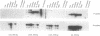Abstract
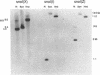
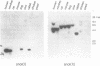
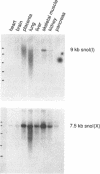
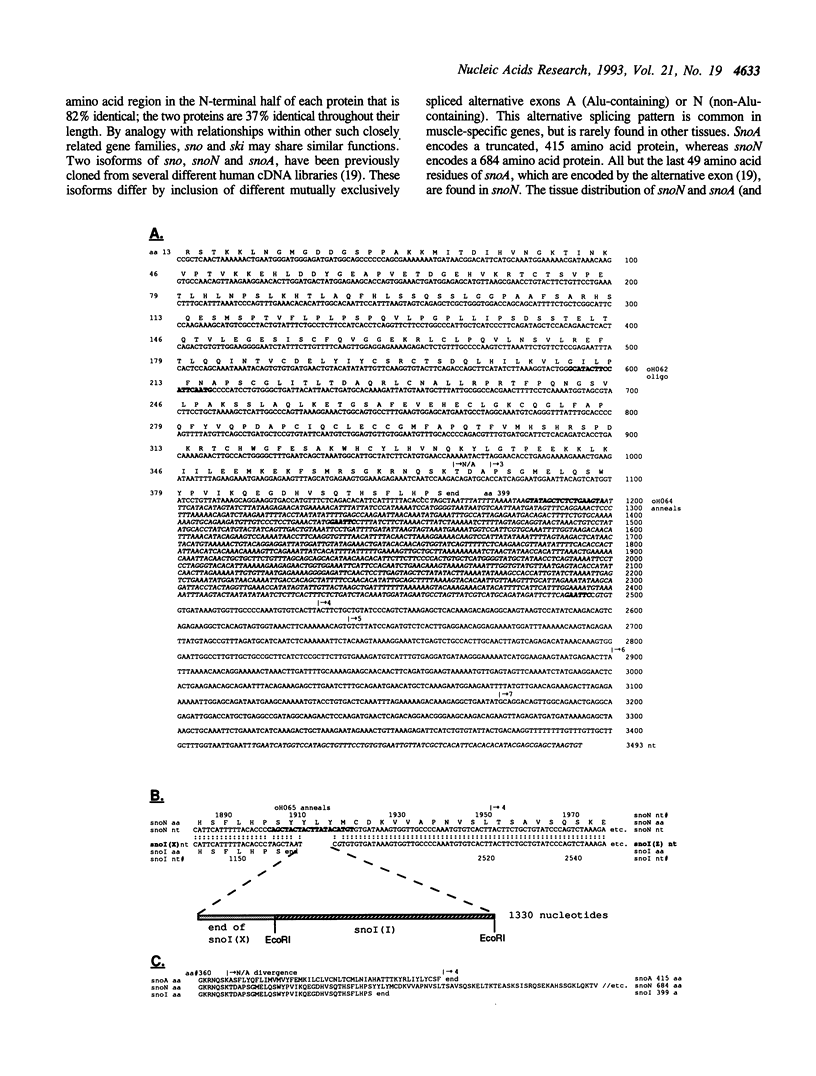
We have cloned and sequenced a novel human isoform of sno, snoI for insertion. SnoI contains 1330 nucleotides inserted in place of 7 nucleotides of the snoN mRNA. Sno is a member of the ski protooncogene family, which has been implicated in muscle development. The two previously known sno alternatively spliced isoforms are snoN (684 amino acids), and snoA (415 amino acids); snoI encodes a truncated isoform of 399 amino acids (44,298 MW). Southern blot experiments show that snoI contains a third alternative exon from the sno gene; a single sno gene can express all three isoforms of sno by alternative splicing. All three isoforms contain the region that is most similar to the ski proto-oncogene. The relationship between snoI and snoN is analogous to that between delta fosB and fosB, where a truncated form of the fosB transcription factor is produced by alternative splicing. We find conservation of human snoI-specific sequences in several mammalian species, in monkey, dog, cow, rabbit and pig, but not in rodents, whereas the common portion of the sno gene is conserved in all vertebrate species tested. SnoN, snoA, and ski mRNAs accumulate in many human tissues including skeletal muscle; the snoI alternative mRNA accumulates more specifically in skeletal muscle. SnoI is also expressed in rhabdomyosarcoma tumor, a tumor that contains differentiated skeletal muscle. The tissue-specific alternative splicing of human snoI, an mRNA in the ski/sno gene family, and the presence of sno mRNAs in muscle are consistent with a proposed role for the sno oncogene in muscle gene regulation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkas A. E., Brodeur D., Stavnezer E. Polyproteins containing a domain encoded by the V-SKI oncogene are located in the nuclei of SKV-transformed cells. Virology. 1986 May;151(1):131–138. doi: 10.1016/0042-6822(86)90111-x. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. L., Colmenares C., Stavnezer E., Hughes S. H. Sequence and biological activity of chicken snoN cDNA clones. Oncogene. 1993 Feb;8(2):457–466. [PubMed] [Google Scholar]
- Braun T., Bober E., Winter B., Rosenthal N., Arnold H. H. Myf-6, a new member of the human gene family of myogenic determination factors: evidence for a gene cluster on chromosome 12. EMBO J. 1990 Mar;9(3):821–831. doi: 10.1002/j.1460-2075.1990.tb08179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Buschhausen-Denker G., Bober E., Tannich E., Arnold H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989 Mar;8(3):701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenares C., Stavnezer E. The ski oncogene induces muscle differentiation in quail embryo cells. Cell. 1989 Oct 20;59(2):293–303. doi: 10.1016/0092-8674(89)90291-2. [DOI] [PubMed] [Google Scholar]
- Colmenares C., Teumer J. K., Stavnezer E. Transformation-defective v-ski induces MyoD and myogenin expression but not myotube formation. Mol Cell Biol. 1991 Feb;11(2):1167–1170. doi: 10.1128/mcb.11.2.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- George E. L., Ober M. B., Emerson C. P., Jr Functional domains of the Drosophila melanogaster muscle myosin heavy-chain gene are encoded by alternatively spliced exons. Mol Cell Biol. 1989 Jul;9(7):2957–2974. doi: 10.1128/mcb.9.7.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes H. L., Szente B. E., Goodenow M. M. C-ski cDNAs are encoded by eight exons, six of which are closely linked within the chicken genome. Nucleic Acids Res. 1992 Apr 11;20(7):1511–1516. doi: 10.1093/nar/20.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Growth-related changes in specific mRNAs of cultured mouse cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4271–4275. doi: 10.1073/pnas.80.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. H., Wold B. Herculin, a fourth member of the MyoD family of myogenic regulatory genes. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1089–1093. doi: 10.1073/pnas.87.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y., Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991 Feb 22;64(4):751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- Nomura N., Sasamoto S., Ishii S., Date T., Matsui M., Ishizaki R. Isolation of human cDNA clones of ski and the ski-related gene, sno. Nucleic Acids Res. 1989 Jul 25;17(14):5489–5500. doi: 10.1093/nar/17.14.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Pownall M. E., Emerson C. P., Jr Sequential activation of three myogenic regulatory genes during somite morphogenesis in quail embryos. Dev Biol. 1992 May;151(1):67–79. doi: 10.1016/0012-1606(92)90214-2. [DOI] [PubMed] [Google Scholar]
- Rhodes S. J., Konieczny S. F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989 Dec;3(12B):2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon D., Lyons G., Wright W. E., Lin V., Lassar A., Weintraub H., Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989 Sep 28;341(6240):303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stavnezer E., Barkas A. E., Brennan L. A., Brodeur D., Li Y. Transforming Sloan-Kettering viruses generated from the cloned v-ski oncogene by in vitro and in vivo recombinations. J Virol. 1986 Mar;57(3):1073–1083. doi: 10.1128/jvi.57.3.1073-1083.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer E., Brodeur D., Brennan L. A. The v-ski oncogene encodes a truncated set of c-ski coding exons with limited sequence and structural relatedness to v-myc. Mol Cell Biol. 1989 Sep;9(9):4038–4045. doi: 10.1128/mcb.9.9.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer E., Gerhard D. S., Binari R. C., Balazs I. Generation of transforming viruses in cultures of chicken fibroblasts infected with an avian leukosis virus. J Virol. 1981 Sep;39(3):920–934. doi: 10.1128/jvi.39.3.920-934.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutrave P., Hughes S. H. Isolation and characterization of three distinct cDNAs for the chicken c-ski gene. Mol Cell Biol. 1989 Sep;9(9):4046–4051. doi: 10.1128/mcb.9.9.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutrave P., Kelly A. M., Hughes S. H. ski can cause selective growth of skeletal muscle in transgenic mice. Genes Dev. 1990 Sep;4(9):1462–1472. doi: 10.1101/gad.4.9.1462. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- de la Brousse F. C., Emerson C. P., Jr Localized expression of a myogenic regulatory gene, qmf1, in the somite dermatome of avian embryos. Genes Dev. 1990 Apr;4(4):567–581. doi: 10.1101/gad.4.4.567. [DOI] [PubMed] [Google Scholar]