Abstract
Antibiotics used by general practitioners frequently appear in adverse-event reports of drug-induced hepatotoxicity. Most cases are idiosyncratic (the adverse reaction cannot be predicted from the drug's pharmacological profile or from pre-clinical toxicology tests) and occur via an immunological reaction or in response to the presence of hepatotoxic metabolites. With the exception of trovafloxacin and telithromycin (now severely restricted), hepatotoxicity crude incidence remains globally low but variable. Thus, amoxicillin/clavulanate and co-trimoxazole, as well as flucloxacillin, cause hepatotoxic reactions at rates that make them visible in general practice (cases are often isolated, may have a delayed onset, sometimes appear only after cessation of therapy and can produce an array of hepatic lesions that mirror hepatobiliary disease, making causality often difficult to establish). Conversely, hepatotoxic reactions related to macrolides, tetracyclines and fluoroquinolones (in that order, from high to low) are much rarer, and are identifiable only through large-scale studies or worldwide pharmacovigilance reporting. For antibiotics specifically used for tuberculosis, adverse effects range from asymptomatic increases in liver enzymes to acute hepatitis and fulminant hepatic failure. Yet, it is difficult to single out individual drugs, as treatment always entails associations. Patients at risk are mainly those with previous experience of hepatotoxic reaction to antibiotics, the aged or those with impaired hepatic function in the absence of close monitoring, making it important to carefully balance potential risks with expected benefits in primary care. Pharmacogenetic testing using the new genome-wide association studies approach holds promise for better understanding the mechanism(s) underlying hepatotoxicity.
Keywords: idiosyncratic, adverse event, clavulanic acid, co-trimoxazole, flucloxacillin
Introduction
Antibiotics are considered as a common cause of drug-induced liver injury (DILI).1–3 Although the frequency of serious antibiotic-induced hepatotoxicity is low compared with the amounts prescribed each year—population-based estimates suggest that it occurs in <5 per 100 000 population4—it remains a main reason for antibiotic withdrawal after product launch. Antibiotic-induced hepatotoxicity is usually asymptomatic, transient and associated with only mild hepatic impairment.5 In rare cases, however, significant morbidity,6,7 the need for liver transplantation7,8 and death from acute liver failure7–9 have been reported. In recent years, the European Medicines Agency (EMA) and the US FDA have addressed these issues by putting emphasis on both pre-clinical (to detect signals) and clinical studies.10 Nonetheless, predicting what hepatotoxicity will be after approval based on data assembled during drug development remains a risky exercise.
Public awareness of antibiotic-induced hepatotoxicity has, however, increased over recent years (following actions of regulatory bodies targeting specific antibiotics),11,12 making it essential for the primary care physician to better identify and minimize the risk of serious liver damage with existing agents.2,5 In the present review, we describe the adverse hepatic effects of antibiotics, including their frequency, severity and clinico-pathological features, and discuss these observations within the context of the primary care setting. Indeed, this is not only where antibiotic consumption is greatest13–15 but also where risks are highest, given the inherent difficulties of rapid access to in-depth biological investigations for prompt diagnosis. As a source of information, we first searched electronically the online version of the US National Library of Medicine (Bethesda, MD, USA; http://www.pubmed.com) for original research papers, case reports and reviews published until the end of February 2011. For antibiotics used primarily for non-tuberculosis indications, the search terms were ‘hepatic adverse event OR hepatotoxicity AND <each of the following terms>’: ‘beta-lactam*’; ‘macrolid*’; ‘(sulfonamide OR sulphonamide)’; ‘tetracyclin*’; ‘fluoroquinolon*’; ‘amoxycillin*’; ‘clavulanic'; ‘telithromycin'; ‘clarithromycin'; ‘azithromycin'; ‘erythromycin'; ‘levofloxacin'; ‘moxifloxacin'; ‘gatifloxacin'; and ‘trovafloxacin', with restriction to papers dealing with antibiotic-induced hepatotoxicity. For hepatotoxicity related to antibiotics used for the treatment of tuberculosis, the search terms were ‘hepatotoxicity AND <each of the following terms>': ‘anti-tuberculosis agents'; ‘ethambutol'; ‘isoniazid'; ‘pyrazinamide'; ‘(rifampicin OR rifampin)’; and ‘streptomycin’, with focus on papers published within the last 10 years. Reference lists from review papers were examined for additional relevant articles. Current prescribing information and regulatory documents from both the FDA and the EMA were also systematically retrieved and examined.
Identifying antibiotic-induced liver injury
Signs and symptoms of hepatotoxic reactions
Most cases of antibiotic-induced hepatotoxicity are idiosyncratic (the adverse reaction occurs in a very small proportion of patients, cannot be predicted either from the drug's pharmacology or from pre-clinical toxicology tests and is host dependent).2,4,16 It is thought to occur either via an immunological reaction, including concomitant liver inflammation associated with viral or bacterial infection of liver or inflammatory disease,17 in response to hepatotoxic metabolites,18 or, as more recently suggested, when the drug synergizes with lipopolysaccharide-induced inflammatory cytokine signalling to cause acute hepatocyte death.17,19 Symptoms are similar to those of other liver diseases, and include jaundice, malaise, abdominal pain, unexplained nausea and anorexia.2 Because antibiotic-induced hepatotoxicity mimics other liver diseases, diagnosis is necessarily one of elimination and is usually based on a high degree of clinical suspicion following exclusion of competing aetiologies, such as viral hepatitis or biliary disease.18 Clues suggestive of drug allergy include rash, fever or eosinophilia, duration of exposure of 1–5 weeks and an often rapid response following re-administration of the antibiotic.2,18,20
Risk factors and early detection
In cases of suspected liver reactions, it is essential to obtain a detailed drug history that includes awareness of the drug's hepatotoxic potential, the timing of drug administration in relation to the emergence of symptoms, previous administration of the antibiotic in question and concomitant drug use (including alternative or herbal medications).18,20,21 Antibiotic-induced hepatotoxicity can often be detected early from elevations in serum alanine aminotransferase (ALT) levels,18 where these exceed twice the upper limit of normal (ULN).20,22–24 Clinically significant rises in ALT accompanied by jaundice (bilirubin level ≥2 × ULN) suggest a worse prognosis compared with elevated ALT alone,9 with the combination of hepatocellular injury (ALT >3 × ULN) and jaundice (bilirubin >2 × ULN) being associated with ∼10% mortality (Hy's Law).7,25 Because of the short-term nature of protocols in clinical trials of antibiotics, these changes may remain unseen for a drug later proven to be hepatotoxic.26
Several factors, however, complicate the picture. First, host factors such as age, pre-existing liver disease, concurrent medications and excessive alcohol consumption may all increase susceptibility to drug-induced hepatotoxicity,20 although the nature of their interaction with antibiotics to increase the risk of hepatotoxicity is uncertain. General practitioners should be aware that while age is often considered a risk factor, children and adolescents may also be affected by the same drugs as adults.27 Predisposing factors, including the concomitant use of acetaminophen (paracetamol), may affect biochemical tests, while infection, especially sepsis, may cause liver toxicity usually in the form of cholestasis. Second, hepatotoxicity seems often related to the administration of large doses of any drug, with 77% of DILI cases included in the Swedish registry occurring with drugs administered at dosages of >50 mg/day.28 In the context of antibiotics, this means that the large daily dosages that may be essential today to keep pace with the decreased susceptibility of the main target organisms (e.g. Streptococcus pneumoniae versus amoxicillin) will increase the risk in a non-specific fashion, dependent on the local epidemiology and guidelines. Third, initial liver injury, as detected by an increase in transaminases, may be transient despite continued treatment, unless the patient has additional factors that will cause mild toxicity to turn into severe hepatic dysfunction.29 Fourth, cases of frank liver failures caused by antibiotics, excluding telithromycin and trovafloxacin (see hereunder), remain very rare and their number appears to be balanced with the large use of these drugs on the one hand, and the rate of idiopathic liver failure seen in normal adults (∼1 case for 1 million adults/year) on the other hand.19
Lastly, the retrospective analysis of DILI cases and drug-causality assessment remains a perilous exercise, because of the inherently subjective nature of this approach and potential observer biases. While instruments have been devised for a more objective approach (the most familiar and comprehensive being the Roussel-Uclaf Causality Assessment Method),22,23 the results obtained may often fit rather poorly with those of the evaluations made by the regulatory authorities,30 creating some confusion among professionals as well as the public. In this context, rechallenge, often considered as a ‘gold standard’ to ascertain causality,31 seems to us impractical and probably unethical with outpatients, not only because of its inherent dangers, but also because the original infection itself (which cannot be recreated) may be an important component in the onset of the toxic reaction.
Timing and treatment of antibiotic-induced liver injury
The interval between drug administration and the onset of hepatic dysfunction is variable: adverse effects may develop almost immediately, late in the course of prolonged antibiotic treatment or several months after the cessation of therapy.5,32 This is frequently observed with amoxicillin/clavulanate and tetracycline,32 and was reported for trovafloxacin,33 although rapid onset is also seen.33,34 Cholelithiasis associated with ceftriaxone–calcium precipitates typically occurs only after 9–11 days of treatment.32 Physicians should therefore alert patients to the warning signs of antibiotic-induced hepatotoxicity and advise them to seek medical attention, even if symptoms appear sometime after completing their treatment.
For all cases of immediate or rapid onset, prompt withdrawal of the suspected antibiotic is indicated.4,5,35 Most patients will then make a full recovery, but chronic liver injury is not infrequent.32,35 Thus, antibiotics were the third most common cause of chronic liver damage in a large, long-term, follow-up registry set up in Spain.35
Frequency and characteristics of antibiotic-induced hepatotoxicity
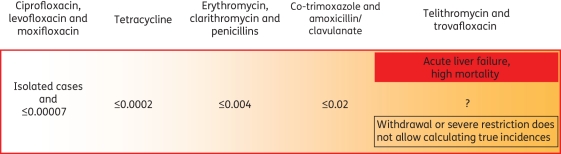
Clinical studies are generally underpowered to identify trends in hepatotoxicity, while studies undertaken to assess hepatic signals are often complicated by numerous variables. With few prospective population-based studies, case histories and registries of spontaneous reports of adverse drug reactions are actually the main source of toxicity data.36 As shown in Figure 1, the incidence and inherent risk of hepatotoxicity varies widely between antibiotics. Table 1 summarizes the classes of antibiotics implicated in hepatotoxic reactions. The clinico-pathological picture can include acute hepatocellular, cholestatic and mixed hepatocellular-cholestatic injury, as well as chronic hepatitis, chronic cholestatic injury, granulomas, and, in the case of intravenous tetracycline, steatosis and steatohepatitis.4
Figure 1.
Hepatotoxicity risk of antibiotics (percentage of prescriptions for antibiotics with main indications for use in the community setting). Derived from references 32, 37, 40, 42, 44, 73, 89, 96 and 108. Excluding antibiotics used mainly for the treatment of tuberculosis.
Table 1.
Frequency and characteristics of hepatotoxicity induced by antibiotics with indications for infections commonly handled initially by general practitioners and/or used for the treatment of tuberculosis
| Antibiotic | Incidence | Liver injury | Onset |
Time to recovery | Risk factors | Comments | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Antibiotics mainly used for non-tuberculosis indications | |||||||||
| β-Lactams | |||||||||
| penicillins | range from 1 per 2 million to 3 per 100 000 prescriptions | primarily hepatocellular | severe and fatal reactions extremely rare but have been reported | 5,37,40,42 | |||||
| oxypenicillins | 1.8 per 100 000 prescriptions | primarily cholestatic hepatitis | both early (1–9 weeks) after the start of treatment but also delayed following treatment cessation | within 12 weeks of cessation of therapy but up to 30% patients have protracted course | older age (>55 years), female sex, longer duration of treatment | flucloxacillin most hepatotoxic | 5,32,43,51,53 | ||
| amoxicillin/clavulanate | 1–17 per 100 000 prescriptions | hepatocellular, cholestatic or mixed hepatocellular-cholestatic hepatitis | within 4 weeks of the start of therapy but typically after drug discontinuation | within 16 weeks of cessation of therapy but some patients have protracted course | older age (>65 years), female sex, prolonged and repeated courses | hepatotoxicity associated with clavulanic acid moiety; although usually benign, fatalities have been reported | 32,40,42,43,56,59,61 | ||
| cephalosporins (ceftriaxone) | up to 25% of adult patients and ∼40% of paediatric patients | cholelithiasis from ceftriaxone–calcium precipitate (so-called biliary sludge) | after 9–11 days of treatment | within 2–3 weeks of treatment cessation | paediatric patients, prolonged treatment | may mirror acute cholecystitis | 32,210 | ||
| Macrolides/ketolides | |||||||||
| erythromycin | <4 cases per 100 000 prescriptions | cholestatic pattern of injury with evidence of portal and bullous inflammation, eosinophilia and mild hepatocellular necrosis | within 10–20 days of treatment | within 8 weeks of treatment cessation but some patients have protracted course | clarithromycin has similar profile and incidence; prognosis generally good with fatalities extremely rare | 32,43,72,73,210 | |||
| telithromycin | unknown | hepatocellular and canalicular bile cholestasis | within a few days of the start of treatment (median 10 days) | significant numbers of affected patients develop severe liver injury, some of which prove fatal | male sex | no published incidence data but risk of hepatotoxicity estimated 82% greater than with other drugs | 88,89,95 | ||
| Fluoroquinolones | |||||||||
| ciprofloxacin | isolated cases | hepatocellular and cholestatic hepatitis | serious cases including fatalities reported, though very rare | 100,102,103,211 | |||||
| levofloxacin | <1 case per 5 million prescriptions | hepatocellular and cholestatic hepatitis | serious cases including fatalities reported, though very rare | 107–110 | |||||
| moxifloxacin | isolated cases | hepatocellular and cholestatic hepatitis | within 3–10 days of starting treatment, although delayed onset up to 30 days after cessation of therapy has also been observed | serious cases including fatalities reported, though very rare | 122 | ||||
| trovafloxacin | hepatic necrosis leading to liver failure | variable but rapid onset within 2 days of the start of treatment seen in some patients | re-exposure | restricted use or market withdrawal due to serious hepatotoxicity | 32,33,128,129 | ||||
| Sulphonamides | |||||||||
| sulfasalazine | 1 per 1000 prescriptions | cholestatic or mixed hepatocellular-cholestatic injury | within the first 4 weeks of treatment | within a few weeks of treatment discontinuation | severe cases of hepatic injury described including fulminant liver failure | 4,43 | |||
| trimethoprim/sulfamethoxazole | <2 per 10 000 prescriptions | cholestatic or mixed hepatocellular-cholestatic injury | within a few weeks of treatment discontinuation | female sex, HIV positive, older age (≥75 years) | sulphonamide component responsible for most side effects | 32,135 | |||
| sulfadimethoxine | necrosis and granulomatous hepatitis | rapid after administration of 2 g | 138 | ||||||
| sulfadoxine + pyrimethamine | granulamatous hepatitis | female sex, older age (>65 years) | sulphonamide component of sulfadoxine + pyrimethamine treatment responsible for most side effects | 139 | |||||
| Tetracyclines | |||||||||
| tetracycline | 1 per 18 million daily doses | microvesicular steatosis (acute fatty liver); cholestatic, ductopenia | long latency period | female sex, pregnancy, large (≥1.5 g daily) intravenous dose, renal disease | 32,143,144 | ||||
| doxycycline | lower than tetracycline | cholestatic liver injury | long latency period | variable with most | re-exposure leads | risk lower than with tetracycline | 145 | ||
| minocycline | microvesicular steatosis (acute fatty liver), autoimmune hepatitis | of over a year | patients recovering on cessation of therapy but serious and occasionally fatal cases reported | to recurrence | 32,146,147 | ||||
| Antibiotics mainly used for tuberculosis | |||||||||
| Ethambutol | isolated cases but increases in combination with isoniazid (6%), rifampicin (30%) and pyrazinamide (50%) | cholestatic hepatitis | within 3–16 weeks of treatment | within a few weeks of treatment cessation | older age (>60 years), female sex, malnutrition | rarely used alone, with most hepatotoxicity attributed to concomitantly administered drugs | 154,182,184 | ||
| Isoniazid | 1%–10% of patients | hepatocellular necrosis | within a few days of treatment | may resolve with continued treatment | older age (>60 years), female sex, malnutrition, slow acetylator status | hepatotoxicity is exacerbated with concomitant rifampicin administration | 178,181,191 | ||
| Pyrazinamide | 6%–20% of patients | centrolobular cirrhosis and cholestasis | within first 5 weeks but may be delayed (>30 days) | gradual decline in serum transaminase levels after prompt cessation of treatment | older age (>60 years), female sex, malnutrition, chronic viral hepatitis | data inconsistent on dose-related hepatotoxicity | 179,181,193–195 | ||
| Rifampicin | <2% of patients | cholestatic hepatitis | within 3–12 weeks | gradual decline in serum transaminase levels after prompt cessation of treatment | rarely used alone, therefore as for other agents | potential to exacerbate hepatotoxicity of co-administered agents | 199,200,203 | ||
| Streptomycin | no hepatotoxic potential | liver disease is a risk factor for nephrotoxicity | 212 | ||||||
In the next sections, we will review what is known about the hepatotoxicity of the main antimicrobials in current clinical use. At the end of each subsection, we will also briefly comment on those antibiotics that were withdrawn because of major hepatotoxic reactions. This may be instructive for a correct assessment of the risks associated with the other molecules in each pharmacological class, and indicate what should be looked for when dealing with drugs that are newly introduced and are therefore less thoroughly investigated.
β-Lactams
Penicillins
Frequency
Liver injury is extremely rare with ampicillin, and rare with benzylpenicillin (penicillin G) and phenoxymethylpenicillin (penicillin V). Amoxicillin has little hepatotoxic potential if administered alone (see hereunder for amoxicillin/clavulanate). Transient increases in ALT have been reported with oxacillin, carbenicillin and ticarcillin.37 Isolated cases of Stevens–Johnson syndrome and chronic cholestasis38 as well as recurrent cholestasis after repeated treatment39 have been described, but these were reversible. In the UK, reported rates of hepatic reactions to amoxicillin vary from 0.1–0.25 to 3 per 100 000 prescriptions.40 Severe reactions include cholestasis41 and acute liver failure, but cases are rare, and deaths due to acute liver failure have not been reported.42 The crude incidence of flucloxacillin-associated acute liver injury has been estimated as 1.8 per 100 000 prescriptions, or 2.6 per 100 000 users,43 and that of flucloxacillin-associated jaundice as 3.6 per 100 000 prescriptions.44
Pathology
Hepatotoxicity associated with penicillins is predominantly hepatocellular,37 although cases of cholestasis with ductopenia have been described.38,39,41 It may frequently be confused with viral hepatitis.45 Phenoxymethylpenicillin has been associated with increased serum alkaline phosphatase activity and mild intrahepatic cholestasis,46 as well as acute hepatitis with elevated ALT.47 Severe and prolonged cholestasis has been reported with benzylpenicillin,48 including following treatment with cloxacillin,49 and with ampicillin in combination with the β-lactamase inhibitor sulbactam, from which recovery took several months.50 Cholestatic hepatitis is more characteristic of the semi-synthetic penicillinase-resistant oxypenicillins oxacillin, cloxacillin, dicloxacillin and, most notably, flucloxacillin.4,5 Case reports of severe and prolonged cholestasis with ductopenia41,51,52 are supported by epidemiological data.40
Risk factors
Older patients and those receiving >2 weeks of treatment appear at significantly increased risk of flucloxacillin-associated jaundice.53 There is also recent evidence to suggest a genetic predisposition to flucloxacillin-associated hepatotoxicity. A genome-wide association study using 866 399 markers in 51 cases of flucloxacillin DILI and 282 controls, which were matched for sex and ancestry, showed an association peak in the major histocompatibility complex (MHC) regions that are part of an extended MHC 57.1 haplotype present in <4% of Europeans.54
Amoxicillin/clavulanate
Frequency
Primary care physicians must be aware that the combination of the β-lactamase inhibitor clavulanic acid with amoxicillin markedly increases the risk of hepatotoxicity.43,55 Thus, amoxicillin/clavulanate is responsible for 13%–23% of drug-induced hepatotoxicity cases7,56,57 and is the leading cause of hospitalization for adverse hepatic events.7 Because symptom onset is usually delayed,58 early diagnosis is difficult.
Hepatotoxicity is clearly linked to the clavulanic acid moiety, with a 5- to 9-fold increase for the combination versus amoxicillin alone.40,59,60 While amoxicillin alone only slightly increases the risk of DILI compared with non-use (adjusted odds ratio, 1.7), the amoxicillin/clavulanate adjusted odds ratio versus non-use reaches 31.9 with recent use and 94.8 with current use.43 A recent retrospective case analysis of 800 patients with drug-induced jaundice suggested that amoxicillin/clavulanate was responsible for 32% of cases, giving an estimated incidence rate of 9.91 cases of jaundice per 100 000 prescriptions.44
Hepatotoxicity associated with amoxicillin/clavulanate usually follows a benign course, with symptoms resolving over several weeks. Some patients, however, have a protracted course that can lead to acute liver failure and death.42 A prospective registry study reported an unfavourable outcome (persistence of liver injury, liver transplantation or death) in 7% of the 69 patients who suffered amoxicillin/clavulanate hepatotoxicity.56
Pathology
Hepatotoxicity associated with amoxicillin/clavulanate is usually characterized by delayed cholestatic or mixed hepatocellular-cholestatic injury.40,59,61 This ‘hepatotoxic signature’ has, however, been challenged with evidence to suggest that while common in older patients, younger patients are more likely to develop hepatocellular injury than cholestatic or mixed injury.56
Risk factors
Prolonged or repeated courses or being >65 years of age increases the risk of developing hepatotoxicity,40,56 and the presence of both can increase the risk of acute liver injury to 1 per 1000 users,40 an incidence that can be detected by primary care physicians in everyday practice. Female sex and age >65 years predispose individuals to amoxicillin/clavulanate-associated jaundice.44 A significant association between the DRB1*1501-DRB5*0101-DQB1*0602 haplotype and cholestatic hepatitis related to amoxicillin/clavulanate was reported in a pioneering Belgian study,62 and was recently confirmed by the DILIGEN group from the UK.63 A wide genome analysis in this population is underway.
Cephalosporins
Cephalosporins are only rarely implicated in hepatotoxic reactions.64–67 Cholestasis featured in all documented cases of cephalosporin-associated hepatotoxicity, with symptoms manifesting within a few days of treatment. With the exception of ceftriaxone, for which a specific mechanism has been identified, hepatotoxicity appears to be immunologically mediated.68 A recent report of cefdinir-induced hepatotoxicity describes jaundice, mild cholestasis with portal and lobular mixed inflammation, and focal bile duct injury.69
Macrolides (including azalides and ketolides)
Erythromycin
Frequency
Erythromycin-related hepatotoxicity, documented >40 years ago, occurs with all ester derivatives.4,5,70 According to Westphal et al.,45 its incidence should be considered as high; elevation of serum aminotransferases is seen in up to 15% of cases and overt hepatitis in ∼2% of patients if treated for >2 weeks.71,72 More recent reports, however, suggest that it remains much lower73 and similar to that of penicillin V.74 A retrospective cohort study using the UK General Practice Research Database (GPRD) estimated the risk of cholestatic hepatitis as 3.6 cases per 100 000 users.73 Hospitalization for symptomatic acute hepatitis is rare (a US study reported 2.28 cases for every 1 million patients receiving a 10 day course of erythromycin).75 To our knowledge, there has been only one documented fatal outcome with erythromycin, which occurred in an elderly male patient treated with intravenous erythromycin lactobionate.76
Pathology
Erythromycin-induced hepatotoxicity probably arises from a combination of intrinsic hepatotoxic effects and the hypersensitivity reactions that characterize most cases of liver injury. A cholestatic pattern of injury is typical, but most cases also have evidence of hepatocellular injury.72 Prognosis is generally good, with symptoms and biochemical abnormalities resolving within 2–5 weeks of treatment discontinuation; very occasionally, cholestasis persists for 3–6 months.4 Actually, all 32 patients with erythromycin-associated hepatocellular injury in the Swedish registry survived, compared with the 40% mortality in halothane- and naproxen-induced hepatocellular damage.25
Clarithromycin
The hepatotoxic profile of clarithromycin appears similar to that of erythromycin.43 The first report of cholestatic hepatitis77 was followed by a brief report of reversible cholestatic hepatitis in 5 of 14 elderly patients taking high-dose clarithromycin.78 Two cases of fulminant hepatic failure have been associated with clarithromycin, one of which required liver transplantation,79 while the second, a fatal case, was attributed to an interaction between clarithromycin and concomitant disulfiram.80 However, infrequent severe hepatic dysfunction and liver failure resulting in death appear in the US labelling for clarithromycin.81
Azithromycin
Adverse hepatic events associated with azithromycin (an azalide closely related to the macrolides but with a considerably longer half-life and extended tissue accumulation) are rare. Reversible azithromycin-related intrahepatic cholestasis has recently been reported in isolated cases,82–85 while association between azithromycin and asymptomatic elevated liver enzymes in treated children is well documented.86
Telithromycin
Telithromycin is the first, and so far the only, ketolide approved for clinical use. It was primarily designed for activity against macrolide-resistant S. pneumoniae87 and, in this context, should have perhaps been restricted to situations where this would have conferred a decisive advantage, i.e. for severe infections in regions where resistance to macrolides is high. In Phase III trials, mild-to-moderate increases in ALT were observed at a higher frequency than in the placebo arm. Subsequent to commercial launch in the USA, three cases of serious liver injury with jaundice and significantly elevated liver enzymes (>10 × ULN in one case) occurred in patients having received telithromycin for minor upper respiratory tract infections. One patient spontaneously recovered, while the second required liver transplantation and the third died.88 An initial analysis of 12 cases provided evidence for a rare, unusual form of hepatotoxicity characterized by short latency, systemic symptoms and, in some cases, significant ascites.89 The FDA then issued a warning about the potential for serious liver toxicity, a drastically restricted indication (sole use in community-acquired pneumonia)11 and a boxed warning in the US product labelling.90 This decision has been reinforced by additional case reports to the FDA's Medwatch.21,96 In parallel, the EMA introduced ‘alterations in hepatic enzymes, cases of severe hepatitis, and liver failure’ in the ‘Special warnings’ section of the ‘Summary of Product Characteristics’ (SPC), but maintained indications for both community-acquired pneumonia and acute exacerbation of chronic bronchitis (for the latter, however, only if the infection is caused by known or suspected β-lactam- and/or macrolide-resistant strains).91–93
Frequency
The warnings discussed above have resulted in a marked reduction in the prescription of telithromycin, making epidemiological studies difficult. Analysis of the FDA post-marketing database indicates a reporting rate of 167 cases of acute liver failure per 1 million person-years of telithromycin use (versus an expected value of 1 case per 1 million person-years).94 The manufacturer reports an incidence of 7 cases of telithromycin-induced hepatitis per 10 000 people treated.90 The FDA Adverse Event Reporting System shows that the risk of hepatotoxicity is 82% greater with telithromycin than with other agents.95 The EMA mentions that an increase in liver enzymes is common, hepatitis uncommon and cholestatic jaundice rare, while the frequency of severe hepatitis and liver failure cannot be estimated from the available data91 (EMA categorizes adverse effects as common, uncommon, rare and very rare based on frequencies of ≥1/100 to <1/10, ≥1/1000 to <1/100, ≥1/10 000 to <1/1000 and <1/10 000 of treated patients, respectively).
Pathology
Telithromycin-related liver injury is characterized by rapid onset of symptoms (consistent with rapid-onset hypersensitivity), including jaundice, fever, abdominal pain and, on occasion, ascites.21,96,97 Of 42 cases of telithromycin-induced hepatotoxicity recently described, 25 developed jaundice and met criteria for Hy's Law, 32 were hospitalized, 14 had severe liver injury (grade 4 and 5), 1 required transplantation, and 4 died.96 Re-exposure to telithromycin caused recurrent acute hepatitis in a patient who had experienced adverse hepatic effects with a previous course.97
Fluoroquinolones
Fluoroquinolones achieve high concentrations in bile, which makes them well suited to the treatment of bacterial cholecystitis and cholangitis. Modest increases in serum ALT levels can be considered as a fluoroquinolone class effect. More severe liver injury remains rare (but see below for information on trovafloxacin). In fact, there have been only few reports of significant hepatotoxicity, even when used in patients with advanced liver disease.5,98 Fluoroquinolones, such as moxifloxacin and levofloxacin, are used off-label as alternative drugs in antituberculosis regimens, but neither drug appears to cause additional hepatotoxicity in this patient population.99
Ciprofloxacin
The literature remains scanty over the potential hepatotoxicity of ciprofloxacin, and the incidence of ciprofloxacin-induced hepatotoxicity is considered very low,4,5 although two fatal cases of acute liver failure in elderly men have been documented.100,101 Cases of hepatocellular injury (n = 51) and cholestatic hepatitis, with or without ductopenia,102,103 have been described following treatment with ciprofloxacin. A recent report also highlights a case of simultaneous acute cholestatic liver injury and renal failure, which resolved within 5 months of discontinuing treatment.104 The recently harmonized European SPC of ciprofloxacin105 mentions only cases of severe hepatotoxicity in the ‘Special warnings’ section, which needs to be seen in the context of a very large use of this drug worldwide. The SPC further mentions that an increase in transaminases and bilirubin is uncommon, hepatic impairment, cholestatic icterus and hepatitis rare, and liver necrosis (very rarely progressing to life-threatening hepatic failure) very rare, based on both oral and intravenous administration of ciprofloxacin.
Levofloxacin
The incidence of adverse hepatic effects associated with levofloxacin, an extended-spectrum fluoroquinolone, is also low. Abnormal liver function occurred in <1% of patients in clinical trials,106 while post-marketing surveillance revealed rates of severe liver injuries (hepatitis, necrosis and hepatic failure) of <1 case per 5 million prescriptions.107 Nonetheless, cases of levofloxacin-related liver injury have been reported, including cases of hepatic failure.108–113 The US prescribing information mentions that severe, and sometimes fatal, hepatotoxicity not associated with hypersensitivity has been reported.114
Moxifloxacin
Frequency
Reviews of clinical trials and post-marketing surveillance studies indicate minimal incidence of hepatic injury, though liver function tests may be abnormal in ∼1%–5% of patients.115,116 While no index case of moxifloxacin-related liver injury has emerged in either clinical trials or post-marketing surveillance, and published data have remained scanty for many years,117,118 there have been spontaneous reports of hepatocellular injury. These include eight deaths due to hepatic failure where a link to moxifloxacin could not be excluded119 and a more recent case where it was considered to be idiosyncratic.120 This has been introduced as an amendment by the EMA in the European SPC,121 but such fatal cases should be seen in the context of 85 million treatments with moxifloxacin worldwide at that time.122 The current, revised SPC mentions elevation of transaminases as being common, hepatic alteration uncommon, and icterus and hepatitis rare. Moxifloxacin is not included in the list of drugs identified in at least five adjudicated cases of DILI.123 Moxifloxacin, as for levofloxacin, was also found to cause no additional hepatotoxicity when used by patients with hepatitis induced by first-line antituberculosis drugs.99
Pathology
Liver injuries induced by moxifloxacin are typically cholestatic or mixed hepatocellular-cholestatic rather than hepatocellular. Symptoms are usually evident within 3–10 days of starting moxifloxacin, although delayed onset of up to 30 days after cessation of therapy has also been observed. A case of moxifloxacin-associated drug hypersensitivity syndrome with toxic epidermal necrolysis and fulminant fatal hepatic failure has been described.124
Gemifloxacin, gatifloxacin and trovafloxacin
Gemifloxacin is approved in the USA and a few other countries, but not in Europe. To date, there has been no report of hepatocellular injury with gemifloxacin, but this may reflect its very limited patient exposure.125 Gatifloxacin was approved in the USA and other countries, but not in Europe. It has been recently withdrawn, mainly for adverse glycaemic effects. Gatifloxacin, however, was also implicated in several cases of hepatotoxicity, with 27 occurrences reported to the FDA (cited in 11 cases as the most probable cause of death).126 Trovafloxacin was approved in the USA in 1998, but post-marketing surveillance quickly revealed significant, serious hepatotoxicity,127 with clear signs of eosinophilic infiltration, hepatocellular vacuolar degeneration and necrosis.128 Thus, within 2 years (with only 2.5 million patient exposures), 140 cases of serious hepatic events, including 14 cases of acute liver failure, 4 cases requiring liver transplantation and 5 deaths, were reported.127,129 The drug was, therefore, restricted to serious and life- or limb-threatening infections.127,129,130 Although approved in Europe, the drug was quickly withdrawn by its registration holder before its actual commercial launch. The greater hepatotoxic potential of trovafloxacin may be due to its difluorophenyl side chain (shared with temafloxacin, also withdrawn for adverse hepatic effects33), but the cyclopropylamine moiety (specific to trovafloxacin) could also play an important role.131 Trovafloxacin was also shown to sensitize hepatocytes to TNF-α-induced death,132 an effect not seen with levofloxacin in comparative studies.133 These clear structural relationships imply that the hepatotoxicity of trovafloxacin is unlikely to be the extreme of a fluoroquinolone class effect.
Sulphonamides
The hepatotoxicity of the sulphonamides, the oldest class of antibiotics, is well documented.134 This class includes sulfamethoxazole, sulfasalazine, sulfamethizole and sulfamethoxypyridazine, as well as the combinations trimethoprim/sulfamethoxazole and pyrimethamine/sulfadoxine.
Frequency
Sulfasalazine emerged as one of the most hepatotoxic drugs in a recent population-based case–control study using the UK-based GPRD, with an incidence approaching 1 per 1000 users,43 i.e. similar to amoxicillin/clavulanate. Sulfasalazine hepatotoxicity is frequent enough to be detected at the primary care level. Most cases of sulphonamide-induced hepatotoxicity are mild and patients usually recover within a few weeks of treatment discontinuation,2 but severe cases, including fulminant liver failure, have been described for the combination trimethoprim/sulfamethoxazole.135,136
Pathology
Sulphonamides most often induce cholestatic or mixed hepatocellular-cholestatic injury,2,137 although cases of necrosis and granulomatous hepatitis have also been described.135,138,139 The sulphonamide component is thought to be responsible for most hepatic side effects with trimethoprim/sulfamethoxazole. The underlying mechanism, explaining the idiosyncratic character of the toxicity, could be its slow acetylation in some patients, preventing its normal elimination and allowing it to enter into other catabolic pathways related to toxicity.139a A case of jaundice and acute liver failure occurred in a patient receiving trimethoprim/sulfamethoxazole for a urinary tract infection; however, liver function returned to normal over a 2 month period, without intervention.140
Tetracyclines
Frequency
Tetracycline hepatotoxicity, first described >50 years ago,141 seems related to the use of large doses142 and, contrary to most other antibiotics, is predictable and reproducible in animal models.45 With normal, low, oral doses, tetracyclines only rarely cause liver injury (∼1 case per 18 million daily doses according to early estimates,143 and 3.7 cases per 100 000 users or 1.5 cases per 100 000 prescriptions in more recent surveys), representing one of the lowest rates for which a significant association of liver injury and antibiotic use has been established.43 Early reports (including one death) of an association between tetracycline hepatotoxicity and pregnancy all related to high-dose intravenous treatment.144 A later survey suggested that female sex was not a risk factor with low-dose oral tetracycline therapy and, importantly, did not increase the risk of death due to hepatic side effects.143
Doxycycline is less hepatotoxic than tetracycline, with no increased risk compared with controls in a retrospective study (current use odds ratio, 1.49; 95% confidence interval, 0.61–3.62).145 A recent fatal case has been described,113 but after exposure to levofloxacin and in association with naproxen. Rarely, minocycline-associated hepatotoxicity has necessitated liver transplantation or proved fatal.5,146 No public report of tigecycline-related DILI has been identified to date.
Pathology
Microvesicular steatosis was the characteristic feature of treatment with intravenous or large oral doses of tetracycline,142 whereas cholestasis was the predominant clinico-pathological pattern with oxytetracycline and minocycline.43 Long-term minocycline use as a treatment for acne has also been associated with autoimmune hepatitis, characterized by antinuclear autoantibodies, a lupus-like syndrome, fatigue, rash and arthralgia,96 and hypersensitivity syndrome.147
Linezolid
Linezolid treatment is most often initiated in the hospital, but, because of its excellent bioavailability in its oral form, linezolid treatment is often extended to home therapy after discharge and, therefore, is under the control of the general practitioner. Prolonged treatment with linezolid has been associated with severe liver failure and lactic acidosis, with liver biopsy showing microvesicular steatosis.148 These alterations were related to the known mitochondrial dysfunction induced by linezolid,149 but no further details have been disclosed so far. In two recently published studies from Japan, chronic liver disorders (liver cirrhosis and hepatitis),150 on the one hand, and high pre-treatment levels of total bilirubin and transaminases and coexistence of chronic liver disease (CLD), on the other hand,151 were found to be significant risk factors for the development of thrombocytopenia, a well-known side effect of linezolid that most commonly develops after ≥14 days of treatment.150 As thrombocytopenia is itself a common complication in patients with CLD,152 it is possible that some sort of synergistic effect and/or an enhancement of linezolid toxicity is occurring due to a reduction in its metabolism.151 Although there is evidence that linezolid accumulates in bile,153 there are insufficient data to provide specific recommendations about potential side effects at this time.
Antituberculosis drugs
Although prescribed by specialist practitioners, antituberculosis drugs are most often used today in outpatients. Thus, primary care physicians are among the first professionals to see potential side effects, including liver damage.
First-line treatment of adult respiratory tuberculosis entails 8 weeks of combination therapy with isoniazid, pyrazinamide and rifampicin, followed by 16 weeks of isoniazid and rifampicin, to which ethambutol is usually added. Streptomycin is used in cases of retreatment,154 with fluoroquinolones reserved for second-line use in drug-resistant tuberculosis or as substitutes in patients unable to tolerate first-line agents.99,155 These drugs have been used for many years, and have known and well-documented adverse effects on the liver that range from asymptomatic increases in liver enzymes to acute hepatitis and fulminant hepatic failure.156 Most commonly associated with isoniazid, pyrazinamide and rifampicin, they usually occur within the first few weeks of the intensive phase of therapy,154,155,157 are among the most common of all adverse events,158 and are responsible for most premature discontinuations due to adverse events.159 The hepatotoxicity of antituberculosis drugs has already been the subject of several recent reviews154,155,160 and will, therefore, be covered here only briefly. Because treatment of active tuberculosis entails concomitant use of more than one drug, it is difficult to identify the hepatotoxic effect of any individual drug in the combination regimen. Thus, we will first review the general risk factors identified in patients receiving antituberculosis treatment and then briefly review each drug individually.
General risk factors
Older age (>60 years), female sex and malnutrition (low body mass index) are widely accepted risk factors for drug-induced hepatotoxicity among tuberculosis patients.154,157,161–166 Pre-existing liver disease, such as chronic viral hepatitis, would seem a likely risk factor and has been identified in some,154,167 but not all,168,169 studies. Similarly, inconsistent findings have been reported for co-infection with HIV.154,170–172 Acetylator status may predispose to an increased risk of drug-induced hepatotoxicity, with several studies showing increased risk in slow versus fast acetylators,173–177 although contradictory but less recent results have also been reported.178,179 In addition to the N-acetyltransferase 2 slow acetylator genotype, other potential risk genotypes for hepatotoxicity include CYP2E1 polymorphisms.176,177 Excess alcohol consumption is thought to predispose to an increased risk of drug-induced hepatotoxicity and, in one study, was found to exacerbate hepatotoxicity in patients with chronic hepatitis C infection.167 Lastly, concomitant administration of other hepatotoxic drugs, such as acetaminophen (paracetamol), can also increase the risk of hepatotoxicity.180
Ethambutol
Frequency
Ethambutol-associated hepatotoxicity is considered rare.181 A recent study, in which ethambutol alone or in combination with fluoroquinolones and streptomycin was used to manage hepatotoxicity secondary to standard first-line drugs, showed no additional drug-induced hepatotoxicity.99 Previous studies suggest that the incidence is lowest when ethambutol is combined with streptomycin and isoniazid,182,183 whereas in one small series, 50% of patients experienced drug-induced hepatotoxicity when ethambutol was combined with pyrazinamide.184
Pathology
Ethambutol is not considered hepatotoxic, although a few isolated cases of hepatic injury and one of cholestatic hepatitis have been described.181
Isoniazid
Frequency
Isoniazid, used for both active treatment and prophylaxis of latent tuberculosis, has known hepatotoxic potential that has been attributed to various metabolites, including hydrazine.154,185 About 10% of patients experience increases in serum transaminase levels during treatment, with ∼1%–2% developing symptomatic hepatitis.178,181 Significantly higher rates of symptomatic hepatitis have been described in comparison with rifampicin (1.8% versus 0.08%, P < 0.001; 4% versus 0.7%, P = 0.003) as well as lower rates of treatment completion due to poorer tolerability.186,187 However, the frequency of severe hepatotoxicity appears significantly less common with isoniazid than with rifampicin in combination with pyrazinamide.188,189 Similarly, rates of hepatotoxicity are reportedly higher when isoniazid is co-administered with rifampicin181 (as a potent inducer of the hepatic CYP450 system, rifampicin can increase production of reactive metabolites of isoniazid and thereby increase the risk of hepatotoxicity154,190,191).
Pathology
Liver injury is characterized by hepatocellular necrosis and degeneration, such as hepatocyte ballooning, as well as mild inflammatory infiltrates within the portal tracts and, less commonly, cholestasis.181 In six cases of fulminant hepatitis following isoniazid/rifampicin combination therapy, centrilobular necrosis was the principal hepatic lesion.190
Pyrazinamide
Frequency
Introduced into clinical practice in the 1950s, pyrazinamide has known hepatotoxic potential; increases in serum transaminase levels and symptomatic hepatitis occur in 10%–20% of patients.181 Originally attributed to high initial dosage regimens, the relationship between pyrazinamide dosage and frequency of hepatotoxicity remains controversial. Early reports of no association between higher dosage and increased hepatotoxicity192 have been supported by a recent meta-analysis193 and a case–control study of dosing schedules.194 While this issue remains unresolved, several studies point to a higher frequency of hepatotoxicity in regimens containing pyrazinamide than in those containing only isoniazid and rifampicin.179,195,196 In one series, drug-induced hepatotoxicity was almost twice as frequent with pyrazinamide as compared with non-pyrazinamide regimens.179 In another study, the estimated risk of hepatotoxicity was 2.8% for pyrazinamide-based regimens, compared with 0.8% for concomitant isoniazid and rifampicin therapy.196 The inclusion of pyrazinamide in retreatment regimens also appears to increase the risk of hepatotoxicity, with rates of 24% in one series as compared with none in the isoniazid, rifampicin, ethambutol and streptomycin treatment arm.197
Pathology
Extensive centrolobular necrosis has been described in one case,198 while the key pathological features in a case of fatal liver failure consisted of bridging necrosis, lymphocyte infiltration, focal cholestasis, increased fibrosis and micronodular cirrhosis.154
Rifampicin
Frequency
Compared with isoniazid and pyrazinamide, rifampicin has a lower propensity to cause hepatotoxicity,181 with <2% of patients experiencing increased serum transaminase levels in a recent study.199 This is consistent with earlier data, in which the incidence of hepatotoxicity was 1.5% for rifampicin versus 4% and 5% for isoniazid and pyrazinamide, respectively.200 In a meta-analysis of pooled data on >3500 patients, severe hepatotoxicity occurred in 0%–0.7% of patients treated with rifampicin versus 1.4%–5.2% of isoniazid-treated patients.201 Although hepatotoxicity attributed to rifampicin itself is rare, it may contribute to increased hepatotoxicity when co-administered with isoniazid and, especially, pyrazinamide.202 In one study, rifampicin in combination with pyrazinamide significantly increased rates of treatment interruption for hepatotoxicity as compared with isoniazid alone (10% versus 2.5%; P = 0.007).203 In another study, mild hepatotoxicity (grade 1 or 2) was more frequent in patients receiving a higher dose of rifampicin in combination with pyrazinamide than with the standard dose (46% versus 20%; P = 0.054).204
Pathology
The histopathological picture is one of spotty-to-diffuse hepatocellular necrosis primarily associated with cholestasis.154,181 In some cases it can lead to liver failure.205
Streptomycin
Frequency
Of the current first-line drugs, streptomycin has little or no hepatotoxic potential; however, it can cause nephrotoxicity,181 as is the case for all aminoglycosides.206 Patients with liver disease are more susceptible to aminoglycoside-induced nephrotoxicity,212 meaning that acute renal failure is prognostic for acute liver failure (in this setting, acute renal failure may precipitate hepatic encephalopathy). Potentially, the inclusion of streptomycin in antituberculosis regimens may alter the course, if not the frequency, of fulminant hepatitis due to isoniazid- or pyrazinamide-induced hepatotoxicity.181
Conclusions
Antibiotic-induced hepatotoxicity produces an array of hepatic lesions that are often clinically indistinguishable from those of hepatobiliary diseases, making causality difficult to establish. While the temporal relationship between drug administration and onset of hepatic symptoms and exclusion of competing aetiologies may help, in most cases diagnosis is largely circumstantial. Delayed onset of hepatic dysfunction after cessation of therapy, which has been reported with several antibiotics, complicates the picture further, especially in cases of fatal liver failure. It is also difficult to determine cause and effect when there are only a few isolated spontaneous reports. Amoxicillin/clavulanate (due to the clavulanic acid component), co-trimoxazole (due to the sulphonamide component) and flucloxacillin appear as the most frequently involved drugs among those in current clinical use by primary care physicians. Conversely, no other currently approved antibiotic that is in use in general practice, except for telithromycin, can be singled out to be overly hepatotoxic.
Antibiotics are a vital weapon in combating serious bacterial infections. To maximize the benefit-to-risk ratio of antibiotic therapy, it is vital to ensure that the appropriate patients are treated with the most suitable drugs. In this respect, it is critical to be vigilant for potential risk factors that may increase the likelihood of a patient experiencing adverse hepatic events, namely the choice of the drug (see above) and patient factors. The latter mainly include a previous experience of hepatic dysfunction with the same antibiotic drug, the co-administration of other drugs known to cause hepatotoxic reactions, a pre-existing hepatic insufficiency without close monitoring of the hepatic function and, depending on the drug, one of several other risk factors, as listed in the paper and summarized in Table 1. Although antibiotic-induced hepatotoxicity is a rare event, and serious events are exceptionally rare, it is important to understand that most antibiotics can cause idiosyncratic hepatotoxic reactions. Both physicians and patients need to be aware of, and monitor for, potential symptoms and take prompt action if signs of hepatotoxicity emerge. This remains probably the only and most effective course of action until pharmacogenetic testing, which several organizations are working on29,207,208 and which already shows promise for a few other drugs,209 becomes available on an ‘easy-to-use’ basis for prospectively identifying those patients susceptible to antibiotic-induced liver injury in daily clinical practice.
Funding
This work was made without specific funding to the authors, who conceived and wrote this review as part of their normal professional activities. The editorial assistance provided by Highfield Communication Consultancy was supported by Bayer HealthCare AG.
Transparency declarations
P. M. T. has received research and educational grants from Sanofi-Aventis-Belgium, GlaxoSmithKline-Belgium, and Bayer HealthCare AG. None of these companies, however, played any role in the selection of material for review and in the writing of this paper. R. J. A.: none to declare.
Dr Claire Inness, Highfield Communication Consultancy (Oxford, UK), provided excellent editorial assistance.
Acknowledgements
We thank Willis C. Maddrey, The University of Texas South-western Medical Center, Texas, USA, for critically reviewing the drafts of this manuscript. We thank Dr Claire Inness, Highfield Communication Consultancy (Oxford, UK) for excellent editorial assistance.
References
- 1.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. doi: 10.1053/j.gastro.2008.09.011. doi:10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade RJ, López-Ortega S, López-Vega MC, et al. Idiosyncratic drug hepatotoxicity: a 2008 update. Expert Rev Clin Pharmacol. 2008;1:261–76. doi: 10.1586/17512433.1.2.261. doi:10.1586/17512433.1.2.261. [DOI] [PubMed] [Google Scholar]
- 3.Robles M, Andrade RJ. Hepatotoxicidad por antibioticos: actualizacion en 2008 [in Spanish (Hepatotoxicity by antibiotics: update in 2008); abstract in English] Rev Esp Quimioter. 2008;21:224–33. [PubMed] [Google Scholar]
- 4.Polson JE. Hepatotoxicity due to antibiotics. Clin Liver Dis. 2007;11:549–61. doi: 10.1016/j.cld.2007.06.009. vi doi:10.1016/j.cld.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Thiim M, Friedman LS. Hepatotoxicity of antibiotics and antifungals. Clin Liver Dis. 2003;7:381–99. doi: 10.1016/s1089-3261(03)00021-7. doi:10.1016/S1089-3261(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 6.George DK, Crawford DH. Antibacterial-induced hepatotoxicity. Incidence, prevention and management. Drug Saf. 1996;15:79–85. doi: 10.2165/00002018-199615010-00007. doi:10.2165/00002018-199615010-00007. [DOI] [PubMed] [Google Scholar]
- 7.Andrade RJ, Lucena MI, Fernandez MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Björnsson E, Jerlstad P, Bergqvist A, et al. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. doi: 10.1080/00365520510023846. doi:10.1080/00365520510023846. [DOI] [PubMed] [Google Scholar]
- 9.Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. doi: 10.1016/j.dld.2005.06.004. doi:10.1016/j.dld.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Hughes B. Industry concern over EU hepatotoxicity guidance. Nat Rev Drug Discov. 2008;7:719. doi: 10.1038/nrd2677. doi:10.1038/nrd2677. [DOI] [PubMed] [Google Scholar]
- 11.FDA. Telithromycin (Marketed as Ketek) Information. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107824.htm. (17 October 2010, date last accessed) [Google Scholar]
- 12.European Medicines Agency Recommends Restricting the Use of Oral Moxifloxacin-containing Medicines. European Medicines Agency; http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2010/08/news_detail_001068.jsp&mid=WC0b01ac058004d5c1&murl=menus/news_and_events/news_and_events.jsp&jsenabled=true. (17 October 2010, date last accessed) [Google Scholar]
- 13.Goossens H, Ferech M, Vander SR, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–87. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 14.Goossens H, Ferech M, Coenen S, et al. Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries. Clin Infect Dis. 2007;44:1091–5. doi: 10.1086/512810. doi:10.1086/512810. [DOI] [PubMed] [Google Scholar]
- 15.McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA. 1995;273:214–9. doi:10.1001/jama.273.3.214. [PubMed] [Google Scholar]
- 16.Uetrecht J. Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol. 2007;47:513–39. doi: 10.1146/annurev.pharmtox.47.120505.105150. doi:10.1146/annurev.pharmtox.47.120505.105150. [DOI] [PubMed] [Google Scholar]
- 17.Ganey PE, Luyendyk JP, Maddox JF, et al. Adverse hepatic drug reactions: inflammatory episodes as consequence and contributor. Chem Biol Interact. 2004;150:35–51. doi: 10.1016/j.cbi.2004.09.002. doi:10.1016/j.cbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Zapater P, Moreu R, Horga JF. The diagnosis of drug-induced liver disease. Curr Clin Pharmacol. 2006;1:207–17. doi: 10.2174/157488406776872514. doi:10.2174/157488406776872514. [DOI] [PubMed] [Google Scholar]
- 19.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489–99. doi: 10.1038/nrd1750. doi:10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 20.Andrade RJ, Camargo R, Lucena MI, et al. Causality assessment in drug-induced hepatotoxicity. Expert Opin Drug Saf. 2004;3:329–44. doi: 10.1517/14740338.3.4.329. doi:10.1517/14740338.3.4.329. [DOI] [PubMed] [Google Scholar]
- 21.Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. 2007;25:1135–51. doi: 10.1111/j.1365-2036.2007.03307.x. doi:10.1111/j.1365-2036.2007.03307.x. [DOI] [PubMed] [Google Scholar]
- 22.Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272–6. doi: 10.1016/0168-8278(90)90124-a. doi:10.1016/0168-8278(90)90124-A. [DOI] [PubMed] [Google Scholar]
- 23.Danan G, Benichou C. Causality assessment of adverse reactions to drugs–I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–30. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 24.Farrell GC. Drug-induced Liver Disease. Edinburgh: Churchill Livingstone; 1994. [Google Scholar]
- 25.Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–9. doi: 10.1002/hep.20800. doi:10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 26.Watkins PB, Seligman PJ, Pears JS, et al. Using controlled clinical trials to learn more about acute drug-induced liver injury. Hepatology. 2008;48:1680–9. doi: 10.1002/hep.22633. doi:10.1002/hep.22633. [DOI] [PubMed] [Google Scholar]
- 27.Ferrajolo C, Capuano A, Verhamme KM, et al. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. doi: 10.1111/j.1365-2125.2010.03754.x. doi:10.1111/j.1365-2125.2010.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lammert C, Einarsson S, Saha C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003–9. doi: 10.1002/hep.22272. doi:10.1002/hep.22272. [DOI] [PubMed] [Google Scholar]
- 29.Wilke RA, Lin DW, Roden DM, et al. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat Rev Drug Discov. 2007;6:904–16. doi: 10.1038/nrd2423. doi:10.1038/nrd2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–26. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–6. doi: 10.1016/0895-4356(93)90102-7. doi:10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 32.Simmons C. Beware: antibiotic-induced hepatotoxicity is rare but deadly. Hosp Pharm. 2002;37:326–30. [Google Scholar]
- 33.Lucena MI, Andrade RJ, Rodrigo L, et al. Trovafloxacin-induced acute hepatitis. Clin Infect Dis. 2000;30:400–1. doi: 10.1086/313680. doi:10.1086/313680. [DOI] [PubMed] [Google Scholar]
- 34.Coleman CI, Spencer JV, Chung JO, et al. Possible gatifloxacin-induced fulminant hepatic failure. Ann Pharmacother. 2002;36:1162–7. doi: 10.1345/aph.1A414. doi:10.1345/aph.1A414. [DOI] [PubMed] [Google Scholar]
- 35.Andrade RJ, Lucena MI, Kaplowitz N, et al. Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–8. doi: 10.1002/hep.21424. doi:10.1002/hep.21424. [DOI] [PubMed] [Google Scholar]
- 36.Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med. 1992;232:133–8. doi: 10.1111/j.1365-2796.1992.tb00562.x. doi:10.1111/j.1365-2796.1992.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 37.Parry MF. The penicillins. Med Clin North Am. 1987;71:1093–112. doi: 10.1016/s0025-7125(16)30799-4. [DOI] [PubMed] [Google Scholar]
- 38.Cavanzo FJ, Garcia CF, Botero RC. Chronic cholestasis, paucity of bile ducts, red cell aplasia, and the Stevens–Johnson syndrome. An ampicillin-associated case. Gastroenterology. 1990;99:854–6. doi: 10.1016/0016-5085(90)90980-f. [DOI] [PubMed] [Google Scholar]
- 39.Köklü S, Yuksel O, Filik L, et al. Recurrent cholestasis due to ampicillin. Ann Pharmacother. 2003;37:395–7. doi: 10.1345/aph.1C273. doi:10.1345/aph.1C273. [DOI] [PubMed] [Google Scholar]
- 40.Garcia Rodriguez LA, Stricker BH, Zimmerman HJ. Risk of acute liver injury associated with the combination of amoxicillin and clavulanic acid. Arch Intern Med. 1996;156:1327–32. doi: 10.1001/archinte.1996.00440110099013. doi:10.1001/archinte.156.12.1327. [DOI] [PubMed] [Google Scholar]
- 41.Davies MH, Harrison RF, Elias E, et al. Antibiotic-associated acute vanishing bile duct syndrome: a pattern associated with severe, prolonged, intrahepatic cholestasis. J Hepatol. 1994;20:112–6. doi: 10.1016/s0168-8278(05)80476-3. doi:10.1016/S0168-8278(05)80476-3. [DOI] [PubMed] [Google Scholar]
- 42.Fontana RJ, Shakil AO, Greenson JK, et al. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci. 2005;50:1785–90. doi: 10.1007/s10620-005-2938-5. doi:10.1007/s10620-005-2938-5. [DOI] [PubMed] [Google Scholar]
- 43.de Abajo FJ, Montero D, Madurga M, et al. Acute and clinically relevant drug-induced liver injury: a population based case–control study. Br J Clin Pharmacol. 2004;58:71–80. doi: 10.1111/j.1365-2125.2004.02133.x. doi:10.1111/j.1365-2125.2004.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussaini SH, O'Brien CS, Despott EJ, et al. Antibiotic therapy: a major cause of drug-induced jaundice in southwest England. Eur J Gastroenterol Hepatol. 2007;19:15–20. doi: 10.1097/01.meg.0000250581.77865.68. doi:10.1097/01.meg.0000250581.77865.68. [DOI] [PubMed] [Google Scholar]
- 45.Westphal JF, Vetter D, Brogard JM. Hepatic side-effects of antibiotics. J Antimicrob Chemother. 1994;33:387–401. doi: 10.1093/jac/33.3.387. doi:10.1093/jac/33.3.387. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein LI, Ishak KG. Hepatic injury associated with penicillin therapy. Arch Pathol. 1974;98:114–7. [PubMed] [Google Scholar]
- 47.Onate J, Montejo M, Aguirrebengoa K, et al. Hepatotoxicity associated with penicillin V therapy. Clin Infect Dis. 1995;20:474–5. doi: 10.1093/clinids/20.2.474. doi:10.1093/clinids/20.2.474. [DOI] [PubMed] [Google Scholar]
- 48.Andrade RJ, Guilarte J, Salmeron FJ, et al. Benzylpenicillin-induced prolonged cholestasis. Ann Pharmacother. 2001;35:783–4. doi: 10.1345/aph.10266. doi:10.1345/aph.10266. [DOI] [PubMed] [Google Scholar]
- 49.Williams CN, Malatjalian DA. Severe penicillin-induced cholestasis in a 91-year-old woman. Dig Dis Sci. 1981;26:470–3. doi: 10.1007/BF01313594. doi:10.1007/BF01313594. [DOI] [PubMed] [Google Scholar]
- 50.Köklü S, Koksal AS, Asil M, et al. Probable sulbactam/ampicillin-associated prolonged cholestasis. Ann Pharmacother. 2004;38:2055–8. doi: 10.1345/aph.1E225. doi:10.1345/aph.1E225. [DOI] [PubMed] [Google Scholar]
- 51.Turner IB, Eckstein RP, Riley JW, et al. Prolonged hepatic cholestasis after flucloxacillin therapy. Med J Aust. 1989;151:701–5. doi: 10.5694/j.1326-5377.1989.tb139652.x. [DOI] [PubMed] [Google Scholar]
- 52.Eckstein RP, Dowsett JF, Lunzer MR. Flucloxacillin induced liver disease: histopathological findings at biopsy and autopsy. Pathology. 1993;25:223–8. doi: 10.3109/00313029309066576. doi:10.3109/00313029309066576. [DOI] [PubMed] [Google Scholar]
- 53.Fairley CK, McNeil JJ, Desmond P, et al. Risk factors for development of flucloxacillin associated jaundice. BMJ. 1993;306:233–5. doi: 10.1136/bmj.306.6872.233. doi:10.1136/bmj.306.6872.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daly AK, Donaldson PT, Bhatnagar P, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–9. doi: 10.1038/ng.379. doi:10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 55.Sabaté M, Ibáñez L, Pérez E, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. doi: 10.1111/j.1365-2036.2007.03338.x. doi:10.1111/j.1365-2036.2007.03338.x. [DOI] [PubMed] [Google Scholar]
- 56.Lucena MI, Andrade RJ, Fernandez MC, et al. Determinants of the clinical expression of amoxicillin–clavulanate hepatotoxicity: a prospective series from Spain. Hepatology. 2006;44:850–6. doi: 10.1002/hep.21324. doi:10.1002/hep.21324. [DOI] [PubMed] [Google Scholar]
- 57.Iravani A, Richard GA. Amoxicillin–clavulanic acid versus cefaclor in the treatment of urinary tract infections and their effects on the urogenital and rectal flora. Antimicrob Agents Chemother. 1986;29:107–11. doi: 10.1128/aac.29.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berg P, Hahn EG. Hepatotoxic reactions induced by β-lactamase inhibitors. Eur J Med Res. 2001;6:535–42. [PubMed] [Google Scholar]
- 59.Larrey D, Vial T, Micaleff A, et al. Hepatitis associated with amoxycillin–clavulanic acid combination report of 15 cases. Gut. 1992;33:368–71. doi: 10.1136/gut.33.3.368. doi:10.1136/gut.33.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salvo F, Polimeni G, Moretti U, et al. Adverse drug reactions related to amoxicillin alone and in association with clavulanic acid: data from spontaneous reporting in Italy. J Antimicrob Chemother. 2007;60:121–6. doi: 10.1093/jac/dkm111. doi:10.1093/jac/dkm111. [DOI] [PubMed] [Google Scholar]
- 61.Brown SJ, Desmond PV. Hepatotoxicity of antimicrobial agents. Semin Liver Dis. 2002;22:157–67. doi: 10.1055/s-2002-30103. doi:10.1055/s-2002-30103. [DOI] [PubMed] [Google Scholar]
- 62.Hautekeete ML, Horsmans Y, Van Waeyenberge C, et al. HLA association of amoxicillin–clavulanate-induced hepatitis. Gastroenterology. 1999;117:1181–6. doi: 10.1016/s0016-5085(99)70404-x. doi:10.1016/S0016-5085(99)70404-X. [DOI] [PubMed] [Google Scholar]
- 63.Donaldson PT, Daly AK, Henderson J, et al. Human leucocyte antigen class II genotype in susceptibility and resistance to co-amoxiclav-induced liver injury. J Hepatol. 2010;53:1049–53. doi: 10.1016/j.jhep.2010.05.033. doi:10.1016/j.jhep.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 64.Ammann R, Neftel K, Hardmeier T, et al. Cephalosporin-induced cholestatic jaundice. Lancet. 1982;2:336–7. doi: 10.1016/s0140-6736(82)90311-7. doi:10.1016/S0140-6736(82)90311-7. [DOI] [PubMed] [Google Scholar]
- 65.Eggleston SM, Belandres MM. Jaundice associated with cephalosporin therapy. Drug Intell Clin Pharm. 1985;19:553–5. doi: 10.1177/106002808501900710. [DOI] [PubMed] [Google Scholar]
- 66.Skoog SM, Smyrk TC, Talwalkar JA. Cephalexin-induced cholestatic hepatitis. J Clin Gastroenterol. 2004;38:833. doi: 10.1097/01.mcg.0000139074.40365.04. doi:10.1097/01.mcg.0000139074.40365.04. [DOI] [PubMed] [Google Scholar]
- 67.Bilici A, Karaduman M, Cankir Z. A rare case of hepatitis associated with cefprozil therapy. Scand J Infect Dis. 2007;39:190–2. doi: 10.1080/00365540600823235. doi:10.1080/00365540600823235. [DOI] [PubMed] [Google Scholar]
- 68.Ramkumar D, LaBrecque D. Drug-induced liver disease and environmental toxins. In: Zakim D, Boyer T, editors. Hepatology: A Textbook of Liver Disease. Vol. 2. Philadelphia: Saunders; 2003. pp. 755–832. [Google Scholar]
- 69.Chen J, Ahmad J. Cefdinir-induced hepatotoxicity: potential hazards of inappropriate antibiotic use. J Gen Intern Med. 2008;23:1914–6. doi: 10.1007/s11606-008-0758-y. doi:10.1007/s11606-008-0758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pessayre D, Benhamou JP. Hepatotoxicity of erythromycin derivatives. Br Med J. 1979;1:1357. doi: 10.1136/bmj.1.6174.1357-b. doi:10.1136/bmj.1.6174.1357-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braun P. Hepatotoxicity of erythromycin. J Infect Dis. 1969;119:300–6. doi: 10.1093/infdis/119.3.300. doi:10.1093/infdis/119.3.300. [DOI] [PubMed] [Google Scholar]
- 72.Zafrani ES, Ishak KG, Rudzki C. Cholestatic and hepatocellular injury associated with erythromycin esters: report of nine cases. Dig Dis Sci. 1979;24:385–96. doi: 10.1007/BF01297126. doi:10.1007/BF01297126. [DOI] [PubMed] [Google Scholar]
- 73.Derby LE, Jick H, Henry DA, et al. Erythromycin-associated cholestatic hepatitis. Med J Aust. 1993;158:600–2. doi: 10.5694/j.1326-5377.1993.tb137625.x. [DOI] [PubMed] [Google Scholar]
- 74.Ginsburg CM. A prospective study on the incidence of liver function abnormalities in children receiving erythromycin estolate, erythromycin ethylsuccinate or penicillin V for treatment of pneumonia. Pediatr Infect Dis. 1986;5:151–3. doi: 10.1097/00006454-198601000-00050. doi:10.1097/00006454-198601000-00050. [DOI] [PubMed] [Google Scholar]
- 75.Carson JL, Strom BL, Duff A, et al. Acute liver disease associated with erythromycins, sulfonamides, and tetracyclines. Ann Intern Med. 1993;119:576–83. doi: 10.7326/0003-4819-119-7_part_1-199310010-00005. [DOI] [PubMed] [Google Scholar]
- 76.Gholson CF, Warren GH. Fulminant hepatic failure associated with intravenous erythromycin lactobionate. Arch Intern Med. 1990;150:215–6. doi:10.1001/archinte.150.1.215. [PubMed] [Google Scholar]
- 77.Yew WW, Chau CH, Lee J, et al. Cholestatic hepatitis in a patient who received clarithromycin therapy for a Mycobacterium chelonae lung infection. Clin Infect Dis. 1994;18:1025–6. doi: 10.1093/clinids/18.6.1025. doi:10.1093/clinids/18.6.1025. [DOI] [PubMed] [Google Scholar]
- 78.Brown BA, Wallace RJ, Jr, Griffith DE, et al. Clarithromycin-induced hepatotoxicity. Clin Infect Dis. 1995;20:1073–4. doi: 10.1093/clinids/20.4.1073. doi:10.1093/clinids/20.4.1073. [DOI] [PubMed] [Google Scholar]
- 79.Shaheen N, Grimm IS. Fulminant hepatic failure associated with clarithromycin. Am J Gastroenterol. 1996;91:394–5. [PubMed] [Google Scholar]
- 80.Masia M, Gutierrez F, Jimeno A, et al. Fulminant hepatitis and fatal toxic epidermal necrolysis (Lyell disease) coincident with clarithromycin administration in an alcoholic patient receiving disulfiram therapy. Arch Intern Med. 2002;162:474–6. doi: 10.1001/archinte.162.4.474. doi:10.1001/archinte.162.4.474. [DOI] [PubMed] [Google Scholar]
- 81.Abbott Laboratories. Biaxin® US Prescribing Information. http://www.rxabbott.com/pdf/biapi.PDF. (5 September 2010, date last accessed) [Google Scholar]
- 82.Longo G, Valenti C, Gandini G, et al. Azithromycin-induced intrahepatic cholestasis. Am J Med. 1997;102:217–8. [PubMed] [Google Scholar]
- 83.Cascaval RI, Lancaster DJ. Hypersensitivity syndrome associated with azithromycin. Am J Med. 2001;110:330–1. doi: 10.1016/s0002-9343(00)00724-5. doi:10.1016/S0002-9343(00)00724-5. [DOI] [PubMed] [Google Scholar]
- 84.Chandrupatla S, Demetris AJ, Rabinovitz M. Azithromycin-induced intrahepatic cholestasis. Dig Dis Sci. 2002;47:2186–8. doi: 10.1023/a:1020170807742. doi:10.1023/A:1020170807742. [DOI] [PubMed] [Google Scholar]
- 85.Lockwood AM, Cole S, Rabinovich M. Azithromycin-induced liver injury. Am J Health Syst Pharm. 2010;67:810–4. doi: 10.2146/ajhp080687. doi:10.2146/ajhp080687. [DOI] [PubMed] [Google Scholar]
- 86.Principi N, Esposito S. Comparative tolerability of erythromycin and newer macrolide antibacterials in paediatric patients. Drug Saf. 1999;20:25–41. doi: 10.2165/00002018-199920010-00004. doi:10.2165/00002018-199920010-00004. [DOI] [PubMed] [Google Scholar]
- 87.Agouridas C, Denis A, Auger JM, et al. Synthesis and antibacterial activity of ketolides (6-O-methyl-3-oxoerythromycin derivatives): a new class of antibacterials highly potent against macrolide-resistant and -susceptible respiratory pathogens. J Med Chem. 1998;41:4080–100. doi: 10.1021/jm980240d. doi:10.1021/jm980240d. [DOI] [PubMed] [Google Scholar]
- 88.Clay KD, Hanson JS, Pope SD, et al. Brief communication: severe hepatotoxicity of telithromycin: three case reports and literature review. Ann Intern Med. 2006;144:415–20. doi: 10.7326/0003-4819-144-6-200503210-00121. [DOI] [PubMed] [Google Scholar]
- 89.FDA. Telithromycin-Associated Hepatotoxicity. http://www.fda.gov/ohrms/dockets/AC/06/slides/2006-4266s1-01-07-FDA-Brinker.ppt. (5 September 2010, date last accessed) [Google Scholar]
- 90.Ketek® Prescribing Information. Sanofi-Aventis US LLC; http://products.sanofi-aventis.us/ketek/ketek.html. (5 September 2010, date last accessed) [Google Scholar]
- 91.Ketek® Summary of Product Characteristics. European Medicines Agency; http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000354/WC500041895.pdf. (17 October 2010, date last accessed) [Google Scholar]
- 92.Ketek 400 mg Tablets - Summary of Product Characteristics. Sanofi-Aventis UK; http://emc.medicines.org.uk/medicine/19852/SPC/Ketek.400mg.Tablets/ (5 September 2010, date last accessed) [Google Scholar]
- 93.European Medicines Agency recommends restricted use and strengthened warnings for Ketek. European Medicines Agency; http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2009/11/news_detail_000146.jsp&mid=WC0b01ac058004d5c1&murl=menus/news_and_events/news_and_events.jsp&jsenabled=true. (5 September 2010, date last accessed) [Google Scholar]
- 94.Graham DJ. Telithromycin and acute liver failure. N Engl J Med. 2006;355:2260–1. doi: 10.1056/NEJMc066372. doi:10.1056/NEJMc066372. [DOI] [PubMed] [Google Scholar]
- 95.Dore DD, DiBello JR, Lapane KL. Telithromycin use and spontaneous reports of hepatotoxicity. Drug Saf. 2007;30:697–703. doi: 10.2165/00002018-200730080-00006. doi:10.2165/00002018-200730080-00006. [DOI] [PubMed] [Google Scholar]
- 96.Brinker AD, Wassel RT, Lyndly J, et al. Telithromycin-associated hepatotoxicity: clinical spectrum and causality assessment of 42 cases. Hepatology. 2009;49:250–7. doi: 10.1002/hep.22620. doi:10.1002/hep.22620. [DOI] [PubMed] [Google Scholar]
- 97.Onur O, Guneysel O, Denizbasi A, et al. Acute hepatitis attack after exposure to telithromycin. Clin Ther. 2007;29:1725–9. doi: 10.1016/j.clinthera.2007.08.004. doi:10.1016/j.clinthera.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 98.Esposito S, Noviello S, Leone S, et al. Clinical efficacy and tolerability of levofloxacin in patients with liver disease: a prospective, non comparative, observational study. J Chemother. 2006;18:33–7. doi: 10.1179/joc.2006.18.1.33. [DOI] [PubMed] [Google Scholar]
- 99.Ho CC, Chen YC, Hu FC, et al. Safety of fluoroquinolone use in patients with hepatotoxicity induced by anti-tuberculosis regimens. Clin Infect Dis. 2009;48:1526–33. doi: 10.1086/598929. doi:10.1086/598929. [DOI] [PubMed] [Google Scholar]
- 100.Grassmick BK, Lehr VT, Sundareson AS. Fulminant hepatic failure possibly related to ciprofloxacin. Ann Pharmacother. 1992;26:636–9. doi: 10.1177/106002809202600504. [DOI] [PubMed] [Google Scholar]
- 101.Fuchs S, Simon Z, Brezis M. Fatal hepatic failure associated with ciprofloxacin. Lancet. 1994;343:738–9. doi: 10.1016/s0140-6736(94)91624-1. doi:10.1016/S0140-6736(94)91624-1. [DOI] [PubMed] [Google Scholar]
- 102.Hautekeete ML, Kockx MM, Naegels S, et al. Cholestatic hepatitis related to quinolones: a report of two cases. J Hepatol. 1995;23:759–60. doi: 10.1016/0168-8278(95)80045-x. doi:10.1016/0168-8278(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 103.Bataille L, Rahier J, Geubel A. Delayed and prolonged cholestatic hepatitis with ductopenia after long-term ciprofloxacin therapy for Crohn's disease. J Hepatol. 2002;37:696–9. doi: 10.1016/s0168-8278(02)00268-4. doi:10.1016/S0168-8278(02)00268-4. [DOI] [PubMed] [Google Scholar]
- 104.Dichiara AJ, Atkinson M, Goodman Z, et al. Ciprofloxacin-induced acute cholestatic liver injury and associated renal failure. Case report and review. Minerva Gastroenterol Dietol. 2008;54:307–15. [PubMed] [Google Scholar]
- 105.Ciprofloxacin Bayer - Article 30 Referral - Annex I, II, III - Harmonized Summary of Product Characteristics. European Medicines Agency; http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Ciprofloxacin_Bayer/human_referral_000024.jsp&mid=WC0b01ac0580024e9a&murl=menus/regulations/regulations.jsp&jsenabled=true#. (17 October 2010, date last accessed) [Google Scholar]
- 106.Croom KF, Goa KL. Levofloxacin: a review of its use in the treatment of bacterial infections in the United States. Drugs. 2003;63:2769–802. doi: 10.2165/00003495-200363240-00008. doi:10.2165/00003495-200363240-00008. [DOI] [PubMed] [Google Scholar]
- 107.Carbon C. Effets indésirables de la lévofloxacine: données des études cliniques et de la pharmacovigilance [In French (Levofloxacin adverse effects, data from clinical trials and pharmacovigilance); abstract in English] Therapie. 2001;56:35–40. [PubMed] [Google Scholar]
- 108.Health Canada. Canadian Adverse Reaction Newsletter. Health Canada. 2007;17:1. [Google Scholar]
- 109.Spahr L, Rubbia-Brandt L, Marinescu O, et al. Acute fatal hepatitis related to levofloxacin. J Hepatol. 2001;35:308–9. doi: 10.1016/s0168-8278(01)00082-4. doi:10.1016/S0168-8278(01)00082-4. [DOI] [PubMed] [Google Scholar]
- 110.Coban S, Ceydilek B, Ekiz F, et al. Levofloxacin-induced acute fulminant hepatic failure in a patient with chronic hepatitis B infection. Ann Pharmacother. 2005;39:1737–40. doi: 10.1345/aph.1G111. doi:10.1345/aph.1G111. [DOI] [PubMed] [Google Scholar]
- 111.Schwalm JD, Lee CH. Acute hepatitis associated with oral levofloxacin therapy in a hemodialysis patient. CMAJ. 2003;168:847–8. [PMC free article] [PubMed] [Google Scholar]
- 112.Karim A, Ahmed S, Rossoff LJ, et al. Possible levofloxacin-induced acute hepatocellular injury in a patient with chronic obstructive lung disease. Clin Infect Dis. 2001;33:2088–90. doi: 10.1086/338156. doi:10.1086/338156. [DOI] [PubMed] [Google Scholar]
- 113.Carrascosa MF, Lucena MI, Andrade RJ, et al. Fatal acute hepatitis after sequential treatment with levofloxacin, doxycycline, and naproxen in a patient presenting with acute Mycoplasma pneumoniae infection. Clin Ther. 2009;31:1014–9. doi: 10.1016/j.clinthera.2009.05.012. doi:10.1016/j.clinthera.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 114.Levaquin Prescribing Information. Ortho-McNeil-Janssen Pharmaceuticals, Inc; http://www.levaquin360.com/levaquin360/shared/pi/levaquin.pdf#zoom=100. (17 October 2010, date last accessed) [Google Scholar]
- 115.Ball P, Stahlmann R, Kubin R, et al. Safety profile of oral and intravenous moxifloxacin: cumulative data from clinical trials and postmarketing studies. Clin Ther. 2004;26:940–50. doi: 10.1016/s0149-2918(04)90170-1. doi:10.1016/S0149-2918(04)90170-1. [DOI] [PubMed] [Google Scholar]
- 116.Andriole VT, Haverstock DC, Choudhri SH. Retrospective analysis of the safety profile of oral moxifloxacin in elderly patients enrolled in clinical trials. Drug Saf. 2005;28:443–52. doi: 10.2165/00002018-200528050-00007. doi:10.2165/00002018-200528050-00007. [DOI] [PubMed] [Google Scholar]
- 117.Soto S, López-Rosés L, Avila S, et al. Moxifloxacin-induced acute liver injury. Am J Gastroenterol. 2002;97:1853–4. doi: 10.1111/j.1572-0241.2002.05873.x. doi:10.1111/j.1572-0241.2002.05873.x. [DOI] [PubMed] [Google Scholar]
- 118.Pugh AJ, Barve AJ, Falkner K, et al. Drug-induced hepatotoxicity or drug-induced liver injury. Clin Liver Dis. 2009;13:277–94. doi: 10.1016/j.cld.2009.02.008. doi:10.1016/j.cld.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 119.EMEA Annex II. The Scientific Conclusions and the Grounds for Amendments of the Summary of Product Characteristics and Package Leaflet (Moxifloxacin) European Medicines Agency; http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Moxifloxacin/human_referral_000114.jsp&mid=WC0b01ac0580024e9a&murl=menus/regulations/regulations.jsp&jsenabled=true#. (5 September 2010, date last accessed) [Google Scholar]
- 120.Verma R, Dhamija R, Batts DH, et al. Moxifloxacin induced fatal hepatotoxicity in a 72-year-old man: a case report. Cases J. 2009;2:8063. doi: 10.4076/1757-1626-2-8063. doi:10.4076/1757-1626-2-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moxifloxacin - Article 107 Procedures - Annex III - Amendments to the Summary of Product Characteristics and Package Leaflet. European Medicines Agency; http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Moxifloxacin/human_referral_000114.jsp&mid=WC0b01ac0580024e9a&murl=menus/regulations/regulations.jsp&jsenabled=true#. (17 October 2010, date last accessed) [Google Scholar]
- 122.Lode HM, Schmidt-Ioanas M. Moxifloxacin: update and perspectives after 8 years of usage. Expert Rev Respir Med. 2008;2:443–53. doi: 10.1586/17476348.2.4.443. doi:10.1586/17476348.2.4.443. [DOI] [PubMed] [Google Scholar]
- 123.Suzuki A, Andrade RJ, Bjornsson E, et al. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase: unified list based on international collaborative work. Drug Saf. 2010;33:503–22. doi: 10.2165/11535340-000000000-00000. doi:10.2165/11535340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 124.Nori S, Nebesio C, Brashear R, et al. Moxifloxacin-associated drug hypersensitivity syndrome with toxic epidermal necrolysis and fulminant hepatic failure. Arch Dermatol. 2004;140:1537–8. doi: 10.1001/archderm.140.12.1537. doi:10.1001/archderm.140.12.1537. [DOI] [PubMed] [Google Scholar]
- 125.Iannini PB. The safety profile of moxifloxacin and other fluoroquinolones in special patient populations. Curr Med Res Opin. 2007;23:1403–13. doi: 10.1185/030079907X188099. doi:10.1185/030079907X188099. [DOI] [PubMed] [Google Scholar]
- 126.Henann NE, Zambie MF. Gatifloxacin-associated acute hepatitis. Pharmacotherapy. 2001;21:1579–82. doi: 10.1592/phco.21.20.1579.34479. doi:10.1592/phco.21.20.1579.34479. [DOI] [PubMed] [Google Scholar]
- 127.Ball P, Mandell L, Niki Y, et al. Comparative tolerability of the newer fluoroquinolone antibacterials. Drug Saf. 1999;21:407–21. doi: 10.2165/00002018-199921050-00005. doi:10.2165/00002018-199921050-00005. [DOI] [PubMed] [Google Scholar]
- 128.Owens RC, Jr, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(Suppl 2):S144–57. doi: 10.1086/428055. doi:10.1086/428055. [DOI] [PubMed] [Google Scholar]
- 129.Bertino J, Jr, Fish D. The safety profile of the fluoroquinolones. Clin Ther. 2000;22:798–817. doi: 10.1016/S0149-2918(00)80053-3. doi:10.1016/S0149-2918(00)80053-3. [DOI] [PubMed] [Google Scholar]
- 130.Mandell L, Tillotson G. Safety of fluoroquinolones: an update. Can J Infect Dis. 2002;13:54–61. doi: 10.1155/2002/864789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun Q, Zhu R, Foss FW, Jr, et al. In vitro metabolism of a model cyclopropylamine to reactive intermediate: insights into trovafloxacin-induced hepatotoxicity. Chem Res Toxicol. 2008;21:711–9. doi: 10.1021/tx7003085. doi:10.1021/tx7003085. [DOI] [PubMed] [Google Scholar]
- 132.Shaw PJ, Beggs KM, Sparkenbaugh EM, et al. Trovafloxacin enhances TNF-induced inflammatory stress and cell death signaling and reduces TNF clearance in a murine model of idiosyncratic hepatotoxicity. Toxicol Sci. 2009;111:288–301. doi: 10.1093/toxsci/kfp163. doi:10.1093/toxsci/kfp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shaw PJ, Fullerton AM, Scott MA, et al. The role of the hemostatic system in murine liver injury induced by coexposure to lipopolysaccharide and trovafloxacin, a drug with idiosyncratic liability. Toxicol Appl Pharmacol. 2009;236:293–300. doi: 10.1016/j.taap.2009.01.018. doi:10.1016/j.taap.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 134.Dujovne CA, Chan CH, Zimmerman HJ. Sulfonamide hepatic injury. Review of the literature and report of a case due to sulfamethoxazole. N Engl J Med. 1967;277:785–8. doi: 10.1056/NEJM196710122771503. doi:10.1056/NEJM196710122771503. [DOI] [PubMed] [Google Scholar]
- 135.Ransohoff DF, Jacobs G. Terminal hepatic failure following a small dose of sulfamethoxazole–trimethoprim. Gastroenterology. 1981;80:816–9. [PubMed] [Google Scholar]
- 136.Alberti-Flor JJ, Hernandez ME, Ferrer JP, et al. Fulminant liver failure and pancreatitis associated with the use of sulfamethoxazole–trimethoprim. Am J Gastroenterol. 1989;84:1577–9. [PubMed] [Google Scholar]
- 137.Mainra RR, Card SE. Trimethoprim–sulfamethoxazole-associated hepatotoxicity - part of a hypersensitivity syndrome. Can J Clin Pharmacol. 2003;10:175–8. [PubMed] [Google Scholar]
- 138.Espiritu CR, Kim TS, Levine RA. Granulomatous hepatitis associated with sulfadimethoxine hypersensitivity. JAMA. 1967;202:985–8. doi:10.1001/jama.202.10.985. [PubMed] [Google Scholar]
- 139.Lazar HP, Murphy RL, Phair JP. Fansidar and hepatic granulomas. Ann Intern Med. 1985;102:722. doi: 10.7326/0003-4819-102-5-722_1. [DOI] [PubMed] [Google Scholar]
- 139a.Nakamura HU, Uetrecht J, Cribb AE, et al. In vitro formation, disposition and toxicity of N-acetoxy-sulfamethoxazole, a potential mediator of sulfamethoxazole toxicity. J Pharmacol Exp Ther. 1995;274:1099–104. doi:10.1186/1757-1626-1-44. [PubMed] [Google Scholar]
- 140.Abusin S, Johnson S. Sulfamethoxazole/trimethoprim induced liver failure: a case report. Cases J. 2008;1:44. doi: 10.1186/1757-1626-1-44. doi:10.1186/1757-1626-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Anon. Tetracycline hepatotoxicity. Br Med J. 1964;2:1545–6. doi:10.1136/bmj.2.5424.1545. [PMC free article] [PubMed] [Google Scholar]
- 142.Zimmerman HJ. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd edn. Philadelphia: Lippincott Williams and Wilkins; 1999. [Google Scholar]
- 143.Björnsson E, Lindberg J, Olsson R. Liver reactions to oral low-dose tetracyclines. Scand J Gastroenterol. 1997;32:390–5. doi: 10.3109/00365529709007690. doi:10.3109/00365529709007690. [DOI] [PubMed] [Google Scholar]
- 144.Whalley PJ, Adams RH, Combes B. Tetracycline toxicity in pregnancy. Liver and pancreatic dysfunction. JAMA. 1964;189:357–62. [PubMed] [Google Scholar]
- 145.Heaton PC, Fenwick SR, Brewer DE. Association between tetracycline or doxycycline and hepatotoxicity: a population based case–control study. J Clin Pharm Ther. 2007;32:483–7. doi: 10.1111/j.1365-2710.2007.00853.x. doi:10.1111/j.1365-2710.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- 146.Gough A, Chapman S, Wagstaff K, et al. Minocycline induced autoimmune hepatitis and systemic lupus erythematosus-like syndrome. BMJ. 1996;312:169–72. doi: 10.1136/bmj.312.7024.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Knowles SR, Shapiro L, Shear NH. Serious adverse reactions induced by minocycline. Report of 13 patients and review of the literature. Arch Dermatol. 1996;132:934–9. doi:10.1001/archderm.132.8.934. [PubMed] [Google Scholar]
- 148.De Bus L, Depuydt P, Libbrecht L, et al. Severe drug-induced liver injury associated with prolonged use of linezolid. J Med Toxicol. 2010;6:322–6. doi: 10.1007/s13181-010-0047-0. doi:10.1007/s13181-010-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.De Vriese AS, Coster RV, Smet J, et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis. 2006;42:1111–7. doi: 10.1086/501356. doi:10.1086/501356. [DOI] [PubMed] [Google Scholar]
- 150.Takahashi Y, Takesue Y, Nakajima K, et al. Risk factors associated with the development of thrombocytopenia in patients who received linezolid therapy. J Infect Chemother. 2010;17:126–38. doi: 10.1007/s10156-010-0182-1. [DOI] [PubMed] [Google Scholar]
- 151.Ikuta SI, Tanimura K, Yasui C, et al. Chronic liver disease increases the risk of linezolid-related thrombocytopenia in methicillin-resistant Staphylococcus aureus-infected patients after digestive surgery. J Infect Chemother. 2010 doi: 10.1007/s10156-010-0188-8. Epub ahead of print 16 December 2010; doi:10.1007/S10156-010-0188-8. [DOI] [PubMed] [Google Scholar]
- 152.Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000–7. doi: 10.1016/j.jhep.2008.03.009. doi:10.1016/j.jhep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 153.Pea F, Viale P, Lugano M, et al. Biliary penetration and pharmacodynamic exposure of linezolid in liver transplant patients. J Antimicrob Chemother. 2009;63:167–9. doi: 10.1093/jac/dkn442. doi:10.2217/14622416.9.11.1617. [DOI] [PubMed] [Google Scholar]
- 154.Tostmann A, Boeree MJ, Aarnoutse RE, et al. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x. doi:10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 155.Yew WW, Leung CC. Antituberculosis drugs and hepatotoxicity. Respirology. 2006;11:699–707. doi: 10.1111/j.1440-1843.2006.00941.x. doi:10.1111/j.1440-1843.2006.00941.x. [DOI] [PubMed] [Google Scholar]
- 156.Idilman R, Ersoz S, Coban S, et al. Antituberculous therapy-induced fulminant hepatic failure: successful treatment with liver transplantation and nonstandard antituberculous therapy. Liver Transpl. 2006;12:1427–30. doi: 10.1002/lt.20839. doi:10.1002/lt.20839. [DOI] [PubMed] [Google Scholar]
- 157.Singh J, Garg PK, Tandon RK. Hepatotoxicity due to antituberculosis therapy. Clinical profile and reintroduction of therapy. J Clin Gastroenterol. 1996;22:211–4. doi: 10.1097/00004836-199604000-00012. doi:10.1097/00004836-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 158.Vilarica AS, Diogo N, Andre M, et al. Adverse reactions to antituberculosis drugs in in-hospital patients: severity and risk factors. Rev Port Pneumol. 2010;16:431–51. doi: 10.1016/s0873-2159(15)30040-4. [DOI] [PubMed] [Google Scholar]
- 159.Sharma SK, Singla R, Sarda P, et al. Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clin Infect Dis. 2010;50:833–9. doi: 10.1086/650576. doi:10.1086/650576. [DOI] [PubMed] [Google Scholar]
- 160.Forget EJ, Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf. 2006;5:231–49. doi: 10.1517/14740338.5.2.231. doi:10.1517/14740338.5.2.231. [DOI] [PubMed] [Google Scholar]
- 161.Krishnaswamy K, Prasad CE, Murthy KJ. Hepatic dysfunction in undernourished patients receiving isoniazid and rifampicin. Trop Geogr Med. 1991;43:156–60. [PubMed] [Google Scholar]
- 162.van den Brande P, van Steenbergen W, Vervoort G, et al. Aging and hepatotoxicity of isoniazid and rifampin in pulmonary tuberculosis. Am J Respir Crit Care Med. 1995;152:1705–8. doi: 10.1164/ajrccm.152.5.7582317. [DOI] [PubMed] [Google Scholar]
- 163.Døssing M, Wilcke JT, Askgaard DS, et al. Liver injury during antituberculosis treatment: an 11-year study. Tuber Lung Dis. 1996;77:335–40. doi: 10.1016/s0962-8479(96)90098-2. doi:10.1016/S0962-8479(96)90098-2. [DOI] [PubMed] [Google Scholar]
- 164.Lee AM, Mennone JZ, Jones RC, et al. Risk factors for hepatotoxicity associated with rifampin and pyrazinamide for the treatment of latent tuberculosis infection: experience from three public health tuberculosis clinics. Int J Tuberc Lung Dis. 2002;6:995–1000. [PubMed] [Google Scholar]
- 165.Fernández-Villar A, Sopeña B, Fernández-Villar J, et al. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2004;8:1499–505. [PubMed] [Google Scholar]
- 166.Kunst H, Khan KS. Age-related risk of hepatotoxicity in the treatment of latent tuberculosis infection: a systematic review. Int J Tuberc Lung Dis. 2010;14:1374–81. [PubMed] [Google Scholar]
- 167.Kaneko Y, Nagayama N, Kawabe Y, et al. [Drug-induced hepatotoxicity caused by anti-tuberculosis drugs in tuberculosis patients complicated with chronic hepatitis] Kekkaku. 2008;83:13–9. [PubMed] [Google Scholar]
- 168.Marzuki OA, Fauzi AR, Ayoub S, et al. Prevalence and risk factors of anti-tuberculosis drug-induced hepatitis in Malaysia. Singapore Med J. 2008;49:688–93. [PubMed] [Google Scholar]
- 169.Nader LA, de Mattos AA, Picon PD, et al. Hepatotoxicity due to rifampicin, isoniazid and pyrazinamide in patients with tuberculosis: is anti-HCV a risk factor? Ann Hepatol. 2010;9:70–4. [PubMed] [Google Scholar]
- 170.Gordin FM, Cohn DL, Matts JP, et al. Hepatotoxicity of rifampin and pyrazinamide in the treatment of latent tuberculosis infection in HIV-infected persons: is it different than in HIV-uninfected persons? Clin Infect Dis. 2004;39:561–5. doi: 10.1086/422724. doi:10.1086/422724. [DOI] [PubMed] [Google Scholar]
- 171.Coca NS, Oliveira MS, Voieta I, et al. Antituberculosis drug-induced hepatotoxicity: a comparison between patients with and without human immunodeficiency virus seropositivity. Rev Soc Bras Med Trop. 2010;43:624–8. doi: 10.1590/s0037-86822010000600004. doi:10.1590/S0037-86822010000600004. [DOI] [PubMed] [Google Scholar]
- 172.Pedral-Sampaio DB, Martins NE, Alcantara AP, et al. Use of standard therapy for tuberculosis is associated with increased adverse reactions in patients with HIV. Braz J Infect Dis. 1997;1:123–30. [PubMed] [Google Scholar]
- 173.Musch E, Eichelbaum M, Wang JK, et al. [Incidence of hepatotoxic side effects during antituberculous therapy (INH, RMP, EMB) in relation to the acetylator phenotype (author's transl.)] Klin Wochenschr. 1982;60:513–9. doi: 10.1007/BF01756097. doi:10.1007/BF01756097. [DOI] [PubMed] [Google Scholar]
- 174.Hussain Z, Kar P, Husain SA. Antituberculosis drug-induced hepatitis: risk factors, prevention and management. Indian J Exp Biol. 2003;41:1226–32. [PubMed] [Google Scholar]
- 175.Lee SW, Chung LS, Huang HH, et al. NAT2 and CYP2E1 polymorphisms and susceptibility to first-line anti-tuberculosis drug-induced hepatitis. Int J Tuberc Lung Dis. 2010;14:622–6. [PubMed] [Google Scholar]
- 176.Wang T, Yu HT, Wang W, et al. Genetic polymorphisms of cytochrome P450 and glutathione S-transferase associated with antituberculosis drug-induced hepatotoxicity in Chinese tuberculosis patients. J Int Med Res. 2010;38:977–86. doi: 10.1177/147323001003800324. [DOI] [PubMed] [Google Scholar]
- 177.Bose PD, Sarma MP, Medhi S, et al. Role of polymorphic N-acetyl transferase2 and cytochrome P4502E1 gene in antituberculosis treatment-induced hepatitis. J Gastroenterol Hepatol. 2011;26:312–8. doi: 10.1111/j.1440-1746.2010.06355.x. doi:10.1111/j.1440-1746.2010.06355.x. [DOI] [PubMed] [Google Scholar]
- 178.Ellard GA. [The potential clinical significance of the isoniazid acetylator phenotype in the treatment of pulmonary tuberculosis] Rev Mal Respir. 1984;1:207–19. [PubMed] [Google Scholar]
- 179.Singh J, Arora A, Garg PK, et al. Antituberculosis treatment-induced hepatotoxicity: role of predictive factors. Postgrad Med J. 1995;71:359–62. doi: 10.1136/pgmj.71.836.359. doi:10.1136/pgmj.71.836.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nolan CM, Sandblom RE, Thummel KE, et al. Hepatotoxicity associated with acetaminophen usage in patients receiving multiple drug therapy for tuberculosis. Chest. 1994;105:408–11. doi: 10.1378/chest.105.2.408. doi:10.1378/chest.105.2.408. [DOI] [PubMed] [Google Scholar]
- 181.Durand F, Jebrak G, Pessayre D, et al. Hepatotoxicity of antitubercular treatments. Rationale for monitoring liver status. Drug Saf. 1996;15:394–405. doi: 10.2165/00002018-199615060-00004. doi:10.2165/00002018-199615060-00004. [DOI] [PubMed] [Google Scholar]
- 182.Taneja DP, Kaur D. Study on hepatotoxicity and other side-effects of antituberculosis drugs. J Indian Med Assoc. 1990;88:278–80. [PubMed] [Google Scholar]
- 183.Akbri MZ, Fatima N, uL Haque E, et al. Liver function tests in patients of pulmonary tuberculosis using four different drug regimens. J Ayub Med Coll Abbottabad. 2001;13:5–10. [PubMed] [Google Scholar]
- 184.Younossian AB, Rochat T, Ketterer JP, et al. High hepatotoxicity of pyrazinamide and ethambutol for treatment of latent tuberculosis. Eur Respir J. 2005;26:462–4. doi: 10.1183/09031936.05.00006205. doi:10.1183/09031936.05.00006205. [DOI] [PubMed] [Google Scholar]
- 185.Woo J, Chan CH, Walubo A, et al. Hydrazine—a possible cause of isoniazid-induced hepatic necrosis. J Med. 1992;23:51–9. [PubMed] [Google Scholar]
- 186.Page KR, Sifakis F, Montes de Oca R, et al. Improved adherence and less toxicity with rifampin vs isoniazid for treatment of latent tuberculosis: a retrospective study. Arch Intern Med. 2006;166:1863–70. doi: 10.1001/archinte.166.17.1863. doi:10.1001/archinte.166.17.1863. [DOI] [PubMed] [Google Scholar]
- 187.Menzies D, Long R, Trajman A, et al. Adverse events with 4 months of rifampin therapy or 9 months of isoniazid therapy for latent tuberculosis infection: a randomized trial. Ann Intern Med. 2008;149:689–97. doi: 10.7326/0003-4819-149-10-200811180-00003. [DOI] [PubMed] [Google Scholar]
- 188.McNeill L, Allen M, Estrada C, et al. Pyrazinamide and rifampin vs isoniazid for the treatment of latent tuberculosis: improved completion rates but more hepatotoxicity. Chest. 2003;123:102–6. doi: 10.1378/chest.123.1.102. doi:10.1378/chest.123.1.102. [DOI] [PubMed] [Google Scholar]
- 189.van Hest R, Baars H, Kik S, et al. Hepatotoxicity of rifampin–pyrazinamide and isoniazid preventive therapy and tuberculosis treatment. Clin Infect Dis. 2004;39:488–96. doi: 10.1086/422645. doi:10.1086/422645. [DOI] [PubMed] [Google Scholar]
- 190.Pessayre D, Bentata M, Degott C, et al. Isoniazid–rifampin fulminant hepatitis. A possible consequence of the enhancement of isoniazid hepatotoxicity by enzyme induction. Gastroenterology. 1977;72:284–9. [PubMed] [Google Scholar]
- 191.Askgaard DS, Wilcke T, Dossing M. Hepatotoxicity caused by the combined action of isoniazid and rifampicin. Thorax. 1995;50:213–4. doi: 10.1136/thx.50.2.213. doi:10.1136/thx.50.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ramakrishnan CV, Janardhanam B, Krishnamurthy DV, et al. Toxicity of pyrazinamide, administered once weekly in high dosage, in tuberculous patients. Bull World Health Organ. 1968;39:775–9. [PMC free article] [PubMed] [Google Scholar]
- 193.Pasipanodya JG, Gumbo T. Clinical and toxicodynamic evidence that high-dose pyrazinamide is not more hepatotoxic than the low doses currently used. Antimicrob Agents Chemother. 2010;54:2847–54. doi: 10.1128/AAC.01567-09. doi:10.1128/AAC.01567-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chang KC, Leung CC, Yew WW, et al. Standard anti-tuberculosis treatment and hepatotoxicity: do dosing schedules matter? Eur Respir J. 2007;29:347–51. doi: 10.1183/09031936.00090306. doi:10.1183/09031936.00090306. [DOI] [PubMed] [Google Scholar]
- 195.Yee D, Valiquette C, Pelletier M, et al. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167:1472–7. doi: 10.1164/rccm.200206-626OC. doi:10.1164/rccm.200206-626OC. [DOI] [PubMed] [Google Scholar]
- 196.Chang KC, Leung CC, Yew WW, et al. Hepatotoxicity of pyrazinamide: cohort and case–control analyses. Am J Respir Crit Care Med. 2008;177:1391–6. doi: 10.1164/rccm.200802-355OC. doi:10.1164/rccm.200802-355OC. [DOI] [PubMed] [Google Scholar]
- 197.Tahaoglu K, Atac G, Sevim T, et al. The management of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2001;5:65–9. [PubMed] [Google Scholar]
- 198.al Sarraf KA, Michielsen PP, Hauben EI, et al. Hepatotoxicity after a short course of low-dose pyrazinamide. Acta Gastroenterol Belg. 1996;59:251–3. [PubMed] [Google Scholar]
- 199.Fountain FF, Tolley EA, Jacobs AR, et al. Rifampin hepatotoxicity associated with treatment of latent tuberculosis infection. Am J Med Sci. 2009;337:317–20. doi: 10.1097/MAJ.0b013e31818c0134. doi:10.1097/MAJ.0b013e31818c0134. [DOI] [PubMed] [Google Scholar]
- 200.Schaberg T, Rebhan K, Lode H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J. 1996;9:2026–30. doi: 10.1183/09031936.96.09102026. doi:10.1183/09031936.96.09102026. [DOI] [PubMed] [Google Scholar]
- 201.Ziakas PD, Mylonakis E. 4 months of rifampin compared with 9 months of isoniazid for the management of latent tuberculosis infection: a meta-analysis and cost-effectiveness study that focuses on compliance and liver toxicity. Clin Infect Dis. 2009;49:1883–9. doi: 10.1086/647944. doi:10.1086/647944. [DOI] [PubMed] [Google Scholar]
- 202.Chan CH, Or KK, Cheung W, et al. Adverse drug reactions and outcome of elderly patients on antituberculosis chemotherapy with and without rifampicin. J Med. 1995;26:43–52. [PubMed] [Google Scholar]
- 203.Tortajada C, Martinez-Lacasa J, Sanchez F, et al. Is the combination of pyrazinamide plus rifampicin safe for treating latent tuberculosis infection in persons not infected by the human immunodeficiency virus? Int J Tuberc Lung Dis. 2005;9:276–81. [PubMed] [Google Scholar]
- 204.Ruslami R, Nijland HM, Alisjahbana B, et al. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob Agents Chemother. 2007;51:2546–51. doi: 10.1128/AAC.01550-06. doi:10.1128/AAC.01550-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kunimoto D, Warman A, Beckon A, et al. Severe hepatotoxicity associated with rifampin–pyrazinamide preventative therapy requiring transplantation in an individual at low risk for hepatotoxicity. Clin Infect Dis. 2003;36:e158–61. doi: 10.1086/375072. doi:10.1086/375072. [DOI] [PubMed] [Google Scholar]
- 206.Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43:1003–12. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Molokhia M, McKeigue P. EUDRAGENE: European collaboration to establish a case–control DNA collection for studying the genetic basis of adverse drug reactions. Pharmacogenomics. 2006;7:633–8. doi: 10.2217/14622416.7.4.633. doi:10.2217/14622416.7.4.633. [DOI] [PubMed] [Google Scholar]
- 208.Liss G, Lewis JH. Drug-induced liver injury: what was new in 2008? Expert Opin Drug Metab Toxicol. 2009;5:843–60. doi: 10.1517/17425250903018904. doi:10.1517/17425250903018904. [DOI] [PubMed] [Google Scholar]
- 209.Tohkin M, Ishiguro A, Kaniwa N, et al. Prediction of severe adverse drug reactions using pharmacogenetic biomarkers. Drug Metab Pharmacokinet. 2010;25:122–33. doi: 10.2133/dmpk.25.122. doi:10.2133/dmpk.25.122. [DOI] [PubMed] [Google Scholar]
- 210.Vial T, Biour M, Descotes J, et al. Antibiotic-associated hepatitis: update from 1990. Ann Pharmacother. 1997;31:204–20. doi: 10.1177/106002809703100213. [DOI] [PubMed] [Google Scholar]
- 211.Zimpfer A, Propst A, Mikuz G, et al. Ciprofloxacin-induced acute liver injury: case report and review of literature. Virchows Arch. 2004;444:87–9. doi: 10.1007/s00428-003-0917-9. doi:10.1007/s00428-003-0917-9. [DOI] [PubMed] [Google Scholar]
- 212.Moore RD, Smith CR, Lipsky JJ, et al. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med. 1984;100:352–7. doi: 10.7326/0003-4819-100-3-352. [DOI] [PubMed] [Google Scholar]