Abstract
Aims
Vascular peroxidase 1 (VPO1) is a newly identified haem-containing peroxidase that catalyses the oxidation of a variety of substrates by hydrogen peroxide (H2O2). Considering the well-defined effects of H2O2 on the vascular remodelling during hypertension, and that VPO1 can utilize H2O2 generated from co-expressed NADPH oxidases to catalyse peroxidative reactions, the aims of this study were to determine the potential role of VPO1 in vascular remodelling during hypertension.
Methods and results
The vascular morphology and the expression of VPO1 in arterial tissues of spontaneously hypertensive rats and Wistar–Kyoto rats were assessed. The VPO1 expression was significantly increased concomitantly with definite vascular remodelling assessed by evaluating the media thickness, lumen diameter, media thickness-to-lumen diameter ratio and mean nuclear area in artery media in spontaneously hypertensive rats. In addition, in cultured rat aortic smooth muscle cells we found that the angiotensin II-mediated cell proliferation was inhibited by knockdown of VPO1 using small hairpin RNA. Moreover, the NADPH oxidase inhibitor, apocynin, and the hydrogen peroxide scavenger, catalase, but not the ERK1/2 inhibitor, PD98059, attenuated angiotensin II-mediated up-regulation of VPO1 and generation of hypochlorous acid.
Conclusion
VPO1 is a novel regulator of vascular smooth muscle cell proliferation via NADPH oxidase–H2O2–VPO1–hypochlorous acid–ERK1/2 pathways, which may contribute to vascular remodelling in hypertension.
Keywords: Vascular peroxidase 1, Vascular remodelling, Rat aortic smooth muscle cells, Proliferation, Hypertension
1. Introduction
In hypertension, the small arteries and arterioles that determine peripheral resistance undergo both structural and functional changes,1 especially structural changes in the media, leading to remodelling and subsequently to other vascular pathologies.2 Vascular NADPH oxidase-derived reactive oxygen species, such as superoxide and hydrogen peroxide (H2O2), have emerged as important molecules in the pathogenesis of hypertension and its vascular complications.3–5 It has been found that H2O2 derived from vascular smooth muscle cells (VSMCs) plays an important role in angiotensin II (Ang II)-induced hypertrophy of the arterial wall.3 Recent evidence has demonstrated that Ang II-mediated activation of NADPH oxidases results in an increase of intracellular H2O2 and causes hypertrophy in VSMCs, suggesting a specific role for H2O2 in the vascular pathogenesis of vascular diseases.4,5
Myeloperoxidase (MPO) is a haem protein derived from neutrophils, monocytes, and macrophages. In physiological conditions, MPO catalyses the reaction of H2O2 and chloride to produce hypochlorous acid (HOCl).6 The evolutionary function of HOCl production is bactericidal, and it is believed to be the major oxidant produced by neutrophils in vivo.7,8 However, localized excess production of HOCl has been implicated in the progress of diseases such as atherosclerosis, which can involve an inflammatory or immunological response.9 HOCl reacts with many biological molecules and can cause damage to tissues.10,11
Vascular peroxidase 1 (VPO1) is a newly identified haem-containing peroxidase. Unlike MPO, which only exists in neutrophils, monocyte and macrophages, VPO1 is mainly distributed in vascular wall, liver, lung etc.12 As a haem-containing peroxidase, VPO1, like MPO, catalyses the reaction of H2O2 and chloride to produce HOCl.12 In addition to its peroxidase domain, VPO1 contains several distinctive domains that are suggestive of its participation in protein complexes. Our previous study demonstrated that VPO1 expressed in HEK293 cells could utilize H2O2 generated from co-expressed NADPH oxidases.12
Considering the well-defined effects of H2O2 on the vascular remodelling during hypertension, and that VPO1 can utilize H2O2 generated from co-expressed NADPH oxidase to catalyse peroxidative reactions, and that NADPH oxidase is normally expressed in the same vascular cells as VPO1, we performed the present study with two related hypotheses. First, we analysed the correlation of VPO1 and vascular remodelling during hypertension. Second, as vascular smooth muscle cell proliferation plays a vital role in vascular remodelling, we therefore evaluated the role of VPO1 in vascular smooth muscle cell proliferation and the underlying mechanisms.
2. Methods
2.1. Materials
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, penicillin, and streptomycin were obtained from Invitrogen (Carlsbad, CA, USA). Dimethyl sulfoxide was purchased from Amresco (Solon, OH, USA). The bromodeoxyuridine (BrDU) cell proliferation assay kit was purchased from Roche (Wettsteinplatz, Basel, Switzerland). Angiotensin II, apocynin, catalase, hydrogen peroxide, PD98059, and HOCl stock solution were purchased from Sigma-Aldrich (St Louis, MO, USA). Rabbit anti-mouse MPO antibody was obtained from Abcam (Cambridge, UK). Goat anti-rabbit immunoglobulin G as the secondary antibody was obtained from Promega (Madison, WI, USA). Hydrogen peroxide assay kits were purchased from Beyotime Institute of Biotechnology (Jiangsu, China). Other chemical reagents were obtained from Beijing Chemical Industry (Beijing, China), unless otherwise mentioned.
2.2. Animals
Male spontaneously hypertensive rats (SHRs) were obtained from SLRC Laboratory Animal Inc. (Shanghai, China). SHRs (n = 10) at 12 weeks of age were randomly assigned to groups. Age- and sex-matched normotensive Wistar–Kyoto (WKY) rats (n = 10) were used as control animals. The study was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the 8 week experimental protocol was approved by the Medicine Animal Welfare Committee of Xiangya Medical School, Central South University (Changsha, China).
Vascular structure of thoracic aorta and mesenteric artery was studied. Systolic blood pressure of rats was measured by the tail-cuff method before the experiment and at weekly intervals. Slices of arteries were stained with haematoxylin and eosin. Media thickness, lumen diameter, media thickness-to-lumen diameter ratio, and mean nuclear area in artery media were assessed with a computer-assisted image analysis system (Image-Pro Plus Version 6.0, Media Cybernetics, Inc, Bethesda, MD, USA).
2.3. Angiotensin II assay
Blood was collected from the right ventricle before the heart was arrested, and 2 mL blood was placed in a chilled tube containing the enzyme inhibitors and centrifuged (within 15 min) at 4°C at 500 g for 10 min. The supernatant plasma was stored at −20°C. The concentration of Ang II was determined by radioimmunoassay using a Type SIV-682 radioimmuno-γ-counter (Shanghai, China). The procedures were performed according to the manufacturer's instructions. The sensitivity was 10 pg/mL with a confidence of variation less than 5%. The rate of cross-reaction with angiotensin I was less than 0.01%.
2.4. Cell culture
Rat aortic smooth muscle cells A10 (A10 VSMCs) were obtained from ATCC (Manassas, VA, USA) and cultured in DMEM containing 10.0% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 μg/mL) and NaHCO3 (3.7 g/L), and grown in a humidified atmosphere of 5.0% CO2 in air at 37°C. Cells were characterized as being VSMCs by immunofluroescence detection of α-smooth muscle actin (Sigma). Cells between passages 7 and 15 were used for the experiments.
2.5. Cell proliferation assays
Cell proliferation was measured by two methods; the DNA synthesis and cell cycle were analysed by BrDU marking and flow cytometry, respectively.
For the BrDU incorporation assay, A10 VSMCs were counted and seeded into 96-well culture plates (6 × 103 cells per well). After 24 h, the medium was changed to DMEM containing 1% newborn calf serum to make them quiescent for 24 h. A10 VSMCs were pre-incubated with apocynin (400 µmol/L, 20 min), catalase (300 nmol/L, 2 h), or PD98059 (10 µmol/L, 30 min), followed by stimulation with Ang II (100 nmol/L) for 24 h. BrDU (0.1 µL/mL; Cell Proliferation ELISA for BrDU; Roche) was added. Cells were fixed and stained after 12 h according to the manufacturer's instructions; colorimetric analysis was performed with an ELISA plate reader (DTX880, Beckman, Miami, FL, USA).
For cell cycle analysis using flow cytometry, A10 VSMCs were counted and seeded into six-well culture plates (1 × 105 cells per well). After 24 h, the medium was changed to DMEM containing 1% fetal bovine serum to make them quiescent for 24 h. A10 VSMCs were pre-incubated with apocynin (400 µmol/L, 20 min), catalase (300 nmol/L, 2 h), or PD98059 (10 µmol/L, 30 min), followed by stimulation with Ang II (100 nmol/L) for 24 h. On the next day, the cells were fixed gently by adding 80% ethanol and placed in a freezer for 2 h. The cells were then treated with 0.25% Triton X-100 for 5 min in an ice bath and resuspended in 300 mL of phosphate-buffered saline containing 40 mg/mL propidium iodide and 0.1 mg/mL RNase. Cells were incubated in a dark room for 20 min at room temperature then subjected to cell cycle analysis using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA) and FlowJo 7.1.0 software (Tree Star, Ashland, OR, USA). For each measurement, at least 10 000 cells were counted.
2.6. Cell treatment with hypochlorous acid
As described previously,13 the concentration of the HOCl stock solution was measured spectrophotometrically at 293 nm using the extinction coefficient of hypochlorite ɛ293 = 349.2 M–1 cm–1 at pH 10–12. The HOCl was diluted in Hank's buffered saline solution (HBSS) to a final concentration of 20 µM. Confluent A10 VSMCs were washed twice with HBSS at 37°C. Fresh HBSS was then added, followed by the addition of HOCl. Cells were treated with HOCl for 1 h, followed by washing and continued incubation in DMEM for up to 24 h, and then were harvested for the proliferation assay.
2.7. Hydrogen peroxide assay
The reactions were carried out using the hydrogen peroxide assay kit (Beyotime Institute of Biotechnology, Haimen, Jiangsu, China). In this kit, ferrous ions (Fe2+) are oxidated to ferric ions (Fe3+) by H2O2. The Fe3+ then forms a purple complex with an indicator dye, xylenol orange (3,3′-bis[N,N-di(carboxymethyl)-aminomethyl]-o-cresolsulfone-phthalein, disodium salt and xylenol orange), which is measurable with a spectrometer at a wavelength of 560 nm.14
For the cells, the lysis buffer solution supplied in the kit was added at a ratio of 100 μL per 106 cells. For the aortic tissue, the lysis buffer solution was added at a ratio of 100 μL per 5 mg. Then the supernatants were gathered by centrifuging at 12 000 g for 5 min for the following tests. All the operations were carried out on ice. Finally, the test-tubes containing 50 µL of the supernatants and 100 µL of test solutions were placed at room temperature for 20 min, and then the absorbance at 560 nm was instantly measured using a microplate reader (DTX880, Beckman). The level of H2O2 in cells and aortic tissues was calculated according to a standard concentration curve originated from standard solutions using an identical protocol.
2.8. Hypochlorous acid assay
Hypochlorous acid production was determined as described by Dypbukt et al.15 In brief, the cells were harvested and suspended in 10 mM phosphate buffer (pH 7.4, 37°C) containing 140 mM sodium chloride, 10 mM potassium chloride, 0.5 mM magnesium chloride, 1 mM calcium chloride, 1 mg/mL glucose, and 5 mM taurine. Reactions were carried out in 1.5 mL Eppendorf centrifuge tubes containing 250 μL of solution. Tubes were rotated gently every 5 min to ensure that the cells were kept in suspension and fully aerated. Reactions were stopped by the addition of 20 µg/mL of catalase and placement of the tubes in melting ice for at least 10 min. The cells were then pelleted by centrifugation (5 min at 14 000 g). The supernatant (200 μL) was rapidly and completely mixed with 50 μL of 2 mM 3,3′,5,5′-tetramethylbenzidine, 100 μM sodium iodide, and 10% dimethylformamide in 400 mM acetate buffer. After 5 min, the absorbance at 650 nm was recorded and related to the standard curve to determine the concentration of HOCl produced. The data were finally expressed as a percentage of the control value.
2.9. RNA interference and cell transfection
High-performance purity grade (90% pure) small hairpin RNAs (shRNAs) against VPO1 (VPO1-shRNA) were obtained from Genechem, Inc. (Shanghai, China). shRNAs with a non-silencing oligonucleotide sequence (non-silencing shRNA) that does not recognize any known homology to mammalian genes were used as a negative control (control-shRNA). A10 VSMCs were seeded at a density of 5 × 104 cells per well in six-well plates and grown in DMEM containing 10% fetal calf serum. Next day, cells were transfected with 100 pmol of control-shRNA or 100 pmol of VPO1-shRNA using lipofectomine NAiFect Transfection Reagent (Qiagen Inc., Sofielundsvägen, Germany) according to the manufacturer's instructions. Four hours after transfection, cells were washed with HBSS, and DMEM medium containing 10% fetal calf serumwas added. Two days later, the cells were trypisnized and split into 100 mm Petri dishes. G418 (400 µg/mL; Gibco, Grand Island, NY, USA) was added to the culture and changed every 1 or 2 days. When the first resistant clones were detected, the concentration of G418 was decreased to 200 µg/mL. Individual clones were picked up, transferred, and grown into clonal lines in medium containing G418 (200 µg/mL).
2.10. Real-time PCR analysis
The mRNA expression of VPO1 in A10 VSMCSs and rats was analysed by using the ABI 7300 real-time PCR system (Foster City, CA, USA). The specific primer pairs were 5′-CTGACCAGCATGCATACGCTGTGG-3′ (forward) and 5′- CACTGTGTGGGCCATGGAGAACAG-3′ (backward). The reverse transcription reaction was performed with 1 μg total RNA isolated from the cells of each group. For PCR amplification, cDNAs were amplified using SYBR Green Real-time PCR Master Mix (Takara) and 0.4 μmol/L of each primer pair. Amplification was carried out starting with an initial step for 30 s at 94°C, followed by 40 cycles of the amplification step (94°C for 30 s, 60°C for 60 s, and 72°C for 1 min) for VPO1 or glyceraldehyde-3-phosphate dehydrogenase. All amplification reaction for each sample was carried out in triplicates and the averages of the threshold cycles were used to interpolate curves using 7300 System SDS Software. Results were expressed as the ratio of VPO1 to glyceraldehyde-3-phosphate dehydrogenase mRNA, and the value of VPO1 expression level in control conditions was set as 100%.
2.11. Anti-VPO1 antibody
A region of the VPO1 peroxidase domain with highly predicted antigenicity was chosen using DNAStar software (Madison, WI, USA). The peptide (corresponding to residues 49–63) was synthesized, purified by reverse-phase high-performance liquid chromatography, and conjugated with keyhole limpet haemocyanin from Sigma–Genosys (The Woodlands, TX, USA). Anti-VPO1 antibody was raised in rabbits against the conjugated peptide (Sigma-Genosys). The antibody was purified by affinity purification according to standard procedures. The specificity of the antiserum was determined by western blotting in our pervious study.12
2.12. Western blot analysis
Cells or homogenized tissues were lysed in RIPA [50mM Tris(pH 7.4), 150mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS] buffer or RIPA buffer with 0.1% CTAB (cetyltrimethyl ammonium bromide) containing protease inhibitor mixture (Sigma). Lysate (50 µg of protein) was resolved by 10% SDS–PAGE and transferred to polyvinylidene difluoride membranes using a Mini Tank Transfer System (Bio-Rad Laboratories, Hercules, CA, USA) at 80 V for 1.5 h. Proteins were detected using western blotting and visualized by chemiluminescence (Pierce, SuperSignal West Pico Chemiluminescent Substrate).
2.13. Histology and immunohistochemistry
Sections were cut from thoracic aorta and mesenteric artery at a thickness of 3 μm and were stained with haematoxylin and eosin for evaluation of remodelling. Immunohistochemistry was carried out by using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). Sections of rat thoracic and mesenteric arteries were incubated with a 1:800 dilution of anti-VPO1 antibody or 1:1000 dilution of anti-MPO antibody and stained with diaminobenzidine tetrahydrochloride and haematoxylin. The negative control was treated with phosphate-buffered saline instead of primary antibody.
2.14. Data analysis
Data are expressed as means ± SD. Quantitative variables were compared by means of Student's paired t-test for two groups or ANOVA followed by Newman–Student–Keuls test for multiple groups. Associations between arterial parameters and quantitative factors were analysed with a general linear model. All of the experiments were performed in triplicate and repeated three or four times. A value of P< 0.05 was considered significant.
3. Results
3.1. Remodelling of thoracic aorta and mesenteric artery in spontaneously hypertensive rats
As expected, systolic blood pressure was significantly higher in SHRs than in WKY rats before the experiment and at weekly intervals (P< 0.01; see Supplementary material online, Figure S1A). As Ang II is an important pathogenic factor in vascular organ damage attendant upon systemic hypertension, we measured plasma Ang II levels in both SHRs and WKY rats, and found that the Ang II level of SHRs was significantly increased compared with that of WKY rats (see Supplementary material online, Figure S1B).
Haematoxylin and eosin staining showed that media thickness, media thickness-to-lumen diameter ratio, and mean nuclear area in artery media of the thoracic aorta and mesenteric artery in SHRs were significantly higher than in WKY rats (P< 0.01), and lumen diameter of mesenteric artery, but not thoracic aorta, in SHRs was significantly lower than in WKY rats (P< 0.01; see Supplementary material online, Figure S1C and D).
3.2. Expression of VPO1 and level of H2O2 in vascular tissues of SHRs
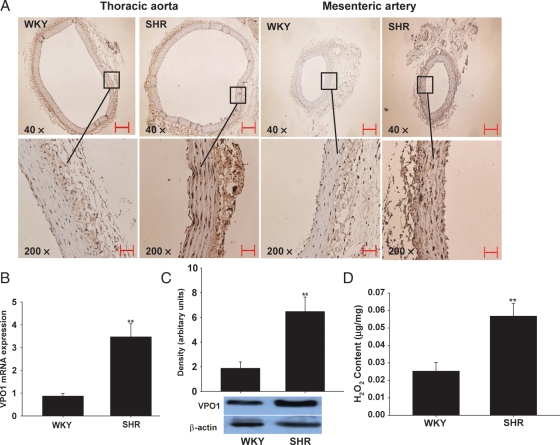
The expression of VPO1 in the wall of the thoracic aorta and mesenteric arteries was detected with immunohistochemical staining, real-time PCR and western blotting, respectively. As shown in Figure 1A, immunohistochemical staining showed that the expression of VPO1 in the thoracic aorta and mesenteric arteries of SHRs was significantly higher than in WKY rats. In keeping with our previous study,12 we found that the distribution of VPO1 was mostly in vascular smooth muscle cells in the vascular tissue of rats, and low in endothelial cells. Likewise, both VPO1 mRNA and protein expression levels in thoracic aorta of SHRs were significantly higher than in WKY rats (Figure 1B and C; and see Supplementary material online, Figure S2; P< 0.01).
Figure 1.
Expression of VPO1 and content of H2O2 in arterial tissues in rats. (A) The immunohistochemistry analysis of VPO1 expression in thoracic aorta and mesenteric artery from SHRs and WKY rats. Scale bar represents 50 µm. (B) The mRNA expression of VPO1 in thoracic aorta by real-time PCR analysis. (C) The protein expression of VPO1 in thoracic aorta by western blot analysis. (D) The H2O2 content in thoracic aorta. SHRs, spontaneously hypertensive rats; WKY, Wistar–Kyoto rats. n = 10; **P< 0.01 vs. SHR.
As H2O2 plays an important role in mediating vascular remodelling during hypertension16 and, like MPO, VPO1 catalyses the reaction of H2O2,12 we therefore measured the H2O2 level in aorta from SHRs and WKY rats. In line with the previous study, which showed a higher H2O2 level in mesenteric arteries of SHRs,17 our results showed that the H2O2 level in aorta is also much higher in SHRs compared with WKY rats (Figure 1D).
In the present study, to determine whether MPO plays a role in the vascular remodelling in SHRs by infiltration or bound to the vascular tissue, we measured the MPO expression in the vascular tissues of WKY rats and SHRs by immunohistochemistry. The result showed that there was no significant expression of MPO in the vascular tissues in WKY rats and SHRs (see Supplementary material online, Figure S3).
3.3. Effect of VPO1 knockdown on cell proliferation induced by Ang II
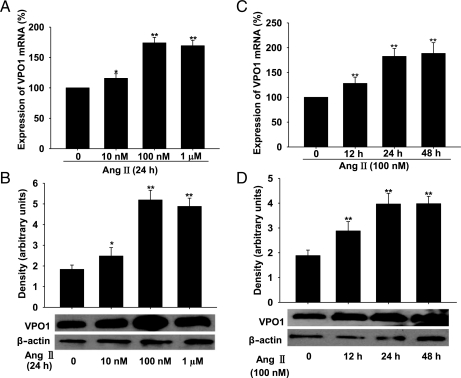
As mentioned above, plasma Ang II level was increased in SHRs, and a great number of studies have demonstrated that Ang II is an important factor in the pathophysiology of hypertension.18,19 We therefore used Ang II as a stimulus to mimic the pathological state of hypertension in in vitro experiments. Interestingly, we found that in A10 VSMCs, Ang II up-regulated VPO1 expression (both mRNA and protein) in a concentration- and time-dependent manner (Figure 2A–D).
Figure 2.
Expression of VPO1 in response to Ang II stimulation in A10 VSMCs and the effect of knockdown of VPO1 by shRNA. (A–D) Ang II up-regulated the expression of VPO1 (mRNA and protein levels analysed by real-time PCR and western blotting, respectively) in a time- and concentration-dependent manner. **P < 0.01 vs. Ang II 0 nM or 0 h. n = 3. Data are representative of three independent experiments.
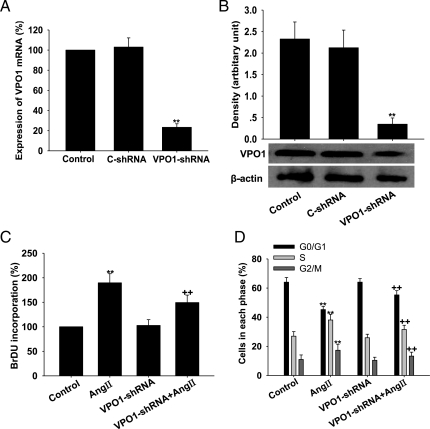
To determine the role of VPO1 in Ang II-mediated proliferation of A10 VSMCs, we developed VPO1-specific shRNA. In our pilot study, we used four shRNA TargetSeq against VPO1 to establish the VPO1-shRNA VSMCs, and compared the effects of the four shRNA TargetSeq. The result showed that the second sequence had the best efficiency to inhibit the VPO1 expression (see Supplementary material online, Figure S4). We therefore used the second shRNA TargetSeq for subsequent experiments. As shown in Figure 3A and B, VPO1-shRNA successfully knocked down VPO1 mRNA and protein levels in A10 VSMCs by 80% (P< 0.01). We then treated A10 VSMCs with Ang II (100 nM) for 24 h to induce proliferation. Our data showed an increase in DNA synthesis ability, an increase the proportion of cells in the S+ G2/M phase, and a decrease in the percentage in the G0/G1 phase (P< 0.01; Figure 3C and D), consistent with previous studies.19 We next examined the effect of VPO1 knockdown by shRNA on cell proliferation. As shown in Figure 3C, VPO1-shRNA blunted the stimulation of cell proliferation induced by Ang II, significantly decreased the DNA synthesis ability of A10 VSMCs, and increased the percentage of cells in the G0/G1 phase, while the proportion of cells in the S+ G2/M phase decreased markedly (P< 0.01; Figure 3D).
Figure 3.
Effect of VPO1 knockdown on Ang II-induced proliferation of A10 VSMCs. (A and B) VPO1-shRNA successfully decreased VPO1 mRNA and protein expression, respectively. **P < 0.01 vs. control (C-shRNA). (C) BrDU incorporation. (D) Cell cycle analysis by flow cytometry. Control, wild-type cells; Ang II, wild-type cells treated with 100 nmol/L Ang II for 24 h; VPO1-shRNA, cells transfected with VPO1-shRNA; VPO1-shRNA + Ang II, VPO1-shRNA-tranfected cells treated with 100 nmol/L Ang II for 24 h. **P < 0.01 vs. control; ++P< 0.01 vs. Ang II. n = 3. Data are representative of three independent experiments.
3.4. Involvement of NADPH oxidase–H2O2–VPO1–ERK1/2 pathway in Ang II-induced cell proliferation
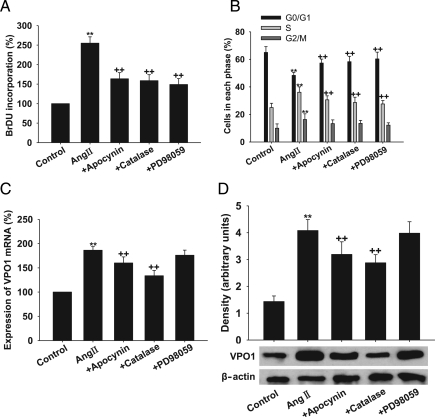
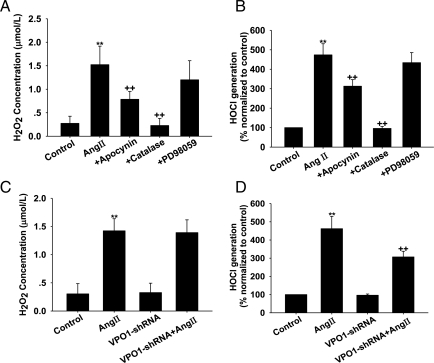
To determine whether the effect of Ang II on cell proliferation is mediated through the NADPH oxidase–H2O2–VPO1–ERK1/2 pathway, we assessed the effect of apocynin (the NADPH oxidase inhibitor), catalase (the specific H2O2 scavenger), and PD98059 (the specific ERK1/2 inhibitor) on Ang II-induced cell proliferation and VPO1 expression. As shown in Figure 4A and B, apocynin, catalase, and PD98059 attenuated the proliferative effect of Ang II (P< 0.01, respectively). Interestingly, apocynin and catalase also down-regulated the expression of VPO1 mRNA and protein, whereas PD98059 had no effect on VPO1 expression (Figure 4C and D). These inhibitors alone at the concentrations used had no effect on cell proliferation and VPO1 expression (data not shown).
Figure 4.
Effect of apocynin, catalase, and PD98059 on VPO1 expression and cell proliferation in Ang II-treated A10 VSMCs. (A) BrdU incorporation. (B) Cell cycle analysis by flow cytometry. (C) VPO1 mRNA measured by real-time PCR analysis. (D) VPO1 expression measured by western blot analysis. Control, wild-type cells; Ang II, wild-type cells treated with 100 nmol/L Ang II for 24 h; +apocynin, cells pre-treated with apocynin (400 µmol/L, 20 min) before 100 nmol/L Ang II for 24 h; +catalase, cells pre-treated with catalase (300 nmol/L, 2 h) before 100 nmol/L Ang II for 24 h; +PD98059, cells pre-treated with PD98059 (10 µmol/L) for 30 min before 100 nmol/L Ang II for 24 h. **P < 0.01 vs. control, ++P< 0.01 vs. Ang II. n = 3. Data are representative of three independent experiments.
It has been demonstrated that chlorotyrosine, the oxidative product of HOCl and l-tyrosine, promotes migration of VSMCs via activation of the ERK1/2 signal pathway.20 VPO1 is highly expressed in the cardiovascular system, and purified VPO1 can catalyse HOCl generation in vitro (data not shown).12 In the present study, we therefore measured the H2O2 and HOCl production in the culture medium. As shown in Figure 5A and B, Ang II treatment significantly increased the H2O2 and HOCl levels, and apocynin and catalase inhibited the effect of Ang II, whereas PD98059 had no effect on H2O2 and HOCl levels.
Figure 5.
Effect of apocynin, catalase, PD98059, and VPO1-shRNA on H2O2 and HOCl levels in Ang II-treated A10 VSMCs. (A) Apocynin, catalase, and PD98059 attenuated H2O2 levels. (B) Apocynin, catalase, and PD98059 attenuated the HOCl levels. (C) VPO1-shRNA attenuated H2O2 levels. (D) VPO1-shRNA attenuated HOCl levels. Control, wild-type cells; Ang II, wild-type cells treated with 100 nmol/L Ang II for 24 h; +apocynin, cells pre-treated with apocynin (400 µmol/L, 20 min) before 100 nmol/L Ang II for 24 h; +catalase, cells pre-treated with catalase (300 nmol/L, 2 h) before 100 nmol/L Ang II for 24 h; +PD98059, cells treated with PD98059 (10 µmol/L) for 30 min before 100 nmol/L Ang II for 24 h; VPO1-shRNA, cells transfected with VPO1-shRNA; VPO1-shRNA + Ang II: VPO1-shRNA transfected cells treated with 100 nmol/L Ang II for 24 h. **P < 0.01 vs. control; ++P< 0.01 vs. Ang II. n = 3. Data are representative of three independent experiments.
We further determined whether knockdown of VPO1 expression in A10 VSMCs would affect H2O2 and HOCl production. As shown in Figure 5C and D, after treatment with Ang II, VPO1-shRNA had no effect on the H2O2 production, but caused a marked decrease in HOCl production compared with wild-type cells.
3.5. Activation of VPO1 in response to H2O2
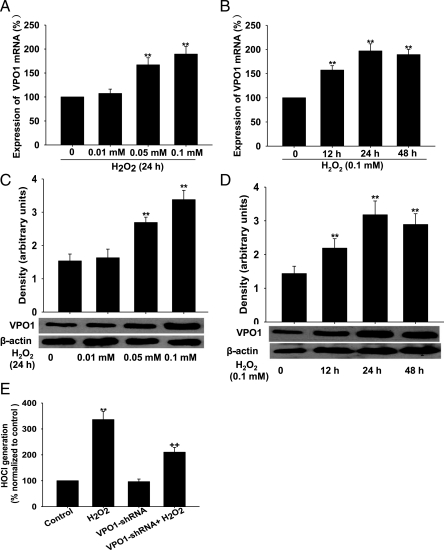
Growing evidence shows that H2O2 serves as a second messenger that activates downstream kinases, such as ERK1/2 mitogen-activated protein kinase, and plays an important role in vascular remodelling.3–5 Whether H2O2 regulates VPO1 expression is largely unknown. We found that VPO1 expression (both mRNA and protein) was significantly increased in A10 VSMCs by H2O2 in a concentration- and time-dependent manner (Figure 6A–D). We next determined the effect of VPO1 knockdown on HOCl production in A10 VSMCs. As shown in Figure 6E, VPO1 shRNA resulted in a significant inhibitory effect on H2O2-induced HOCl production compared with wild-type cells.
Figure 6.
VPO1 expression and HOCl levels in response to H2O2 in A10 VSMCs. (A–D) H2O2 up-regulated the expression of VPO1 (mRNA and protein analysed by real-time PCR and western blot, respectively) in a time- and concentration-dependent manner. **P < 0.01 vs. H2O2 0 mM or 0 h. (E) VPO1-shRNA successfully decreased HOCl generation. **P < 0.01 vs. control, ++P< 0.01 vs. H2O2. n = 3. Data are representative of three independent experiments.
3.6. Effect of VPO1 knockdown on cell proliferation induced by HOCl
We also examined the effect of VPO1 knockdown by shRNA on cell proliferation of A10 VSMCs induced by HOCl. As shown in Supplementary material online, Figure S5A and B, HOCl itself induced cell proliferation as shown by the increase in DNA synthesis ability and in the proportion of cells in the S+ G2/M phase, as well as the decrease in the percentage of cells in the G0/G1 phase (P< 0.01). VPO1-shRNA had no effect on cell proliferation of A10 VSMCs stimulated by HOCl.
4. Discussion
The main findings of the present study are as follows: (1) remodelling of thoracic aorta and mesenteric arteries occurs in SHRs, while the expression level of VPO1 is elevated in the vascular walls; (2) knockdown of VPO1 attenuates the Ang II-mediated proliferative effects of VSMCs; and (3) Ang II induces cell proliferation via the NADPH oxidase–H2O2–VPO1–HOCl–ERK1/2 pathway. Collectively, these findings suggest, for the first time, that VPO1 plays an important role in the mediation of proliferation of VSMCs, which may contribute to vascular remodelling in SHRs.
Previous studies have demonstrated that not only can infiltrated leucocytes release MPO into the vascular wall, but blood-derived MPO can also bind and infiltrate into the vascular wall directly.21,22 In vivo, the vascular-bound MPO can use vascular non-leucocyte oxidase-derived H2O2 to induce vascular injury.18 In vitro, transcytosis of MPO, both in cultured endothelial cells and in rat aortic tissues, occurring independently of leucocyte emigration, confers specificity to nitration of vascular matrix proteins.23 In the present study, we measured the MPO expression in the vascular tissues of WKY rats and SHRs and found that there was no significant expression of MPO in the vascular tissues in WKY rats and SHRs. This finding is in line with previous conclusions that macrophages in murine atherosclerotic tissue do not express immunoreactive MPO, and the mouse model is of uncertain relevance for studying the role of MPO in vascular disease.24,25 Resistance arteries exhibit mainly inward eutrophic remodelling in the pathogenesis of hypertension.1 These changes involve media thickening, reduced lumen diameter and consequent increased media-to-lumen ratio.2 In the present study, we found that the lumen diameter of mesenteric artery in SHRs was significantly lower than that of WKY rats, and the lumen diameter, media thickness, media thickness-to-lumen diameter ratio, and mean nuclear area in artery media, as well as the expression of VPO1 in SHRs were higher than those of WKY rats. These results suggest that VPO1, but not MPO, may have a strong relation with vascular diseases, and is a sensitive index to reflect the vascular remodelling in SHRs.
In pathological states of vascular remodelling, it is generally considered that medial smooth muscle cell proliferation is a key component.26,27 A number of studies have demonstrated that Ang II-mediated vascular injury in hypertension is mainly mediated by NADPH oxidase-derived reactive oxygen species.3,28–30 An increasing amount of evidence has shown that H2O2 plays an important role in mediating vascular remodelling during hypertension.4,5,16 In the present study, we also found that H2O2 levels in aorta are much higher in SHRs compared with WKY rats. One of the possible mechanisms of vascular injury induced by haem peroxidases is proposed to be through its reaction with H2O2 derived from NADPH oxidases to produce HOCl and its chlorinating species.12 VPO1, which is intrinsically expressed in the cardiovascular system in mice and rats, may utilize NADPH oxidase-derived H2O2 for peroxidative reactions.12 In the present study, we found that Ang II-mediated stimulation had dose- and time-dependent up-regulatory effects on VPO1 expression in A10 VSMCs concomitantly with enhanced cell proliferation. Furthermore, Ang II treatment significantly increased H2O2 and HOCl levels, while both the NADPH oxidase inhibitor and catalase attenuated the cell proliferation and VPO1 expression. Knockdown of VPO1 expression had no effect on the H2O2 production, but caused a marked decrease in HOCl production concomitantly with decreased cell proliferation. We also found that VPO1 expression in VSMCs was increased when exposed to H2O2 in a dose- and time-dependent manner, and VPO1 shRNA resulted in a significant inhibitory effect on H2O2-induced HOCl production. Our present data revealed that NADPH oxidase-derived H2O2 was a potential source for VPO1-generated HOCl, and HOCl amplified vascular NADPH oxidase-derived reactive oxygen species-induced vascular smooth cell proliferation. It is of note that in the present study we used a non-specific NADPH oxidase inhibitor, apocynin; the other sources of H2O2 for VPO1-generated HOCl cannot be ruled out.
It has been reported that HOCl is able to activate the mitogen-activated protein kinase pathway, especially the extracellular regulated kinase (ERK1/2), and enhances cell survival,30 and that chlorotyrosine, the oxidative product of HOCl and l-tyrosine, promotes migration of VSMCs via activating the ERK1/2 signal pathway.20 In the present study, we found that Ang II treatment significantly increased the HOCl level and the proliferation rate, whereas pre-treatment with the ERK1/2 inhibitor, PD98059, inhibited the Ang II-mediated proliferation effect without affecting VPO1 expression. Interestingly, we also found that VPO1-shRNA had no effect on exogenous HOCl-induced cell proliferation. These results suggest that Ang II stimulates VSMC proliferation at least in part through the NADPH oxidase–H2O2–VPO1–HOCl–ERK1/2 pathway.
The specific tissue distributions and properties to catalyse peroxidative reactions determine the importance of VPO1 in the cardiovascular system. The present study describes a novel function of VPO1 in the regulation of proliferation of A10 VSMCs and the correlation with vascular remodelling in SHRs. These findings imply that the expression of VPO1 provokes an exaggerated oxidative stress, leading to enhanced susceptibility to vascular remodelling and hypertension.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
The work was supported by the National Natural Science Foundation of China (nos 30871052 and 30971194), Graduate Education Innovation Project of Central South University (nos 2960-71131100010 and 2008yb028) and the National Institute of Health of USA (NIH/NHLBI) Grant HL086836 (to G.C.).
Supplementary Material
References
- 1.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. doi:10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ, Gibbons GH, Morishita R, Pratt RE. New perspectives in hypertention research: potentials of vascular biology. Hypertension. 1994;23:1132–1140. doi: 10.1161/01.hyp.23.6.1132. [DOI] [PubMed] [Google Scholar]
- 3.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, et al. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 4.Zhang M, Dong Y, Xu J, Xie Z, Wu Y, Song P, et al. Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide. Circ Res. 2008;102:328–337. doi: 10.1161/CIRCRESAHA.107.163253. doi:10.1161/CIRCRESAHA.107.163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanc A, Pandey NR, Srivastava AK. Synchronous activation of ERK 1/2, p38mapk and PKB/Akt signaling by H2O2 in vascular smooth muscle cells: potential involvement in vascular disease. Int J Mol Med. 2003;11:229–234. [PubMed] [Google Scholar]
- 6.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. doi:10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 7.Kettle AJ, Winterbourn CC. Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep. 1997;3:3–15. doi: 10.1080/13510002.1997.11747085. [DOI] [PubMed] [Google Scholar]
- 8.McKenna SM, Davies KJ. The inhibition of bacterial growth by hypochlorous acid. Possible role in the bactericidal activity of phagocytes. Biochem. 1988;254:685–692. doi: 10.1042/bj2540685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss SJ, Slivka A. Monocyte and granulocyte-mediated tumor cell destruction. A role for the hydrogen peroxide-myeloperoxidase-chloride system. J Clin Invest. 1982;69:255–262. doi: 10.1172/JCI110447. doi:10.1172/JCI110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinecke JW. Mechanisms of oxidative damage by myeloperoxidase in atherosclerosis and other inflammatory disorders. J Lab Clin Med. 1999;133:321–325. doi: 10.1016/s0022-2143(99)90061-6. doi:10.1016/S0022-2143(99)90061-6. [DOI] [PubMed] [Google Scholar]
- 11.Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985;840:204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 12.Cheng G, Salerno JC, Cao Z, Pagano PJ, Lambeth JD. Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic Biol Med. 2008;45:1682–1694. doi: 10.1016/j.freeradbiomed.2008.09.009. doi:10.1016/j.freeradbiomed.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juliet MP, Christine CW, Margret CMV. The effect of hypochlorous acid on the expression of adhesion molecules and activation of NF-κB in cultured human endothelial cells. Antioxid Redox Signal. 2002;4:5–15. doi: 10.1089/152308602753625807. doi:10.1089/152308602753625807. [DOI] [PubMed] [Google Scholar]
- 14.Deiana L, Carru C, Pes G, Tadolini B. Spectrophotometric measurement of hydroperoxides at increased sensitivity by oxidation of Fe2+ in the presence of xylenol orange. Free Radic Res. 1999;31:237–244. doi: 10.1080/10715769900300801. doi:10.1080/10715769900300801. [DOI] [PubMed] [Google Scholar]
- 15.Dypbukt JM, Bishop C, Brooks WM, Thong B, Eriksson H, Kettle AJ. A sensitive and selective assay for chloramine production by myeloperoxidase. Free Radic Biol Med. 2005;39:1468–1477. doi: 10.1016/j.freeradbiomed.2005.07.008. doi:10.1016/j.freeradbiomed.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Griendling KK, Dikalova A, Owens GK, Taylor WR. Vascular hypertrophy in angiotensin II-induced hypertension is mediated by vascular smooth muscle cell-derived H2O2. Hypertension. 2005;46:732–737. doi: 10.1161/01.HYP.0000182660.74266.6d. doi:10.1161/01.HYP.0000182660.74266.6d. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Bohlen HG, Miller SJ, Unthank JL. NAD(P)H oxidase-derived peroxide mediates elevated basal and impaired flow-induced NO production in SHR mesenteric arteries in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H1008–H1016. doi: 10.1152/ajpheart.00114.2008. doi:10.1152/ajpheart.00114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heeneman S, Sluimer JC, Daemen MJ. Angiotensin-converting enzyme and vascular remodeling. Circ Res. 2007;101:441–454. doi: 10.1161/CIRCRESAHA.107.148338. doi:10.1161/CIRCRESAHA.107.148338. [DOI] [PubMed] [Google Scholar]
- 19.Guo J, Chen H, Ho J, Mancini J, Sontag T, Laporte SA, et al. TGFβ-induced GRK2 expression attenuates AngII-regulated vascular smooth muscle cell proliferation and migration. Cell Signal. 2009;21:899–905. doi: 10.1016/j.cellsig.2009.01.037. doi:10.1016/j.cellsig.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Mu H, Wang X, Lin PH, Yao Q, Chen C. Chlorotyrosine promotes human aortic smooth muscle cell migration through increasing superoxide anion production and ERK1/2 activation. Atherosclerosis. 2008;201:67–75. doi: 10.1016/j.atherosclerosis.2007.12.049. doi:10.1016/j.atherosclerosis.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, et al. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Yang J, Jacobs JD, Jennings LK. Interaction of myeloperoxidase with vascular NAD(P)H oxidase-derived reactive oxygen species in vasculature: implications for vascular diseases. Am J Physiol Heart Circ Physiol. 2003;285:H2563–H2572. doi: 10.1152/ajpheart.00435.2003. [DOI] [PubMed] [Google Scholar]
- 23.Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, et al. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107:419–430. doi: 10.1172/JCI8797. doi:10.1172/JCI8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castellani LW, Chang JJ, Wang X, Lusis AJ, Reynolds WF. Transgenic mice express human MPO –463G/A alleles at atherosclerotic lesions, developing hyperlipidemia and obesity in –463G males. J Lipid Res. 2006;47:1366–1377. doi: 10.1194/jlr.M600005-JLR200. doi:10.1194/jlr.M600005-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Q, Ishibashi M, Hiasa K, Tan C, Takeshita A, Egashira K. Essential role of vascular endothelial growth factor in angiotensin II-induced vascular inflammation and remodeling. Hypertension. 2004;44:264–270. doi: 10.1161/01.HYP.0000138688.78906.6b. doi:10.1161/01.HYP.0000138688.78906.6b. [DOI] [PubMed] [Google Scholar]
- 26.Ginnan R, Guikema BJ, Halligan KE, Singer HA, Jourd'heuil D. Regulation of smooth muscle by inducible nitric oxide synthase and NADPH oxidase in vascular proliferative diseases. Free Radic Biol Med. 2008;44:1232–1245. doi: 10.1016/j.freeradbiomed.2007.12.025. doi:10.1016/j.freeradbiomed.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol. 2005;90:449–455. doi: 10.1113/expphysiol.2005.030080. doi:10.1113/expphysiol.2005.030080. [DOI] [PubMed] [Google Scholar]
- 28.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. doi:10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens. 2004;22:535–542. doi: 10.1097/00004872-200403000-00016. doi:10.1097/00004872-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Midwinter RG, Vissers MC, Winterbourn CC. Hypochlorous acid stimulation of the mitogen-activated protein kinase pathway enhances cell survival. Arch Biochem Biophys. 2001;394:13–20. doi: 10.1006/abbi.2001.2530. doi:10.1006/abbi.2001.2530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.