Abstract
Objectives
It is widely believed that persistent Mycobacterium tuberculosis inhabits necrotic lung granulomas in humans and that the microenvironmental conditions encountered therein render the bacilli phenotypically tolerant to antibiotics, accounting for the long duration required for successful treatment of tuberculosis (TB). To validate this belief, we directly compared the activity of rifampicin/isoniazid/pyrazinamide (RHZ) against chronic TB infection in guinea pigs, which exhibit caseous granulomas histologically resembling human caseous foci, and in mice, which lack necrotic granulomas.
Methods
Guinea pigs and mice were aerosol-infected with M. tuberculosis CDC1551 and twice weekly treatment with RHZ was started 4 weeks later. Culture-positive relapse was assessed in subgroups of guinea pigs after 3 months and 4 months of treatment.
Results
All guinea pig lungs exhibited histological evidence of granulomas with central caseation, while mouse lungs exhibited cellular lesions at the initiation of antibiotic treatment. Guinea pig lungs became culture-negative after 2 months of RHZ given twice weekly at human-equivalent doses. Relapse rates in guinea pigs were 0% (0/10) both after 3 months and 4 months of treatment. In contrast, all mouse lungs remained culture-positive after 4 months of equivalent RHZ exposures.
Conclusions
Caseous necrosis does not reduce the sterilizing activity of the standard antituberculosis regimen of RHZ. Our findings have important implications for the use of alternative animal models in testing novel TB drug regimens and for modelling M. tuberculosis persistence.
Keywords: chronic tuberculosis, guinea pig, mouse, antibiotics, chemotherapy, caseous necrosis, hypoxia, granuloma, persistence
Introduction
Efforts to control tuberculosis (TB) in many parts of the world have been hampered by the lengthy and cumbersome drug regimens currently available, which have contributed to medical non-adherence and the emerging problems of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB.1 The need for such lengthy treatment is believed to reflect the unique ability of a subpopulation of bacilli to remain in a non-replicating persistent state within caseous lung granulomas.2 Although genetically drug susceptible, these ‘persisters’ exhibit phenotypic tolerance3 to currently available first-line drugs.
Historically, the mouse has been the preferred model for the study of antituberculous drugs because of its relatively reduced cost, ease of handling and housing, robustness and ability to accurately recapitulate the activity of the short-course antituberculosis regimen in humans.4 However, a major deficiency of the mouse model of TB is the lack of caseous necrosis, which is the pathological hallmark of human TB.5,6 Guinea pig TB granulomas more closely approximate their human counterparts with respect to cellular composition, granuloma architecture and presence of caseation necrosis.5 Microenvironmental conditions associated with caseous necrosis, including hypoxia,7–9 are thought to interfere with the sterilizing activity of the standard antituberculosis regimen by rendering bacilli phenotypically tolerant to the killing activity of bactericidal drugs like isoniazid.10 Based on this reasoning, we hypothesized that M. tuberculosis infection in the guinea pig is more refractory to anti-TB treatment than in the mouse.
We recently performed pharmacokinetics studies with rifampicin, isoniazid and pyrazinamide, establishing the human-equivalent dose of each of these drugs in guinea pigs.11,12 We also demonstrated that rifampicin/isoniazid/pyrazinamide (RHZ) treatment administered daily for the first 2 weeks and then twice weekly was much more active against acute TB infection in guinea pigs than in mice.12 In the latter study, however, guinea pigs and mice were infected with a high-dose inoculum, and treatment was initiated 14 days after infection, prior to the development of caseous granulomas in the lungs of guinea pigs.11 Therefore, in order to directly test the hypothesis that caseous necrosis interferes with the sterilizing activity of the standard antituberculosis regimen, we designed the current study in which guinea pigs and mice were aerosol-infected with a low-dose inoculum of 102 bacilli and treatment was initiated 28 days post-infection, during the chronic phase of infection in each species, and after the development of caseous necrosis in guinea pigs. Each species received 2 months of RHZ, followed by 2 months of rifampicin/isoniazid. The principal endpoints included sterility of the lungs at the completion of treatment, as well as the percentage of animals showing culture-positive relapse at 3 months of follow-up after having completed 3 months or 4 months of this treatment schedule.
Materials and methods
Bacterial strain
An M. tuberculosis wild-type CDC1551 strain was passaged once in mice and guinea pigs, and frozen in aliquots at −80°C before use. Aliquots were thawed and grown to logarithmic phase in Middlebrook 7H9 broth (Fisher, Pittsburgh, PA, USA) with 10% oleic acid-albumin-dextrose-catalase (Difco, Detroit, MI, USA) and 0.1% Tween 80 (Sigma, St Louis, MO, USA) prior to aerosol infections. The MICs of isoniazid and rifampicin for this strain were determined to be 0.03 mg/L and 0.12 mg/L, respectively.
Antibiotic preparation
Isoniazid and rifampicin were purchased from Sigma-Aldrich (St Louis, MO, USA) and pyrazinamide was purchased from Acros Organics (Morris Plains, NJ, USA). Drugs were prepared in distilled water containing 40% sucrose (weight/volume) in final volumes of 0.2 and 0.5 mL for mice and guinea pigs, respectively. Solutions were prepared weekly and stored at 4°C until use.
Animals
Female outbred Hartley guinea pigs (250–300 g) and female BALB/c mice (6–8 weeks old) were purchased from Charles River (Wilmington, MA, USA). All procedures related to mice or guinea pigs followed protocols approved by the Institutional Animal Care and Use Committee at Johns Hopkins.
Aerosol infections
Seventy-two guinea pigs and 82 mice were aerosol-infected separately with diluted log-phase (OD600 = 0.5) cultures of M. tuberculosis CDC1551 using the Madison chamber aerosol generation device (College of Engineering Shops, University of Wisconsin, Madison, WI, USA) and Inhalation Exposure System (Glas-Col, Terre Haute, IN, USA), respectively. Each aerosol chamber was calibrated to deliver ∼100 cfu into the lungs of individual animals.
Antibiotic treatment
Chemotherapy was initiated 28 days after aerosol infection in each species. During the intensive phase, i.e. for the first 2 months of treatment, both species were given RHZ, and during the continuation phase, i.e. for the remaining 2 months, only rifampicin/isoniazid (2RHZ/2RH) was given. Dosing was twice weekly throughout the treatment in order to avoid toxicity associated with five times weekly administration of the drugs in guinea pigs, as previously reported.12 Mice received 10 mg/kg rifampicin, 25 mg/kg isoniazid and 150 mg/kg pyrazinamide by oesophageal cannula. Guinea pigs received 100 mg/kg rifampicin, 60 mg/kg isoniazid and 300 mg/kg pyrazinamide in the posterior oropharynx by automatic pipette with disposable tip, as previously described.11 Drug doses in each species were selected to yield similar exposures to those following single doses of each drug in humans.11,12 During the study period, mice and guinea pigs were weighed weekly and drug doses were adjusted accordingly based on mean animal body weights. In each species, the rifampicin dose preceded that of the other drugs by at least 1 h to limit drug–drug interactions.
Study endpoints
The basic experimental scheme is shown in Table 1. In order to determine the number of implanted bacilli, the growth of organisms prior to immune containment and the bacillary burden at the onset of treatment, mice and guinea pigs were sacrificed on the day after infection, 2 weeks after infection and 1 month after infection, respectively. The bactericidal activity of RHZ in each species was assessed 2 weeks and 4 weeks after treatment initiation, as well as monthly thereafter until the completion of 4 months of treatment. At each timepoint, the lungs were aseptically removed and the right lung was homogenized and plated on Middlebrook 7H11 plates (Becton Dickinson, Sparks, MD, USA) for cfu enumeration, as previously described.13 Log-transformed cfu values were used to calculate averages and standard deviations for graphing purposes. Data are derived from 5 mice and 4 guinea pigs for each timepoint.
Table 1.
Basic experimental schemea
| Month |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animals/therapy | −1 | −0.5 | 0 | 0.5 | 1 | 2 | 3 | 4 | 3(+3)b | 4(+3)b | total |
| Mice | |||||||||||
| untreated | 5 | 5 | 5 | 5 | 5 | 5 | — | — | — | — | 30 |
| 2RHZ + 2RHc | — | — | 5 | 5 | 5 | 5 | 5 | 5 | 11 | 11 | 52 |
| total | 5 | 5 | 10 | 10 | 10 | 10 | 5 | 5 | 11 | 11 | 82 |
| Guinea pigs | |||||||||||
| untreated | 4 | 4 | 4 | 4 | 4 | 4 | — | — | — | — | 24 |
| 2RHZ + 2RHc | — | — | 4 | 4 | 4 | 4 | 4 | 4 | 11 | 11 | 46 |
| total | 4 | 4 | 8 | 8 | 8 | 8 | 4 | 4 | 11 | 11 | 70 |
aValues in the table are numbers of animals.
b3(+3) and 4(+3) indicate that animals were killed 3 months after completing 3 and 4 months of treatment, respectively.
cIn mice, 2 months of 10 mg/kg rifampicin (R) + 25 mg/kg isoniazid (H) + 150 mg/kg pyrazinamide (Z), followed by 2 months of RH. In guinea pigs, 2 months of 100 mg/kg rifampicin (R) + 60 mg/kg isoniazid (H) + 300 mg/kg pyrazinamide (Z), followed by 2 months of RH. Doses of each drug were determined to be equivalent in each species based on area under the serum concentration-time curve (AUC),11,12 and were given twice weekly in each species throughout.
In order to assess the sterilizing activity of RHZ, treatment was discontinued for groups of 11 guinea pigs after 3 months and 4 months of therapy, and animals were sacrificed 3 months after discontinuation of treatment in order to assess the relapse rate. Relapse was defined as a positive culture upon plating the entire undiluted lung and spleen homogenates from each of 10 animals. The limit of detection for relapse studies was 1 cfu/organ. Since all mouse lungs were found to be culture-positive after 4 months of RHZ treatment, the relapse arms of the mouse study were terminated prematurely.
At each timepoint, lungs and spleens were weighed, photographed and examined for gross pathology. In order to account for the physiological increase in organ weights in ageing animals, organ weights were normalized using the following formula: sacrificed animal organ weight on day of sacrifice × (mean body weight on day after infection/sacrificed animal body weight on day of sacrifice). At each timepoint the left lung from each animal was fixed in 10% buffered formaldehyde for histopathology. Lungs were sectioned in a standardized fashion along the longitudinal axis (apex to lower lobe), traversing the maximum horizontal dimension (through the hilum). The tissue was embedded in paraffin wax, sectioned and stained with both haematoxylin-eosin and Kinyoun stain for acid-fast bacilli (AFB) detection. Secondary granulomas were differentiated from primary granulomas based on their smaller size, preponderance of lymphocytes and scarcity of histiocytes, as well as absence of necrosis.14,15 The tissue sections were evaluated by a single pathologist with expertise in pulmonary pathology (M. M. F.). Images were obtained using an Olympus DP72 camera (Olympus USA, Center Valley, PA, USA).
Results
Morbidity and mortality during treatment
No deaths were observed in either the treated or untreated groups of mice, and no significant change in mean body weight was seen in either mouse group during the course of the experiment (data not shown).
Although no deaths were observed in the treated guinea pigs, two of the untreated guinea pigs died 3 months after aerosol infection due to TB-related disease. During the first 2 weeks of treatment, treated guinea pigs were found to have lower mean body weights relative to the untreated control group, perhaps due to gavage-induced stress (data not shown). However, mean body weights of treated guinea pigs increased thereafter and continued to increase even after completion of treatment in animals kept for relapse studies. In contrast, all untreated guinea pigs began to lose weight by the completion of the experiment, consistent with TB-related morbidity (data not shown).
Organ weights, gross pathology and histology during treatment
As in the case of body weights, mean lung weights of untreated and treated mice remained constant throughout the course of the experiment (ranging from 0.2 ± 0.02 g to 0.34 ± 0.1 g). On the day after infection, normalized mean guinea pig lung weights were 2.1 ± 0.5 g, increasing to 3.0 ± 0.4 g at the initiation of treatment (Figure 1a). Normalized mean lung weights of untreated guinea pigs continued to increase throughout, reaching 7.5 ± 3.9 g at the completion of the experiment (Figure 1a). In contrast, the normalized mean lung weights of treated guinea pigs decreased over time to 1.5 ± 0.2 g at month 4, remaining relatively stable in animals kept for relapse 3 months after treatment completion (Figure 1a). Similar trends were observed for normalized mean spleen weights of treated and untreated guinea pigs (Figure 1b).
Figure 1.
Antibiotic treatment reduces lung and spleen weights in M. tuberculosis-infected guinea pigs. Normalized lung (a) and spleen (b) weights are shown in untreated guinea pigs (continuous lines) and those treated with 2 months of human-equivalent doses of rifampicin, isoniazid and pyrazinamide, followed by 2 months of rifampicin and isoniazid (2RHZ/2RH) (broken lines). n = 5 mice and 4 guinea pigs per timepoint.
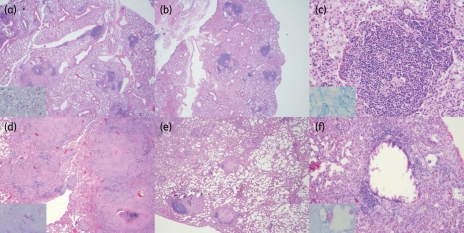
At the initiation of antibiotic treatment, 28 days after aerosol infection, 4/4 guinea pig lungs exhibited grossly visible lesions measuring 1–2 mm in diameter. By the completion of the experiment, tubercle lesions coalesced to cover >70% of the lung surface of all untreated guinea pigs, while the lungs of three treated animals showed fewer than 10 tubercle lesions measuring <1 mm diameter, and one set of lungs showed no visible lesions (data not shown). Histological evaluation at treatment onset revealed well-circumscribed granulomas comprising aggregates of epithelioid histiocytes admixed with a few lymphocytes and plasma cells with central necrosis (Figure 2d). The average number of granulomas at treatment initiation was 46 per lung, with an average diameter of 2400 μm (range 600–4700 μm) and central necrosis averaging 880 μm in diameter. Acid-fast organisms were localized within histiocytes and in the necrotic debris of granulomas. After 4 months of treatment, the average number of primary and secondary granulomas was 6.5 and 10 per lung, respectively. Primary granulomas were predominantly peripheral in location and had areas of necrosis or prominent epithelioid and giant cell reaction (Figure 2e and f). The average diameter of the primary granulomas at treatment completion declined to 365 μm and areas of necrosis measured 245 μm in diameter on average, while secondary granulomas averaged approximately 95 μm and lacked necrosis. The ratio of granulomatous reaction to necrosis was lower before treatment and higher after treatment was completed (Figure 2f). Upon treatment completion, few AFB could still be detected in the necrotic centres of the remaining primary granulomas (Figure 2f, inset), which were present in the periphery of the lungs. Dystrophic calcification and cavitation were absent in all samples.
Figure 2.
Mouse and guinea pig histopathology following antibiotic treatment. (a) Mouse lungs at treatment initiation; lesions are loose aggregates of primarily lymphocytes with few histiocytes and plasma cells, and AFB were detected primarily within foamy macrophages (inset). Low power (b) and high power (c) magnification of mouse lungs after 4 months of treatment; lesions are smaller and cell composition is maintained, and rare intracellular AFB were detected (c, inset). (d) Guinea pig lungs at treatment initiation; large, well-circumscribed granulomas comprising mainly histiocytes and a few lymphocytes are seen, with central necrosis. Acid-fast staining revealed the presence of organisms within histiocytes and in the necrotic debris of granulomas (inset). Low-power (e) and high-power (f) magnification of guinea pig lungs at month 4 of treatment; necrotic granulomas are smaller and confined mostly to the periphery of the lungs, and bacilli can be seen within the necrotic centre of one such granuloma (f, inset). Sections were stained with haematoxylin-eosin and images were obtained at low power (a, b, d and e; 2× magnification) and high power (c and f; 20× magnification). Insets: images of acid-fast-stained sections were obtained at 60× magnification. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Tubercle lesions measuring <1 mm in diameter were grossly visible in the lungs of 3/5 untreated mice 28 days after infection and histological evaluation of mouse lungs revealed loose aggregates of mononuclear cells, including primarily lymphocytes with few histiocytes and plasma cells, which were surrounded by pulmonary oedema in the intra-alveolar spaces (Figure 2a). AFB were predominantly localized to foamy macrophages (Figure 2a, inset). By month 4 of antibiotic treatment, pulmonary oedema improved and mouse lung lesions became smaller, although the cellular composition remained unchanged (Figure 2b and c), and rare intracellular AFB were detected (Figure 2c, inset). Although cellular lesions were present, granulomas with central necrosis were not detected in the lungs of any of the treated or untreated mice.
Bactericidal activity
On the day after aerosol infection, the average lung bacillary counts in mice and guinea pigs were 2.1 ± 0.1 log10 and 2.1 ± 0.2 log10, respectively (Figure 3). One month later, at treatment initiation, lung counts increased to 5.6 ± 0.5 log10 and 5.6 ± 0.3 log10 in mice and guinea pigs, respectively, indicating growth of ∼3.5 log10 in each species. Untreated mice and guinea pigs maintained a relatively stable lung bacillary burden throughout the duration of the experiment. Mice treated with 2RHZ/2RH administered twice weekly experienced an ∼1.0 log10 kill during the first month of treatment, and the killing rate remained constant for the remainder of the treatment course, such that mouse lungs had ∼1 log10 bacilli at month 4 (Figure 3). On the other hand, guinea pigs treated with similar exposures of RHZ showed an ∼1.0 log10 reduction in lung bacillary counts after just 2 weeks of treatment, and a further ∼2.0 log10 bacillary kill over the next 2 weeks, i.e. by month 1 of treatment. By month 2, all guinea pig lungs were culture-negative and similar results were observed after 3 months and 4 months of treatment (Figure 3).
Figure 3.
Equivalent exposures of the combination regimen comprising rifampicin, isoniazid and pyrazinamide (RHZ) show greater bactericidal activity against M. tuberculosis in guinea pig lungs relative to mouse lungs. Twice weekly administration of RHZ was initiated 28 days after aerosol infection (M0) in each species for a total of 2 months (2RHZ). Animals received human-equivalent doses of RH during the remaining 2 months of treatment (2RH). In guinea pigs, the following doses were used: 50 mg/kg rifampicin; 60 mg/kg isoniazid; and 300 mg/kg pyrazinamide. In mice, the following doses were used: 10 mg/kg rifampicin; 25 mg/kg isoniazid; and 150 mg/kg pyrazinamide. Data represent mean cfu counts per lung ± SD. n = 5 mice and 4 guinea pigs per timepoint. GP, guinea pigs.
Sterilizing activity
Relapse after 2RHZ/2RH was not studied in mice, as all mouse lungs remained culture-positive after 4 months of treatment. Relapse rates in guinea pigs were 0% (0/10) after both 3 months and 4 months of treatment, as the lungs and spleens of each of the animals kept for relapse assessment remained culture-negative.
Discussion
The major finding of this study was the rapid cure of chronic TB infection in guinea pigs despite the presence of numerous caseous foci in their lungs. Compared with its activity in the mouse chronic TB model, RHZ treatment was much more bactericidal and sterilizing in the guinea pig, as assessed by time to lung culture conversion and by lung and spleen culture relapse data, respectively. The differences in the responses of the two species to the same drug regimen cannot be explained by differences in serum drug exposures or by bacillary burden at treatment initiation.
According to the ‘volume theory’ of inflammation, which postulates that small hosts tend to trigger more limited inflammatory responses to maintain their body integrity, mice are considered relatively ‘tolerant’ of M. tuberculosis infection, allowing greater bacillary multiplication relative to larger animals such as guinea pigs or humans.16 Thus the relatively robust delayed-type hypersensitivity responses in the more ‘resistant’ guinea pig14,16 may have contributed to the more rapid clearance of M. tuberculosis in this species compared with mice following equivalent RHZ exposures. However, the findings of the current study appear incongruous with clinical data in humans, which would be considered more resistant hosts relative to guinea pigs. In our study, guinea pig lungs became culture-negative after only 2 months of twice weekly treatment with RHZ and were relapse-free after only 3 months of treatment, while 2 months of daily treatment with RHZ and ethambutol followed by 4 months of intermittent rifampicin/isoniazid are required to achieve clinically acceptable relapse rates for TB patients. The more rapid sterilization of guinea pig lungs may be due to less severe necrosis in guinea pig lesions relative to human lesions, and especially its absence within secondary granulomas of guinea pigs. Also, it is important to note that at the time of clinical presentation most human pulmonary cavities, in which there is uninhibited multiplication of M. tuberculosis,16 harbour bacillary burdens exceeding by several orders of magnitude those of the guinea pigs in our study,17 and likely necessitate a longer duration of antibiotic treatment to achieve stable cure in humans. In support of this hypothesis, studies have shown that smear- and culture-negative patients with TB, i.e. those with lower bacillary burdens, achieve acceptably low relapse rates when treated with regimens containing RHZ for as few as 3 months.18,19 Alternatively, some elements of the immune response to M. tuberculosis infection may be more effective in guinea pigs compared with humans, leading to enhanced bacillary killing in the face of systemic TB chemotherapy. For example, it has been suggested that guinea pig phagocytes mount a more vigorous oxidative burst than do their human counterparts, as katG-deficient, isoniazid-resistant mutants cannot be isolated following isoniazid monotherapy in guinea pigs,11 whereas such mutants are readily detectable from the sputum of infected patients. The concept that the immune system positively contributes to sterilization of tissues by TB chemotherapy is further supported by the observation that boosting of immune responses following vaccination of mice with DNA vaccines20 or detoxified fragments of M. tuberculosis formed into liposomes21 leads to enhanced clearance of murine TB infection in combination with chemotherapy. It is important to note, however, that RHZ also showed potent bactericidal and sterilizing activity against acute TB infection in guinea pigs lacking caseous foci,12 suggesting that immune-based clearance of M. tuberculosis infection is not dependent on the development of caseation necrosis. Paradoxically, in the latter study, in which treatment was initiated 2 weeks after infection, guinea pig lungs were rendered culture-negative after 3 months of treatment, while in the current study, in which RHZ was delayed until the formation of necrotic granulomas 4 weeks after infection, only 2 months of treatment were required to reach this endpoint. One possible explanation for these findings is that the peak bacillary burden in the acute infection model was 1000-fold higher than in the current study, thus necessitating prolonged treatment.
Our data showing a reduction in the number and size of guinea pig lung TB lesions following RHZ treatment are consistent with those of Ordway et al.,22 who made similar observations by histological evaluation and magnetic resonance imaging. However, our data differ in that Ordway et al.22 observed a biphasic killing curve associated with RHZ treatment of M. tuberculosis-infected guinea pigs, with a profound ∼5 log10 reduction in lung cfu during the first 30 days of treatment, followed by a very slow second phase of killing, such that cfu could be detected in the lungs of animals even after 120 days of treatment. Important methodological differences between the two studies might explain the different findings. These include the M. tuberculosis strains used for infection of animals, the bacillary burden at treatment initiation, and the drug doses administered. Treatment was initiated in our study at a peak lung bacillary burden of ∼6 log10 cfu, while the corresponding number of bacilli in the study by Ordway et al.22 was at least 10-fold higher, likely contributing to the lengthier treatment course required to achieve bacillary clearance in the latter study. Drug doses in the latter study were significantly lower than those we administered, which were chosen to match the area under the serum concentration–time curve (AUC) following standard human doses of each of these drugs.11,12 The AUC/MIC appears to be the parameter most closely correlated with the bactericidal activity of isoniazid,23 rifampicin24 and pyrazinamide.25 In particular, the lower doses of rifampicin and pyrazinamide used by Ordway et al.22 may have contributed to the reduced sterilizing activity of RHZ observed in their study. Although treatment in the current study was administered twice weekly as compared with five times weekly in the study by Ordway et al.,22 it is likely that the long post-antibiotic effect of each of the drugs26–28 contributed to the potent bactericidal activity of the RHZ regimen in our study.
Intriguingly, AFB were detectable within the necrotic core of peripheral granulomas of ‘sterile’ guinea pig lungs after 4 months of treatment in our study, suggesting either delayed clearance of antibiotic-killed bacilli, which continued to stain positively,29 or the presence of uncultivable persisters. Although guinea pigs were kept for 3 months following completion of treatment to assess the sterilizing activity of RHZ, it is possible the lungs harvested at relapse timepoints were not truly sterile if the immune system of these animals can more readily maintain persisters in a non-cultivable state. In addition, although guinea pig lungs and spleens were cultured for relapse, it is possible that persistent bacilli were present in the intrathoracic lymph nodes or other organs. In support of this hypothesis, Shang et al.30 recently showed the persistence of cultivable bacilli and progressive granulomatous pathology in the thoracic lymph nodes of guinea pigs treated with a combination of the experimental compound TMC207 plus rifampicin and pyrazinamide. In addition, although this regimen reduced lung cfu to undetectable levels by 8 weeks of treatment, more than one-fifth of animals developed clinical relapse as late as 11 months after discontinuation of treatment.30 Future studies will use immunomodulatory agents following the completion of treatment in order to reactivate any potential persisters and assess true sterilization of RHZ and other combination regimens in guinea pig lungs.
The precise role of the guinea pig in the preclinical screening of novel antituberculous drugs and drug combinations remains to be determined. Interestingly, the biphasic kill curve observed following isoniazid11 and streptomycin31 monotherapy in the guinea pig model of TB infection, which is not attributable to the selection of drug-resistant mutants, supports the conclusion that host immune containment in this model rapidly renders bactericidal drugs poorly active. Therefore, the dramatic efficacy of the RHZ combination regimen against chronic TB infection in guinea pigs compared with mice suggests that the activity of the sterilizing drugs rifampicin and pyrazinamide is more highly represented in the guinea pig. Consistent with this hypothesis, and contrary to earlier studies in which pyrazinamide was given at doses well below the human-equivalent dose,32,33 we recently found that pyrazinamide has dose-dependent activity against chronic TB infection in guinea pigs and exhibits synergy with rifampicin,34 as in humans.35
In conclusion, we found no evidence that necrotizing granulomas interfere with the antituberculous activity of the combination regimen RHZ. In contrast, our data raise the interesting possibility that the immune system may enhance antibiotic-mediated killing of M. tuberculosis. Whether such enhancement, if it occurs, is limited to sterilizing drugs, such as rifampicin and pyrazinamide, or applies to all antibiotics remains to be seen. Studies comparing mice able to mount potent delayed-type hypersensitivity responses with otherwise genetically identical mouse strains are currently under way in order to directly address the role of necrotic granulomas in bacillary clearance by TB chemotherapy. These research questions have important implications for the treatment of patients with HIV/TB co-infection, as well as the role of immunotherapy in shortening the duration of TB treatment and/or overcoming drug resistance.
Funding
This work was supported by the Bill and Melinda Gates Foundation (TB Accelerator grant #42851 to E. L. N., J. H. G. and P. C. K.) and the National Institutes of Health (AI064229 and AI083125 to P. C. K.).
Transparency declarations
None to declare.
References
- 1.Fauci AS. Multidrug-resistant and extensively drug-resistant tuberculosis: the National Institute of Allergy and Infectious Diseases Research agenda and recommendations for priority research. J Infect Dis. 2008;197:1493–8. doi: 10.1086/587904. doi:10.1086/587904. [DOI] [PubMed] [Google Scholar]
- 2.Vandiviere HM, Loring WE, Melvin I, et al. The treated pulmonary lesion and its tubercle bacillus. II. The death and resurrection. Am J Med Sci. 1956;232:30–7. doi: 10.1097/00000441-195607000-00006. passim doi:10.1097/00000441-195607000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Tomasz A, Albino A, Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970;227:138–40. doi: 10.1038/227138a0. doi:10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- 4.McCune RM, Jr, McDermott W, Tompsett R. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med. 1956;104:763–802. doi: 10.1084/jem.104.5.763. doi:10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn J, Chan J. Animal models of tuberculosis. In: Rom W, Garay S, editors. Tuberculosis. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 237–50. [Google Scholar]
- 6.McMurray DN. Disease model: pulmonary tuberculosis. Trends Mol Med. 2001;7:135–7. doi: 10.1016/s1471-4914(00)01901-8. doi:10.1016/S1471-4914(00)01901-8. [DOI] [PubMed] [Google Scholar]
- 7.Lenaerts AJ, Hoff D, Aly S, et al. Location of persisting mycobacteria in a guinea pig model of tuberculosis revealed by r207910. Antimicrob Agents Chemother. 2007;51:3338–45. doi: 10.1128/AAC.00276-07. doi:10.1128/AAC.00276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai MC, Chakravarty S, Zhu G, et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–32. doi: 10.1111/j.1462-5822.2005.00612.x. doi:10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 9.Aly S, Wagner K, Keller C, et al. Oxygen status of lung granulomas in Mycobacterium tuberculosis-infected mice. J Pathol. 2006;210:298–305. doi: 10.1002/path.2055. doi:10.1002/path.2055. [DOI] [PubMed] [Google Scholar]
- 10.Karakousis PC, Williams EP, Bishai WR. Altered expression of isoniazid-regulated genes in drug-treated dormant Mycobacterium tuberculosis. J Antimicrob Chemother. 2008;61:323–31. doi: 10.1093/jac/dkm485. doi:10.1093/jac/dkm485. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad Z, Klinkenberg LG, Pinn ML, et al. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J Infect Dis. 2009;200:1136–43. doi: 10.1086/605605. doi:10.1086/605605. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad Z, Nuermberger EL, Tasneen R, et al. Comparison of the ‘Denver regimen’ against acute tuberculosis in the mouse and guinea pig. J Antimicrob Chemother. 2010;65:729–34. doi: 10.1093/jac/dkq007. doi:10.1093/jac/dkq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinkenberg LG, Sutherland LA, Bishai WR, et al. Metronidazole lacks activity against Mycobacterium tuberculosis in an in vivo hypoxic granuloma model of latency. J Infect Dis. 2008;198:275–83. doi: 10.1086/589515. doi:10.1086/589515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMurray DN. Guinea pig model of tuberculosis. In: Bloom B, editor. Tuberculosis: Pathogenesis, Protection, and Control. Washington, DC: American Society for Microbiology; 1994. pp. 135–47. [Google Scholar]
- 15.Ly LH, Russell MI, McMurray DN. Cytokine profiles in primary and secondary pulmonary granulomas of guinea pigs with tuberculosis. Am J Respir Cell Mol Biol. 2008;38:455–62. doi: 10.1165/rcmb.2007-0326OC. doi:10.1165/rcmb.2007-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardona PJ. Revisiting the natural history of tuberculosis. The inclusion of constant reinfection, host tolerance, and damage-response frameworks leads to a better understanding of latent infection and its evolution towards active disease. Arch Immunol Ther Exp (Warsz) 2010;58:7–14. doi: 10.1007/s00005-009-0062-5. doi:10.1007/s00005-009-0062-5. [DOI] [PubMed] [Google Scholar]
- 17.Canetti G. Present aspects of bacterial resistance in tuberculosis. Am Rev Respir Dis. 1965;92:687–703. doi: 10.1164/arrd.1965.92.5.687. [DOI] [PubMed] [Google Scholar]
- 18.Leibert E, Rom W. Principles of tuberculosis management. In: Rom W, Garay S, editors. Tuberculosis. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 713–28. [Google Scholar]
- 19.British Medical Research Council. A controlled trial of 3-month, 4-month, and 6-month regimens of chemotherapy for sputum-smear-negative pulmonary tuberculosis. Results at 5 years. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. Am Rev Respir Dis. 1989;139:871–6. doi: 10.1164/ajrccm/139.4.871. [DOI] [PubMed] [Google Scholar]
- 20.Ha SJ, Jeon BY, Youn JI, et al. Protective effect of DNA vaccine during chemotherapy on reactivation and reinfection of Mycobacterium tuberculosis. Gene Ther. 2005;12:634–8. doi: 10.1038/sj.gt.3302465. doi:10.1038/sj.gt.3302465. [DOI] [PubMed] [Google Scholar]
- 21.Cardona PJ, Amat I, Gordillo S, et al. Immunotherapy with fragmented Mycobacterium tuberculosis cells increases the effectiveness of chemotherapy against a chronical infection in a murine model of tuberculosis. Vaccine. 2005;23:1393–8. doi: 10.1016/j.vaccine.2004.09.008. doi:10.1016/j.vaccine.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Ordway DJ, Shanley CA, Caraway ML, et al. Evaluation of standard chemotherapy in the guinea pig model of tuberculosis. Antimicrob Agents Chemother. 2010;54:1820–33. doi: 10.1128/AAC.01521-09. doi:10.1128/AAC.01521-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayaram R, Shandil RK, Gaonkar S, et al. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2004;48:2951–7. doi: 10.1128/AAC.48.8.2951-2957.2004. doi:10.1128/AAC.48.8.2951-2957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayaram R, Gaonkar S, Kaur P, et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2003;47:2118–24. doi: 10.1128/AAC.47.7.2118-2124.2003. doi:10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumbo T, Dona CS, Meek C, et al. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother. 2009;53:3197–204. doi: 10.1128/AAC.01681-08. doi:10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan CY, Au-Yeang C, Yew WW, et al. Postantibiotic effects of antituberculosis agents alone and in combination. Antimicrob Agents Chemother. 2001;45:3631–4. doi: 10.1128/AAC.45.12.3631-3634.2001. doi:10.1128/AAC.45.12.3631-3634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan CY, Au-Yeang C, Yew WW, et al. In vitro postantibiotic effects of rifapentine, isoniazid, and moxifloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2004;48:340–3. doi: 10.1128/AAC.48.1.340-343.2004. doi:10.1128/AAC.48.1.340-343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchison DA, Davies GR. Assessment of the efficacy of new anti-tuberculosis drugs. Open Infect Dis J. 2008;2:59–76. doi: 10.2174/1874279300802010059. doi:10.2174/1874279300802010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rees RJ, Hart PD. Analysis of the host-parasite equilibrium in chronic murine tuberculosis by total and viable bacillary counts. Br J Exp Pathol. 1961;42:83–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Shang S, Shanley CA, Caraway ML, et al. The activity of TMC 207, rifampin, and pyrazinamide against Mycobacterium tuberculosis infection in guinea pigs. Antimicrob Agents Chemother. 2011;55:124–31. doi: 10.1128/AAC.00978-10. doi:10.1128/AAC.00978-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad Z, Pinn ML, Nuermberger EL, et al. The potent bactericidal activity of streptomycin in the guinea pig model of tuberculosis ceases due to the presence of persisters. J Antimicrob Chemother. 2010;65:2172–5. doi: 10.1093/jac/dkq277. doi:10.1093/jac/dkq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dessau FI, Yeager RL, Burger FJ, et al. Pyrazinamide (aldinamide) in experimental tuberculosis of the guinea pig. Am Rev Tuberc. 1952;65:519–22. [PubMed] [Google Scholar]
- 33.Steenken W, Jr, Wolinsky E. The antituberculous activity of pyrazinamide in vitro and in the guinea pig. Am Rev Tuberc. 1954;70:367–9. doi: 10.1164/art.1954.70.2.367. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad Z, Fraig MM, Bisson GP, et al. Dose-dependent activity of pyrazinamide in animal models of intracellular and extracellular tuberculosis infections. Antimicrob Agents Chemother. 2011;55:1527–32. doi: 10.1128/AAC.01524-10. doi:10.1128/AAC.01524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]