Abstract
Chronic active EBV disease (CAEBV) is a lymphoproliferative disorder characterized by markedly elevated levels of antibody to EBV or EBV DNA in the blood and EBV RNA or protein in lymphocytes in tissues. We present our experience with CAEBV during the last 28 years, including the first 8 cases treated with hematopoietic stem cell transplantation in the United States. Most cases of CAEBV have been reported from Japan. Unlike CAEBV in Japan, where EBV is nearly always found in T or natural killer (NK) cells in tissues, EBV was usually detected in B cells in tissues from our patients. Most patients presented with lymphadenopathy and splenomegaly; fever, hepatitis, and pancytopenia were common. Most patients died of infection or progressive lymphoproliferation. Unlike cases reported from Japan, our patients often showed a progressive loss of B cells and hypogammaglobulinemia. Although patients with CAEBV from Japan have normal or increased numbers of NK cells, many of our patients had reduced NK-cell numbers. Although immunosuppressive agents, rituximab, autologous cytotoxic T cells, or cytotoxic chemotherapy often resulted in short-term remissions, they were not curative. Hematopoietic stem cell transplantation was often curative for CAEBV, even in patients with active lymphoproliferative disease that was unresponsive to chemotherapy. These studies are registered at http://www.clinicaltrials.gov as NCT00032513 for CAEBV, NCT00062868 and NCT00058812 for EBV-specific T-cell studies, and NCT00578539 for the hematopoietic stem cell transplantation protocol.
Introduction
Approximately 95% of adults are infected with EBV. Although most infections occur during childhood and are asymptomatic, infection in adolescents or young adults often results in infectious mononucleosis. Mononucleosis often presents with fever, pharyngitis, lymphadenopathy, and splenomegaly. Most patients have an uncomplicated course; however, some develop complications, including upper airway obstruction, rupture of the spleen, neurologic disease, severe hematologic cytopenias, or hepatitis. In most cases these symptoms resolve without sequelae.
Rare persons infected with EBV develop a life-threatening condition termed chronic active EBV disease (CAEBV).1–4 Most cases of CAEBV have been reported from Japan. These patients often have some of the complications found in otherwise-healthy patients with acute EBV infection, but unlike healthy patients, these complications persist and progress. These patients have markedly elevated levels of EBV DNA in the blood and viral RNA and proteins in tissues. Most patients present with fever, hepatic dysfunction, splenomegaly, lymphadenopathy, and thrombocytopenia.2 Other features that appear in > 10% of patients include hepatomegaly, anemia, hypersensitivity to mosquito bites, rash, oral ulcers, hemophagocytic syndrome, coronary artery aneurysms, liver failure, lymphoma, and interstitial pneumonia. Less common features include uveitis, CNS disease, intestinal perforation, and myocarditis.5
Although EBV is present in the B cells of healthy persons infected with EBV, in most cases of CAEBV reported in Asians or Native Americans, EBV has been detected in T or natural killer (NK) cells.2,6 The virus was present in the B cells of lesions from rare patients with CAEBV in Japan5 and in the United States.7 Some patients had defective cytotoxic T-cell (CTLs)8,9 or NK-cell10 activity against EBV-infected cells. Recently, we reported one patient with mutations in both alleles of his perforin gene that impaired maturation of the protein and reduced killing by T cells.11 At an international workshop,4 participants concluded that CAEBV should be classified as a B, T, or NK cell in origin, and although the authors of one study compared T- and NK-cell disease,5 no reports have compared T- and B-cell disease.
Therapy for CAEBV, in the absence of hematopoietic stem cell transplantation (HSCT), is often unsatisfactory and at best transiently delays the progression of disease. Antiviral therapy and immunomodulatory agents usually are ineffective. Corticosteroids or other immunosuppressive agents often reduce symptoms, but over time patients become refractory to therapy, develop progressive immunodeficiency, and usually succumb to opportunistic infections or lymphoproliferative disease. Cytotoxic chemotherapy and autologous EBV specific CTLs are usually unsuccessful. In contrast, allogeneic HSCT has been successful in several cases reported from Japan.12–14
We report our experience with 19 patients with CAEBV. Sixteen consecutive patients were followed at the National Institutes of Health (NIH) Clinical Center during the past 28 years, and 3 patients were seen at Baylor College of Medicine. We describe the features of CAEBV in the United States that differ from those cases reported in Japan and report that the only effective therapy in our patients with CAEBV is allogeneic HSCT.
Methods
Entry criteria
CAEBV was defined as (1) a severe progressive illness of > 6 months' duration usually with fever, lymphadenopathy, and splenomegaly that either began as a primary EBV infection or was associated with markedly elevated antibody titers to EBV viral capsid antigen (VCA ≥ 1:5120) or early antigen (≥ 1:640), or markedly elevated EBV DNA in the blood; (2) infiltration of tissues (eg, lymph nodes, lungs, liver, CNS, bone marrow, eye, skin) with lymphocytes; (3) elevated EBV DNA, RNA, or proteins in affected tissues; and (4) the absence of any other immunosuppressive condition.2,15 Our definition is similar to that used in most Japanese studies,2 but we also required evidence of lymphocytic infiltration (and EBV) in the tissues to ensure that the organ disease was attributable to EBV-infected lymphocytes. This research was approved by Institutional Review Boards at NIH and at Baylor College of Medicine, and all patients or guardians provided written informed consent in accordance with the Declaration of Helsinki. Patient 3,11 patient 10,6 and patient 1516 were reported previously.
Pathology
All cases of CAEBV from the NIH Clinical Center from 1982 until the present (patients 1-8, 10-16) and an additional 4 patients from Baylor College of Medicine in Houston who underwent HSCT (patients 9, 17-19) were reviewed at the Hematopathology Section of the National Cancer Institute at the NIH. Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue sections as described previously.6
In situ hybridization for EBV-encoded RNA-1 (EBER1) was performed on fixed paraffin-embedded sections as previously described with the use of an EBER1 riboprobe.6 Double staining for EBER1 and CD20 or CD3 was performed with in situ hybridization and then immunohistochemistry. In selected cases, the distribution of EBER1 positivity in T cells versus B cells was inferred by comparison of sequential sections, on the basis of the observed distribution of cells positive for EBER, CD3, and CD20.
Molecular studies were performed by extracting genomic DNA from paraffin-embedded sections. Clonal rearrangements of the IgH genes and the TCR γ chain genes were investigated by the use of appropriate primers and PCR as described previously.6
EBV viral DNA loads
EBV DNA was quantified from peripheral blood lymphocytes of patients 8-11 by the use of serial dilutions of DNA and PCR as described previously.17 EBV DNA was quantified from PBMCs in patients 12-16 by real-time PCR as described previously.18 EBV DNA was measured in patients 17-19 by isolating DNA from PBMCs with an anion exchange column followed by real-time PCR as described previously.19
Serum cytokines and EBV-specific antibodies
Serum cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IFNγ, GM-CSF, and TNF-α) were measured by the use of the Multiplex MAP high sensitivity human cytokine panel (Millipore), following the manufacturer's instructions. To account for the multiple cytokine tests that were performed, only P values < .01 were considered significant.
To measure EBV-specific antibody responses, fusion proteins were constructed in which EBV genes were fused to the Renilla luciferase gene plasmid vectors pREN3S or pREN2 (see supplemental data, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Antibodies were measured against latency (EBNA-1, LMP2A), immediate-early (BZLF1), early (BALF1, BHRF1, BMRF1, SM), and late (BCRF1, p18 capsid protein, p23 capsid, protein, gp25, gp350, and gp42) viral proteins. Cos1 cells were transfected with plasmids containing EBV-luciferase fusion proteins and activity of lysates was determined by measuring light units (LU) with a luminometer.20 Plasma was diluted 1:10 and 10 μL was added to 1 × 107 LU of transfected Cos1 cell extract, immunoprecipitations were performed with addition of protein A/G beads, and LU were determined by a plate luminometer. A cutoff threshold limit was derived from the mean value plus 2 SD of background LU. Controls for cytokine and antibody measurements were persons with chronic inflammatory disorders and with chronic fatigue syndrome on protocols approved by the Institutional Review Board at NIH.
Results
Clinical characteristics and pathology of patients with CAEBV
Nineteen patients with CAEBV were evaluated—16 patients at the NIH Clinical Center and 3 at Baylor College of Medicine (Table 1). Sixty-eight percent were men, and 32% were women. Twelve (63%) were white, 3 (16%) were Asian, 3 (16%) were Hispanic, and 1 (5%) was African American. None of the patients had family histories suggestive of similar disease. The mean age at the onset of disease was 19 years (range, 4-51 years). Eleven had B-cell CAEBV, 3 T-cell CAEBV, 1 had NK-cell CAEBV, and 4 were undetermined. Of the patients with B-cell CAEBV, 4 patients (patients 3, 6, 9, and 11) had a prominent reactive T-cell infiltrate in biopsy sections and fewer numbers of EBER+ and CD20+ lymphocytes demonstrating a similar distribution in sequential sections. Many of the patients with EBV in B cells had virus in both CD20+ and CD20− B cells on their initial biopsy, and the frequency of CD20− B cells increased after rituximab therapy (eg, patient 16, Figure 1); the latter would not be sensitive to rituximab.
Table 1.
Outcome of patients with chronic active EBV disease
| Patient | Race/sex | Age at onset, y | Age at death, y | Outcome |
|---|---|---|---|---|
| Unknown whether B-, T-, or NK-cell disease | ||||
| 1 | WM | 28 | 38 | Died, LPD, MDS/acute myelomonocytic leukemia |
| 2 | WM | 13 | 30 | Died, infection |
| 4 | AF | 11 | 14 | Died, LPD, pneumonia |
| 5 | WM | 22 | 23 | Died, LPD, pulmonary thrombosis |
| B-cell disease | ||||
| 3 | WM | 9 | 17 | Died, progressive disease, disseminated candidiasis |
| 6 | WM | 18 | 24 | Died, progressive disease, Aspergillus pneumonia, brain abscess |
| 7 | BF | 13 | 17 | Died, B-cell lymphoma |
| 8 | WM | 29 | 36 | Died, cirrhosis (alcohol-related) |
| 9 | WM | 17 | Alive (37 y) | Alive 11 years after BMT, EBV negative |
| 11 | WF | 20 | 31 | Died, progressive disease |
| 12 | WM | 51 | Alive (64 y) | Alive 6 years after HSCT, EBV positive, mild GVHD, infections |
| 13 | WM | 44 | 45 | Died, central pontine myelinolysis after HSCT, residual B-cell lymphoma |
| 14 | AF | 22 | Alive (30 y) | Alive with B-cell LPD, 1 year after autologous EBV CTLs (LCL-specific and LMP1/2- specific cells) |
| 16 | WM | 29 | 31 | Died, residual B-cell lymphoma after HSCT × 3 |
| 17 | HF | 5 | Alive (16 y) | Alive 6 years after HSCT, EBV negative |
| T-cell disease | ||||
| 10 | WF | 9 | 15 | Died, T-cell lymphoma |
| 15 | HM | 8 | Alive (15 y) | Alive 2 years after HSCT |
| 18 | AM | 4 | 9 | Died, 4 years after HSCT, T-cell lymphoma |
| NK-cell disease | ||||
| 19 | HM | 7 | Alive (14 y) | Alive 2 years after HSCT in complete remission |
A indicates Asian; B, black; BMT, bone marrow transplant; CTLs, cytotoxic T lymphocytes; F, female; H, Hispanic; HSCT, hematopoietic stem cell transplant; LCL, lymphoblastoid cell line (EBV-transformed B-cell line); LMP, latent membrane protein; LPD, lymphoproliferative disease; M, male; MDS, myelodysplastic syndrome; and W, white.
Figure 1.
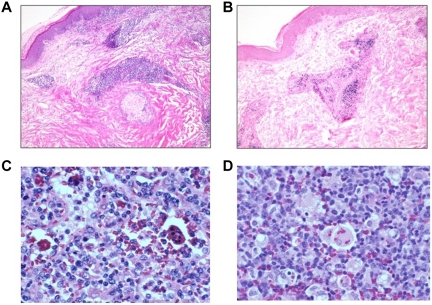
EBV RNA in both CD20+ and CD20− B cells in a lymph node from patient 16. (A) Lymph node biopsy shows polymorphic B-cell lymphoma. (B) In situ hybridization shows that the tumor cells are positive for EBER. Few cells express the CD20 B-cell marker (C), whereas many cells express the CD3 T-cell (D), CD79 B-cell (E), and Pax-5 B-cell (F) markers.
Patients with T-cell CAEBV were younger at presentation (mean age, 7 years) than those with B-cell disease (mean age, 23 years). Of the 11 patients followed at NIH for at least 5 years who did not undergo HSCT, only one is alive and she has B-cell lymphoproliferative disease resembling posttransplantation lymphoproliferative disease (PTLD). The mean time to death after onset of disease was 6.2 years; this finding was similar in those with T-cell CAEBV and those with B-cell disease. Death was most often because of uncontrolled lymphoproliferative disease or opportunistic infection.
The most common signs and symptoms were lymphadenopathy in 79% of patients, splenomegaly in 68%, fever in 47%, hepatitis in 47%, hypogammaglobulinemia in 44%, pancytopenia in 42%, hematophagocytosis in 32%, hepatomegaly in 32%, interstitial pneumonitis in 26%, central nervous system disease in 21%, and peripheral neuropathy in 21% (Table 2). The most frequent organs involved with EBV+ lymphocytes were lymph nodes in 63% of patients, BM in 37%, liver in 26%, spleen in 21%, lung in 21%, and skin in 16% (Figure 2, Table 3). Patients with T-cell CAEBV more often had fever (100% for T-cell vs 45% for B-cell), hepatitis (100% vs 36%), and hepatomegaly (66% vs 27%), whereas patients with B-cell CAEBV more often had hypogammaglobulinemia (55% for B-cell vs 0% for T-cell).
Table 2.
Symptoms of chronic active EBV
| Patient | Fever | Lymphadenopathy | Splenomegaly | Hepatomegaly | CNS disease | Peripheral neuropathy | Interstitial pneumonitis | Pancytopenia | Hepatitis | Hemophagocytosis | Hypogammaglobulinemia | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unknown whether B-, T-, or NK-cell disease | ||||||||||||
| 1 | + | + | + | + | + | + | + | |||||
| 2 | + | + | + | + | + | |||||||
| 4 | + | + | ||||||||||
| 5 | + | + | + | + | Autoimmune hemolytic anemia, sinusitis | |||||||
| B-cell disease | ||||||||||||
| 3 | + | + | + | + | Autoimmune hemolytic anemia, neutropenia, skin disease, vasculitis, sinusitis, thrombocytopenia | |||||||
| 6 | + | + | + | + | + | + | + | + | Uveitis | |||
| 7 | + | + | + | Sinusitis, vasculitis | ||||||||
| 8 | + | + | + | |||||||||
| 9 | + | + | + | + | + | + | + | + | + | |||
| 11 | + | + | + | + | + | + | Autoimmune hemolytic anemia | |||||
| 12 | + | + | + | + | + | + | Eye disease, skin disease | |||||
| 13 | + | + | + | + | + | Skin disease | ||||||
| 14 | + | + | + | |||||||||
| 16 | + | + | + | |||||||||
| 17 | + | + | + | + | Sinusitis | |||||||
| T-cell disease | ||||||||||||
| 10 | + | + | + | + | + | + | + | Sinusitis, thrombocytopenia | ||||
| 15 | + | + | + | Hydroa vacciniforme | ||||||||
| 18 | + | + | + | + | + | Conjunctivitis | ||||||
| NK-cell disease | ||||||||||||
| 19 | Skin disease, mosquito bite hypersensitivity |
Figure 2.
EBV disease involving skin, lymph nodes, and spleen. Skin from patient 12 shows a perivascular lymphocytic infiltrate (A) that is positive for EBER (B). Lymph node (C) and spleen (D) with hemophagocytosis from patient 9.
Table 3.
Organs infiltrated with EBV+ cells and clonality
| Organs infiltrated with EBV+ cells and clonality |
Clonality | ||||||
|---|---|---|---|---|---|---|---|
| Lymph node | BM | Liver | Spleen | Lung | Other | ||
| Unknown whether B-, T-, or NK-cell disease | |||||||
| 1 | + | + | + | Intestine | ND | ||
| 2 | ND* | ND | |||||
| 4 | + | ND | |||||
| 5 | + | ND | |||||
| B-cell disease | |||||||
| 3 | + | + | ND | ||||
| 6 | + | + | + | ND | |||
| 7 | + | + | Sinus | Yes | |||
| 8 | + | Brain | ND | ||||
| 9 | + | + | + | + | Yes | ||
| 11 | + | + | Yes | ||||
| 12 | + | Skin | No | ||||
| 13 | + | CSF | Yes | ||||
| 14 | + | Tonsil | No | ||||
| 16 | + | + | No | ||||
| 17 | + | + | Yes | ||||
| T-cell disease | |||||||
| 10 | + | + | + | Kidney | Yes | ||
| 15 | + | Skin | Yes | ||||
| 18 | + | + | Yes | ||||
| NK-cell disease | |||||||
| 19 | Skin | ND | |||||
ND indicates not done; and NK, natural killer.
The patient had a brain biopsy that showed encephalitis; however, insufficient material was available for EBV EBER staining.
Clonality was determined in tumors from 11 of the patients (Table 3). Sixty-three percent of patients with B-cell CAEBV had clonal lymphoid proliferations, and 100% of those with T-cell CAEBV had clonal lesions. Thirty-eight percent (3/8) of those with clonal tumors were alive at last follow-up, whereas 67% (2/3) with nonclonal lymphoproliferative lesions were alive at follow-up.
Absence of mutations in cellular genes involved in control of EBV
SAP is mutated in patients with X-linked lymphoproliferative disease; other studies of Japanese patients have shown no evidence of SAP mutations in patients with CAEBV.2 Because CAEBV shares some of the features of X-linked lymphoproliferative disease, we sequenced genes encoding proteins (SLAM, 2B4, NTB-A) that interact with SAP. In addition we determined the sequence of perforin (because patient 3 was previously reported to have mutations in the gene),11 as well as granulysin, which like perforin is also present in cytotoxic granules of NK and CTLs. Finally we sequenced X-linked inhibitor of apoptosis protein, which has been reported to be mutated in some patients with severe EBV infections. None of the 10 patients tested (patients 2, 6, 7, 8, 9, 10, 12, 13, 14, and 16) had mutations in these genes.
Antibodies to EBV proteins and loss of Igs and B cells in patients with CAEBV evaluated at NIH
Antibody to EBV VCA IgG was elevated to ≥ 1:5260 in 53% of those tested; 100% with T-cell CAEBV and 50% with B-cell disease had antibody titers this high (Table 4). Antibody to EBV VCA IgM was elevated at the time of referral in 5 of 17 patients tested; the antibody became negative in 3 of 4 patients who were initially positive and were subsequently retested (supplemental data and supplemental Table 1). Antibody to EBV VCA IgA was elevated in 8 of 8 patients tested with a conventional immunofluorescence assay; in 6 of 8 patients the titer was ≥ 1:256. Of the 5 patients whose blood was tested by real-time PCR for EBV DNA, the median viral load was 320 000 copies per million cells (range, 5700-1.7 million copies per million cells).
Table 4.
Hematologic data for patients with chronic active EBV seen at NIH
| Pt | CD4% | CD8% | CD19% | CD20% | NK% | EBV VCA IgG titer | EBV load* | Serum IgG |
|
|---|---|---|---|---|---|---|---|---|---|
| High | Low | ||||||||
| Unknown whether B-, T-, or NK- cell disease | |||||||||
| 1 | ND | ND | ND | ND | ND | 20 480 | ND | 2830 | 540 |
| 2 | 60 | 30 | 0 | 0.1 | 5.5 | 40 960 | ND | 5440 | 504 |
| 4 | 50 | 21 | ND | ND | 10 | 2560 | ND | 3080 | 682 |
| 5 | 20 | 45 | 5.6 | 4.6 | 0.7 | 1280 | ND | 3148 | 745 |
| B-cell disease | |||||||||
| 3 | 59 | 38 | 0.3 | 0.5 | 3 | 20 480 | ND | 3570 | 907 |
| 6 | 83 | 12 | 0 | 0 | 1.2 | 10 240 | ND | 914 | 756 |
| 7 | 22 | 59 | 2.9 | 2.9 | 16.4 | 20 480 | ND | 2680 | 328 |
| 8 | 22 | 23 | 13 | 6 | 16 | 2560 | 1:1000† | 1640 | 617 |
| 9 | 79 | 13 | 0.5 | 0.5 | 1.5 | 2560 | 1:10‡ | 2180 | 225 |
| 11 | 50 | 41 | ND | ND | 7 | 163 840 | 1:100§ | 6530 | ND |
| 12 | 56 | 36 | 0.8 | 0.7 | 5 | 640 | 5.0 × 105 | 643 | 479 |
| 13 | 64 | 8 | 17 | ND | 5 | ND | 1.7 × 106 | 734 | ND |
| 14 | 25 | 55 | 11 | 10 | 5.1 | 1280 | 5.7 × 103 | 793 | 553 |
| 16 | 42 | 32 | 5 | 5 | 17 | 320 | 4.6 × 104 | 916 | 910 |
| 17 | ND | ND | ND | ND | ND | > 10 240 | 2.2 × 106 | 432 | ND |
| T-cell disease | |||||||||
| 10 | 43 | 24 | 21 | 21 | 8 | 40 960 | 1:10 000¶ | 2330 | 2120 |
| 15 | 24 | 59 | 8 | ND | 4 | ND | 3.2 × 105 | 1910 | ND |
| 18 | ND | ND | ND | ND | ND | > 10 240 | 1.2 × 105 | 824 | ND |
| NK-cell disease | |||||||||
| 19 | 23 | 14 | 16 | ND | 47 | 320 | > 2.6 × 105 | 1010 | 817 |
Normal values are CD4 362-1275 (29%-57%), CD8 344-911 (25%-51%), CD19 47-409 (3.5%-17%), CD20 49-424 (3.7%-16%), and CD56 87-505 (4.6%-30%); normal values for serum IgG for patients 1-16 at NIH are 642-1730 mg/dL, and for patients 17-19 at Baylor College of Medicine are 641-1353 mg/dL.
ND indicates not done; NIH, National Institutes of Health; Pt, patient; and VCA, dilution of antibody to EBV viral capsid antigen that is positive in the serum.
All laboratory data are shown before rituximab therapy given; low IgG and low CD19% were present before cytotoxic chemotherapy was given except for patient 3.
EBV load expressed as dilution of peripheral blood lymphocytes in which EBV PCR is positive (patients 8-11) or EBV copies per 106 genomes from PBMCs (patients 12-16) or EBV copies per μg of DNA (patients 17-19). The lower limit of detection was 10 EBV copies per 106 cellular genomes (assay used for patients 12-16) and 4 genomes of EBV per μg of DNA (assay used for patients 17-19).
Estimated to be ∼ 11 000 copies/mL.
Estimated to be ∼ 1200 copies/mL.
Estimated to be ∼ 4400 copies/mL.
Estimated to be > 11 000 copies/mL.
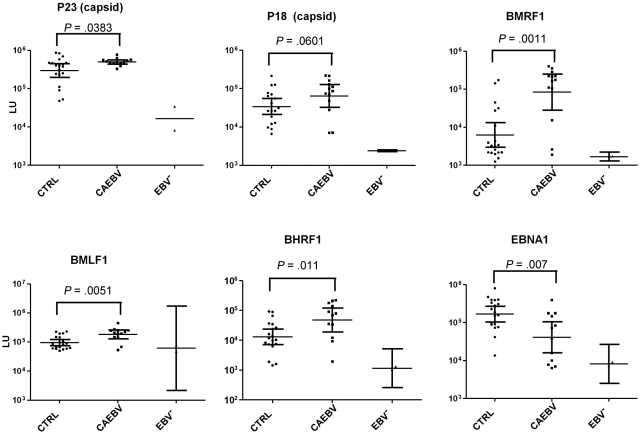
Titers of antibodies to early EBV proteins (BMRF1-DNA polymerase processivity factor, BHRF1-bcl-2 homolog, BMLF1-transporter of intronless RNAs) were significantly greater (P < .011) in CAEBV patients than in EBV-seropositive control patients (Figure 3). Antibodies to immediate-early (BZLF1-Z transactivator) and late (BFRF3-p18 capsid, BLRF2-p23 capsid) EBV proteins were greater in CAEBV patients than EBV-seropositive control patients, but the difference did not reach statistical significance. Antibody titers to EBV early antigen (diffuse) were elevated in 10 of 11 patients tested with a conventional immunofluorescence assay; in 7 of 11 patients the titer was ≥ 1:5120 (supplemental data). In contrast, EBV titers to latent protein EBNA1 were significantly lower in CAEBV patients than in EBV-seropositive control patients (P = .007). Antibody titers to each of the aforementioned immediate-early, early, and late proteins (except for BLRF2-p23 capsid) were greater in patients with T-cell CAEBV than in patients with B-cell CAEBV. In contrast, antibody to EBNA1 was greater in patients with B-cell CAEBV than in those with T-cell CAEBV. Thus, patients with CAEBV have greater titers of antibodies to certain EBV lytic proteins and lower titers of antibody to EBNA1 than control patients.
Figure 3.
Levels of antibody to EBV lytic proteins and EBNA1 by the luciferase immunoprecipitation system assay in EBV-seropositive control patients (CTRL), patients with CAEBV, and EBV-seronegative control patients. Each point represents an individual sample from an uninfected control or a person infected with EBV. Antibody titers are expressed in LU. P values were calculated with the Mann-Whitney U test. The solid horizontal lines indicate the geometric mean antibody titers in each group, and the vertical lines show the 95% confidence interval.
A striking feature of our patients was a progressive loss of B cells and hypogammaglobulinemia, even in the absence of rituximab therapy. Forty-two percent developed hypogammaglobulinemia, 43% had low numbers of CD19 cells in the absence of rituximab therapy, 31% had low NK cells, 38% had low CD4 cells, and 44% had low CD8 cells (Table 4). Patients with B-cell CAEBV more often had CD19 cell numbers less than normal (56% of B-cell CAEBV patients vs 0% of T-cell CAEBV patients), and the mean percent of CD19 cells in patients with B-cell CAEBV (5.6%) was lower than that for patients with T-cell CAEBV (14.5%).
Cytokine levels in patients with CAEBV evaluated at NIH
The mean serum levels of IL-6, IL-10, TNF-α, and IFN-γ were significantly greater for patients with CAEBV (B- and T-cell) than control patients, or for patients with B-cell CAEBV than controls (all P < .006, Figure 4). Although serum levels of IL-12 were 2.4-fold greater in patients with CAEBV (B- and T-cell) than controls, and 3.3-fold greater in patients with B-cell CAEBV than control patients, the results did not reach statistical significance. Similarly, levels of GM-CSF were 1.3-fold greater in patients with CAEBV (B- and T-cell) than control patients and 1.8-fold greater in patients with B-cell CAEBV than control patients, but the results did not reach statistical significance. In contrast, levels of IL-5 were similar in those with CAEBV as in control patients.
Figure 4.
Cytokine levels in patients with CAEBV. Levels of IL-6, IL-10, TNF-α, and IFN-γ are shown in pg/mL. CTRL indicates healthy EBV-seropositive control patients; B, patients with B-cell CAEBV; B + T, combined patients with B-cell and T-cell CAEBV.
Response to treatment in patients with CAEBV
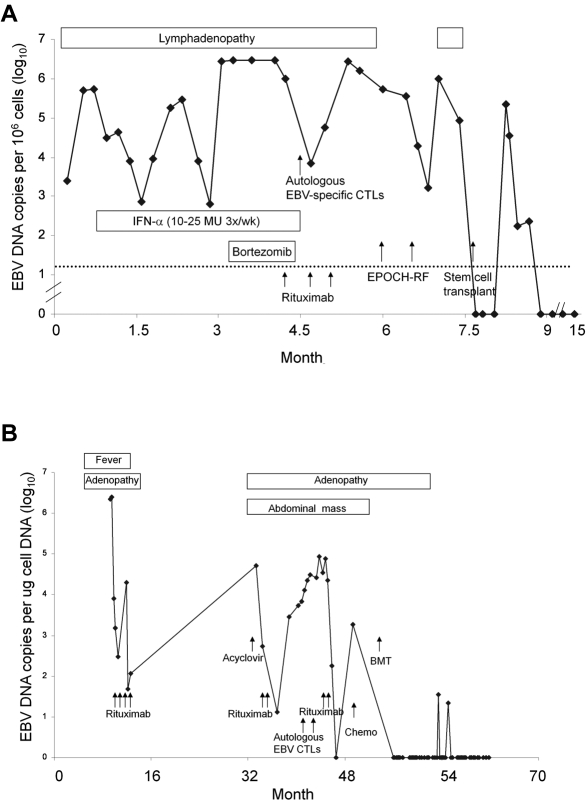
Patients were treated with a variety of agents (Table 5), including antiviral therapy with acyclovir or valacyclovir (63%), intravenous IgG (42%), IFN-α (32%), or other IFNs (11%) without improvement. Several patients were treated with immunosuppressive therapy, including corticosteroids (79%), cyclosporine (26%), or azathioprine (21%). Although immunosuppressive therapy transiently relieved symptoms in most patients, it did not provide a lasting effect in any patient. Five patients (26%) received rituximab alone or as part of their chemotherapeutic regimen; in 3 (patients 12, 13, 16), CD20-negative lymphoproliferative lesions developed after rituximab therapy. Twelve patients (63%) received cytotoxic chemotherapy, resulting in transient or no response. 3 patients (16%) received autologous EBV-specific CTLs21 before HSCT and one in the absence of transplantation; these patients either had no response (patients 12, 17, and 19; Figure 5A-B) or a transient response (patient 14). Patient 14 had widespread lymphadenopathy; a biopsy showed an EBV B-cell lymphoproliferative disorder, and her EBV DNA was 953 copies/μg in peripheral blood before her first infusion of EBV-specific CTLs. After the infusion, her viral load remained elevated, but her lymphadenopathy partially diminished. When her lymphadenopathy increased and a biopsy showed progressive disease with features of polymorphic B-cell lymphoma 2 years later, she received 2 infusions of LMP1/2-specific CTLs, but her disease did not respond to treatment. She is currently being considered for HSCT. Other treatments included plasmapheresis (1 patient) and the combination of bortezomib and ganciclovir in an attempt to induce EBV lytic replication and killing of replicating cells (1 patient); these were ineffective. Although some of these treatments, particularly immunosuppressive therapy, rituximab, cytotoxic chemotherapy, and autologous virus-specific CTLs, resulted in transient improvement, none had a lasting effect.
Table 5.
Treatment for CAEBV
| Patient | Acyclovir or valacyclovir | Steroids | Cyclosporine | Azathioprine | IFN-α | IVIG | Rituximab | Cytotoxic chemotherapy | Other |
|---|---|---|---|---|---|---|---|---|---|
| Unknown whether B-, T-, or NK-cell disease | |||||||||
| 1 | + | + | Desciclovir, plasmapheresis | ||||||
| 2 | + | + | + | + | Cy | ||||
| 4 | + | + | |||||||
| 5 | + | + | Cy | Splenectomy | |||||
| B-cell disease | |||||||||
| 3 | + | + | + | + | + | Cy, Vin, Adria | Splenectomy | ||
| 6 | + | + | + | + | + | MTX, Vin | IFN-γ, 2-chlorodeoxyadenosine, splenectomy | ||
| 7 | + | + | + | + | Cy, MTX, Vin | ||||
| 8 | + | + | + | ||||||
| 9 | + | + | + | + | + | Cy, AraC, VP16 | IFN-β, ATG, splenectomy, allogeneic HSCT | ||
| 11 | + | + | + | + | Ganciclovir, plasmapheresis, splenectomy | ||||
| 12 | + | + | + | + | EPOCH | Bortezomib, autologous EBV-specific cytotoxic T cells followed by allogeneic HSCT | |||
| 13 | + | + | EPOCH-R, Cy, Flu | Bortezomib/ganciclovir, allogeneic HSCT | |||||
| 14 | + | Autologous EBV LCL-specific and LMP 1/2-specific cytotoxic T cells, lenalidomide | |||||||
| 16 | + | + | + | EPOCH-R | Allogeneic HSCT | ||||
| 17 | + | + | + | EPOH, MTX | Autologous EBV-specific cytotoxic T cells followed by allogeneic HSCT | ||||
| T-cell disease | |||||||||
| 10 | + | + | Splenectomy | ||||||
| 15 | CHOEP, hyper CVAD, MTX | High-dose cytarabine with L-asparaginase, allogeneic HSCT | |||||||
| 18 | + | + | + | + | VP16 | Syngeneic HSCT | |||
| NK-cell disease | |||||||||
| 19 | Autologous EBV-specific cytotoxic T cells followed by allogeneic HSCT |
Adria indicates adriamycin; AraC, cytarabine; ATG, antithymocyte globulin; CHOEP, cyclophosphamide-doxorubicin-vincristine-etoposide-prednisolone; CTL, cytotoxic T lymphocyte; Cy, cyclophosphamide; EPOCH, etoposide-prednisone-vincristine-cyclophosphamide-doxorubicin; EPOCH-R, EPOCH-rituximab; Flu, fludarabine; EPOH, etoposide-prednisone-vincristine-doxorubicin; HSCT, hematopoietic stem cell transplantation; hyperCVAD, cyclophosphamide-vincristine-doxorubicin-dexamethasone; IVIG, intravenous immunoglobulin; LCL, lymphoblastoid cell line (EBV-transformed B cell line); LMP, latent membrane protein; MTX, methotrexate; NK, natural killer; Vin, vincristine; and VP16, etoposide.
Figure 5.
Clinical course. Clinical course for patients 12 (A) and 17 (B) is shown. Time from initial clinic visit is shown on the x-axis and EBV DNA viral load is on the y-axis. Chemo indicates chemotherapy; EPOCH-RF, etoposide-vincristine-doxorubicin-cyclophosphamide-prednisone-rituximab-fludarabine.
HSCT for CAEBV
Although patients were first seen in 1982 and various therapies were tried, HSCT was not begun in our patients until 1999. Most of the patients who underwent HSCT, like those who did not receive this therapy, had active disease involving vital organs (lung, CNS, BM). Despite active disease at the time of HSCT several were cured. Eight patients received HSCT. Three patients were at the NIH, 1 at New York Presbyterian Morgan Stanley Children's Hospital, and 4 at Baylor; 7 were allogeneic transplantations, and 1 was a syngeneic transplantation (Table 6 and supplemental data). Four patients received donor-derived EBV-specific CTLs after undergoing transplantation.22 Patients 15, 17, 18, and 19 received EBV- or LMP-specific T cells after undergoing allogeneic transplantation on clinical trials at Baylor College of Medicine,21,22 which were open to patients receiving allogeneic transplantation for an EBV-associated disease. The goal of these studies was to boost their cellular immune response to EBV and prevent or treat disease recurrence. Patients 15 and 17 received prophylactic EBV- or EBV-LMP1/2–specific CTLs after HSCT as consolidation therapy at a time when the EBV DNA load was undetectable. Patient 18 received both EBV- and EBV-LMP1/LMP2–specific CTLs because of persistently elevated EBV DNA in the blood and lymphadenopathy after transplantation; the patient's disease progressed and he died of EBV+ T-cell lymphoma. Patient 19 received EBV-specific CTLs for elevated EBV DNA in blood after transplantation; the EBV DNA decreased and it has remained undetectable.
Table 6.
Outcome of hematopoietic stem cell transplant for patients with chronic active EBV
| Patient | EBV+ cells | Age at Tx, y | Duration disease before Tx, y | Previous therapy | Donor | Disease status at transplant | Conditioning | GVHD prophylaxis | Donor EBV CTLs posttransplant | EBV PCR posttransplant | Outcome, time after transplant |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Transplanted at the NIH Clinical Center | |||||||||||
| 12 | B cell | 58 | 4 | IFNα, IVIG, bortezomib, EPOCH, rituximab, fludarabine, autologous CTLs | 6/6 sibling | Persistent disease | Cyclophosphamide, fludarabine | MTX, CyA | No | PCR−, then +, then − | Alive, 6 years |
| 13 | B cell | 45 | 0.5 | IFNα, EPOCH, rituximab, ganciclovir bortezomib, | 6/6 sibling | Persistent disease | Cyclophosphamide, fludarabine | CyA sirolimus | No | PCR+, then − | Died, 20 days, central pontine myelinolysis, residual B-cell lymphoma, multiorgan failure |
| 16 | B cell | 30 | 1 | IFNα, steroids, EPOCH, rituximab | Sibling/URD | Persistent disease | EPOCH-FR; fludarabine/TBI | CyA, sirolimus, tacrolimus | No | PCR+ | Died, 16 months, residual B-cell lymphoma |
| Transplanted at New York Presbyterian Morgan Stanley Children's Hospital | |||||||||||
| 15 | T cell | 12 | 4 | CHOEP, hyperCVAD, cytarabine, asparaginase, MTX | 5/6 sibling | Persistent disease | Thiotepa, cyclophosphamide, fludarabine | Tacrolimus, MMF | Yes; 2 infusions of 2 × 107/m2 EBV LMP1/2-specific CTLs | PCR− | Alive, 2 years |
| Transplanted at Baylor College of Medicine | |||||||||||
| 9 | B cell | 25 | 8 | Acyclovir, CyA, steroids, etoposide | 6/6 URD | Persistent T-cell lymphoma | Cyclophosphamide /cytarabine /ATG/TBI | CD6/8 T cell depletion, CyA | No | PCR- | Alive, CR 11 years |
| 17 | B cell | 10 | 5 | CyA, steroids, etoposide, rituximab, VAMP, autologous CTLs | 5/6 URD | 4th CR | Busulphan/ cyclophosphamide /cytarabine/campath | CyA | Yes; 1 infusion of 2 × 107/m2 EBV-specific CTLs | PCR Low positive /negative | Alive, CR 6 years |
| 18 | T cell | 6 | 2 | IVIG, acyclovir, steroids, etoposide, CyA | Syngeneic | Refractory T-cell lymphoma | Busulphan/ cyclophosphamide /alemtuzumab | MTX, Tacrolimus | Yes; 1 infusion of 2 × 107/m2 EBV-specific CTLs and 6 infusions of 2 × 107/m2 EBV LMP1/LMP2-specific CTLs | PCR+ | Died, 4 years, relapsed T-cell lymphoma |
| 19 | NK cell | 12 | 4 | autologous CTLs | 5/6 URD | Persistent disease | Cyclophosphamide/ cytarabine /alemtuzumab/TBI | MTX, tacrolimus | Yes; 1 infusion of 2 × 107/m2 EBV-specific CTLs | PCR−, then +, then − | Alive, CR 2 years |
ATG indicates antithymocyte globulin; CHOEP, cyclophosphamide-doxorubicin-vincristine-etoposide-prednisone; CR, complete remission; CTLs, cytotoxic T lymphocytes; CVAD, cyclophosphamide-vincristine-doxorubicin-dexamethasone; CyA, cyclosporine; EPOCH, etoposide-prednisone-vincristine-cyclophosphamide-doxorubicin; FR, fludarabine-rituximab; IVIG, intravenous immunoglobulin; LMP, latent membrane protein; MMF, mycophenolate mofetil; MTX, methotrexate; NK, natural killer; TBI, total body irradiation; TX, transplant; URD, unrelated donor; and VAMP, vincristine-doxorubicin-methotrexate-prednisone.
Five patients are still alive 2-11 years after transplantation. Of these 5 patients, 2 (patients 9 and 15) were persistently EBV PCR− after undergoing transplantation (one of whom received donor derived EBV-specific CTLs after transplantation). Patient 12 was initially EBV PCR− after transplantation, transiently became positive, and is negative now 6 years after transplantation. Patient 17 has had low positive-to-negative PCR results, received EBV-specific CTLs after transplantation, and is now 5.5 years posttransplantation. Patient 19, who was initially negative after transplantation, received EBV-specific CTLs when the blood EBV PCR results came back positive and currently is EBV PCR− 2 years after transplantation. Of the 5 patients who are alive after HSCT, 4 received CTLs either before or after transplantation; of the 3 who died after HSCT, only 1 received CTLs.
Three patients died after transplantation. Patient 13 died of multiorgan failure, had a single-negative EBV PCR near the time of death, and at autopsy had primarily necrotic lymphoma. Patient 16 remained EBV PCR+ and died of refractory EBV B-cell lymphoma. Patient 18 who received a syngeneic transplantation remained PCR+ after transplantation despite receipt of donor EBV-specific CTLs after transplantation, and died 4 years later of recurrent T-cell lymphoma.
In summary, 5 of 8 patients who underwent HSCT survived, and the other 3 patients died with EBV-positive lymphomas (of B- or T-cell lineage). Three of the 4 patients who underwent HSCT with myeloablative pretransplantation conditioning are alive, and 1 died of progressive EBV-positive lymphoma. Two of the 3 patients who underwent nonmyeloablative HSCT survived, whereas one died with central pontine myelinolysis. One patient received 2 transplantations, the first with nonmyeloablative and the second with myeloablative conditioning; he died with refractory EBV-positive B-cell lymphoma.
Discussion
We have reviewed our 28-year experience with CAEBV and report that patients in the United States have several important differences from previously reported cases in Japan and South or Central America. Nearly all of the patients in the literature with CAEBV have been in Asians from Japan, Taiwan, or Korea, or native Americans from Mexico, Central or South America.2,4–6,23 In contrast, 68% (13/19) of patients in our study were white or African Americans. In the largest review of patients with CAEBV, which included 82 patients from Japan, the mean age at disease onset was 11 years,5 whereas the mean age at presentation in our study was 19 years. Kimura et al5 reported that older age (≥ 8 years) at onset of disease correlated with a poor prognosis for survival. Signs and symptoms for CAEBV in Japan and in the United States were similar, although interstitial pneumonitis, CNS disease, and peripheral neuropathy were more common in US patients, whereas hypersensitivity to mosquito bites (a finding usually associated with NK cell CAEBV) were more commonly reported in Japan.
Antibody titers to several EBV lytic proteins were greater in patients with CAEBV compared with control patients. The increase in antibody titers to EBV lytic proteins in patients with CAEBV disease compared with EBV-seropositive control patients is probably because of the uncontrolled proliferation of EBV-infected cells with expression of viral lytic proteins and subsequent production of antibody to these proteins. This is likely because of a cellular immune disorder with failure to regulate EBV latently infected lymphocytes. Although the authors of other studies have reported markedly high titers to EBV viral capsid antigen, early antigen-diffuse and early antigen-restricted in approximately two-thirds of patients with CAEBV,1,2 we identified the individual viral proteins that were the target of these antibodies in T-cell and B-cell CAEBV. Furthermore, we found that patients with T-cell CAEBV have greater antibody titers to lytic proteins than those with B-cell CAEBV. Antibody to the EBNA1 latency protein has been reported to be lacking in approximately 20% of patients with CAEBV2,5; 33% (4/12) of our patients lacked antibody to EBNA1, and patients with B-cell CAEBV had greater levels of EBNA1 antibody than those with T-cell CAEBV. Although patients with CAEBV have high titers of antibodies to EBV, which might neutralize cell-free virus, CAEBV disease is because of proliferation of EBV latently infected lymphocytes. Cellular immunity, not antibody, is important in controlling proliferating virus-infected lymphocytes, as evidenced by the effectiveness of infusions of EBV-specific CTLs to treat PTLD in transplantation recipients.22
Low B-cell numbers have not been reported previously in CAEBV patients. We found that 43% had low levels of B cells in the absence of rituximab, and 42% of our patients developed hypogammaglobulinemia during the course of their disease.
Plasma levels of several cytokines, including IL-1β, IL-10, and IFN-γ, were reported to be elevated in persons with T-cell or NK-cell CAEBV; IL-13 was elevated in patients with NK-cell CAEBV.23 Transcripts for IL-1β, IL-2, IL-10, IL-12p35, IL-13, IL-15, IFN-γ, and TNF-α were also observed to be elevated in PBMCs from patients with CAEBV.23,24 We found that both Th1 (TNF-α, and IFN-γ) and Th2 (IL-6, IL-10) cytokines were significantly elevated in the serum of our patients with B-cell CAEBV compared with control patients. This finding is indicative of the “unbalanced cytokine profile” that has been reported previously in these patients.24 Elevated levels of each of these cytokines has been reported in the serum of patients with hemophagocytosis, which is common in CAEBV disease.
Approximately 33% of our patients had low NK-cell numbers. In a study of 81 patients with CAEBV in Japan, none was reported to have low NK-cell numbers, and 19% (15/81) had elevated NK-cell numbers.5 One patient in our study (patient 16) had reduced NK-cell activity, despite a normal number of NK cells. Reduced NK-cell activity has been reported in 8 of 9 patients with CAEBV from Japan25 and in 2 patients from Canada.10
Most Asians or Native Americans reported with CAEBV have EBV involving T cells or NK cells. In a review of patients with CAEBV in Japan, 54% of patients had EBV in T cells, 39% in NK cells, 4% in T and NK cells, and 3% in B cells.5 In our patients in whom tissue was available, 73% had EBV in B cells and 20% in T cells in tissues. Four of the 11 patients with EBV in B cells had prominent T-cell infiltrates in their tissues and in some cases the T cells were clonal. A similar clonal T-cell expansion in response to EBV-infected B cells has been reported in infectious mononucleosis.26
Kimura et al2,5 have shown that T-cell CAEBV has a poorer prognosis than NK-cell CAEBV. The probability of survival at 5 years after disease onset was 0.59 for patients with T-cell CAEBV and 0.87 for those with NK-cell CAEBV. In our study, at 5 years after the onset of disease, 66% of patients with T-cell CAEBV were alive and 73% of those with B-cell CAEBV were alive. Overall, however, 66% of the patients with T-cell CAEBV died and 64% of those with B-cell CAEBV died.
Patients with CAEBV received several therapies before HSCT, including antiviral agents (acyclovir and valacyclovir). Acyclovir acts by inhibiting the viral DNA polymerase, which is expressed during EBV lytic, but not latent gene expression. Although there have been anecdotal reports that acyclovir may have activity in some cases,7 the observation that EBV-infected cells from patients with CAEBV have a type 2 or 3 latency pattern without lytic gene expression11,23,27 is consistent with the lack of response to antiviral therapy that we and most others have observed. Similarly, although anecdotal reports suggest that CAEBV may respond to immunomodulatory agents (eg, IFN-α,28 IL-229), we and others have found these agents to be ineffective. Immunosuppressive agents (eg, corticosteroids, cyclosporine) have been effective in the treatment of EBV-associated hemophagocytosis,30 which was seen in 32% of our patients and was reported in 24% of patients in Japan5; however, these agents did not result in long-term remissions in our patients. Rituximab has been effective in some cases of X-linked lymphoproliferative disease and was temporarily effective in several patients with B-cell CAEBV; however, in most of our patients EBV was found in both CD20+ and CD20− B cells in the initial lesions, and after treatment with rituximab several patients had lesions that were EBV-positive solely in CD20− B cells. Cytotoxic chemotherapy has been effective for treatment of B-cell lymphomas in patients without CAEBV; however, combination chemotherapy (including EPOCH [etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin] with rituximab), bortezomib, and the combination of bortezomib and ganciclovir were ineffective in our patients (Table 5).
Transfer of autologous EBV-specific CTLs or HLA-identical EBV-specific CTLs from an HLA-identical sibling have been used to treat CAEBV. A transient decrease in EBV DNA in blood in a patient with CAEBV was reported with EBV-specific CTLs.31 In patients with a milder form of EBV-associated disease, defined as ≥ 6 months of symptoms (usually fever and fatigue) and either elevated EBV DNA in the blood, free EBV DNA in serum/cerebrospinal fluid, or EBV VCA antibody titer > 1:640, autologous EBV-specific CTLs resulted in improvement of symptoms.32 However, these patients had milder disease than that seen with CAEBV and did not have biopsy-proven evidence of tissue infiltration with EBV-positive lymphocytes. Four patients in our study (patients 12, 14, 17, and 19) received autologous EBV-specific CTLs. Patients 12, 17, and 19 did not respond to treatment; patient 14 had a transient response after receiving 3 courses of CTLs. All 4 patients eventually relapsed and 3 underwent allogeneic HSCT. The lack of long-term benefit from infusions of autologous EBV-specific T cells is likely because of impaired function of the patient's CTLs. In contrast, infusions of virus-specific T cells after transplantation from donors that are able to control EBV infection have been shown to be useful in prevention or treatment of EBV lymphoproliferative disease after transplantation.22 In a review of 101 HSCT recipients given EBV-specific CTLs either prophylactically after high-risk transplantations (eg, transplantations from matched unrelated donors with CD8 T-cell depletion or transplantations for EBV lymphoma) none of the patients developed PTLD.22 In the same review 11 of 13 HSCT recipients given EBV-specific CTLs for EBV PTLD or increasing levels of EBV DNA in the blood and symptoms consistent with EBV PTLD had sustained long-term remissions. Thus, in patients with CAEBV disease who may not have fully engrafted and who have moderate or high levels of EBV DNA in the blood after transplantation, infusions of donor-derived EBV-specific T cells may be effective.
Fifty-six individual cases of CAEBV treated with allogeneic HSCT from Japan12,14,33–47 and 1 case from the Netherlands48 have been reported in the literature (Table 7). Forty-six percent (26/57) were T cell in origin, 42% (24/57) were NK cell, 5% (3/57) were NK/T, and 7% (4/57) were of undetermined origin; none were B cell in origin. In contrast, of the 8 CAEBV patients who underwent HSCT in this report, 5 were B-cell CAEBV, 2 were T-cell, and 1 was NK-cell. Of the 57 patients in the literature, 25 (44%) received HSCT from related donors, 20 (35%) from matched unrelated donors, 7 (12%) underwent cord blood transplantations, 1 underwent an allogeneic-related transplantation followed by unrelated cord blood, 1 underwent an autologous transplantation followed by unrelated cord blood, and 3 were reported only to receive peripheral blood CD34+ cells. In our series, 4 received sibling donor, nonmyeloablative transplantation (1 was followed by an unrelated donor nonmyeloablative transplantation), 3 underwent unrelated donor myeloablative transplantation, and 1 underwent transplantation from his identical twin donor.
Table 7.
Previous reports of hematopoietic stem cell transplant for patients with chronic active EBV
| Reference | EBV+ cells | Age at Tx, y | Duration of disease before Tx, y | Type of transplant | Follow-up after transplant |
|---|---|---|---|---|---|
| 33 | T/NK | 7 | 3 | Allo sib, MA | A 134 mo, EBV PCR− |
| 34 | NK | 24 | 2 | Allo sib, MA | D 19 d, infection, PCR− |
| 35 | T | 9 | 0.1 | Allo sib, MA | A 47 mo, PCR− |
| 36 | T | 8 | 3 | Allo, dad, MA | D 26 d, VOD, MOF, PCR+ |
| 36 | T | 5 | 4 | Allo, dad, MA | A 124 mo, PCR− |
| 37 | NK | 4 | 11 | Allo, MUD, MA | D d 14, VOD, infection, RF |
| 37 | NK | 3 | 4 | Allo, MUD, MA | A 110 mo, PCR− |
| 37 | NK | 11 | 8 | Allo, MUD, MA | A 110 mo, PCR− |
| 38 | T | 31 | 1 | Allo, sib, NMA | A 86 mo, PCR− |
| 39 | NK | 10 | 5 | Allo, sib, MA | A 24 mo, PCR− |
| 40 | T | 14 | 1 | Allo, sib, NMA | A 27 mo, PCR− |
| 41 | ND | 31 | 0.5 | Allo, sib, MA | D 3 mo, liver failure, pancreatitis, PCR− |
| 42 | T | 11 | 4 | Allo, mom, NMA | Rejection, carditis, PCR+ |
| Allo, mom, NMA | Graft failure, GVHD, PCR+ | ||||
| Unrelated cord blood | A 15 mo, GVHD, zoster, PCR− | ||||
| 43 | T | 8 | 5 | Unrelated cord blood | A 15 mo, GVHD, PCR− |
| 44 | ND | 53 | 3 | Auto HSCT | Progressed |
| Unrelated cord blood | A 16 mo, acute then chronic GVHD, PCR− | ||||
| 12 | T | 8 | 7 | Allo, mom, NMA | A 67 mo |
| 12 | T | 2 | 1 | Allo, MUD, NMA | A 23 mo |
| 12 | NK | 6 | 4 | Allo, MUD, NMA | A 13 mo |
| 12 | NK | 20 | 18 | Allo, sib, NMA | D 2 mo, relapse |
| 12 | NK | 15 | 12 | Allo, sib, MA | D 1 mo, VOD, intracranial bleed |
| 12 | ND | 31 | 27 | Allo, sib, MA | D 67 mo, DIC, acute pancreatitis |
| 12 | T | 9 | 5 | Allo, sib, MA | A 49 mo, rejection, relapse, 2nd Tx |
| 12 | NK | 9 | 5 | Allo, MUD, MA | A 12 mo |
| 12 | NK | 16 | 6 | Allo, MUD, NMA | A 57 mo |
| 12 | NK | 19 | 7 | Allo, MUD, NMA | A 9 mo |
| 12 | T | 24 | 11 | Allo, MUD, NMA | D 10 mo, relapse |
| 12 | T | 18 | 4 | Allo, sib, MA | D 1 mo, intracranial bleed |
| 12 | NK | 15 | 1 | Allo, sib, NMA | D 16 mo, relapse |
| 12 | T | 24 | 5 | Allo, MUD, NMA | D 4 mo, encephalomyelitis |
| 12 | T | 27 | 1 | Allo, MUD, NMA | A 46 mo |
| 45 | T | 30 | 0.1 | Allo, sib, NMA | A 10 mo, rejected donor T cells, reinfused donor T cells twice, PCR− |
| 46 | T | 5 | 0.5 | Allo, mom, MA | A 4 mo, PCR+ |
| 46 | NK | 2 | 0.5 | Allo, MUD, MA | A 3 mo, PCR+ |
| 47 | NK | 13 | 10 | Allo, MUD, NMA | A 27 mo, PCR− |
| 14 | T | 10 | 0.5 | Allo, MUD, MA | A 101 mo |
| 14 | T | 18 | 6 | Allo, MUD, MA | D, 35 mo |
| 14 | T | 13 | 1 | Allo, CD34, MA | D, 198 d |
| 14 | NK | 5 | 1 | Allo, MUD, MA | A, 69 mo |
| 14 | T/NK | 18 | 0.3 | Allo, rel, MA | D, 1 y |
| 14 | T | 4 | 1 | Unrelated cord blood, NMA | A, 71 mo |
| 14 | NK | 19 | 3 | Allo, rel, NMA | A, 70 mo |
| 14 | NK | 5 | 1 | Allo, rel, NMA | A, 68 mo |
| 14 | NK | 17 | 1 | Allo, CD34, NMA | D, 19 d |
| 14 | T | 7 | 1 | Allo, rel, NMA | Relapse |
| 1.75 | Allo, rel, NMA | A, 51 mo | |||
| 14 | NK | 47 | 19 | Unrelated cord blood, NMA | A, 45 mo |
| 14 | T | 19 | 0.5 | Allo, CD34, NMA | A, 43 mo |
| 14 | T | 11 | 2.5 | Unrelated cord blood, NMA | Graft failure |
| 3 | Unrelated cord blood, NMA | A, 27 mo | |||
| 14 | NK | 13.5 | 1.5 | Allo, MUD, NMA | A, 27 mo |
| 14 | T | 9 | 2 | Unrelated cord blood, NMA | A, 27 mo |
| 14 | T | 36 | 13 | Allo, rel, NMA | A, 27 mo |
| 14 | ND | 3 | 1 | Unrelated cord blood, NMA | A, 23 mo |
| 14 | NK | 10 | 3 | Unrelated cord blood, NMA | A, 22 mo |
| 14 | NK | 18.5 | 0.5 | Allo, rel, NMA | A, 21 mo |
| 14 | T/NK | 7 | 6 | Allo, MUD, NMA | A, 21 mo |
| 14 | T | 21 | 1 | Allo, MUD, NMA | A, 14 mo |
| 14 | NK | 38 | 1 | Allo, rel, NMA | A, 12 mo |
| 48 | NK | 59 | 1 | Allo, MUD, NMA | D 30 d, GI bleed, PCR+ |
A indicates alive; Allo, allogeneic; Auto, autologous; D, dead; DIC, disseminated intravascular coagulation; GI, gastrointestinal; HSCT, hematopoietic stem cell transplant; MA, myeloablative; mo, months; MOF, multiorgan failure; MUD, matched unrelated donor; ND, not determined; NK, natural killer; NMA, nonmyeloablative; rel, related; RF, renal failure; sib, sibling; Tx, transplant; and VOD, veno-occlusive disease.
Seventy-two percent (41/57) of CAEBV patients who underwent HSCT in the literature survived after transplantation, whereas 63% (5/8) of our patients survived. Of the patients reported in the literature that were transplanted who died versus those who survived, there was no difference in the frequency of T-cell or NK-cell disease; however, survivors presented at an earlier age (mean, 14.4 years vs 21.2 years), had a shorter duration of disease before transplantation (3.5 years vs 6.5 years), and were more likely to have undergone nonmyeloablative transplantations (69% vs 38%) than myeloablative transplantations. Deaths reported in the literature and in our study were primarily because of progressive disease, relapsed disease, or transplantation-related complications. All but one of our patients whom we have followed for more than 5 years and who did not receive an allogeneic HSCT died from complications related to CAEBV.
In conclusion, CAEBV in the United States is most often because of uncontrolled EBV infection in B cells, and many of these patients have a progressive loss of B cells and hypogammaglobulinemia. Antiviral therapy is usually ineffective; although patients may have a temporary response to rituximab, immunosuppressive therapy, cytotoxic chemotherapy, autologous CTLs, or syngeneic HSCT, these treatments are not curative, and most patients succumb to their disease. Allogeneic HSCT is often curative for CAEBV disease. Both myeloablative and nonmyeloablative conditioning regimens have been successful even with progressive CAEBV disease at the time of transplantation. Unfortunately, death from progressive CAEBV disease remains the primary cause of failure after transplantation. Therefore, allogeneic HSCT should be considered early in the course of the disease when the patient is better able to tolerate transplantation.
Supplementary Material
Acknowledgments
We thank Gary Fahle of the NIH Clinical Center and Greg Storch of Washington University for measuring EBV DNA in blood, Stacey Rickleffs and Steve Porcella of Rocky Mountain Laboratories NIAID for DNA sequence analysis, Karen Thatcher and Ronald Hornung of SAIC Frederick for cytokine measurements, and Thomas Fleisher of the NIH Clinical Center for peripheral blood flow cytometry measurements The paper is dedicated to Stephen Straus, who initiated the work and inspired us.
This work was supported by the intramural research programs of the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the National Institute of Dental and Craniofacial Research and extramural grants CA094237 and P50CA126752.
Note added in proof.
Patient 14 has recently undergone allogeneic HSCT.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.I.C. and S.E.S. designed and supervised the study; J.I.C. wrote the manuscript; E.S.J. and S.P. were responsible for diagnosis and reviewing pathology; J.K.D., H.E.H., C.M.R., S.G., C.M.B., V.K.R., A.M., A.S.W., R.F.L., M.S.C., D.F., C.S., M.R.B., and W.W. provided data on patients, participated in clinical care and decision-making on patients, and reviewed the manuscript; S-P.T., R.F., and N.K.E. assisted with patient care; and P.D.B. performed antibody assays.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey I. Cohen, Laboratory of Infectious Diseases, 50 South Dr, Bldg 50, Rm 6134, MSC 8007, National Institutes of Health, Bethesda, MD 20892-8007; e-mail: jcohen@niaid.nih.gov.
References
- 1.Okano M, Matsumoto S, Osato T, Sakiyama Y, Thiele GM, Purtilo DT. Severe chronic active Epstein-Barr virus infection syndrome. Clin Microbiol Rev. 1991;4(1):129–135. doi: 10.1128/cmr.4.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura H, Hoshino Y, Kanegane H, et al. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood. 2001;98(2):280–286. doi: 10.1182/blood.v98.2.280. [DOI] [PubMed] [Google Scholar]
- 3.Okano M, Kawa K, Kimura H, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005;80(1):64–69. doi: 10.1002/ajh.20398. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann Oncol. 2009;20(9):1472–1482. doi: 10.1093/annonc/mdp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura H, Morishima T, Kanegame H, et al. Prognostic factors for chronic active Epstein-Barr virus infection. J Infec Dis. 2003;187(4):527–533. doi: 10.1086/367988. [DOI] [PubMed] [Google Scholar]
- 6.Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96(2):443–451. [PubMed] [Google Scholar]
- 7.Schooley RT, Carey RW, Miller G, et al. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis. Clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med. 1986;104(5):636–643. doi: 10.7326/0003-4819-104-5-636. [DOI] [PubMed] [Google Scholar]
- 8.Fujieda M, Wakiguchi H, Hisakawa H, Kubota H, Kurashige T. Defective activity of Epstein-Barr virus (EBV) specific cytotoxic T lymphocytes in children with chronic active EBV infection and in their parents. Acta Paediatr Jpn. 1993;35(5):394–399. doi: 10.1111/j.1442-200x.1993.tb03079.x. [DOI] [PubMed] [Google Scholar]
- 9.Sugaya N, Kimura H, Hara S, et al. Quantitative analysis of Epstein-Barr virus (EBV)-specific CD8+ T cells in patients with chronic active EBV infection. J Infect Dis. 2004;190(5):985–988. doi: 10.1086/423285. [DOI] [PubMed] [Google Scholar]
- 10.Joncas J, Monczak Y, Ghibu F, et al. Brief report: killer cell defect and persistent immunological abnormalities in two patients with chronic active Epstein-Barr virus infection. J Med Virol. 1989;28(2):110–117. doi: 10.1002/jmv.1890280211. [DOI] [PubMed] [Google Scholar]
- 11.Katano H, Ali MA, Patera AC, et al. Chronic active Epstein-Barr virus infection associated with mutations in perforin that impair its maturation. Blood. 2004;103(4):1244–1252. doi: 10.1182/blood-2003-06-2171. [DOI] [PubMed] [Google Scholar]
- 12.Gotoh K, Ito Y, Shibata-Watanabe Y, et al. Clinical and virological characteristics of 15 patients with chronic active Epstein-Barr virus infection treated with hematopoietic stem cell transplantation. Clin Infect Dis. 2008;46(10):1525–1534. doi: 10.1086/587671. [DOI] [PubMed] [Google Scholar]
- 13.Sato E, Ohga S, Kuroda H, et al. Allogeneic hematopoietic stem cell transplantation for Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disease in Japan. Am J Hematol. 2008;83(9):721–727. doi: 10.1002/ajh.21247. [DOI] [PubMed] [Google Scholar]
- 14.Kawa K, Sawada A, Sato M, et al. Excellent outcome of allogeneic hematopoietic SCT with reduced-intensity conditioning for the treatment of chronic active EBV infection. Bone Marrow Transplant. 2011;46(1):77–83. doi: 10.1038/bmt.2010.122. [DOI] [PubMed] [Google Scholar]
- 15.Straus SE. Acute progressive Epstein-Barr virus infections. Annu Rev Med. 1992;43:437–449. doi: 10.1146/annurev.me.43.020192.002253. [DOI] [PubMed] [Google Scholar]
- 16.El-Mallawany NK, Geller L, Bollard CM, et al. Long term remission in a child with refractory EBV(+) hydroa vacciniforme-like T-cell lymphoma through sequential matched EBV(+)-related allogeneic hematopoietic SCT followed by donor-derived EBV-specific cytotoxic T-lymphocyte immunotherapy [published online ahead of print July 26, 2010]. Bone Marrow Transplant. doi: 10.1038/bmt.2010.176. doi: 10.1038/bmt.2010-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenagy DN, Schlesinger Y, Weck K, Ritter JH, Gaudreault-Keener MM, Storch GA. Epstein-Barr virus DNA in peripheral blood leukocytes of patients with posttransplant lymphoproliferative disease. Transplantation. 1995;60(6):547–554. doi: 10.1097/00007890-199509270-00005. [DOI] [PubMed] [Google Scholar]
- 18.Calattini S, Sereti I, Scheinberg P, Kimura H, Childs RW, Cohen JI. Detection of EBV genomes in plasmablasts/plasma cells and non-B cells in the blood of most patients with EBV lymphoproliferative disorders using Immuno-FISH. Blood. 2010;116(22):4546–4559. doi: 10.1182/blood-2010-05-285452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savoldo B, Goss J, Liu Z, et al. Generation of autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells (CTL) for adoptive immunotherapy in solid organ transplant recipients. Transplantation. 2001;72(6):1078. doi: 10.1097/00007890-200109270-00017. [DOI] [PubMed] [Google Scholar]
- 20.Sashihara J, Burbelo PD, Savoldo B, Pierson TC, Cohen JI. Human antibody titers to Epstein-Barr Virus (EBV) gp350 correlate with neutralization of infectivity better than antibody titers to EBV gp42 using a rapid flow cytometry-based EBV neutralization assay. Virology. 2009;391(2):249–256. doi: 10.1016/j.virol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110(8):2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura H, Hoshino Y, Hara S, et al. Differences between T cell-type and natural killer cell-type chronic active Epstein-Barr virus infection. J Infect Dis. 2005;191(4):531–539. doi: 10.1086/427239. [DOI] [PubMed] [Google Scholar]
- 24.Ohga S, Nomura A, Takada H, et al. Epstein-Barr virus (EBV) load and cytokine gene expression in activated T cells of chronic active EBV infection. J Infect Dis. 2001;183(1):1–7. doi: 10.1086/317653. [DOI] [PubMed] [Google Scholar]
- 25.Wakiguchi H, Fujieda M, Matsumoto K, Ohara Y, Wakiguchi A, Kurashige T. Defective killer cell activity in patients with chronic active Epstein-Barr virus infection. Acta Med Okayama. 1988;42(3):137–142. doi: 10.18926/AMO/31028. [DOI] [PubMed] [Google Scholar]
- 26.Callan MF, Steven N, Krausa P, et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2(8):906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 27.Iwata S, Wada K, Tobita S, et al. Quantitative analysis of Epstein-Barr virus (EBV)-related gene expression in patients with chronic active EBV infection. J Gen Virol. 2010;91(Pt 1):42–50. doi: 10.1099/vir.0.013482-0. [DOI] [PubMed] [Google Scholar]
- 28.Sakai Y, Ohga S, Tonegawa Y, et al. Interferon-alpha therapy for chronic active Epstein-Barr virus infection: potential effect on the development of T-lymphoproliferative disease. J Pediatr Hematol Oncol. 1998;20(4):342–346. doi: 10.1097/00043426-199807000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Kawa-Ha K, Franco E, Doi S, et al. Successful treatment of chronic active Epstein-Barr virus infection with recombinant interleukin-2. Lancet. 1987;1(8525):154. doi: 10.1016/s0140-6736(87)91980-5. [DOI] [PubMed] [Google Scholar]
- 30.Imashuku S, Hibi S, Ohara T, et al. Effective control of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis with immunochemotherapy. Histiocyte Society. Blood. 1999;93(6):1869–1874. [PubMed] [Google Scholar]
- 31.Kuzushima K, Yamamoto M, Kimura H. Establishment of anti-Epstein-Barr virus (EBV) cellular immunity by adoptive transfer of virus-specific cytotoxic T cells from an HLA-matched sibling to a patients with severe chronic active EBV infection. Clin Exp Immunol. 1996;103(2):192–198. doi: 10.1046/j.1365-2249.1996.d01-619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savoldo B, Huls MH, Liu Z, et al. Autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for the treatment of persistent active EBV infection. Blood. 2002;100(12):4059–4066. doi: 10.1182/blood-2002-01-0039. [DOI] [PubMed] [Google Scholar]
- 33.Okamura T, Hatsukawa Y, Arai H, Inoue M, Kawa K. Blood stem-cell transplantation for chronic active Epstein-Barr virus with lymphoproliferation. Lancet. 2000;356(9225):223–234. doi: 10.1016/S0140-6736(00)02488-0. [DOI] [PubMed] [Google Scholar]
- 34.Fujii N, Takenaka K, Hiraki A, et al. Allogeneic peripheral blood stem cell transplantation for the treatment of chronic active Epstein-Barr virus infection. Bone Marrow Transplant. 2000;26(7):805–808. doi: 10.1038/sj.bmt.1702600. [DOI] [PubMed] [Google Scholar]
- 35.Taketani T, Kikuchi A, Inatomi J, et al. Chronic active Epstein-Barr virus infection (CAEBV) successfully treated with allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2002;29(6):531–533. doi: 10.1038/sj.bmt.1703392. [DOI] [PubMed] [Google Scholar]
- 36.Yasui M, Okamura T, Sakata N, et al. CD34+ progenitor cell transplantation from HLA-mismatched donors to two patients with chronic active Epstein-Barr virus infection. Rinsho Ketsueki. 2001;42(11):1111–1116. [PubMed] [Google Scholar]
- 37.Okamura T, Kishimoto T, Inoue M, et al. Unrelated bone marrow transplantation for Epstein-Barr virus-associated T/NK-cell lymphoproliferative disease. Bone Marrow Transplant. 2003;31(2):105–111. doi: 10.1038/sj.bmt.1703796. [DOI] [PubMed] [Google Scholar]
- 38.Sakata N, Sato E, Sawada A, Yasui M, Inoue M, Kawa K. Chronic active Epstein-Barr virus infection treated with reduced intensity stem cell transplantation. Rinsho Ketsueki. 2004;45(5):393–396. [PubMed] [Google Scholar]
- 39.Ebihara Y, Manabe A, Tanaka R, et al. Successful treatment of natural killer (NK) cell leukemia following a long-standing chronic active Epstein-Barr virus (CAEBV) infection with allogeneic bone marrow transplantation. Bone Marrow Transplant. 2003;31(12):1169–1171. doi: 10.1038/sj.bmt.1704078. [DOI] [PubMed] [Google Scholar]
- 40.Uehara T, Nakaseko C, Hara S, et al. Successful control of Epstein-Barr virus (EBV)-infected cells by allogeneic nonmyeloablative stem cell transplantation in a patient with the lethal form of chronic active EBV infection. Am J Hematol. 2004;76(4):368–372. doi: 10.1002/ajh.20121. [DOI] [PubMed] [Google Scholar]
- 41.Takeoka Y, Nakao Y, Ueda M, et al. A case of a long-time survivor with chronic active Epstein-Barr virus infection. Eur J Haematol. 2004;72(1):73–76. doi: 10.1046/j.0902-4441.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 42.Ishimura M, Ohga S, Nomura A, et al. Successful umbilical cord blood transplantation for severe chronic active Epstein-Barr virus infection after the double failure of hematopoietic stem cell transplantation. Am J Hematol. 2005;80(3):207–212. doi: 10.1002/ajh.20430. [DOI] [PubMed] [Google Scholar]
- 43.Iguchi A, Kobayashi R, Sato TZ, Nakajima M, Kaneda M, Ariga T. Successful report of reduced-intensity stem cell transplantation from unrelated umbilical cord blood in a girl with chronic active Epstein-Barr infection. J Pediatr Hematol Oncol. 2006;28(4):254–256. doi: 10.1097/01.mph.0000212916.29889.c2. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa M, Hashino S, Takahata M, et al. Successful reduced-intensity stem cell transplantation with cord blood for a poor-prognosis adult with refractory chronic active Epstein-Barr virus infection. Int J Hematol. 2007;85(5):443–445. doi: 10.1532/IJH97.06115. [DOI] [PubMed] [Google Scholar]
- 45.Yoshiba F, Hagihara M, Tazume K, et al. Complete resolution of severe chronic active Epstein-Barr virus infection by cultured, activated donor T lymphocyte infusion after nonmyeloablative stem cells allografting. Bone Marrow Transplant. 2003;32(1):107–110. doi: 10.1038/sj.bmt.1704074. [DOI] [PubMed] [Google Scholar]
- 46.Miyamura T, Chayama K, Wada T, et al. Two cases of chronic active Epstein-Barr virus infection in which EBV-specific cytotoxic T lymphocyte was induced after allogeneic bone marrow transplantation. Pediatr Transplant. 2008;12(5):588–592. doi: 10.1111/j.1399-3046.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- 47.Matsunaga T, Kurosawa H, Okuya M, et al. Chronic active Epstein-Barr virus infection with mosquito allergy successfully treated with reduced-intensity unrelated allogeneic bone marrow transplantation in a boy. Pediatr Transplant. 2009;13(2):231–234. doi: 10.1111/j.1399-3046.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- 48.Sonke GS, Ludwig I, van Oosten H, et al. Poor outcomes of chronic active Epstein-Barr virus infection and hemophagocytic lymphohistiocytosis in non-Japanese adult patients. Clin Infect Dis. 2008;47(1):105–108. doi: 10.1086/588790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.