Abstract
Aims
Angiotensin II (Ang II) type AT1 receptors expressed on vascular smooth muscle cells (VSMCs) couple to the Jak2 signalling pathway. However, the importance of this tissue-specific coupling is poorly understood. The purpose of this investigation was to determine the importance of VSMC-derived Jak2 in angiotensin II-mediated hypertension.
Methods and results
The Cre-loxP system was used to conditionally eliminate Jak2 tyrosine kinase expression within the smooth muscle cells of mice. Following chronic Ang II infusion, the resulting increase in mean arterial pressure (MAP) was significantly attenuated in the Jak2 null mice when compared with littermate controls. The VSMC Jak2 null mice were also protected from the Ang II-induced vascular remodelling. Aortic rings from the VSMC Jak2 null mice exhibited reduced Ang II-induced contraction and enhanced endothelial-dependent relaxation via increased nitric oxide (NO) bioavailability. When compared with controls, the VSMC Jak2 nulls also had lower levels of hydrogen peroxide, Rho kinase activity, and intracellular Ca2+ in response to Ang II.
Conclusions
The data indicate that VSMC Jak2 expression is involved in the pathogenesis of Ang II-dependent hypertension due to the increased presence of reactive oxygen species (ROS). As such, VSMC-derived Jak2 tyrosine kinase modulates overall vascular tone via multiple, non-redundant mechanisms.
Keywords: Jak2 tyrosine kinase, Angiotensin II, Vascular smooth muscle cell, Hypertension
1. Introduction
Hypertension is a major risk factor for cardiovascular disease (CVD) and death.1 Angiotensin II (Ang II) plays a major role in the regulation of normal physiological responses of the cardiovascular system and the pathogenesis of hypertension. Previous studies have shown that Ang II stimulation of the AT1 receptor leads to increased activation of the Jak2 signalling pathway, and increased Jak2 activity has been correlated with various Ang II-mediated CVDs.2–12 Recent work demonstrated that the Rho exchange factor, Arhgef1, mediates the effects of Ang II on vascular tone and blood pressure.13 It was demonstrated in this work that Jak2 may be involved in this in vivo process; however, this correlation was made using the Jak2 pharmacological inhibitor, AG490. Although AG490 is a potent inhibitor of Jak2, it also inhibits other tyrosine kinases.14,15 To wit, it inhibits EGFR phosphorylation 1000 times more potently than it inhibits Jak2.16 Moreover, systemic administration of a pharmacological inhibitor is unable to discriminate between potential Jak2 target tissues such as brain, kidney, heart, or the vasculature.
Reactive oxygen species (ROS) have been implicated in the pathogenesis of hypertension and Ang II is involved in mediating ROS-dependent signalling.17–21 One mechanism by which ROS trigger hypertension is via scavenging of nitric oxide (NO).22 ROS also increase intracellular free Ca2+ levels, which contribute to increased vascular tone.17 In VSMC and endothelial cells (ECs), Ang II stimulates the activity of membrane-bound NAD(P)H oxidase producing ROS, such as superoxide and hydrogen peroxide (H2O2).23–29 Superoxide generation in response to Ang II inactivates NO in both these cell types.30–32 Interestingly, the Ang II-induced formation of ROS is not dependent on its haemodynamic effects, since this phenomenon is not observed in norepinephrine-induced hypertension.24,33,34 Furthermore, work in human leukaemia cells demonstrated that Jak2 is involved in ROS production as inhibition of Jak2 resulted in decreased ROS levels.35
We hypothesized that the mechanism of Ang II-induced hypertension is by Jak2 tyrosine kinase activation within VSMC leading to increased ROS generation. Utilizing a conditional knockout mouse approach in which Jak2 was deleted from VSMC, we identified Jak2 as a key modulator of Ang II-induced vascular contraction via a ROS-dependent mechanism.
2. Methods
Detailed methods are contained in the Supplementary material online.
2.1. Mice
Animals were maintained according to NIH standards established in the Guidelines for the Care and Use of Experimental Animals in a specific pathogen-free facility at the Laboratory Animal Center of University of Florida. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Florida. Mice harboring a floxed Jak2 allele were crossed with mice expressing Cre recombinase under the control of the SM22α promoter and the Jak2 null allele was identified using primers 5′-GTCTATACACCACCACTCCTG and 5′-GAGCTGGAAAGATAGGTCAGC.
2.2. Blood pressure measurements
Mice were anaesthetized by isoflurane (2–5%, aerosolized) and surgically fitted with telemetry probes placed in the left carotid artery. Buprenorphine was the post-operative analgesia (0.05–0.1 mg/kg every 6–12 h for 48 h, ip). After 10 days of recovery, baseline blood pressure measurements were made. Mice were again anaesthetized with isoflurane and micro-osmotic pumps were placed subcutaneously for infusion of 1000 ng/kg/min Ang II. Radio telemetry recordings were then performed over the ensuing 4 week period.
2.3. Aortic contraction/relaxation
Mice were euthanized via CO2 asphyxiation followed by cervical dislocation. Two millimetre abdominal aorta ring segments were then mounted on a wire myograph in Krebs-bicarbonate buffer equilibrated with 95% O2, 5% CO2 at 37°C. The rings were allowed to equilibrate for 45 min, stimulated with different pharmacological agents and changes in contraction/relaxation were recorded. Following treatment with each vasoactive agent, the rings were allowed to recover for 30 min, with six washes during this time period.
2.4. Histology
Tissue samples were prepared and stained with haematoxylin and eosin or Masson's trichrome. Immunohistological detection of anti-smooth muscle α-actin was carried out using the Rat on Mouse AP-Polymer Kit. The anti-Jak2 antibody was purchased commercially (Abcam ab39636).
2.5. NO measurements
Aortic rings were incubated with 7 µM fluorescent dye 4-amino-5-methylamino-2′,7′-difluorescein (DAF-FM) aerated with 95% O2–5% CO2 at 37°C for 45 min. Samples for basal accumulation of NO were taken. The rings were then treated with Ang II (10−7 M) or Ach (10−6 M) for 30 min, removed, dried, and weighed. Fluorescence was measured at an excitation wavelength of 495 nm and an emission wavelength of 520 nm and normalized to tissue weight.
2.6. H2O2 detection
H2O2 production was measured using the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit and presented as the catalase-inhabitable signal normalized to total cellular protein.
2.7. Rho kinase activity
Rho kinase activity was determined using the CycLex Rho kinase Assay Kit or by directly measuring the phosphorylation levels of myosin phosphatase subunit 1 (MYPT1), a down stream target of Rho kinase.
2.8. Calcium imaging
Ca2+ levels within individual cells were determined via fura-2 loading and a cooled charge-coupled device camera fitted to a fluorescence microscope.
2.9. Statistics
Comparison of genotypes and treatments was performed by unpaired/paired Student's t-test, analysis of variance followed by the Bonferroni t-test, or Friedman's test.
3. Results
3.1. Generation of mice with VSMC deletion of Jak2
The Cre-loxP system was used to ablate Jak2 within VSMCs. The schematic representation of the floxed Jak2 allele (Supplementary material online, Figure S1A) and the breeding strategy used to generate such mice (Supplementary material online, Figure S1B) are shown. The genotypes of all offspring were analysed by PCR (Supplementary material online, Figure S1C). Mice homozygous for the floxed Jak2 allele were identified by the presence of a 310 bp band as opposed to the 230 bp band of the WT allele. Identification of SM22αCre was confirmed by the presence of a 201 bp Cre-specific amplicon. The site-specific recombination event to obtain the null Jak2 allele was verified by a PCR assay of the abdominal aorta using primers 278 and 279; the 355 bp band could only be generated after Cre-mediated excision of the Jak2 start codon. In order to confirm that the SM22α promoter targets Cre expression to VSMC, our mice were crossed with the ubiquitously expressed Rosa26 β-galactosidase reporter mouse36 and X-Gal staining was analysed in tissue sections. VSMCs of renal arteries showed no staining in the SM22αCre(−)Jak2fl/fl/Rosa26 control mice (Supplementary material online, Figure S1D, left). However, there was efficient Cre expression and recombination within VSMC leading to intensive X-Gal staining in the renal arteries of SM22αCre(+)Jak2fl/fl/Rosa26 mice (Supplementary material online, Figure S1D, right). Overall, these data demonstrate a high degree of Cre activity in VSMC.
Having confirmed the appropriate genetic manipulations within these mice, we next wanted to confirm the specific absence of Jak2 protein within VSMC. For this, immuno histochemistry was carried out on renal arteries of several genotypes to demonstrate differential Jak2 staining patterns (Supplementary material online, Figure S2). Collectively, these data demonstrate that the Cre-mediated conditional deletion of the first coding exon of Jak2 within VSMC gives rise to mice that correspondingly lack Jak2 protein in VSMC.
3.2. Deletion of VSMC Jak2 attenuates Ang II-induced hypertension
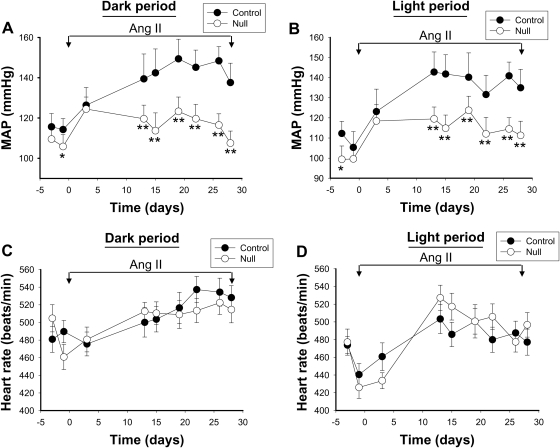
While it is well accepted that the Ang II type AT1 receptor couples to Jak2 signalling,2–8 the functional role of this interaction is not clearly understood and little is known about the direct role of Jak2 in blood pressure regulation. We hypothesized that Jak2 within VSMC plays a critical role in Ang II-mediated hypertension. For all subsequent studies, we used two genotypes; SM22αCre(−)Jak2fl/fl herein designated as control and SM22αCre(+)Jak2fl/fl herein designated as the VSMC Jak2 null. Using telemetry blood pressure recordings, we found that the basal mean arterial pressure (MAP) was slightly lower in VSMC Jak2 null mice, when compared with controls (Figure 1A and B). After two baseline recordings, the mice were implanted with osmotic minipumps infusing Ang II at a rate of 1000 ng/kg/min for 28 days. Infusion of Ang II resulted in an increase in MAP in both groups. However, the increase in VSMC Jak2 null mice was significantly lower (P< 0.001) compared with that observed in the control group, both during the dark (Figure 1A) and the light (Figure 1B) periods. Examination of the heart rate data indicated that the heart rates were not significantly different between the two groups during these same periods (Figure 1C and D). These results indicate that deletion of VSMC Jak2 attenuates Ang II-induced hypertension, but has no effect on the heart rate.
Figure 1.
Deletion of vascular smooth muscle cell Jak2 attenuates Ang II-induced hypertension. Long-term radio-telemetric recording of mean arterial pressure was performed in VSMC Jak2 null (n= 6) and age-matched controls (n= 6). Two base line recordings were performed before the mice were implanted with Ang II mini pumps infusing 1000 ng/kg/min of Ang II (day 0). Further recording was continued over the ensuing 28 days. The values shown represent daily average 12 h mean arterial pressure recordings for the active dark period (A) and the resting light period (B). Heart rates were also plotted as a function of both genotype and time. The average 12 h mean heart rate recordings for the active dark period (C) and the resting light period (D) are shown. Data represent means ± SE (*P< 0.05, **P< 0.01, ANOVA).
3.3. VSMC Jak2 null mice are protected from Ang II-induced aortic wall thickening
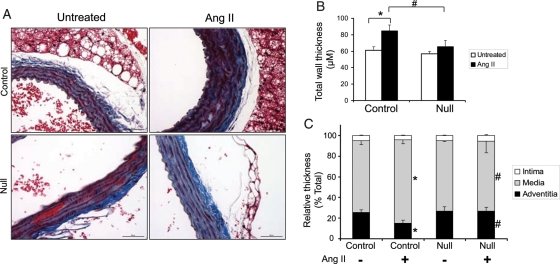
Chronically elevated Ang II levels promote pathological vascular wall remodelling.37,38 To determine the affects of Ang II infusion on vascular remodelling in our mice, aortas from both genotypes were prepared for the analysis. Representative sections are shown as Figure 2A, while the aggregate data for all animals are shown in Figure 2B. In the controls, Ang II infusion increased the aortic wall thickness relative to untreated littermate controls. However, this Ang II-mediated pathological thickening was significantly reduced in the null mice. Computer-assisted morphometric analysis indicated that the tunica intima thickness as a percentage of the total thickness was similar in VSMC Jak2 null mice and controls (Figure 2C). In contrast, the per cent tunica media thickness was significantly increased and the per cent tunica adventitia thickness was significantly reduced in aortic wall sections of controls when compared with the null mice (Figure 2C). Collectively, these data indicate that VSMC Jak2 null mice are protected from pathological vascular remodelling that occurs as a consequence of chronic Ang II infusion.
Figure 2.
VSMC Jak2 null mice are protected from Ang II-induced aortic wall thickening. (A) Aortic histological sections from control and VSMC Jak2 null mice were stained with trichrome. (B) The total aortic wall thickness from each animal was measured and then plotted as a function of treatment and genotype. (C) Aortic wall layers of the intima, media, and adventitia were defined, and their thickness is presented as a percentage of the total wall thickness. Data represent means ± SE; n = 6. *P< 0.05 vs. untreated control; #P< 0.05 vs. Ang II-treated control, paired Student's t-test.
3.4. Deletion of VSMC Jak2 correlates with reduced Ang II contraction of aortic rings and increased endothelium-derived nitric oxide
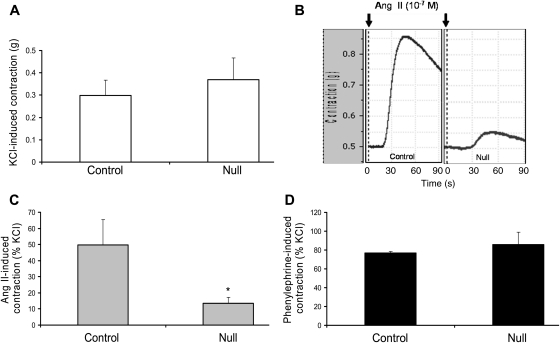
Next, we wanted to determine whether VSMC Jak2 null mice have reduced Ang II-mediated aortic contraction when compared with controls. For this, abdominal aortic rings from control and null genotypes were isolated and their constrictive properties were measured. In response to KCl, there was no significant difference in the absolute contraction between the control and VSMC Jak2 null rings, suggesting that the contractile machinery was similar in both genotypes (Figure 3A). In contrast, rings constricted with Ang II showed a marked difference with Ang II inducing a forceful contraction in the control rings, but not in the null rings; a representative response is shown as Figure 3B while the aggregate data from all experiments are shown as Figure 3C. To determine whether this effect was specific for Ang II or common to vasoactive molecules, aortic rings from both genotypes were constricted with the α1-adrenergic receptor agonist, phenylephrine. No significant difference in the phenylephrine-induced contraction between the two genotypes was observed (Figure 3D). Additionally, pretreatment with the AT2 receptor blocker PD123319 or the Ang-(1–7) selective antagonist A-779, had no significant effect on the per cent change between the Ang II-induced constriction in Jak2 null and the control rings, indicating that the reduced contractile property of the VSMC Jak2 null rings in response to Ang II was independent of AT2 and Mas receptor signalling (data not shown).
Figure 3.
Deletion of vascular smooth muscle cell Jak2 correlates with reduced Ang II-induced contraction of aortic rings. (A) KCl-induced absolute contraction of aortic rings from both genotypes. (B) Representative profiles showing Ang II-induced contraction (10−7 M) in aortic rings obtained from control and VSMC Jak2 null mice. (C) Aggregate Ang II-induced contraction data plotted as a per cent of KCl. (D) Phenylephrine-induced contraction plotted as a per cent of KCl. Data represent means ± SE (*P <0.05; n =8, paired Student's t-test).
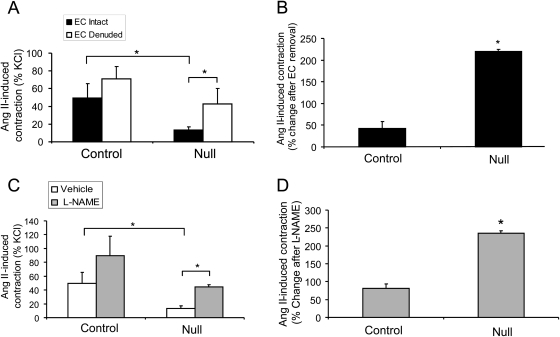
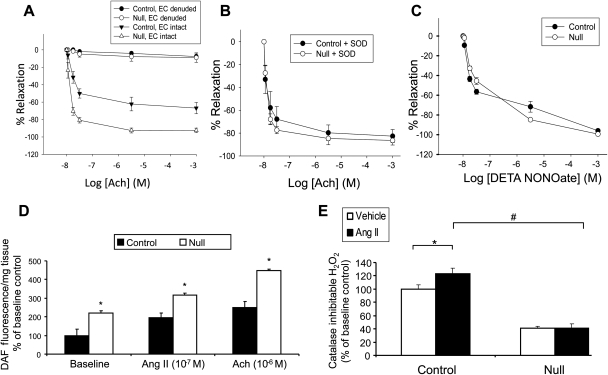
In order to determine whether the endothelium played a role in the observed differences in the Ang II-mediated contraction of the vessels, aortic rings from both genotypes were either left intact or the endothelium was denuded prior to Ang II-mediated constriction. Removal of the endothelium increased the Ang II-induced aortic contraction of the null rings to levels that were similar to controls (Figure 4A). When the data were plotted as the per cent change in constriction after EC removal, we found that the per cent change between the endothelium intact and endothelium denuded aortic rings was significantly greater in VSMC Jak2 null mice, when compared with controls (Figure 4B). These results suggest that the inability of the VSMC Jak2 null rings to contract in response to Ang II is likely due to endothelium-derived inhibitory factors. In order to determine whether NO was one such factor, rings were pretreated with either vehicle or 1 mM of the nitric oxide synthase (NOS) inhibitor L-NAME, followed by Ang II treatment. Pretreatment with L-NAME led to a partial increase in the Ang II-dependent contraction of the null rings (Figure 4C). When the data were plotted as the per cent change in contraction after L-NAME treatment, we found that rings from the null mice had a significantly greater increase in contraction when compared with controls (Figure 4D).
Figure 4.
Deletion of vascular smooth muscle cell Jak2 correlates with increased levels of nitric oxide. (A) Ang II-induced contraction (10−7 M) data plotted as a per cent of KCl either in the presence or absence of the endothelium. (B) The per cent change in Ang II-induced contraction between endothelial cell (EC) intact and EC denuded aortic rings. (C) Ang II-induced contraction of aortic rings pretreated with vehicle or N(G)-nitro-L-arginine methyl ester (l-NAME) and plotted as a per cent of KCl. (D) The per cent change in Ang II-induced contraction between vehicle treated and L-NAME treated aortic rings for both genotypes. Data represent means ± SE (* P <0.05; n =8, paired Student's t-test).
Overall, these results indicate that deletion of VSMC-derived Jak2 correlates with reduced Ang II-mediated contraction. Furthermore, the data support that there is increased bio-available NO in the rings of the null mice, which is likely responsible for part of the reduced Ang II-induced contraction in the aortic rings of these animals.
3.5. Deletion of VSMC Jak2 enhances endothelium-dependent aortic relaxation due to reduced ROS and increased NO availability
To understand the relationship between NO and ROS in this process, aortic rings were first constricted with phenylephrine (Phe) (10−6 M) to elicit an initial Phe-induced maximal contraction. Increasing concentrations of acetylcholine (Ach) were then added, and the per cent of Ach-mediated relaxation of the rings was determined. Ach caused a concentration-dependent relaxation of the rings, which was significantly enhanced in the null rings when compared with the controls (Figure 5A). However, when the endothelium was first denuded prior to Ang II stimulation, there was no relaxation in either set of rings, indicating that the relaxation was entirely endothelium dependent. Accordingly, these results support that deletion of VSMC Jak2 enhances endothelium-dependent vascular relaxation in response to Ach.
Figure 5.
Deletion of vascular smooth muscle cell Jak2 enhances endothelium-dependent vascular relaxation due to reduced reactive oxygen species and increased nitric oxide availability. (A) Dose-dependent acetylcholine-induced relaxation, plotted as a per cent of maximum phenylephrine-induced contraction (10−6 M) with or without the endothelium. Data represent means ± SE (n =8). (B) Dose-dependent acetylcholine-induced relaxation, plotted as a per cent of maximum phenylephrine-induced contraction (10−6 M) with superoxide dismutase pretreatment. Data represent means ± SE (n =8). (C) Per cent DETA NONOate-induced relaxation in aortic rings preincubated with L-NAME. (D) DAF fluorescence nitric oxide measurements in aortic rings at baseline, or following Ang II (10−7) and acetylcholine (10−6) treatment (*P< 0.05 vs. control, paired Student's t-test). (E) Catalase inhibitable H2O2 in vascular smooth muscle cells obtained from control and Jack2 null mice at baseline, and in response to Ang II treatment (*P< 0.05 vs. vehicle control, #P< 0.05 vs. control, unpaired Student's t-test).
ROS have been implicated in the pathogenesis of hypertension via the scavenging of NO.17–22 In order to determine whether reduced levels of ROS were responsible for the improved endothelium-dependent relaxation observed in the null mice, aortic rings were pretreated with the antioxidant, superoxide dismutase (SOD), followed by treatment with increasing concentrations of Ach. Preincubation with SOD improved aortic relaxation of the control rings to levels that were comparable to that observed with the Jak2 null rings, indicating that the control rings had higher levels of ROS (Figure 5B). To determine whether there were inherent differences in the ability of VSMC to relax, aortic rings were pretreated with the NOS inhibitor L-NAME, and then treated with increasing concentrations of the exogenous NO donor, DETA NONOate, a compound that will directly relax the VSMC (Figure 5C). There was no significant difference in aortic relaxation between control and null rings indicating that the impaired endothelium-dependent aortic relaxation observed in the control mice was not due to an impaired ability of the VSMC to relax per se.
We next measured NO levels directly and found that basal levels of NO were significantly greater in the aortic rings taken from null mice when compared with controls (Figure 5D). Similarly, Ang II (10−7) and Ach (10−6) caused greater increases in NO production in aortic rings of VSMC Jak2 nulls when compared with the controls (Figure 5D). Finally, levels of ROS were measured in cultured VSMC from both genotypes. Both at baseline and in response to Ang II, more H2O2 was detected in VSMC obtained from control mice than VSMC Jak2 nulls (Figure 5E). Collectively, the results in Figure 5 indicate that the aortic rings from VSMC Jak2 null mice have enhanced endothelium-dependent relaxation when compared with controls. Furthermore, this enhanced relaxation correlates with decreased ROS and increased NO.
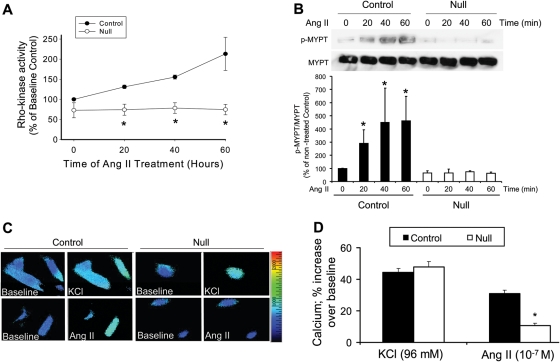
3.6. Deletion of VSMC Jak2 results in reduced Rho kinase activity and intracellular Ca2+ levels in response to Ang II
Rho kinase is a critical mediator of VSMC contraction.39–41 As such, Ang II-dependent Rho kinase activity was measured in cultured VSMC derived from control and null mice. While Ang II treatment caused a time-dependent increase in Rho kinase activity in the control cells, it was completely lacking in the VSMC Jak2 null cells (Figure 6A). To demonstrate this result another way, western blot analysis was used to analyse the activation of MYPT1, a down stream target of Rho kinase. As expected, Ang II treatment increased the phosphorylation of MYPT1 in a time-dependent manner in the control cells (Figure 6B, top). However, VSMC Jak2 null cells completely lacked this effect (Figure 6B, top). Densitometry was then performed on all blots so that quantitative values could be compared between the two genotypes (Figure 6B, bottom). Control cells exhibited a robust Ang II-dependent phosphorylation of MYPT1, which was completely lacking in the VSMC Jak2 null cells.
Figure 6.
Deletion of vascular smooth muscle cell Jak2 results in reduced Rho kinase activity and intracellular Ca2+ levels in response to Ang II. (A) Time-dependent Ang II-induced Rho kinase activity in vascular smooth muscle cells obtained from control and VSMC Jak2 null mice. (B) Representative immunoprecipitation/western blot analysis of phospho-MYPT showing an Ang II-induced phosphorylation of MYPT by Rho kinase (top). All blots were quantitated via densitometric analysis and graphed as a function of genotype and time (bottom) (*P< 0.05 vs. untreated condition, Friedman's test). (C) KCl and Ang II-induced increase in intracellular Ca2+ as measured by fura-2 fluorescent imaging in individual cells. (D) The average Ca2+ responses plotted as a function of treatment and genotype (P< 0.05 vs. control, unpaired Student's t-test).
Lastly, given that ROS can increase intracellular Ca2+ levels and this can contribute to increased vascular tone,17 we hypothesized that the VSMC Jak2 null cells would have decreased intracellular Ca2+ levels compared with controls. To test this, control and VSMC Jak2 null cells were first depolarized with KCl and intracellular Ca2+ levels were determined. Both cells types generated a peak Ca2+ response that was virtually identical, indicating that the Ca2+ signalling machinery is comparable in both cell types (Figure 6C, top panels). However, when VSMC Jak2 null cells were treated with Ang II, intracellular Ca2+ elevation was significantly blunted when compared with control cells (Figure 6C, bottom panels). All responses were then graphed as a function of both treatment and genotype (Figure 6D). We found that deletion of Jak2 within VSMC significantly blunted, but did not prevent Ang II-mediated intracellular Ca2+ elevation. In summary, the data in Figure 6 demonstrate that deletion of Jak2 within VSMC results in a less contractile phenotype when compared with control cells; namely, in response to Ang II, the Jak2 nulls cells have reduced Rho kinase activity, reduced MYPT1 phosphorylation, and reduced intracellular Ca2+ levels.
4. Discussion
Ang II-induced actions via the AT1 receptor are considered to play a major role in the pathogenesis of hypertension. However, the downstream signalling mechanisms through which Ang II exerts its actions are not fully understood. Here, we investigated the role of Jak2 in mediating Ang II-induced vasoconstriction and hypertension by using mice whose VSMC are devoid of Jak2. This study identified Jak2 as a mediator of Ang II-induced vasoconstriction and hypertension through multiple, non-redundant mechanisms that are all ROS dependent. To our knowledge, this is the first study to report that Jak2 is involved in the production of ROS in VSMC with subsequent modulation of blood pressure via changes in NO, Ca2+, and Rho kinase.
Recent studies have demonstrated that the Rho exchange factor, Arhgef1, mediates the effects of Ang II on vascular tone and blood pressure, and that Jak2, by phosphorylating Arhgef1 on Tyr 738, plays a role in this process.13 Our data here both confirm and extend those observations as we show that VSMC Jak2 null cells are unable to activate Rho in response to Ang II (Figure 6A). In addition to phosphorylation-dependent Rho kinase activation, previous studies have shown that Rho kinase can be activated by increased ROS.42,43 The cartoon in Supplementary material online, Figure S3 integrates the signalling modalities involved in Ang II and Jak2-mediated vascular contraction. Our work here indicates that Jak2 generates ROS. The higher levels of ROS reduce the levels of NO in the endothelium and increase the levels of Ca2+ in the VSMC, both of which lead to enhanced contraction. Furthermore, Jak2 can activate Rho kinase via both ROS-dependent42,43 and phosphorylation-dependent mechanisms.13 Collectively, these data indicate that while Jak2 is not required for Ang II contraction per se, it plays an absolutely critical role in modulating overall Ang II-induced vascular tone via multiple, non-redundant mechanisms. We believe that by contributing to the production of ROS, Jak2 is centrally located to mediate most of the mechanisms which are known to be mediated by Ang II and this might explain the dramatically reduced in vivo hypertensive response to Ang II in VSMC Jak2 null mice when compared with controls.
It is possible that the reduced vascular pathology observed in the null mice (Figure 2) is due to the lower blood pressure in these animals. Previous studies, however, have shown that Ang II has growth factor-like properties that act independent of its haemodynamic effects. For example, Ang II induces ROS production leading to endothelial dysfunction through mechanisms that are independent of its pressor actions and norepinephrine-induced hypertension fails to elicit these same deleterious outcomes.33,34 Additionally, administration of renin–angiotensin system inhibitors provide numerous cardio protective effects that are independent of blood pressure reduction.44 Based on these observations, we hypothesize that the Ang II-induced vascular pathology observed in the control mice occurs via a mechanism that is independent of its pressor effect and instead mediated by its growth factor-like properties acting through Jak2. Clearly however, this needs to be demonstrated experimentally.
With respect to the dosage of Ang II used in this work, previous dose response studies in rats revealed that infusion of a dose as low as 200 ng/kg/min can induce patho-physiological effects such as increased systolic blood pressure.45 Studies in mice, however, have shown that infusion doses of 1000 ng/kg/min are required to achieve similar effects.46,47 We therefore used the 1000 ng/kg/min dosage of Ang II in this current work and this dosage produced discernible patho-physiological effects similar to those observed in humans suffering from Ang II-mediated pathogenesis.48 That said, this is well in excess of circulating levels of Ang II that are ∼20 pmol/L.49 Thus, the levels of Ang II required to induce hypertension in mice are in the pharmacological rather than the physiological range. The high levels of Ang II in these studies may therefore be leading to additional non-specific vasoactive effects independent of Ang II receptors. As such, there is an overall need for caution when interpreting the relevance of Jak2 in the regulation of patho-physiological hypertension. However, the slightly lower blood pressure at baseline in the Jak2 null mice may suggest an effect of Jak2 even at normal circulating Ang II levels, but clearly, this needs to be elucidated further.
Previous work has shown that increased levels of ROS within VSMC leads to the activation of Jak2.50 On the other hand, more recent work in human leukaemia cells demonstrated that Jak2 is capable of increasing the levels of ROS.35 Our work here supports this later observation as genetic ablation of Jak2 from VSMC lead to decreased levels of ROS. An important question that remains, however, is by what mechanism does Jak2 increase ROS? Given the well known and critical role that Jak2 plays in modulating gene transcription, perhaps Jak2 regulates the expression of redox-sensitive genes and the ablation of Jak2 disturbs this critical balance. Current studies are attempting to identify such gene candidates.
Gain-of-function, somatic mutations in the Jak2 allele are known to cause various human diseases including the classical myeloproliferative neoplasms.51 As a consequence, great effort has been made to develop small molecule inhibitors that target Jak2 and a limited number of these inhibitors are currently in clinical trials.52 While changes in parameters such as spleen size, blood counts, cytokine levels, fatigue, neurotoxicity, and gastrointestinal disturbances were monitored, our results here suggest that changes in blood pressure may also serve as a key clinical parameter to follow.
In conclusion, these studies identify a novel role of Jak2 tyrosine kinase in regulating vascular tone by increasing ROS. Elevated ROS leads to increased vasoconstriction and hypertension by scavenging endothelial NO, increasing intracellular Ca2+, and increasing Rho kinase activity. Hence, this work strongly supports the consideration of Jak2 as a new therapeutic target for the management of hypertension.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by National Institutes of Health Award R01-HL67277 and American Heart Association Awards #0855361E and 10PRE4310065.
Supplementary Material
Acknowledgements
We thank Ms. Naime Fliess for assisting with the immunohistochemistry, Dr Alvaro N. Gurovich for assisting with statistical analysis, Mr Bruce Cunningham and the UF Hypertension Center for assisting with the telemetry.
Conflict of interest: none declared.
References
- 1.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 2.Bhat GJ, Thekkumkara TJ, Thomas WG, Conrad KM, Baker KM. Angiotensin II stimulates sis-inducing factor-like DNA binding activity. Evidence that the AT1A receptor activates transcription factor-Stat91 and/or a related protein. J Biol Chem. 1994;269:31443–31449. [PubMed] [Google Scholar]
- 3.Bhat GJ, Thekkumkara TJ, Thomas WG, Conrad KM, Baker KM. Activation of the STAT pathway by angiotensin II in T3CHO/AT1A cells. Cross-talk between angiotensin II and interleukin-6 nuclear signaling. J Biol Chem. 1995;270:19059–19065. doi: 10.1074/jbc.270.32.19059. [DOI] [PubMed] [Google Scholar]
- 4.Marrero MB, Schieffer B, Paxton WG, Heerdt L, Berk BC, Delafontaine P, et al. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–250. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 5.Marrero MB, Schieffer B, Li B, Sun J, Harp JB, Ling BN. Role of Janus kinase/signal transducer and activator of transcription and mitogen-activated protein kinase cascades in angiotensin II- and platelet-derived growth factor-induced vascular smooth muscle cell proliferation. J Biol Chem. 1997;272:24684–24690. doi: 10.1074/jbc.272.39.24684. [DOI] [PubMed] [Google Scholar]
- 6.Ali MS, Sayeski PP, Dirksen LB, Hayzer DJ, Marrero MB, Bernstein KE. Dependence on the motif YIPP for the physical association of Jak2 kinase with the intracellular carboxyl tail of the angiotensin II AT1 receptor. J Biol Chem. 1997;272:23382–23388. doi: 10.1074/jbc.272.37.23382. [DOI] [PubMed] [Google Scholar]
- 7.McWhinney CD, Hunt RA, Conrad KM, Dostal DE, Baker KM. The type I angiotensin II receptor couples to Stat1 and Stat3 activation through Jak2 kinase in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 1997;29:2513–2524. doi: 10.1006/jmcc.1997.0489. [DOI] [PubMed] [Google Scholar]
- 8.Dostal DE, Hunt RA, Kule CE, Bhat GJ, Karoor V, McWhinney CD, et al. Molecular mechanisms of angiotensin II in modulating cardiac function: intracardiac effects and signal transduction pathways. J Mol Cell Cardiol. 1997;29:2893–2902. doi: 10.1006/jmcc.1997.0524. [DOI] [PubMed] [Google Scholar]
- 9.Pan J, Fukuda K, Kodama H, Makino S, Takahashi T, Sano M, et al. Role of angiotensin II in activation of the JAK/STAT pathway induced by acute pressure overload in the rat heart. Circ Res. 1997;81:611–617. doi: 10.1161/01.res.81.4.611. [DOI] [PubMed] [Google Scholar]
- 10.Kodama H, Fukuda K, Pan J, Makino S, Sano M, Takahashi T, et al. Biphasic activation of the JAK/STAT pathway by angiotensin II in rat cardiomyocytes. Circ Res. 1998;82:244–250. doi: 10.1161/01.res.82.2.244. [DOI] [PubMed] [Google Scholar]
- 11.Pan J, Fukuda K, Saito M, Matsuzaki J, Kodama H, Sano M, et al. Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res. 1999;84:1127–1136. doi: 10.1161/01.res.84.10.1127. [DOI] [PubMed] [Google Scholar]
- 12.Seki Y, Kai H, Shibata R, Nagata T, Yasukawa H, Yoshimura A, et al. Role of the JAK/STAT pathway in rat carotid artery remodeling after vascular injury. Circ Res. 2000;87:12–18. doi: 10.1161/01.res.87.1.12. [DOI] [PubMed] [Google Scholar]
- 13.Guilluy C, Bregeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, et al. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med. 2010;16:183–190. doi: 10.1038/nm.2079. [DOI] [PubMed] [Google Scholar]
- 14.Kleinberger-Doron N, Shelah N, Capone R, Gazit A, Levitzki A. Inhibition of Cdk2 activation by selected tyrphostins causes cell cycle arrest at late G1 and S phase. Exp Cell Res. 1998;241:340–351. doi: 10.1006/excr.1998.4061. [DOI] [PubMed] [Google Scholar]
- 15.Oda Y, Renaux B, Bjorge J, Saifeddine M, Fujita DJ, Hollenberg MD. cSrc is a major cytosolic tyrosine kinase in vascular tissue. Can J Physiol Pharmacol. 1999;77:606–617. [PubMed] [Google Scholar]
- 16.Levitzki A. Tyrphostins–potential antiproliferative agents and novel molecular tools. Biochem Pharmacol. 1990;40:913–918. doi: 10.1016/0006-2952(90)90474-y. [DOI] [PubMed] [Google Scholar]
- 17.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells—implications in cardiovascular disease. Braz J Med Biol Res. 2004;37:1263–1273. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- 19.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 20.Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, et al. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 21.Yan C, Kim D, Aizawa T, Berk BC. Functional interplay between angiotensin II and nitric oxide: cyclic GMP as a key mediator. Arterioscler Thromb Vasc Biol. 2003;23:26–36. doi: 10.1161/01.atv.0000046231.17365.9d. [DOI] [PubMed] [Google Scholar]
- 22.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 23.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 24.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, et al. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 26.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 27.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Schmeisser A, Garlichs CD, Plotze K, Damme U, Mugge A, et al. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc Res. 1999;44:215–222. doi: 10.1016/s0008-6363(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 29.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 30.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 31.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 32.Dzau VJ. Theodore Cooper Lecture: tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 33.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 34.Harrison DG. Endothelial function and oxidant stress. Clin Cardiol. 1997;20:II-17. [PubMed] [Google Scholar]
- 35.Walz C, Crowley BJ, Hudon HE, Gramlich JL, Neuberg DS, Podar K, et al. Activated Jak2 with the V617F point mutation promotes G1/S phase transition. J Biol Chem. 2006;281:18177–18183. doi: 10.1074/jbc.M600064200. [DOI] [PubMed] [Google Scholar]
- 36.Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci USA. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falkenhahn M, Gohlke P, Paul M, Stoll M, Unger T. The renin-angiotensin system in the heart and vascular wall: new therapeutic aspects. J Cardiovasc Pharmacol. 1994;24(Suppl. 2):S6–S13. [PubMed] [Google Scholar]
- 38.Tham DM, Martin-McNulty B, Wang YX, Da Cunha V, Wilson DW, Athanassious CN, et al. Angiotensin II injures the arterial wall causing increased aortic stiffening in apolipoprotein E-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1442–R1449. doi: 10.1152/ajpregu.00295.2002. [DOI] [PubMed] [Google Scholar]
- 39.Muthalif MM, Parmentier JH, Benter IF, Karzoun N, Ahmed A, Khandekar Z, et al. Ras/mitogen-activated protein kinase mediates norepinephrine-induced phospholipase D activation in rabbit aortic smooth muscle cells by a phosphorylation-dependent mechanism. J Pharmacol Exp Ther. 2000;293:268–274. [PubMed] [Google Scholar]
- 40.Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, et al. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- 41.Begum N, Duddy N, Sandu O, Reinzie J, Ragolia L. Regulation of myosin-bound protein phosphatase by insulin in vascular smooth muscle cells: evaluation of the role of Rho kinase and phosphatidylinositol-3-kinase-dependent signaling pathways. Mol Endocrinol. 2000;14:1365–1376. doi: 10.1210/mend.14.9.0522. [DOI] [PubMed] [Google Scholar]
- 42.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–H1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 43.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boos CJ, Dawes M. ACE cardiovascular protection: EUROPA versus HOPE. Cardiovasc Drugs Ther. 2004;18:179–180. doi: 10.1023/b:card.0000033657.01493.6e. [DOI] [PubMed] [Google Scholar]
- 45.Huang F, Thompson JC, Wilson PG, Aung HH, Rutledge JC, Tannock LR. Angiotensin II increases vascular proteoglycan content preceding and contributing to atherosclerosis development. J Lipid Res. 2008;49:521–530. doi: 10.1194/jlr.M700329-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Henriques TA, Huang J, D'Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology. 2004;145:3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 47.Cassis LA, Marshall DE, Fettinger MJ, Rosenbluth B, Lodder RA. Mechanisms contributing to angiotensin II regulation of body weight. Am J Physiol. 1998;274:E867–E876. doi: 10.1152/ajpendo.1998.274.5.E867. [DOI] [PubMed] [Google Scholar]
- 48.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skinner S, Bouhnik J, Huang H, Gonzalez MF, Ménard J, Corvol P. Plasma angiotensin in binephrectomised mice. Clin Exp Hypertens. 1995;17:847–860. doi: 10.3109/10641969509033639. [DOI] [PubMed] [Google Scholar]
- 50.Shaw S, Wang X, Redd H, Alexander GD, Isales CM, Marrero MB. High glucose augments the angiotensin II-induced activation of JAK2 in vascular smooth muscle cells via the polyol pathway. J Biol Chem. 2003;278:30634–30641. doi: 10.1074/jbc.M305008200. [DOI] [PubMed] [Google Scholar]
- 51.Delhommeau F, Pisani DF, James C, Casadevall N, Constantinescu S, Vainchenker W. Oncogenic mechanisms in myeloproliferative disorders. Cell Mol Life Sci. 2006;63:2939–2953. doi: 10.1007/s00018-006-6272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verstovsek S. Therapeutic potential of JAK2 inhibitors. Hematology Am Soc Hematol Educ Program. 2009;1:636–642. doi: 10.1182/asheducation-2009.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.