Abstract
Objectives
To investigate whether the major fungal multidrug efflux systems (MESs) affect the efficiency of methylene blue (MB)-mediated antimicrobial photodynamic inactivation (APDI) in pathogenic fungi and test specific inhibitors of these efflux systems to potentiate APDI.
Methods
Candida albicans wild-type and mutants that overexpressed two classes of MESs [ATP-binding cassette (ABC) and major facilitator superfamily (MFS)] were tested for APDI using MB as the photosensitizer with and without addition of MES inhibitors. The uptake and cytoplasm localization of photosensitizer were achieved using laser confocal microscopy.
Results
ABC MES overexpression reduced MB accumulation and APDI killing more than MFS MES overexpression. Furthermore, by combining MB APDI with the ABC inhibitor verapamil, fungal killing and MB uptake were potentiated, while by combining MB APDI with the MFS inhibitor INF271, fungal killing and MB uptake were inhibited. This latter surprising finding may be explained by the hypothesis that the MFS channel can also serve as an uptake mechanism for MB.
Conclusions
The ABC pumps are directly implicated in MB efflux from the cell cytoplasm. Both the influx and efflux of MB may be regulated by MFS systems, and blocking this gate before incubation with MB can decrease the uptake and APDI effects. An ABC inhibitor could be usefully combined with MB APDI for treating C. albicans infections.
Keywords: ABC transporters, major facilitator superfamily, photodynamic therapy, photosensitizer, verapamil
Introduction
Infections caused by fungi can be serious, especially in immunocompromised and debilitated patients (HIV infection, transplantation, corticosteroid therapy and lymphoma).1 The incidence of invasive mycoses has increased significantly over the last 3 decades and now represents an exponentially growing threat for human health due to a combination of difficult diagnosis and a shortage of effective antifungal drugs. Thus, systemic fungal infections result in high attributable mortality. Candida albicans is the most common pathogenic yeast species, representing about 60% of all yeasts isolated in clinical samples. Azole antifungals are commonly used for fungal infections; however, recurrence of clinical signs is a common observation.2,3 High-level, clinically significant azole resistance usually involves overexpression of plasma membrane multidrug efflux systems (MESs) belonging to the ATP-binding cassette (ABC) or the major facilitator superfamily (MFS) classes of transporters.4–7 Phylogenetic analysis of the ABC family has provided a new understanding of this important class of efflux pumps. Several approaches have been proposed to tackle efflux-mediated antifungal drug resistance, including (i) the use of alternative antifungal drugs that are not efflux pump substrates (such as the echinocandins); (ii) targeting efflux pump transcriptional regulators and fungal stress response pathways; (iii) blockade of energy supply; and (iv) direct pharmacological inhibition of efflux pumps.
Photodynamic therapy (PDT) combines a non-toxic photoactivatable dye or photosensitizer (PS) with harmless visible light of the correct wavelength to excite the dye to its reactive triplet state, which will then generate reactive oxygen species such as singlet oxygen and hydroxyl radicals that are toxic to cells.8–10 PDT, discovered more than 100 years ago, has a killing effect on microorganisms,11 and has been successfully employed in clinics as a treatment for cancer12 and age-related macular degeneration.13 The exponentially increasing threat of microbial multidrug resistance has highlighted antimicrobial photodynamic inactivation (APDI) as a promising alternative treatment for localized infections.10,14,15 APDI involves the direct application of the PS to the infected tissue rather than being injected intravenously, as is usual with PDT for cancer.
A frequently employed class of antimicrobial PSs are the blue dyes known as phenothiazinium salts, including toluidine blue O (TBO),7 methylene blue (MB)9 and azure dyes.16 Phenothiazinium salts are amphipathic tricyclic planar molecules that possess one intrinsic quaternary nitrogen atom and have phototoxic efficiency against a broad range of microorganisms,17,18 including Escherichia coli,17 Staphylococcus aureus,18 streptococci,19 Listeria monocytogenes,20 Vibrio vulnificus21 and Candida albicans.9,22 At the present time, the only PSs used clinically for antimicrobial treatments are phenothiazinium salts. For instance, the combination of MB or TBO together with red light is used to disinfect blood products, sterilize dental cavities and root canals, and for the treatment of periodontitis.23
APDI has been shown to have a microbicidal effect against a broad range of pathogens.15,22,24–28 It is well known that there is a fundamental difference in susceptibility between Gram-positive bacteria, Gram-negative bacteria and fungi due to physiological differences in the cell walls in these classes of microorganisms.15 In addition, different members of the same class of microbes seem to have different susceptibility patterns to APDI due to intrinsic metabolic determinants (enzymatic defence against reactive oxygen species and efflux pumps that recognize PS molecules).
It has been suggested that amphipathic cations represent the existing natural substrates of microbial efflux pumps (MEPs),29 and it has been established that disabling MEPs by employing either efflux mutants or synthetic efflux pump inhibitors (EPIs) leads to a striking increase in the activity of a wide array of plant secondary metabolites, including natural efflux substrates.30 We previously showed that phenothiazinium salts, which are structurally characterized as amphipathic cations, are substrates of efflux systems in Gram-positive and Gram-negative bacteria.31 These observations led to a study that demonstrated that specific EPIs could potentiate APDI in bacteria.31
The main aim of this study was to investigate whether the major fungal efflux systems (MFSs and ABCs) affect the efficiency of MB-mediated APDI in pathogenic fungi. We report that both MESs in C. albicans do affect the efficiency of APDI using MB and red light, and that the fungicidal effect of MB APDI can be potentiated by the ABC inhibitor verapamil, but not by the MFS inhibitor INF271.
Materials and methods
Microbial strains
The C. albicans strains used in this study are listed in Table 1.32–39 C. albicans DAY185 is not an azole-resistant strain, similar to YEM12. However, the resistance of strains YEM13, 14 and 15 to azoles is higher (64–128 × MIC) than YEM12 and DAY185.33,39 Cells were grown by shaking in yeast extract/peptone/dextrose (YPD) liquid medium at 30°C and cell density was assessed with a spectrophotometer (Mini 1240, Shimadzu, Columbia, MD, USA) at 600 nm (OD600).
Table 1.
C. albicans strains used in this study and their fluconazole susceptibility
| Strain name | Characteristics | Fluconazole MIC (mg/L) |
|---|---|---|
| DAY185 | wild-type32 | 0.533 |
| YEM12 | parent of YEM1333 | 139 |
| YEM13 | overexpressing MDR133 | 6439 |
| YEM14 | parent of YEM1533 | 6439 |
| YEM15 | overexpressing CDR1, CDR233–35 | 6439 |
MDR1, multidrug resistance (protein) 1.
Incubation with compounds
Yeast suspensions in PBS (initial concentration 107 cfu/mL) were incubated with the appropriate pump inhibitors for 30 min and then the cells were centrifuged at 4000 g and washed twice in PBS. Verapamil (+) (Sigma-Aldrich, St Louis, MO, USA) was designated as an EPI for ABCs, and INF271 (Chembridge, San Diego, CA, USA) has been extensively used to block MFSs mostly in Gram-positive bacteria.7,40,41 Stock solutions were prepared in DMSO (INF271) or ethanol (verapamil) at a concentration of 10 mg/mL and stored at −20°C. The inhibitors were incubated with yeast cells at final concentrations of 10, 25 and 50 μg/mL. Subsequently the washed cells were incubated with PS for another 30 min, then centrifuged (as described earlier), washed twice and resuspended in sterile PBS. A stock solution of MB (Sigma-Aldrich) was prepared in distilled water at a concentration of 10 mM, filtered through a sterile 0.22 μm filter membrane and stored for a maximum of 2 weeks at 4°C in the dark before use. In some cases the yeast cells were incubated with PS alone, without inhibitors.
APDI studies
The yeast suspensions were placed in 96-well microtitre plates (Fisher Scientific, Pittsburgh, PA, USA) and illuminated using a GaAlAs diode laser (Photon Lase III, DMC, São Carlos, Brazil) with a wavelength of 660 nm and a total power of 50 mW. Yeast strains were continually irradiated from the top of the flat-bottom microtitre plate with fluences ranging from 0 to 60 J/cm2 at a fluence rate of 165 mW/cm2. During illumination, aliquots of 20 μL were taken to determine the cfus. The contents of the wells were constantly stirred during illumination (to ensure that yeast cells did not settle to the bottom of the wells) and were mixed before sampling. The aliquots were serially diluted 10-fold in PBS to give dilutions of 10−1 to 10−5 times the original concentrations and were streaked horizontally on square YPD agar plates as described by Jett et al.42 This allowed a maximum of 7 logs of killing to be measured. Plates were incubated at 37°C overnight. Two types of control conditions were used: illumination in the absence of PS and incubation with PS in the dark.
PS uptake
C. albicans cells were incubated with EPI as previously described and then rhodamine 123 (R123; Eastman Kodak, Rochester, NY, USA), a mitochondrial localizing dye,43 and MB were added into sample tubes at a final concentration of 10 μM and 100 μM, respectively. Suspensions were incubated at room temperature for 30 min, washed twice and fixed in 2% formaldehyde and 10% glycerol. Aliquots of 4 μL were taken from the pellet, placed on a slide and covered with a cover slip for analysis. A confocal laser microscope (Leica TCS NT, Leica Mikroskopie und System GmBH, Wetzlar, Germany) with excitation at 488 nm from an argon laser was used. The cells were observed with an ×100 oil immersion objective and images at 512 × 512 pixels resolution were recorded with a dimension of 0.13 μm on each side of one pixel. Two channels collected fluorescence signals in either the green range (580 nm dichroic mirror plus 525/50 nm bandpass filter) from R123 or in the red range (580 nm dichroic mirror plus 665 nm longpass filter) from MB. The false output colour green and red images were superimposed for the figures. The images were analysed by counting the red pixels for cells in the fields (related to MB fluorescence). The cells were analysed individually using image processing software (ImageJ 1.41o, National Institutes of Health, Bethesda, MD, USA) and red, green, blue (RGB) data were measured in the selected fields.
Statistics
Values are means from three separate experiments and bars are standard deviations (SDs). Statistical analysis of the cfu data was performed using one-way analysis of variance (ANOVA). Mean comparisons were carried out with Tukey's test with a significance level at 5% (P < 0.05).44 Differences between RGB data means were compared using a t-test with the overall significance level set at 5%.
Results
The survival fraction of C. albicans strains treated only with laser irradiation or with MB alone did not show a significant difference compared with the control cells in all experiments (data not shown). There were substantial differences in the killing effect and dye uptake after MB-mediated APDI. Due to the efflux-specific characteristics of each strain and the experimental methodology adopted, the data will be presented in different sections.
Impact of MFS efflux systems in MB-mediated APDI
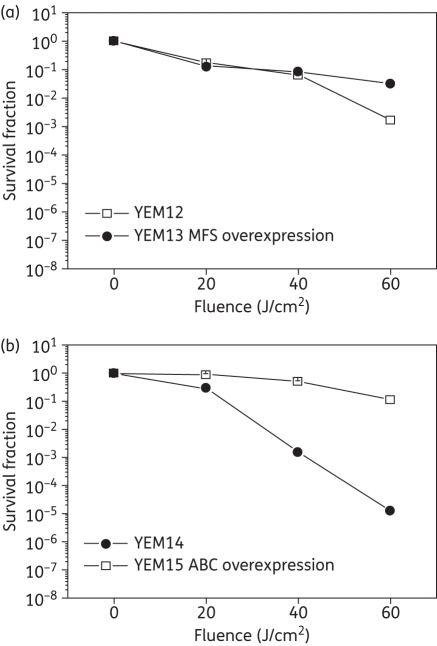
In order to test this hypothesis we employed an isogenic pair of C. albicans strains: the YEM13-overexpressing MFS system and the parent YEM12. Incubation with 100 μm MB and subsequent APDI revealed identical phototoxicity between both strains up to 40 J/cm2 (Figure 1a). An increase in fluence (60 J/cm2) led to a statistically significant reduction of 3 logs in C. albicans YEM12 whereas YEM13 exhibited only 1.5 logs of inactivation. This observation indicates that MFS system overexpression contributed slightly to a protective effect of MB APDI phototoxicity in yeast cells after 6 min of irradiation.
Figure 1.
Effect of efflux pumps on photodynamic inactivation of C. albicans. (a) MFS pump. (b) ABC pump. Yeast strains were incubated with 100 μM MB for 30 min, and PS was washed from cells before irradiation with 165 mW/cm2 (λ = 660 nm). Data are the means of three independent experiments and the bars are the SDs.
Impact of the ABC systems in MB-mediated APDI
We tested this hypothesis by comparing APDI with MB for two isogenic C. albicans strains: the parent YEM14 and the YEM15 mutant overexpressing CDR1/CDR2. The effect of ABC overexpression was more prominent when compared with MFS overexpression in protecting against MB-mediated APDI. YEM15 was resistant to APDI killing (no killing after 6 min irradiation, 60 J/cm2 with 100 μM MB) whereas YEM14 showed a fluence-dependent killing, leading to 5 logs of killing (P = 0.008) under the same APDI conditions (Figure 1b). These experiments showed that ABC transporter overexpression effectively abolished the effect of MB-mediated APDI on C. albicans.
Effect of EPIs in MB-mediated APDI
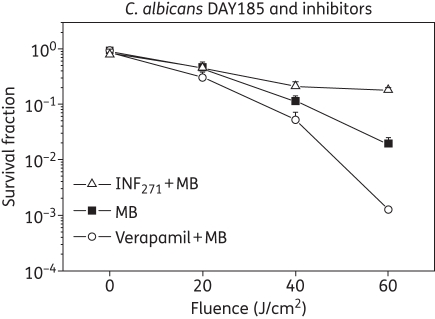
Since efflux systems affect APDI of MB in C. albicans, a logical strategy was to explore whether EPIs could potentiate APDI with MB. We used two well-documented EPIs (one for each corresponding efflux system), INF271 (targeting MFS) and verapamil (+) (known to target ABCs both in yeast and mammalian systems), and used the wild-type reference C. albicans strain DAY185. APDI with MB did not show any significant phototoxicity at 20 J/cm2, but killing increased in a dose–response manner, reaching 2 logs after 6 min of irradiation (60 J/cm2). Pre-exposure to INF271 followed by MB-mediated PDI, resulted in a paradoxical protection rather than enhancement of phototoxicity (Figure 2).
Figure 2.
Photodynamic inactivation of C. albicans DAY strain in the presence of pump inhibitors. Verapamil (circles) was used to inhibit ABC pumps and INF271 (triangles) was used for MFS pumps. Cells were incubated with verapamil or INF271 before MB was added to the suspensions. The MB experiments (squares) were performed without pump inhibitors. Data are the means of three independent experiments and the bars are the SDs.
On the other hand pre-exposure of DAY185 cells to verapamil (+) and subsequent MB-mediated APDI led to a moderate increase in phototoxicity when compared with the PDI efficacy of MB in the absence of EPIs (1 log, 60 J/cm2, P = 0.00053; Figure 2).
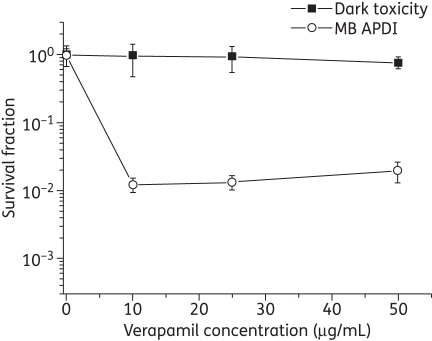
In addition, pre-exposure of the CDR1/CDR2-overexpressing mutant YEM15 in verapamil (+) and subsequent APDI revealed 2 logs of cell reduction in the presence of the EPI at 60 J/cm2, compared with virtually no effect in the absence of the ABC pump inhibitor (Figure 3). However, the increase in verapamil (+) concentration did not enhance the killing effect. Moreover, verapamil (+) did not exhibit apparent direct toxicity towards YEM15 at concentrations as high as 50 μg/mL (Figure 3).
Figure 3.
ABC pump inhibitor (verapamil) increases the MB APDI effect on CDR1/CDR2-overexpressing mutant YEM15. Data are the mean survival fractions and the bars are the SDs.
Effect of efflux systems on PS uptake
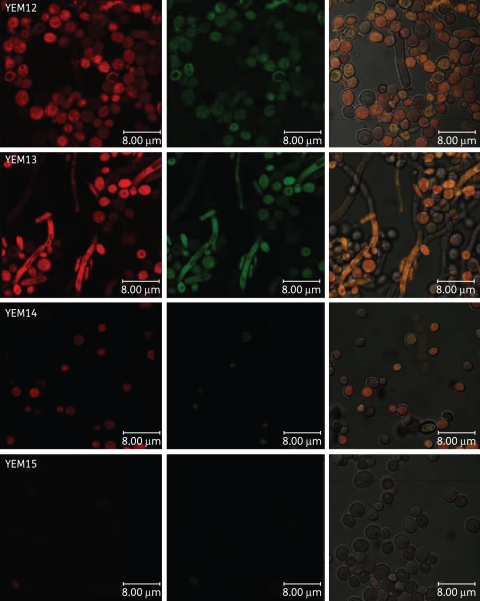
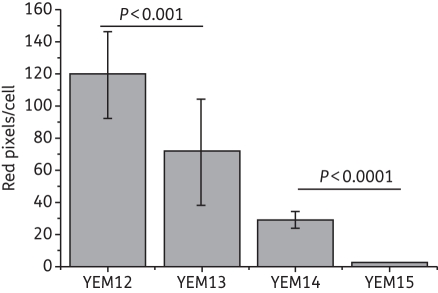
We employed the same isogenic pairs used in the APDI studies—MB and R123 (a designated tracker for mitochondria that is also a substrate of yeast efflux systems)—and confocal laser scanning microscopy (CLSM). Images obtained from the isogenic pair YEM12/YEM13 showed intense red and green fluorescence, indicating the localization of both MB and R123 in the yeast cell (Figure 4). MB accumulation in cytoplasm and nucleus were observed in both strains by the superimposition of green and red fluorescence images. MB did not appear to accumulate in the mitochondria. Comparing the red intensity of the strains, C. albicans YEM12 presented a slight difference compared with YEM13, seen as a more intense red image, suggesting a higher concentration of MB in YEM12. On the other hand, a remarkable difference was observed between CLSM images for accumulation of MB/R123 in the pair YEM14/YEM15 (Figure 4). C. albicans YEM14 showed both red and green fluorescence whereas no detectable MB or R123 fluorescence was observed in YEM15. Furthermore, we employed the CLSM images to determine cellular uptake by the quantification of MB fluorescence (red pixels) (Figure 5). The concentration of intracellular MB was significantly reduced in both mutant strains YEM13 and YEM15 compared with the wild-type strains. However, ABC overexpression promoted an ∼90% (P < 0.001) reduction in the intracellular concentration of MB whereas a 40% (P < 0.01) reduction was observed for the MFS-overexpressing strain. This finding supported further the primary observation from the APDI studies that MB efflux is predominantly affected by the ABC efflux systems in C. albicans.
Figure 4.
Confocal microscopy image of YEM strains. Cells were incubated with 10 μM R123 and 100 μM MB and excited at 488 nm. We present three pictures of the same field. Red corresponds to the fluorescence of MB and green corresponds to the fluorescence of R123. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 5.
Cellular uptake of MB under different conditions of efflux pump expression. The cells were analysed individually using ImageJ software and RGB data were measured in the selected fields. Data are the mean values of red pixels/cell (related to MB fluorescence) and the bars are the SDs.
EPIs and MB uptake in C. albicans DAY185
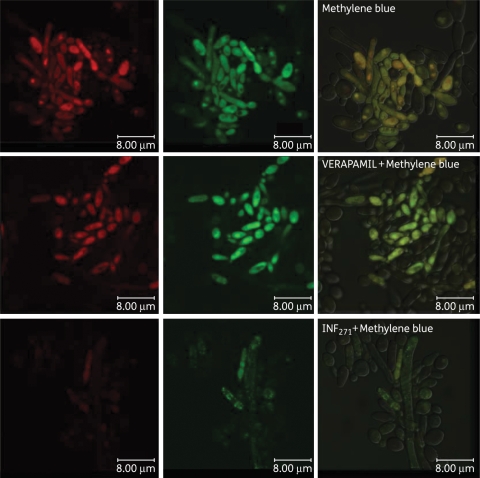
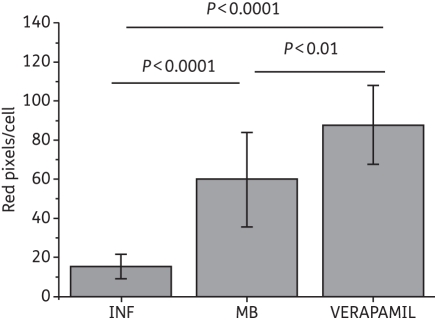
Using CLSM and the pair of EPIs employed in the APDI studies, we assessed differences in accumulation for both MB and R123 in C. albicans DAY185. Incubation with EPIs was performed as described in the Materials and methods section and applied in the APDI experiments. C. albicans DAY185 pre-exposure to verapamil (+) or single incubation with MB and R123 showed intense red and green fluorescence (Figure 6). Superimposed images of MB and R123 fluorescence show MB uptake in mitochondria in the presence or absence of verapamil (+). On the other hand, INF271 pre-exposure reduced the intracellular fluorescence intensity of MB. In addition, quantification of MB fluorescence (red pixels) in CLSM images showed that pre-incubation with each EPI had different effects on MB uptake (Figure 7). The verapamil (+) inhibition of ABC pumps increased the amount of intracellular MB by 50% (P<0.01); however, INF271 reduced MB uptake by 70% (P < 0.0001).
Figure 6.
Confocal microscopy image of the DAY wild-type strain. Cells were incubated with the major facilitator inhibitor (INF271) or the ABC transporter inhibitor (verapamil) before incubation with 10 µM R123 and 100 µM MB and excited at 488 nm. Green corresponds to the fluorescence of R123 and red corresponds to the fluorescence of MB. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 7.
Cellular uptake of MB in the presence of MEP inhibitors. The cells were analysed individually using ImageJ software and RGB data were measured in the selected fields. Data are the mean values of red pixels/cell (related to MB fluorescence) and the bars are the SDs.
Discussion
There have been a substantial number of published APDI studies that target pathogenic fungi employing an array of PSs, including phenothiazinium salts (MB and TBO), under a range of conditions.8,9,23,45–47 Nevertheless, in the case of C. albicans there is minimal information implicating phenotypic cell physiology determinants concerning the uptake and localization of MB as well as information on the APDI mechanism. Giroldo et al.48 suggested that MB-mediated APDI may be related to damage to the plasma membranes. The present study demonstrated a role of the predominant yeast MESs (MFSs and ABCs) in both MB uptake and phototoxicity towards C. albicans. As we have previously reported, MB is a substrate for bacterial efflux systems and the use of specific EPIs enhances its phototoxicity.7,40 MB behaves as a substrate of the phylogenetically distant yeast efflux systems, and these can dramatically alter MB APDI efficacy. Overexpression of both systems reduced MB uptake, as well as the killing effect of MB-mediated APDI, suggesting that MFSs and ABCs are involved in MB export. Moreover, the protective effect of the MFS system in APDI killing was less pronounced than ABCs, and it was observed only after several minutes of irradiation. Since the particular MFS system in C. albicans MDR1 is a proton antiporter and substrate transport is dependent on chemiosmotic ion gradients,6 both MB concentrations inside and outside the cell are important in this process. In the present study, the excess of MB was washed out and the cells were resuspended in PBS before irradiation. Incorporating a wash step before illumination has been shown to dramatically reduce the APDI effect of TBO in C. albicans.49 Thus, the absence of MB outside the cells probably induced a slow efflux of MB through the MFS system, providing a plausible explanation for the relation of MB APDI efficacy and MFS overexpression. On the other hand, evidence of the effect of ABC on MB efflux is compelling, considering both the extremely low uptake and the protection from APDI in the ABC-overexpressing mutant.
Distinct killing effects were observed for wild-type strains DAY185, YEM12 and YEM14 under the same MB concentration and irradiation parameters. In addition, C. albicans DAY185 and YEM12 are more susceptible to azole (MIC 0.5 mg/L and 1 mg/L, respectively) than YEM14 (MIC 64 mg/L).33,39 In contrast, the MB APDI killing was greater for YEM14 compared with the other wild-type strains. Although the azole resistance is also related to efflux pump expression,5 the MB APDI effect is dependent on other phenotypic determinants related to oxidative stress responses (expression of enzymes that detoxify reactive oxygen species and production of scavengers). One possible explanation for the differences in MB APDI effect may be the variation in response to oxidative stress among several C. albicans strains, resulting in different susceptibility patterns to oxidant agents.50 Although each strain can be inactivated under a different set of parameters, we used a sub-lethal dose of APDI to accentuate the differences between parent strains.
Cells were washed after incubation with both EPIs based on our previous observations that pre-incubation with EPIs and subsequent washing enhances antimicrobial PDI.7 It is quite possible that this phenomenon is either species- or EPI-specific, and there is no direct mechanistic evidence regarding whether EPI washing prevents or promotes potential interaction between EPIs and PSs. Regarding the direct antimicrobial effect of both EPIs, there is extensive published information for INF271 in Gram-positive bacteria and non-pathogenic yeasts.30,51 Moreover, verapamil is routinely used worldwide in yeast susceptibility testing as well as rhodamine uptake and efflux experiments with a variety of C. albicans phenotypes at the same or higher concentrations (50–100 μM) without any toxic effect.52
The results of both MB APDI and uptake in DAY185 employing two EPIs (INF271 and verapamil) that have reported activity in each efflux system further supported the initial observations with the efflux-overexpressing phenotypes. Verapamil increased the amount of intracellular MB and improved APDI efficiency. The INF271 contribution was rather puzzling, as it essentially dramatically reduced MB uptake and phototoxicity in DAY185 cells. A possible explanation for this observation involves a more active entry door for MB uptake mediated by MFS. In fact, in this experiment, cells were first incubated with INF271 that blocked MFS systems and then MB was introduced in the suspension. We observed that DAY185 pre-treated with INF271 was not killed and MB uptake was reduced. MFS pump families are reported to be responsible for the uptake and export of several substances in C. albicans.6 The low overall effect of MFS overexpression could be explained if MFS was responsible for both uptake and efflux of MB and the effects of increasing MFS levels effectively cancelled each other out.
Antifungal therapy is a challenging problem in the management of infections caused by clinically relevant fungal pathogens because of the high incidence of resistance developed during therapy, especially in immunocompromised individuals.5 The use of EPIs has been proposed as a viable combination with antimicrobials in a wide variety of pathogenic efflux systems, including those primary suspects in C. albicans.4,7,30,40 The current study demonstrates that designated ABC EPIs (verapamil) substantially potentiated APDI with MB, even against a C. albicans strain overexpressing CDR1/CDR2. Our results also suggest that EPIs may provide a viable and promising combination with antifungal chemotherapy. This pump inhibitor was reported to strongly reduce the MICs for fluconazole and itraconazole resistance of an azole-resistant strain.53
It is proposed that antifungal resistance can be reversed by an ABC pump-dependent mechanism.4 In conclusion, our results demonstrated that the influx as well as the efflux of MB is regulated by MFS systems and blocking this gate before incubation with MB can decrease the uptake and APDI effects. On the other hand, ABC pumps are directly implicated in MB efflux from the cell cytoplasm.
Funding
This work was partially supported by the Brazilian National Commission on Research (CNPq) and FAPESP (2010/13313-9). Research in the Hamblin laboratory is supported by NIH grants (R01A1050875 to M. R. H. and R01CA137108 to Long Y. Chiang) and the US Air Force MFEL Program (FA9550-04-1-0079).
Transparency declarations
None to declare.
Acknowledgements
We thank Dr Olga Lomovskaya (MPEX Pharmaceuticals, San Diego, CA, USA) and Professor Dominique Sanglard (Institute of Microbiology, University Hospital Lausanne, Lausanne, Switzerland) for providing C. albicans strains (YEM12, YEM13, YEM14 and YEM15). We also thank Professor Aaron Mitchell (Department of Biological Sciences, Carnegie Mellon University, Pittsburgh, PA, USA) for the C. albicans DAY185 strain.
References
- 1.Espinel-Ingroff A. Novel antifungal agents, targets or therapeutic strategies for the treatment of invasive fungal diseases: a review of the literature (2005–2009) Rev Iberoam Micol. 2009;26:15–22. doi: 10.1016/S1130-1406(09)70004-X. doi:10.1016/S1130-1406(09)70004-X. [DOI] [PubMed] [Google Scholar]
- 2.d'Enfert C. Hidden killers: persistence of opportunistic fungal pathogens in the human host. Curr Opin Microbiol. 2009;12:358–64. doi: 10.1016/j.mib.2009.05.008. doi:10.1016/j.mib.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Freydiere AM, Guinet R, Boiron P. Yeast identification in the clinical microbiology laboratory: phenotypical methods. Med Mycol. 2001;39:9–33. doi: 10.1080/mmy.39.1.9.33. [DOI] [PubMed] [Google Scholar]
- 4.Schuetzer-Muehlbauer M, Willinger B, Egner R, et al. Reversal of antifungal resistance mediated by ABC efflux pumps from Candida albicans functionally expressed in yeast. Int J Antimicrob Agents. 2003;22:291–300. doi: 10.1016/s0924-8579(03)00213-9. doi:10.1016/S0924-8579(03)00213-9. [DOI] [PubMed] [Google Scholar]
- 5.Cowen LE, Anderson JB, Kohn LM. Evolution of drug resistance in Candida albicans. Annu Rev Microbiol. 2002;56:139–65. doi: 10.1146/annurev.micro.56.012302.160907. doi:10.1146/annurev.micro.56.012302.160907. [DOI] [PubMed] [Google Scholar]
- 6.Pao SS, Paulsen IT, Saier MH., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tegos GP, Masago K, Aziz F, et al. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob Agents Chemother. 2008;52:3202–9. doi: 10.1128/AAC.00006-08. doi:10.1128/AAC.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai TH, Tegos GP, Zhiyentayev T, et al. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg Med. 2010;42:38–44. doi: 10.1002/lsm.20887. doi:10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prates RA, da Silva EG, Yamada AM, et al. Light parameters influence cell viability in antifungal photodynamic therapy in a fluence and rate fluence-dependent manner. Laser Physics. 2009;19:1038–44. doi:10.1134/S1054660X09050284. [Google Scholar]
- 10.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections—state of the art. Photodiagnosis Photodyn Ther. 2009;6:170–88. doi: 10.1016/j.pdpdt.2009.10.008. doi:10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003;23:3591–600. [PubMed] [Google Scholar]
- 12.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–7. doi: 10.1038/nrc1071. doi:10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 13.Cruess AF, Zlateva G, Pleil AM, et al. Photodynamic therapy with verteporfin in age-related macular degeneration: a systematic review of efficacy, safety, treatment modifications and pharmacoeconomic properties. Acta Ophthalmol. 2009;87:118–32. doi: 10.1111/j.1755-3768.2008.01218.x. doi:10.1111/j.1755-3768.2008.01218.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–50. doi: 10.1039/b311900a. doi:10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jori G, Fabris C, Soncin M, et al. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 2006;38:468–81. doi: 10.1002/lsm.20361. doi:10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 16.Wainwright M, Phoenix DA, Laycock SL, et al. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett. 1998;160:177–81. doi: 10.1111/j.1574-6968.1998.tb12908.x. doi:10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- 17.Wainwright M, Phoenix DA, Marland J, et al. A study of photobactericidal activity in the phenothiazinium series. FEMS Immunol Med Microbiol. 1997;19:75–80. doi: 10.1111/j.1574-695X.1997.tb01074.x. doi:10.1111/j.1574-695X.1997.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 18.Phoenix DA, Sayed Z, Hussain S, et al. The phototoxicity of phenothiazinium derivatives against Escherichia coli and Staphylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:17–22. doi: 10.1016/S0928-8244(03)00173-1. doi:10.1016/S0928-8244(03)00173-1. [DOI] [PubMed] [Google Scholar]
- 19.Wilson M, Burns T, Pratten J, et al. Bacteria in supragingival plaque samples can be killed by low-power laser light in the presence of a photosensitizer. J Appl Bacteriol. 1995;78:569–74. doi: 10.1111/j.1365-2672.1995.tb03101.x. [DOI] [PubMed] [Google Scholar]
- 20.Romanova NA, Brovko LY, Moore L, et al. Assessment of photodynamic destruction of Escherichia coli O157:H7 and Listeria monocytogenes by using ATP bioluminescence. Appl Environ Microbiol. 2003;69:6393–8. doi: 10.1128/AEM.69.11.6393-6398.2003. doi:10.1128/AEM.69.11.6393-6398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong TW, Wang YY, Sheu HM, et al. Bactericidal effects of toluidine blue-mediated photodynamic action on Vibrio vulnificus. Antimicrob Agents Chemother. 2005;49:895–902. doi: 10.1128/AAC.49.3.895-902.2005. doi:10.1128/AAC.49.3.895-902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demidova TN, Hamblin MR. Photodynamic therapy targeted to pathogens. Int J Immunopathol Pharmacol. 2004;17:245–54. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wainwright M. The use of dyes in modern biomedicine. Biotech Histochem. 2003;78:147–55. doi: 10.1080/10520290310001602404. doi:10.1080/10520290310001602404. [DOI] [PubMed] [Google Scholar]
- 24.Demidova TN, Gad F, Zahra T, et al. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J Photochem Photobiol B. 2005;81:15–25. doi: 10.1016/j.jphotobiol.2005.05.007. doi:10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monfrecola G, Procaccini EM, Bevilacqua M, et al. In vitro effect of 5-aminolaevulinic acid plus visible light on Candida albicans. Photochem Photobiol Sci. 2004;3:419–22. doi: 10.1039/b315629j. doi:10.1039/b315629j. [DOI] [PubMed] [Google Scholar]
- 26.O'Neill JF, Hope CK, Wilson M. Oral bacteria in multi-species biofilms can be killed by red light in the presence of toluidine blue. Lasers Surg Med. 2002;31:86–90. doi: 10.1002/lsm.10087. doi:10.1002/lsm.10087. [DOI] [PubMed] [Google Scholar]
- 27.Garcez AS, Ribeiro MS, Tegos GP, et al. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg Med. 2007;39:59–66. doi: 10.1002/lsm.20415. doi:10.1002/lsm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Usacheva MN, Teichert MC, Usachev YM, et al. Interaction of the photobactericides methylene blue and toluidine blue with a fluorophore in Pseudomonas aeruginosa cells. Lasers Surg Med. 2008;40:55–61. doi: 10.1002/lsm.20593. doi:10.1002/lsm.20593. [DOI] [PubMed] [Google Scholar]
- 29.Lewis K. Multidrug resistance: versatile drug sensors of bacterial cells. Curr Biol. 1999;9:R403–7. doi: 10.1016/s0960-9822(99)80254-1. doi:10.1016/S0960-9822(99)80254-1. [DOI] [PubMed] [Google Scholar]
- 30.Tegos G, Stermitz FR, Lomovskaya O, et al. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob Agents Chemother. 2002;46:3133–41. doi: 10.1128/AAC.46.10.3133-3141.2002. doi:10.1128/AAC.46.10.3133-3141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tegos GP, Anbe M, Yang C, et al. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob Agents Chemother. 2006;50:1402–10. doi: 10.1128/AAC.50.4.1402-1410.2006. doi:10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobile CJ, Andes DR, Nett JE, et al. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006;2:e63. doi: 10.1371/journal.ppat.0020063. doi:10.1371/journal.ppat.0020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otzen T, Wempe EG, Kunz B, et al. Folate-synthesizing enzyme system as target for development of inhibitors and inhibitor combinations against Candida albicans-synthesis and biological activity of new 2,4-diaminopyrimidines and 4'-substituted 4-aminodiphenyl sulfones. J Med Chem. 2004;47:240–53. doi: 10.1021/jm030931w. doi:10.1021/jm030931w. [DOI] [PubMed] [Google Scholar]
- 34.Watkins WJ, Lemoine RC, Chong L, et al. Quinazolinone fungal efflux pump inhibitors. Part 2: In vitro structure-activity relationships of (N-methyl-piperazinyl)-containing derivatives. Bioorg Med Chem Lett. 2004;14:5133–7. doi: 10.1016/j.bmcl.2004.07.071. doi:10.1016/j.bmcl.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 35.Lemoine RC, Glinka TW, Watkins WJ, et al. Quinazolinone-based fungal efflux pump inhibitors. Part 1: Discovery of an (N-methylpiperazine)-containing derivative with activity in clinically relevant Candida spp. Bioorg Med Chem Lett. 2004;14:5127–31. doi: 10.1016/j.bmcl.2004.07.070. doi:10.1016/j.bmcl.2004.07.070. [DOI] [PubMed] [Google Scholar]
- 36.Sanglard D, Ischer F, Monod M, et al. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40:2300–5. doi: 10.1128/aac.40.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barchiesi F, Arzeni D, Del Prete MS, et al. Fluconazole susceptibility and strain variation of Candida albicans isolates from HIV-infected patients with oropharyngeal candidosis. J Antimicrob Chemother. 1998;41:541–8. doi: 10.1093/jac/41.5.541. doi:10.1093/jac/41.5.541. [DOI] [PubMed] [Google Scholar]
- 38.Sanglard D, Ischer F, Calabrese D, et al. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob Agents Chemother. 1999;43:2753–65. doi: 10.1128/aac.43.11.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruno VM, Mitchell AP. Regulation of azole drug susceptibility by Candida albicans protein kinase CK2. Mol Microbiol. 2005;56:559–73. doi: 10.1111/j.1365-2958.2005.04562.x. doi:10.1111/j.1365-2958.2005.04562.x. [DOI] [PubMed] [Google Scholar]
- 40.Tegos GP, Hamblin MR. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob Agents Chemother. 2006;50:196–203. doi: 10.1128/AAC.50.1.196-203.2006. doi:10.1128/AAC.50.1.196-203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaatz GW. Bacterial efflux pump inhibition. Curr Opin Investig Drugs. 2005;6:191–8. [PubMed] [Google Scholar]
- 42.Jett BD, Hatter KL, Huycke MM, et al. Simplified agar plate method for quantifying viable bacteria. BioTechniques. 1997;23:648–50. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 43.Ball DJ, Luo Y, Kessel D, et al. The induction of apoptosis by a positively charged methylene blue derivative. J Photochem Photobiol B. 1998;42:159–63. doi: 10.1016/s1011-1344(98)00061-x. doi:10.1016/S1011-1344(98)00061-X. [DOI] [PubMed] [Google Scholar]
- 44.Pfaller MA, Burmeister L, Bartlett MS, et al. Multicenter evaluation of four methods of yeast inoculum preparation. J Clin Microbiol. 1988;26:1437–41. doi: 10.1128/jcm.26.8.1437-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ragas X, Sanchez-Garcia D, Ruiz-Gonzalez R, et al. Cationic porphycenes as potential photosensitizers for antimicrobial photodynamic therapy. J Med Chem. 2010;53:7796–803. doi: 10.1021/jm1009555. doi:10.1021/jm1009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang L, Huang YY, Mroz P, et al. Stable synthetic cationic bacteriochlorins as selective antimicrobial photosensitizers. Antimicrob Agents Chemother. 2010;54:3834–41. doi: 10.1128/AAC.00125-10. doi:10.1128/AAC.00125-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donnelly RF, McCarron PA, Tunney MM, et al. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J Photochem Photobiol B. 2007;86:59–69. doi: 10.1016/j.jphotobiol.2006.07.011. doi:10.1016/j.jphotobiol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Giroldo LM, Felipe MP, de Oliveira MA, et al. Photodynamic antimicrobial chemotherapy (PACT) with methylene blue increases membrane permeability in Candida albicans. Lasers Med Sci. 2009;24:109–12. doi: 10.1007/s10103-007-0530-2. doi:10.1007/s10103-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 49.Demidova TN, Hamblin MR. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother. 2005;49:2329–35. doi: 10.1128/AAC.49.6.2329-2335.2005. doi:10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sampaio P, Nogueira E, Loureiro AS, et al. Increased number of glutamine repeats in the C-terminal of Candida albicans Rlm1p enhances the resistance to stress agents. Antonie Van Leeuwenhoek. 2009;96:395–404. doi: 10.1007/s10482-009-9352-5. doi:10.1007/s10482-009-9352-5. [DOI] [PubMed] [Google Scholar]
- 51.Markham PN, Westhaus E, Klyachko K, et al. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:2404–8. doi: 10.1128/aac.43.10.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pina-Vaz C, Rodrigues AG, Costa-de-Oliveira S, et al. Potent synergic effect between ibuprofen and azoles on Candida resulting from blockade of efflux pumps as determined by FUN-1 staining and flow cytometry. J Antimicrob Chemother. 2005;56:678–85. doi: 10.1093/jac/dki264. doi:10.1093/jac/dki264. [DOI] [PubMed] [Google Scholar]
- 53.Stringaro A, Molinari A, Calcabrini A, et al. Detection of human P-glycoprotein-like molecule in azole-resistant Candida albicans from HIV+ patients. Microb Drug Resist. 2002;8:235–44. doi: 10.1089/107662902760326968. doi:10.1089/107662902760326968. [DOI] [PubMed] [Google Scholar]