Abstract
Aims
Regulator of G protein signalling (RGS) proteins act as molecular ‘off switches’ that terminate G protein signalling by catalyzing the hydrolysis of Gα-bound GTP to GDP. Many different Gαi-coupled receptors have been implicated in the cardioprotective effects of ischaemic preconditioning. However, the role of RGS proteins in modulating cardioprotection has not been previously investigated. We used mice that were homozygous (GS/GS) or heterozygous (GS/+) for a mutation in Gαi2 rendering it RGS-insensitive (G184S) to determine whether interactions between endogenous RGS proteins and Gαi2 modulate Gαi-mediated protection from ischaemic injury.
Methods and results
Langendorff-perfused mouse hearts were subjected to 30 min global ischaemia and 2 h reperfusion. Infarcts in GS/GS (14.5% of area at risk) and GS/+ (22.6% of AAR) hearts were significantly smaller than those of +/+ hearts (37.2% of AAR) and recovery of contractile function was significantly enhanced in GS/GS and GS/+ hearts compared with +/+ hearts. The cardioprotective phenotype was not reversed by wortmannin or U0126 but was reversed by 5-hydroxydecanoic acid and HMR 1098, indicating that RGS-insensitive Gαi2 protects the heart through a mechanism that requires functional ATP-dependent potassium channels but does not require acute activation of extracellular-regulated kinase or Akt signalling pathways.
Conclusions
This is the first study to demonstrate that Gαi2-mediated cardioprotection is suppressed by RGS proteins. These data suggest that RGS proteins may provide novel therapeutic targets to protect the heart from ischaemic injury.
Keywords: Ischaemic preconditioning, Ischaemia reperfusion injury, Regulator of G protein signalling (RGS), Gi2
1. Introduction
Brief periods of ischaemia and reperfusion protect the heart from injury that is caused by a subsequent episode of prolonged ischaemia through a process called ischaemic preconditioning. This phenomenon was initially discovered by Murry et al.1 who found that ischaemic preconditioning decreases infarct size in the ischaemic dog heart. Subsequent studies demonstrated that this endogenous cardioprotective mechanism also occurs in many other mammalian species. More recent studies have revealed that ischaemic preconditioning is not limited to protection of the myocardium, but also protects the brain, liver, kidney, and skin2–5 from ischaemic injury. Intense effort has been made to understand the endogenous signalling mechanisms that mediate ischaemic preconditioning and to identify a practical way to exploit this process for the therapeutic benefit of patients who have ischaemic heart disease, ischaemic stroke, or other ischaemia-related diseases.
Ischaemic preconditioning can be pharmacologically mimicked by exogenous adenosine, acetylcholine, bradykinin, opioids, angiotensin II, endothelin, and other agonists that act at Gαi-coupled receptors.6 Three Gαi isoforms have been cloned (Gαi1, Gαi2, and Gαi3), and at least two isoforms (Gαi2 and Gαi3) are present in the heart.7 Ischaemic injury is exacerbated in both Gαi2 knockout mice and in transgenic mice expressing a dominant negative Gαi2 minigene construct. One goal of this study was to determine whether enhancement of Gαi2 signalling through loss of its negative regulation protects the heart from ischaemic injury.
Signalling through the Gαi-family of G proteins is under strong negative regulation by regulator of G protein signalling (RGS) proteins which might represent a point of pharmacological modulation. G protein-coupled receptors (GPCRs) activate G proteins by inducing the release of GDP from the Gα subunit followed immediately by GTP binding and Gα dissociation from Gβγ. Both Gα and Gβγ interact with downstream signalling proteins to produce biochemical changes within the cell. G protein signalling is terminated when Gα-bound GTP is hydrolyzed to GDP and Gα reassociates with Gβγ. RGS proteins enhance the GTPase activity of G proteins leading to more rapid G protein deactivation and termination of G protein signalling. mRNA transcripts for at least 17 RGS proteins have been identified in the heart.8 However, the lack of selective RGS inhibitors and the redundant functions of some RGS proteins have made it difficult to study the functional roles of individual RGS proteins by traditional pharmacological methods or by using RGS knockout animals. Huang et al.9 developed a knock-in mouse model in which the endogenous Gαi2 gene is replaced with an RGS-insensitive G184S Gαi2 mutant that is unable to interact with RGS proteins.10 This mutation provides a unique tool to study the effect of inhibiting interactions between RGS proteins and Gαi2 in the heart. This mouse model has been previously used to study the role of endogenous RGS proteins in modulating muscarinic and adenosine receptor-mediated chronotropic effects11 and to investigate the role of endogenous RGS proteins on sinoatrial and atrioventricular node function.12 In the present study, we found that hearts expressing RGS-insensitive Gαi2 show enhanced Gαi2 signalling in the ventricles and are protected from ischaemic injury. This is the first study to demonstrate that enhancement of Gαi2 signalling by reversal of its negative regulation by RGS proteins protects the heart from ischaemic injury.
2. Methods
2.1. Mice expressing RGS-insensitive Gαi2
Generation of mice expressing RGS-insensitive Gαi2 from their genomic locus has been previously described.9 All animals used in this study were backcrossed for at least four generations onto a C57BL/6 background. Mice were either wild type (+/+) or were heterozygous (GS/+) or homozygous (GS/GS) for the Gαi2G184S RGS-insensitive mutation. Animals were housed with a 12 h light/dark cycle with ad libitum access to food and water. Male mice 2–10 months of age were used for all experiments except for experiments using isolated ventricular myocytes, which were isolated from female mice. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). This study was performed with the approval of the Institutional Animal Care and Use Committees of Ohio Northern University and the University of Michigan.
2.2. Ventricular myocyte contractility assay
Cardiac myocytes were isolated from adult female mice as described by O'Connell et al.13 Cells were stimulated with 200 nM isoproterenol, and the contractile response to isoproterenol was recorded using the Ionoptix system (IonOptix, Milton, MA, USA). Carbachol was added to produce cumulative concentrations of 100 nM–30 μM. Details are provided in the Supplementary material online, Methods.
2.3. Langendorff-isolated heart preparation
Mice were anesthetized with a single injection containing sodium pentobarbital (100 mg/kg, ip) and heparin. Hearts were rapidly removed and cannulated while bathed in ice-cold Krebs solution. Krebs solution was perfused through the aortic cannula, and contractile function of the left ventricle was measured using an intraventricular balloon as described in the Supplementary material online, Methods. Infarct size was measured by triphenyltetrazolium chloride staining as described in the Supplementary material online, Methods.
2.4. Western blots
Hearts were either removed from mice and immediately flash-frozen in liquid nitrogen or perfused on the Langendorff apparatus for 55 min prior to flash freezing in liquid nitrogen. Frozen hearts were then prepared for western blotting as described in the Supplementary material online, Methods.
2.5. Statistical analysis
The non-linear least squares method with global fitting was used to fit concentration–response curves for carbachol. IC50 values were reported as the mean ±95% confidence interval (CI). pIC50 values and the maximum effect of carbachol were compared using an F-test on the extra sum-of-squares of the global fit in GraphPad Prism.14 Data from Langendorff-isolated heart experiments were reported as mean ± SEM and were compared by one-way ANOVA and Tukey post hoc analysis. Comparisons involving two variables (genotype and drug treatment) were performed using two-way ANOVA and the Bonferroni post test. Graphpad Prism software (San Diego, CA, USA) was used for statistical analysis, and P-values ≤0.05 were considered statistically significant.
3. Results
3.1. RGS proteins regulate Gαi2 signalling in isolated ventricular myocytes
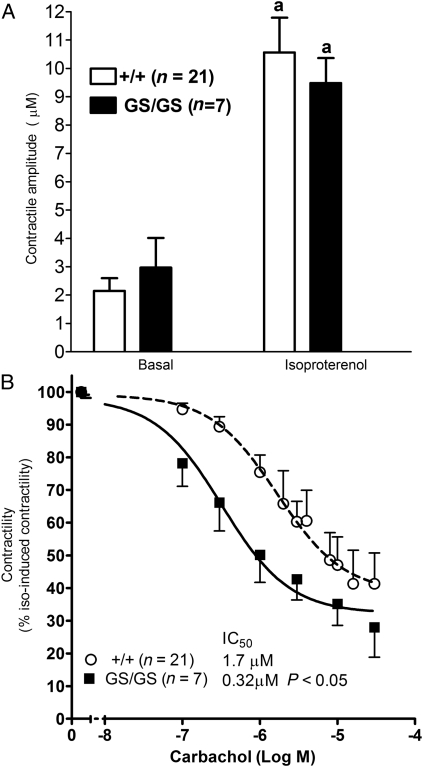
We previously demonstrated that RGS-insensitive Gαi2 mutants exhibit enhanced muscarinic effects in SA and AV node.11,12 Here, ventricular myocytes from +/+ and GS/GS hearts were used to determine whether endogenous RGS proteins modulate the negative inotropic effect of muscarinic receptor stimulation. There were no differences in the morphology or basal contractility of +/+ and GS/GS myocytes, and isoproterenol significantly (P < 0.001) increased the contractile amplitude of myocytes from both genotypes (Figure 1A). The maximum effect of carbachol was similar in GS/GS and +/+ myocytes, but carbachol decreased the contractility of isoproterenol-stimulated GS/GS myocytes with ∼five-fold greater potency than +/+ myocytes (GS/GS, IC50 = 0.37 μM; 95% CI = 0.13–0.80 μM; +/+ IC50 = 1.7 μM; 95% CI = 0.8–3.4 μM, P < 0.05) (Figure 1B). These data indicate that expression of RGS-insensitive Gαi2 increases signalling by agonists acting through Gαi2-coupled receptors in ventricular myocytes.
Figure 1.
Expression of RGS-insensitive Gαi2 enhances the potency of carbachol in ventricular myocytes. The ability of carbachol to inhibit isoproterenol-induced contractility was measured in ventricular myocytes isolated from Gαi2 G184S homozygote (GS/GS) and wild-type (+/+) mice. Isoproterenol significantly increased the contractility of GS/GS and +/+ cardiomyocytes (A). Carbachol decreased isoproterenol-induced contractility with significantly greater potency in GS/GS cardiomyocytes compared with +/+ cardiomyocytes. Data were normalized to contractility induced by 200 nM isoproterenol in the absence of carbachol which was not different between genotypes. ‘a’ indicates a significant difference (P < 0.001) compared with basal contractile function in the absence of isoproterenol.
3.2. Enhanced Gαi2 signalling protects the heart from ischaemic injury
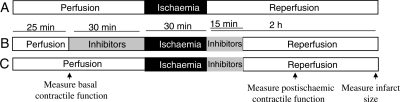
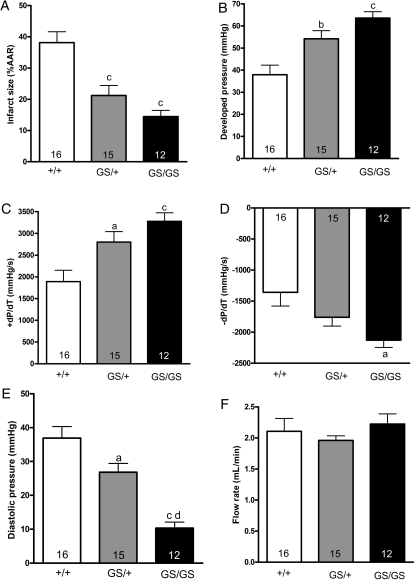
Adenosine, opioids, bradykinin, and other agonists that act at Gαi-coupled receptors protect the heart from ischaemic injury. Gαi2 signalling is enhanced in GS/+ and GS/GS hearts because the Gαi2G184S mutation prohibits RGS proteins from catalyzing the hydrolysis of Gαi2-bound GTP. Therefore, we used the Langendorff-isolated heart model to determine whether GS/+ and GS/GS hearts are protected from ischaemic injury. Hearts were subjected to 30 min global ischaemia and 2 h reperfusion as shown in Figure 2A. Preischaemic parameters of cardiac function were measured in isolated hearts following a 25 min equilibration period. Developed pressure, diastolic pressure, and coronary flow rates were similar in +/+, GS/+, and GS/GS hearts (Table 1). Preischaemic +dP/dT and −dP/dT values were similar in +/+ and GS/+ hearts, but both values were ∼10% lower in GS/GS hearts compared with +/+ hearts, indicating that ventricular pressure develops slightly more slowly during systole and that the ventricle relaxes more slowly during diastole in GS/GS hearts compared with +/+ hearts. (Table 1).
Figure 2.
Perfusion protocol for GS/GS, GS/+, and +/+ hearts. Control hearts were perfused for 55 min prior to 30 min global ischaemia and 2 h reperfusion (A). Hearts treated with DMSO, wortmannin, U0126, or 5-hydroxydecanoic acid were perfused with the respective agent for 30 min immediately prior to ischaemia and for the first 15 min of reperfusion (B). Experiments were also performed in which either 5-hydroxydecanoic acid or HMR 1098 were perfused only during the initial 15 min of reperfusion (C). Basal contractile function was measured after 25 min perfusion. Postischaemic recovery of contractile function was measured after 1 h of reperfusion, and infarct size was measured after 2 h reperfusion.
Table 1.
Basal parameters of contractile function
| +/+ (n = 16) | GS/+ (n = 15) | GS/GS (n = 12) | |
|---|---|---|---|
| Developed pressure (mmHg) | 107 ± 1 | 108 ± 3 | 107 ± 3 |
| +dP/dT (+ mmHg/s) | 5740 ± 129 | 5593 ± 113 | 5029 ± 148a,b |
| −dP/dT (− mmHg/s) | −4195 ± 83 | −4035 ± 81 | −3741 ± 99a |
| Diastolic pressure (mmHg) | 4.7 ± 0.4 | 4.7 ± 0.4 | 4.6 ± 0.4 |
| Flow rate (mL/min) | 3.8 ± 0.2 | 3.4 ± 0.1 | 3.4 ± 0.2 |
Data were analysed by one-way analysis of variance and post hoc Tukey test.
aSignificant difference (P < 0.01) compared with +/+ hearts.
bSignificant difference (P < 0.05) compared with GS/+ hearts.
Infarct size induced by 30 min global ischaemia was decreased by 39% in GS/+ hearts and by 61% in GS/GS hearts compared with +/+ hearts (Figure 3A), indicating that disruption of interactions between endogenous RGS proteins and Gαi2 promotes the survival of myocardial tissue during ischaemia and reperfusion. Recovery of developed pressure and +dP/dT were significantly enhanced in both GS/+ and GS/GS hearts compared with +/+ hearts after 1 h of reperfusion (Figure 3B and C). Recovery of −dP/dT was significantly enhanced in GS/GS hearts (Figure 3D). Furthermore, diastolic pressure was significantly reduced in GS/+ and GS/GS hearts compared with +/+ hearts, indicating that hearts expressing the Gαi2G184S mutation were protected from ischaemia-induced contracture (Figure 3E). There were no significant differences in postischaemic coronary flow rates among +/+, GS/+, and GS/GS hearts following 1 h of reperfusion (Figure 3F). Ischaemic preconditioning with three cycles of 5 min ischaemia and 5 min reperfusion administered prior to 30 min ischaemia significantly decreased the infarct size of +/+ hearts, but did not cause a further reduction in infarct size of GS/+ or GS/GS hearts (Supplementary material online, Figure S1).
Figure 3.
Expression of RGS insensitive Gαi2 decreases infarct size and enhances postischaemic recovery of contractile function. Hearts were subjected to 30 min ischaemia and 2 h reperfusion as described in Figure 2A, and triphenyltetrazolium chloride staining was used to identify infarcted myocardial tissue. (A) Recovery of developed pressure (B), +dP/dT (C), −dP/dT (D), diastolic pressure (E), and coronary flow rate (F) was measured after 1 h of reperfusion. Data were analysed by one-way analysis of variance and post hoc Tukey test. Data represent the mean ± SEM of 12–16 separate hearts. ‘a’, ‘b’, and ‘c’ indicate significant differences (P < 0.05, P < 0.01, and P < 0.001, respectively) compared with +/+ hearts, and ‘d’ indicates a significant difference (P < 0.001) between GS/+ and GS/GS hearts.
3.3. ERK does not mediate cardioprotection in hearts expressing RGS insensitive Gαi2
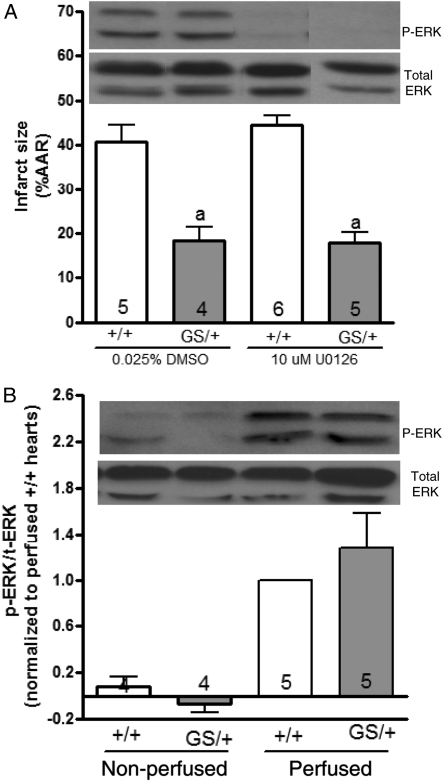
Signalling through the mitogen-activated protein kinase kinase (MEK)/extracellular-regulated kinase (ERK) pathway has been previously shown to mediate the cardioprotective effect of ischaemic preconditioning.15,16 Since we did not know whether the cardioprotective signalling events in GS/+ and GS/GS hearts occurred prior to the onset of ischaemia, during ischaemia, or during the initial moments of reperfusion, we perfused +/+ and GS/+ hearts with 10 μM U0126 (MEK inhibitor) for 30 min prior to ischaemia and during the first 15 min of reperfusion (Figure 2B) to determine whether the cardioprotective phenotype of GS/+ hearts could be reversed by inhibiting ERK phosphorylation. Western blots confirmed that 30 min of U0126 perfusion completely abolished ERK phosphorylation in +/+ and GS/+ hearts, but U0126 did not reverse the cardioprotective phenotype of GS/+ hearts (Figure 4A). Thus, activation of the MEK/ERK pathway immediately prior to ischaemia, during ischaemia, or during the initial moments of reperfusion is not required for cardioprotection in GS/+ hearts.
Figure 4.
ERK does not mediate cardioprotection of hearts expressing RGS-insensitive Gαi2. Hearts were perfused with 10 μM U0126 or 0.025% DMSO (vehicle) according to the protocol shown in Figure 2B. Histograms represent the mean ± SEM of 4–6 hearts. Two-way ANOVA revealed a significant effect [F = 61.2 (1,16) P < 0.0001] of genotype but no significant effect [F = 0.27 (1,16) P = 0.61] of DMSO/U0126 treatment on infarct size. ‘a’ indicates a significant difference compared with DMSO- or U0126-treated +/+ hearts. Inhibition of ERK phosphorylation was confirmed by perfusing hearts with 10 μM U0126 or 0.025% DMSO for 30 min prior to homogenization and subsequent western blot analysis. This western blot is representative of three separate experiments (A). In another set of experiments, hearts were either removed from the mice and immediately flash-frozen (non-perfused) or perfused for 55 min prior to being flash-frozen (perfused) and homogenized. Western blots using non-perfused hearts indicate that +/+ and GS/+ hearts both exhibit very low levels of ERK phosphorylation in vivo. Perfusion increased ERK phosphorylation in +/+ and GS/+ hearts. ERK phosphorylation of perfused hearts was quantified by densitometery using NIH Image J software. The band density of phospho-ERK was divided by that of total ERK, and the phospho-ERK/total-ERK ratio of GS/+ hearts was normalized to that of perfused +/+ hearts (B).
Western blot analysis of +/+ and GS/+ hearts subjected to 55 min of perfusion (without U0126) demonstrated that ERK phosphorylation was similar in hearts of both genotypes at the time point immediately prior to the onset of ischaemia (Figure 4B), providing additional evidence that the cardioprotective phenotype of GS/+ hearts does not result from enhanced MEK/ERK signalling. We also investigated the possibility that protection of GS/+ hearts might result from MEK/ERK signalling that occurred in vivo prior to perfusion by comparing ERK phosphorylation in GS/+ and +/+ hearts that were flash-frozen in liquid nitrogen immediately after being removed from the mice. Phospho-ERK bands were very faint in both non-perfused +/+ and GS/+ hearts suggesting that in vivo levels of ERK phosphorylation were very low in hearts of both genotypes (Figure 4B). Collectively, the inability of U0126 to reverse the cardioprotective phenotype of GS/+ hearts and the observation that phospho-ERK quantities were similar in hearts of both genotypes suggests that ERK does not mediate the cardioprotective phenotype of hearts expressing RGS insensitive Gαi2.
3.4 The phosphatidylinositol-3-kinase (PI-3-K)/Akt/glycogen synthase kinse-3β (GSK-3β) signalling pathway does not mediate cardioprotection in hearts expressing RGS-insensitive Gαi2
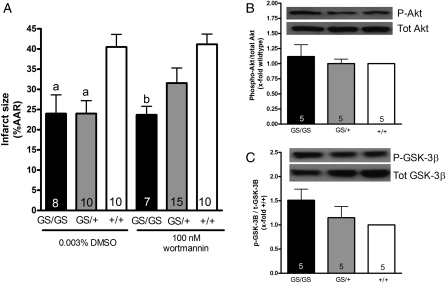
The PI-3-K/Akt/GSK-3β signalling pathway has also been implicated in ischaemic preconditioning. We used wortmannin, an irreversible PI-3-K inhibitor, to determine whether this signalling pathway mediates the cardioprotective phenotype of GS/+ and GS/GS hearts. Hearts were perfused with 100 nM wortmannin for 30 min prior to ischaemia and for the first 15 min of reperfusion as shown in Figure 2B. Previous studies have demonstrated that 100 nM wortmannin inhibits Akt phosphorylation in the Langendorff model.17,18 However, wortmannin had no significant effect on infarct size in GS/+ or GS/GS hearts (Figure 5A) suggesting that signalling through PI-3-K is not required for cardioprotection in GS/+ or GS/GS hearts. Consistent with these data, western blot analysis demonstrated very little phosphorylation of Akt or GSK-3β (downstream substrate of Akt) in perfused +/+ or GS/+ hearts (data not shown).
Figure 5.
Signalling through Akt and GSK-3β does not mediate cardioprotection of hearts expressing RGS-insensitive Gαi2. Hearts were perfused with 100 nM wortmannin or 0.003% DMSO (vehicle) according to the protocol shown in Figure 2B. Two-way ANOVA revealed a significant effect [F = 11.98 (2,54) P < 0.0001] of genotype but no significant effect [F = 0.80 (1,54) P = 0.37] of DMSO/wortmannin treatment on infarct size in +/+, GS/+, and GS/GS hearts. Data represent the mean ± SEM of 7–15 hearts. ‘a’ indicates a significant difference (P < 0.01) compared with DMSO-treated +/+ hearts, and ‘b’ indicates a significant difference (P < 0.01) compared with wortmannin-treated +/+ hearts (A). Akt phosphorylation (B) and GSK-3β phosphorylation (C) were analysed by western blot. There were no significant differences (P > 0.05) in Akt or GSK-3β phosphorlyation between +/+, GS/+, and GS/GS hearts. Panels (B and C) represent the mean ± SEM of five experiments and were analysed by one-way ANOVA. Western blots in (A and B) are representative of five independent experiments.
We investigated the possibility that cardioprotection of GS/+ and GS/GS hearts might result from enhanced Akt or GSK-3β signalling that occurs in vivo by comparing Akt and GSK-3β phosphorylation in +/+, GS/+, and GS/GS hearts that were flash-frozen in liquid nitrogen immediately after being removed from the mice. There were no significant differences in Akt or GSK-3β phosphorylation between non-perfused +/+, GS/+, and GS/GS hearts (Figure 5B and C). These data are consistent with the observation that wortmannin did not reverse the cardioprotective phenotype of GS/+ or GS/GS hearts (Figure 5A), and they provide additional evidence that signalling though the PI-3-K/Akt/GSK-3β pathway does not mediate the cardioprotective phenotype of GS/+ and GS/GS hearts.
3.5. Functional mitochondrial and sarcolemmal potassium channels are required for protection of hearts expressing RGS-insensitive Gαi2
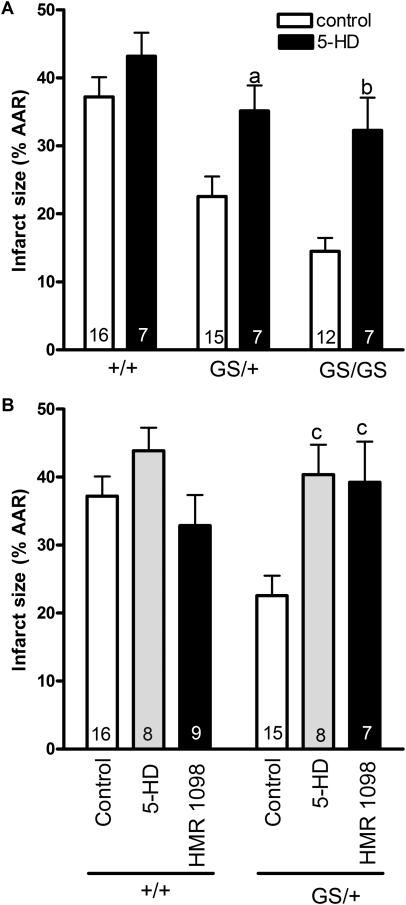
Previous work has demonstrated that the opening of 5-hydroxydecanoic acid (5-HD)-sensitive mitochondrial KATP channels is required for the cardioprotective benefits of ischaemic preconditioning.19,20 Therefore, we perfused hearts with 200 μM 5-HD for 30 min prior to ischaemia and for the first 15 min of reperfusion (Figure 2B) to determine whether mitochondrial KATP channels are required for the cardioprotective effect of RGS-insensitive Gαi2 expression. 5-HD reversed the cardioprotective phenotype of both GS/+ and GS/GS hearts but had no effect on +/+ hearts (Figure 6A). Subsequent experiments revealed that perfusion of 5-HD (200 µM) during the reperfusion phase alone (perfusion protocol shown in Figure 2C) was sufficient to reverse cardioprotection in hearts expressing RGS-insensitive Gαi2 (Figure 6B). Previous work has also implicated HMR 1098-sensitive sarcolemmal KATP channels in protection from ischaemic injury.21 This is consistent with our observation that perfusion of HMR 1098 during the initial 15 min of reperfusion also reversed the cardioprotective phenotype of GS/+ hearts (Figure 6B). These data suggest that disruption of interactions between endogenous RGS proteins and Gαi2 protects the heart through a mechanism that requires both sarcolemmal and mitochondrial KATP channels. However, it is important to note that the putative mitochondrial KATP channel has not been cloned or identified at the molecular level, making it difficult to assign a specific molecular identity to this functionally defined channel.
Figure 6.
ATP-dependent K+ channels mediate cardioprotection in hearts expressing RGS insensitive Gαi2. Hearts were perfused with Krebs solution alone (control) or Krebs solution containing 200 μM 5-hydroxydecanoic acid (5-HD) for 30 min prior to ischaemia and for the initial 15 min of reperfusion according to the protocols shown in Figure 2A and B. 5-HD had no significant effect (P > 0.05) on infarct size in +/+ hearts. However, the cardioprotective effect of RGS-insensitive Gαi2 was reversed by 5-HD in GS/+ hearts and GS/GS hearts. Data were analysed by two-way analysis of variance with genotype and drug treatment) as variables. There was a significant effect [F = 19.56 (1, 54) P < 0.0001] of 5-HD treatment in both GS/+ and GS/GS hearts (A). The cardioprotective effect of RGS-insensitive Gαi2 was also inhibited by either 5-HD (200 µM) or HMR 1098 (30 µM) perfused only during the initial 15 min of reperfusion (perfusion protocol shown in Figure 2C) (B). There was a significant effect [F = 5.47 (2,57) P < 0.01] of 5-HD and HMR 1098 treatment in GS/+ hearts. Data represent the mean ± SEM of 7–16 separate hearts. ‘a’ indicates a significant difference (P < 0.05) compared with control GS/+ hearts, ‘b’ indicates a significant difference (P < 0.01) compared with control GS/GS hearts, and ‘c’ indicates a significant difference (P < 0.01) compared with control GS/+ hearts.
4. Discussion
The ability of Gαi-coupled receptors to protect the heart from ischaemic injury is well established. Three Gαi isoforms (Gαi1, Gαi2, and Gαi3) have been cloned and characterized, and at least two isoforms (Gαi2 and Gαi3) are expressed in the heart.7 Previous work has demonstrated that inhibition of Gαi signalling by expression of a minigene encoding the carboxyl terminal tail of Gαi2 worsens ischaemia-induced injury.22 In the present study, we report that enhanced signalling through the Gαi2 isoform protects the heart from ischaemic injury. Importantly, we found that eliminating the endogenous ‘brake’ on Gαi2 signalling (mediated by interaction with RGS proteins) is sufficient to cause substantial cardiac protection. These data suggest that RGS proteins may provide novel therapeutic targets to reduce ischaemic injury in the heart.
Gαi2G184S results in increased signalling but it is not constitutively active. Rather, agonist-induced Gα activation is prolonged and/or occurs at lower concentrations of agonist due to loss of RGS action. The observations that the Gαi2G184S mutation increases the potency of carbachol (Figure 1) and that GS/+ and GS/GS hearts are protected from ischaemic injury (Figure 3) suggests that disruption of interactions between Gαi2 and RGS proteins enhances the sensitivity of Gαi2-coupled receptors so that they become stimulated by basal levels of endogenous cardioprotective agonists that are too low to activate sufficient Gαi2 signalling in +/+ hearts. Cohen et al.23 reported that the individual amounts of opioids, bradykinin, and adenosine released individually from the heart during ischaemic preconditioning are too small to protect the heart from injury but that the contribution of each of these agonists to a common downstream signalling pathway causes a threshold to be reached that enables cardioprotection to occur. Thus, it is possible that the cardioprotection observed in GS/+ and GS/GS hearts results from signalling through multiple types of Gαi2-coupled receptors which converge at Gαi2G184S and have additive effects on downstream signalling pathways. The present study (Figure 1) as well as previous studies12,24 have demonstrated that Gαi2G184S expression enhances agonist potency but does not always alter agonist efficacy. Our finding that the cardioprotective phenotype of GS/+ and GS/GS hearts was not enhanced by ischaemic preconditioning (Supplementary material online, Figure S1) provides evidence that Gαi2G184S expression permits a maximal effect to occur with low amounts of endogenous cardioprotective agonists. Additional studies are needed to identify the receptor(s) responsible for Gαi2G184S-mediated protection from ischaemic injury.
Two phases of ischaemic preconditioning have been described. Early preconditioning protects the heart from ischaemic injury within 1–2 h after the preconditioning stimulus and requires activation of the PI-3-K/Akt/GSK-3β and MEK/ERK signalling pathways.15,16,25 These kinases lead to the opening of mitochondrial KATP channels which inhibit mitochondrial proapoptotic signalling and prevent opening of the mitochondrial permeability transition pore (MPTP) which would otherwise be lethal to myocardial cells.20 Delayed preconditioning is a second phase of cardioprotection that occurs 24–72 h after the preconditioning stimulus and requires the synthesis of new proteins such as cyclooxygenase-2, inducible nitric oxide synthase, superoxide dismutase, and others.26 In addition, brief periods of ischaemia and reperfusion that follow a prolonged period of ischaemia protect the heart from reperfusion-induced injury through a phenomenon called ‘ischaemic postconditioning’, a process that also requires Akt and ERK phosphorylation.15 Our finding that 5-HD and HMR 1098 reverse the cardioprotective phenotype of hearts expressing RGS-insensitive Gαi2 is consistent with previous studies which have identified mitochondrial and sarcolemmal KATP channels as mediators of ischaemic preconditioning.19,21 However, we were surprised to find that inhibition of PI-3-K or MEK did not reverse the cardioprotective phenotype of GS/+ or GS/GS hearts and that the amounts of Akt, GSK-3-β, and ERK phosphorylation in hearts expressing RGS-insensitive Gαi2, were similar to those of +/+ hearts. Hearts from GS/+ and GS/GS mice have most likely experienced chronically enhanced Gαi2 signalling throughout their entire lives as a result of genetic disruption of RGS–Gαi2 protein interactions. It is possible that a delayed preconditioning mechanism protects GS/+ and GS/GS hearts and that Akt, GSK-3-β, or ERK had already triggered the activation of KATP channels and other downstream cardioprotective signalling processes hours or days prior to the onset of ischaemia. Another potential explanation is that chronic enhancement of Gαi2 signalling may induce alternative cardioprotective signalling pathways that activate KATP channels through a mechanism that does not require PI-3-K/Akt or MEK/ERK signalling. Further work is necessary to determine whether GS/+ and GS/GS hearts are protected by an early preconditioning, delayed preconditioning, or postconditioning mechanism and to more clearly understand the mechanism by which chronic disruption of RGS–Gαi2 interactions protects the heart from ischaemic injury.
Previous studies have demonstrated that ischaemia induces changes in the expression of some RGS proteins. RGS2 expression is decreased and RGS4, RGS7, RGS8, and RGS9 are increased in the ischaemic brain.27,28 Ischaemia also increases RGS1 gene transcription in the ischaemic retina.29 mRNA transcripts encoding at least 17 RGS proteins have been identified in the heart,8 but the effects of ischaemia and reperfusion on cardiac RGS protein expression and function are unknown. Our finding that disruption of interactions between RGS proteins and Gαi2 protects the heart from ischaemic injury raises the possibility that ischaemia-induced changes in RGS protein expression or function might play a role in ischaemic preconditioning. The ability of RGS proteins to be acutely regulated by proteosomal degradation, phosphorylation, translocation, and transcription8,30 provides potential mechanisms for ischaemia and reperfusion to alter the ability of RGS proteins to interact with Gαi2. Further work is needed to determine whether ischaemia-induced changes in RGS proteins occur in the heart and whether these potential changes alter the myocardial response to an ischaemic insult.
One of the challenges to the development of therapeutically useful drug treatments that mimic the effects of ischaemic preconditioning is the necessity to predict when myocardial ischaemia will occur. The ability of RGS inhibitors to potentiate the effects of endogenous agonists might provide a novel mechanism to selectively target the ischaemic heart on a temporal basis so that patients at high risk for myocardial infarction could be prophylactically treated prior to the onset of ischaemia. Adenosine, bradykinin, opioids, and other cardioprotective Gi-coupled agonists are released from the heart during ischaemia. RGS inhibitors may enable signalling through cardioprotective receptors to be enhanced during ischaemia when the concentrations of these agonists are elevated while avoiding enhanced signalling when the heart is adequately perfused and these cardioprotective agonists are present at low basal concentrations. This hypothesis is speculative. However, it is supported by the fact that preischaemic contractile function of +/+ and GS/+ hearts were similar (Table 1), yet GS/+ hearts were protected from ischaemic injury. Further work is needed to determine which RGS proteins modulate the cardioprotective effect of Gαi2 signalling and to develop selective RGS inhibitors that can be used to test this hypothesis.
In conclusion, these data demonstrate that signalling through Gαi2 protects the heart from ischaemic injury and that this process is modulated by endogenous RGS proteins. Our data suggest that the development of chemical inhibitors of RGS proteins31,32 may provide new pharmacotherapies to protect the heart from ischaemic injury.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by Ohio Northern University [Bower, Bennet, and Bennet Endowed Research Award to B.R.R.]; the National Institutes of Health [GM39561 to R.R.N.]; the American Association of Colleges of Pharmacy [New Investigator Award to J.N.T.]; and the American Foundation for Pharmaceutical Education [Gateway to Research Scholarship to R.E.W.].
Supplementary Material
Acknowledgements
HMR1098 was a generous gift from Sanofi Aventis.
Conflict of interest: none declared.
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Cochrane J, Williams BT, Banerjee A, Harken AH, Burke TJ, Cairns CB, et al. Ischemic preconditioning attenuates functional, metabolic, and morphologic injury from ischemic acute renal failure in the rat. Ren Fail. 1999;21:135–145. doi: 10.3109/08860229909066978. doi:10.3109/08860229909066978. [DOI] [PubMed] [Google Scholar]
- 3.Kume M, Yamamoto Y, Saad S, Gomi T, Kimoto S, Shimabukuro T, et al. Ischemic preconditioning of the liver in rats: implications of heat shock protein induction to increase tolerance of ischemia-reperfusion injury. J Lab Clin Med. 1996;128:251–258. doi: 10.1016/s0022-2143(96)90026-8. doi:10.1016/S0022-2143(96)90026-8. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Kato H, Nakata N, Kogure K. Protection of rat hippocampus against ischemic neuronal damage by pretreatment with sublethal ischemia. Brain Res. 1992;586:121–124. doi: 10.1016/0006-8993(92)91380-w. doi:10.1016/0006-8993(92)91380-W. [DOI] [PubMed] [Google Scholar]
- 5.Zahir KS, Syed SA, Zink JR, Restifo RJ, Thomson JG. Ischemic preconditioning improves the survival of skin and myocutaneous flaps in a rat model. Plast Reconstr Surg. 1998;102:140–150. doi: 10.1097/00006534-199807000-00022. doi:10.1097/00006534-199807000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Eisen A, Fisman EZ, Rubenfire M, Freimark D, McKechnie R, Tenenbaum R, et al. Ischemic preconditioning: nearly two decades of research. A comprehensive review. Atherosclerosis. 2004;172:201–210. doi: 10.1016/S0021-9150(03)00238-7. doi:10.1016/S0021-9150(03)00238-7. [DOI] [PubMed] [Google Scholar]
- 7.Ye C, Sowell MO, Vassilev PM, Milstone DS, Mortensen RM. Galpha(i2), Galpha(i3)and Galpha(o) are all required for normal muscarinic inhibition of the cardiac calcium channels in nodal/atrial-like cultured cardiocytes. J Mol Cell Cardiol. 1999;31:1771–1781. doi: 10.1006/jmcc.1999.1015. doi:10.1006/jmcc.1999.1015. [DOI] [PubMed] [Google Scholar]
- 8.Riddle EL, Schwartzman RA, Bond M, Insel PA. Multi-tasking RGS proteins in the heart: the next therapeutic target? Circ Res. 2005;96:401–411. doi: 10.1161/01.RES.0000158287.49872.4e. doi:10.1161/01.RES.0000158287.49872.4e. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Fu Y, Charbeneau RA, Saunders TL, Taylor DK, Hankenson KD, et al. Pleiotropic phenotype of a genomic knock-in of an RGS-insensitive G184S Gnai2 allele. Mol Cell Biol. 2006;26:6870–6879. doi: 10.1128/MCB.00314-06. doi:10.1128/MCB.00314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan KL, Sarvazvan NA, Taussig R, Mackenzie RG, DiBello PR, Dohlman HG, et al. A point mutation in Gαo and Gαi1 blocks interaction with regulator of G protein signaling proteins. J Biol Chem. 1998;273:1794–1797. doi: 10.1074/jbc.273.21.12794. doi:10.1074/jbc.273.3.1794. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y, Huang X, Piao L, Lopatin AN, Neubig RR. Endogenous RGS proteins modulate SA and AV nodal functions in isolated heart: implications for sick sinus syndrome and AV block. Am J Physiol Heart Circ Physiol. 2007;292:H2532–H2539. doi: 10.1152/ajpheart.01391.2006. doi:10.1152/ajpheart.01391.2006. [DOI] [PubMed] [Google Scholar]
- 12.Fu Y, Huang X, Zhong H, Mortensen RM, D'Alecy LG, Neubig RR. Endogenous RGS proteins and Galpha subtypes differentially control muscarinic and adenosine-mediated chronotropic effects. Circ Res. 2006;98:659–666. doi: 10.1161/01.RES.0000207497.50477.60. doi:10.1161/01.RES.0000207497.50477.60. [DOI] [PubMed] [Google Scholar]
- 13.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 14.Motulsky HJ, Neubig RR. Current Protocols in Neuroscience. Hoboken, NJ: John Wiley & Sons, Inc; 2010. Analyzing binding data; pp. 7.5.1–7.5.65. [DOI] [PubMed] [Google Scholar]
- 15.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. doi:10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. doi:10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 17.Wynne AM, Mocanu MM, Yellon DM. Pioglitazone mimics preconditioning in the isolated perfused rat heart: a role for prosurival kinases PI3K and P42/P44 MAPK. J Cardiovas Pharmacol. 2005;46:817–822. doi: 10.1097/01.fjc.0000188365.07635.57. doi:10.1097/01.fjc.0000188365.07635.57. [DOI] [PubMed] [Google Scholar]
- 18.Bell RM, Clark JE, Hearse DJ, Shattock MJ. Reperfusion kinase phosphorylation is essential but not sufficient in the mediation of pharmacological preconditioning: characterisation in the bi-phasic profile of early and late preconditioning. Cardiovasc Res. 2007;73:153–163. doi: 10.1016/j.cardiores.2006.10.013. doi:10.1016/j.cardiores.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Oldenturg O, Cohen MV, Yellon DM, Downey JM. Mitochondrial K(ATP) channels: role in cardioprotection. Cardiovasc Res. 2002;55:429–437. doi: 10.1016/s0008-6363(02)00439-x. doi:10.1016/S0008-6363(02)00439-X. [DOI] [PubMed] [Google Scholar]
- 20.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Ann Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. doi:10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, et al. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeGeorge BR, Jr, Gao E, Boucher M, Vinge LE, Martini JS, Raake PW, et al. Targeted inhibition of cardiomyocyte Gi signaling enhances susceptibility to apoptotic cell death in response to ischemic stress. Circulation. 2008;117:1378–1387. doi: 10.1161/CIRCULATIONAHA.107.752618. doi:10.1161/CIRCULATIONAHA.107.752618. [DOI] [PubMed] [Google Scholar]
- 23.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Annu Rev Physiol. 2000;62:79–109. doi: 10.1146/annurev.physiol.62.1.79. doi:10.1146/annurev.physiol.62.1.79. [DOI] [PubMed] [Google Scholar]
- 24.Talbot JN, Jutkiewicz EM, Graves SM, Clemans CF, Nicol MR, Mortensen RM, et al. RGS inhibition at G(alpha)i2 selectively potentiates 5_HT1A-mediated antidepressant effects. Proc Natl Acad Sci USA. 2010;107:11086–11091. doi: 10.1073/pnas.1000003107. doi:10.1073/pnas.1000003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juhaszova M, Zoroy DB, Yaniv Y, Nuss HB, Wang S, Sollot SJ. Role of glycogen synthase kinase-3 beta in cardioprotection. Circ Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. doi:10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein AB, Tang XL, Guo Y, Xuan YT, Dawn B, Bolli R. Delayed adaptation of the heart to stress: late preconditioning. Stroke. 2004;35:2676–2679. doi: 10.1161/01.STR.0000143220.21382.fd. doi:10.1161/01.STR.0000143220.21382.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen DM, Xiao L, Cai X, Zeng R, Zhu XZ. Involvement of multitargets in paeoniflorin-induced preconditioning. J Pharmacol Exp Ther. 2006;319:165–180. doi: 10.1124/jpet.106.104380. doi:10.1124/jpet.106.104380. [DOI] [PubMed] [Google Scholar]
- 28.Shelat PB, Coulibaly AP, Wang Q, Sun AY, Sun GY, Simonyi A. Ischemia-induced increase in RGS7 mRNA expression in gerbil hippocampus. Neurosci Lett. 2006;403:157–161. doi: 10.1016/j.neulet.2006.04.037. doi:10.1016/j.neulet.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 29.Cervia D, Martini D, Ristori C, Catalani E, Timperio AM, Bagnoli P, et al. Modulation of the neuronal response to ischaemia by somatostatin analogues in wild-type and knock-out mouse retinas. J Neurochem. 2008;106:2224–2235. doi: 10.1111/j.1471-4159.2008.05556.x. doi:10.1111/j.1471-4159.2008.05556.x. [DOI] [PubMed] [Google Scholar]
- 30.Bodenstein J, Sunahara RK, Neubig RR. N-terminal residues control proteasomal degradation of RGS2, RGS4, and RGS5 in human embryonic kidney 293 cells. Mol Pharmacol. 2007;71:1040–1050. doi: 10.1124/mol.106.029397. doi:10.1124/mol.106.029397. [DOI] [PubMed] [Google Scholar]
- 31.Blazer LL, Roman DL, Chung A, Larsen MJ, Greedy BM, Husbands SM, et al. Reversible allosteric small-molecule inhibitors of regulator of G protein signaling proteins. Mol Pharmacol. 2010;78:524–533. doi: 10.1124/mol.110.065128. doi:10.1124/mol.110.065128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roman DL, Blazer LL, Monroy CA, Neubig RR. Allosteric inhibition of the regulator of G protein signaling Gα protein-protein interaction by CCG-4986. Mol Pharmacol. 2010;78:360–365. doi: 10.1124/mol.109.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.