koch SE, Fuller-Bicer GA, Butler KL. Regulating RISK: a role for JAK-STAT signaling in postconditioning? Am J Physiol Heart Circ Physiol. 2008;295:H1649–H1656. doi: 10.1152/ajpheart.00692.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opie LH. Reperfusion injury and its pharmacologic modification. Circulation. 1989;80:1049–1062. doi: 10.1161/01.cir.80.4.1049. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Dorado D, Ruiz-Meana M, Piper HM. Lethal reperfusion injury in acute myocardial infarction: facts and unresolved issues. Cardiovasc Res. 2009;83:165–168. doi: 10.1093/cvr/cvp185. [DOI] [PubMed] [Google Scholar]
- 19.Heusch G. Postconditioning: old wine in a new bottle? J Am Coll Cardiol. 2004;44:1111–1112. doi: 10.1016/j.jacc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Krajewska WM, Maslowska I. Caveolins: structure and function in signal transduction. Cell Mol Biol Lett. 2004;9:195–220. [PubMed] [Google Scholar]
- 21.Tsutsumi YM, Horikawa YT, Jennings MM, Kidd MW, Niesman IR, Yokoyama U, et al. Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning. Circulation. 2008;118:1979–1988. doi: 10.1161/CIRCULATIONAHA.108.788331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Xie W, Zhang Y, Lin P, Han L, Han P, et al. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res. 2010;107:76–83. doi: 10.1161/CIRCRESAHA.109.215822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.