Abstract
TLR2, a functional, inflammatory-related receptor, is known to be expressed on megakaryocytes and platelets and to lead to infection and immune-mediated activation of platelets; however, the role of this receptor in megakaryocytes is not understood. Using Meg-01 cells and mouse megakaryocytes, we found that NFκB, ERK-MAPK, and PI3K/Akt pathways, known downstream pathways of TLRs, are activated by Pam3CSK4, a TLR2-specific ligand. In addition, transcription factors associated with megakaryocyte maturation, GATA-1, NF-E2, and mammalian target of rapamycin (mTOR), are all increased in the presence of Pam3CSK4. The effect of Pam3CSK4 on megakaryocyte maturation was verified by the increase in DNA content and adhesion to extracellular matrix proteins by TLR2-dependent stimulation. In addition, TLR2 stimulation resulted in an increase in reactive oxygen species (ROS) production. Gene expression and protein levels of GP1b, CD41, MCP-1, COX2, NFκB1, and TLR2 were up-regulated in megakaryocytes after TLR2 stimulation through NFκB, PI3K/Akt, and ERK-MAPK pathways. Treatment of wild-type mice with Pam3CSK4 resulted in a return to normal platelet levels and an increase in megakaryocyte maturation, which did not occur in the TLR2−/− mice. Therefore, inflammation, through TLR2, can increase maturation and modulate the phenotype of megakaryocytes, contributing to the interrelationship between inflammation and hemostasis.
Introduction
Megakaryocytes mature through a process called megakaryopoiesis, ultimately leading to the production of platelets. To create platelets, thrombopoietin (TPO)1–3 and other factors, such as IL-1,4 IL-6,5 and stromal cell-derived factor-1,6 trigger the enlargement of megakaryocytes. During the process of thrombopoiesis, megakaryocytes increase their DNA content from 4N to 128N per cell by not undergoing endocytosis.7 RNA and protein production are increased to generate the necessary cellular contents for each platelet.7 Platelets are then released from pseudopod-like extensions called proplatelets, which contain enhanced platelet-specific granules.7 Inflammatory conditions are known to increase TPO levels, thereby increasing the number of circulating platelets. IL-6 has been shown to increase TPO levels in vitro and in vivo.8 In addition, it has been reported that TPO levels and platelet counts increase after 24 hours of exposure to low-grade endotoxemia, resulting in an 8% increase in circulating platelets 7 days after exposure.9 Individuals with pulmonary tuberculosis also have increases in thrombopoiesis and highly reactive platelets.10
As part of the innate immune system, TLRs are activated upon interaction with different pathogen-associated molecular patterns (PAMPs). There are 11 human TLRs.11 TLR2, which forms heterodimers with TLR1 and TLR6, recognizes triacylated and diacylated lipoproteins, peptidoglycans, and lipoteichoic acid from gram-positive bacteria, lipoarabinomannan from mycobacteria, glycosylphosphatidylinositol anchors, phenol-soluble modulin, zymosan from fungi, glycolipids, and lipopolysaccharide from non-enterobacteria.11 Upon stimulation of TLR2 by one of its PAMPs, several different signaling proteins, such as MyD88 and IRAK, are activated, eventually resulting in the activation of the NF-κB pathway.12–13 In innate immune cells, this signaling cascade results in the increased expression and release of inflammatory cytokines such as interleukins and tumor necrosis factor-α.11 TLR2 has also been implicated in other inflammatory processes associated with vascular disease, such as atherosclerosis.14
Recent studies have shown that functional TLRs, including TLR1, TLR2, TLR4, and TLR6, are expressed on megakaryocytes and platelets.15–19 The binding of an agonist to TLR2 on platelets results in activation, as shown by an increase in surface expression of P-selectin, activation of αIIbβ3, adhesion to collagen, and the formation of heterotypic aggregates with neutrophils.15,20 Flow cytometry and immunohistochemistry data have shown that megakaryocytes express TLR1, TLR2, TLR4, and TLR6,16–17 but the functional significance of TLR signaling in megakaryocytes has not been reported previously. TLR2 is expressed on megakaryocytes, and it is possible that this immune-related receptor can modulate megakaryocyte cellular function and affect gene expression through various signaling pathways. In the present study, we show that upon stimulation of megakaryocyte TLR2 by a specific synthetic ligand, Pam3CSK4, there is an increase in phosphorylation of the NFκB p65 subunit, Akt, and ERK1/2, which alters gene expression, protein levels, and megakaryocyte cell function. Therefore, we hypothesize that inflammation, through TLR2, can affect megakaryopoiesis, promoting increased platelet development.
Methods
Cell culture
Meg-01 cells, a megakaryoblast cell line (American Type Culture Collection), were grown in suspension in RPMI 1640 medium (GIBCO) supplemented with l-glutamine, 10% heat-inactivated FBS (GIBCO), and 1% antibiotic/antimycotic (GIBCO).
Animal care and Pam3CSK4 treatment
Wild-type (WT; C57BL/6J) and TLR2−/− (B6.129-Tlr2tm1Kir/J) mice were purchased from The Jackson Laboratory and housed and maintained in the Boston University Laboratory Animal Facility. All animal studies were approved by the Boston University School of Medicine Institutional Animal Care and Use Committee. Mice were injected intraperitoneally with 4 mg/kg of Pam3CSK4 (InvivoGen) in saline. Blood and bone marrow were collected on days 3, 5, and 7 for analysis.
Western blots
Lysates (25 μg as determined by Bio-Rad DC Protein Assay) were run on 12% polyacrylamide gels, transferred onto PVDF (Millipore), and probed using the following antibodies: phospho-NFκB p65 and total NFκB p65 (Cell Signaling Technology), phospho-Akt and total Akt (Cell Signaling Technology), phospho-ERK and total ERK (Cell Signaling Technology), COX2 (Cell Signaling Technology), NFκB1 (Cell Signaling Technology), NF-E2 (Santa Cruz Biotechnology), GATA-1 (Cell Signaling Technology), phospho-mTOR and total mTOR (Cell Signaling Technology), and actin (Santa Cruz Biotechnology). Densitometry was performed using ImageJ v1.43u software.
Flow cytometry
For the surface expression of GP1b, TLR2, and CD41, Meg-01 cells were treated for 3 hours with 1 μg/mL of Pam3CSK4 (InvivoGen). Intact cells were stained with anti-human GP1b-FITC, TLR2-FITC, CD41-FITC, and the corresponding isotype controls (all from eBioscience) and run on a FACScan analyzer (BD Biosciences) using CellQuest Pro v5.2 Software (BD Biosciences).
MCP-1 ELISA
Conditioned medium collected from Meg-01 cells after being treated for 3 hours with 1 μg/mL of Pam3CSK4 was concentrated using Amicon Ultra Centrifugal Filter Devices (Millipore). Samples were then tested for the presence of MCP-1 using an ELISA (R&D Systems).
Real-time PCR
Meg-01 cells or murine bone marrow was treated with 1 μg/mL of Pam3CSK4 for 3 hours at 37°C and 5% CO2. For the pharmacologic inhibitors, Meg-01 cells were pretreated with 50μM LY294002 (Calbiochem), 50μM U0126 (Biomol), 50μM BAY 11-7082 (Calbiochem), or DMSO for 45 minutes. Cells were then treated for 3 hours with Pam3CSK4 (1 μg/mL).
Total RNA was isolated from Meg-01 cells using the RNeasy Mini Kit (QIAGEN). RNA was converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Gene expression of GAPDH, MCP-1, GP1b, COX2, TLR2, CD41, and NFκB1 (primers and probes from Applied Biosystems) using the TaqMan Gene Expression Master Mix (Applied Biosystems) was assessed with real-time PCR (Applied Biosystems 7900 HT Fast Real-Time PCR System with SDS 2.2.2 software).
Total RNA was isolated from mouse megakaryocytes using the miRNeasy Mini Kit (QIAGEN). RNA was converted to cDNA using the QuantiTect Reverse Transcription Kit (QIAGEN). Preamplification of cDNA was performed using TaqMan PreAmp Master Mix (Applied Biosystems), and then assayed for gene expression as described previously.
Mouse bone marrow isolation and megakaryocyte preparation
Bone marrow from WT and TLR2−/− mice was flushed out of femurs and tibias using CATCH buffer (1× HBSS, 0.38% Na citrate, 1mM adenosine, 2mM theophylline, and 5% heat-inactivated FBS) and isolated as described previously.21 Cells were initially seeded at 5 × 106/mL and grown for up to 4 days in medium (IMDM with l-glutamine, 25mM HEPES, 1% antibiotic/antimycotic, and 10% heat-inactivated FBS) supplemented with 50 ng/mL of murine TPO (mTPO) daily. Megakaryocytes were isolated using magnetic bead separation of lineage-positive cells (Miltenyi Biotec). Flow cytometry was performed to verify the purity of the megakaryocyte sample. Samples were stained with mouse CD45R-FITC, Ter119-FITC, Ly-6G-PE-Cy7 (all from eBioscience), CD41-FITC (BD Biosciences), and corresponding isotype controls (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Adhesion
Meg-01 cells (5 × 104) were treated with Pam3CSK4 and plated onto glass coverslips coated with 10 μg/mL of fibronectin (Sigma) for 1 hour at 37°C. Adherent cells were fixed in 2% paraformaldehyde and stained with anti-human CD41-FITC antibody. Adherent cells were counted from 5 separate 20× views using a fluorescent microscope (Nikon) with Open Lab v5.5.2 software.
For fibrinogen, a 24-well plate was coated with 10 μg/mL of fibrinogen (Enzyme Research Laboratories) and run as previously described. Adherent cells were then treated with serum-free medium and 0.5 mg/mL of MTT (Sigma-Aldrich). The absorbance of each well was read at 595 nm.
Ploidy
Bone marrow isolated from WT and TLR2−/− mice was cultured for 3 days in medium supplemented daily with 50 ng/mL of mTPO, 1 μg/mL of Pam3CSK4, 50μM U0126, 50μM BAY 11-7082, 50μM LY294002, or buffer control. On day 3, bone marrow samples were stained with anti-mouse CD41 FITC antibody (or isotype control), 0.05 mg/mL of propidium iodide, and 100 μg/mL of RNase A, and then analyzed by flow cytometry gating for the CD41-positive cells.
ROS production
Meg-01 cells were treated with Pam3CSK4, stained with 5-(and-6)-carboxy-2′,7′-difluorodihydrofluorescein diacetate (Invitrogen), and analyzed for ROS by flow cytometry.
Statistical analysis
Data are reported as the average ± SD. Some results are reported as the percentage of no treatment (NT), which was set to 100%. Statistical analysis was performed using a 1-sample t test with a normal distribution, a theoretical mean of 100, and significance of P < .05. Other results were analyzed using a Student t test or ANOVA with a normal distribution and a significance of P < .05. All statistical analysis was done using Prism 5 software (GraphPad).
Results
Megakaryocytes express TLRs and TLR2 ligand binding activates the NFκB pathways
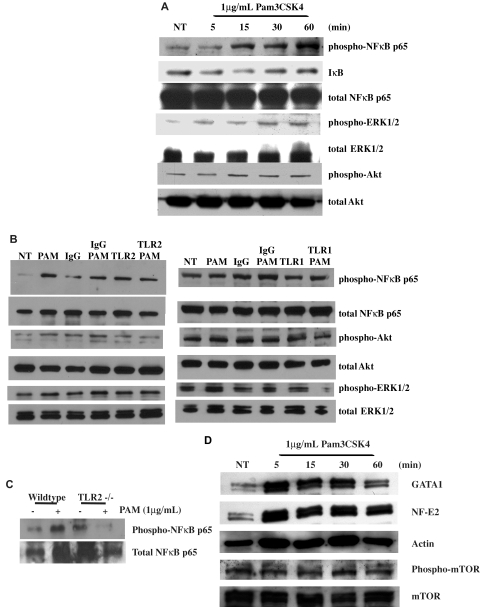
Megakaryocytes express TLRs16–17 on their cell surfaces (supplemental Figure 2). Focusing on TLR2, Meg-01 cells were treated with 1 μg/mL of the TLR2-specific ligand Pam3CSK4 for up to 1 hour. Lysates from each time point were immunoblotted for the phosphorylated p65 subunit of NFκB, a known downstream signaling pathway of TLR2.13 As shown in Figure 1A, treatment with Pam3CSK4 increased the level of phospho-NFκB p65 at 15 minutes by 176.4% ± 69.6% compared with NT, which continued at 60 minutes to 254.2% ± 95.8%. In addition, lysates were immunoblotted for the loss of IκB, the negative regulator of NFκB subunits.22 As shown in Figure 1A, there was a loss of IκB by 5 minutes (4.9% ± 7.5%) compared with NT, which continued to decrease for 15 minutes (8.6% ± 2.6%), and then returned back to baseline by 60 minutes.
Figure 1.
TLR2 activates the NFκB, PI3K/Akt, and ERK pathways in Meg-01 cells. (A) Meg-01 cells were treated for up to 1 hour with 1μg/mL of Pam3CSK4 (PAM). Western blots were run with samples at each time point and probed for phospho-NFκBp65, total NFκBp65, IκB, phospho-Akt, total Akt, phospho-ERK, and total ERK. Western blots are representative of 3 independent experiments, and results were compared with NT. (B) Meg-01 cells were pretreated for 1 hour with 25 μg/mL of IgG, anti-TLR2, or anti-TLR1 antibodies. Cells were then treated for 30 minutes with 1μg/mL of Pam3CSK4. Western blots are representative of 4 experiments. (C) Isolated megakaryocytes from WT and TLR2−/− mice that were treated for 30 minutes with 1 μg/mL of Pam3CSK4 were run on Western blots and probed for phospho-NFκBp65 and total NFκBp65. Western blots are representative of 3 independent experiments. (D) Meg-01 cells were treated for up to 1 hour with 1 μg/mL of Pam3CSK4 (PAM). Western blots were probed for GATA-1, NF-E2, phospho-mTOR, total mTOR, and actin (loading control). Western blots are representative of 4 independent experiments.
To verify that the activation of the NFκB pathway was specific to the binding of Pam3CSK4 to TLR2, Meg-01 cells were preincubated with TLR2-blocking antibodies or mouse IgG before treatment with Pam3CSK4 for 30 minutes. As shown in Figure 1B, there was an increase in the phosphorylation of NFκB p65 after treatment with Pam3CSK4 alone (130.7% ± 22.2%) or Pam3CSK4 with IgG (209.2% ± 81.5%). Conversely, in the absence of Pam3CSK4 binding to TLR2, there was no increase in NFκB p65 phosphorylation (138.5% ± 43.6%). TLR2 forms a dimer with TLR1 to recognize Pam3CSK4. Experiments using blocking antibodies to TLR1 (Figure 1B) also showed that the Pam3CSK4-induced increase in the phosphorylation of NFκBp65 (222.8% ± 87.4%) required TLR1 (116.5% ± 33.5%). Further verification was done with murine megakaryocytes isolated from WT and TLR2−/− mice. In vitro, bone marrow was treated for 30 minutes with 1 μg/mL of Pam3CSK4. Isolated WT megakaryocytes responded to Pam3CSK4 and showed an increase in phosphorylated NFκB p65 (173.7%; Figure 1C). However, in TLR2−/− megakaryocytes, there was no increase in NFκB p65 phosphorylation (20.5%; Figure 1C).
Activation of the PI3K/Akt and ERK MAPK pathways by TLR2 in megakaryocytes
Upon activation of the TLR2 receptor, adaptor proteins and proteases such as MyD88, IRAKs, and TRAFs leads to the phosphorylation and activation of IKK. This protease then phosphorylates IκB, which allows NFκB to localize into the nucleus. In addition, these same pathways that activate IKK will also activate other signaling pathways, including the ERK-MAPK pathway.22 To determine whether the ERK-MAPK pathway was also activated by TLR2 in megakaryocytes, lysates of Meg-01 cells were treated with 1 μg/mL of Pam3CSK4 for up to 1 hour, and then immunoblotted for phosphorylated ERK1/2. As shown in Figure 1A, there was an increase in the phosphorylation of ERK beginning at 5 minutes, which peaked at 30 minutes (105.8% ± 3.9%). Blocking Pam3CSK4 binding to TLR2 resulted in the reduction of ERK1/2 phosphorylation (98.4% ± 27.8%) at 30 minutes compared with Pam3CSK4 alone (202.7% ± 52.8%) or Pam3CSK4 with IgG (171.5% ± 26.4%; Figure 1B). The results with TLR1-blocking antibody (Figure 1B) were similar, suggesting that both receptors are needed for activation of the ERK-MAPK pathway.
Another signaling pathway activated by TLR2 is the PI3K/Akt pathway. Previous studies have shown that the p85 subunit of PI3K can bind to the cytoplasmic domain of TLRs and lead to the activation of the NFκB pathway.23 PI3K/Akt was shown to phosphorylate the p65 subunit of NFκB.22 To determine whether this pathway is also activated by TLR2 in megakaryocytes, lysates treated for up to 1 hour with 1 μg/mL of Pam3CSK4 were also immunoblotted for the phosphorylation of Akt, a downstream target of PI3K. As shown in Figure 1A, there was an increase in the phosphorylation of Akt beginning at 30 minutes (139.4% ± 38.5%), which was maintained for up to 1 hour (129.3% ± 20.1%). Similar to the previous results, when Pam3CSK4 was blocked from binding to TLR2, there was no increase in the phosphorylation of Akt (73.5% ± 44.5%) compared with Pam3CSK4 alone (202.8% ± 120.5%) or Pam3CSK4 with IgG (180.1% ± 106.6%; Figure 1B). In addition, blocking TLR1 also led to a loss of Pam3CSK4-induced phosphorylation of Akt (68.3% ± 32.8%) compared with Pam3CSK4 alone (178.4% ± 57.2%) or Pam3CSK4 with IgG (108.3% ± 6.1%; Figure 1B). Therefore, not only was the NFκB pathway activated by TLR2/1 in megakaryocytes, but also the ERK-MAPK and PI3K/Akt pathways, which may independently alter megakaryocyte cellular function and/or regulate the NFκB signaling pathway.
TLR2 binding increases NF-E2, GATA-1, and phospho-mTOR levels
Various transcription factors also regulate megakaryocyte maturation and thrombopoiesis by up-regulating and suppressing gene-expression levels. GATA-1 is a transcription factor that directs all stages of megakaryocyte development and up-regulation of platelet-specific genes, including CD41.24 Knocking out GATA-1 results in thrombocytopenia and dysregulation of proliferation and maturation of megakaryocytes.25 As shown through Western blots, Meg-01 cells treated with 1 μg/mL of Pam3CSK4 had an increased level of GATA-1 compared with NT starting at 5 minutes (133.4% ± 12.7%) and continuing for up to 1 hour (137.2% ± 25.4%; Figure 1D). GATA-1 is known to up-regulate the expression of another transcription factor, NF-E2, which controls late-stage megakaryopoiesis and platelet production, including granule formation and platelet development. Without NF-E2, mice have severe thrombocytopenia26 because the maturation process is halted and megakaryocytes do not form platelets.27 As seen in Figure 1D, treatment with 1 μg/mL of Pam3CSK4 increased NF-E2 levels in the Meg-01 cells starting at 30 minutes (132.0% ± 19.3%) and continuing through to 1 hour (208.0% ± 63.2%).
Another transcription factor known to regulate cell-cycle progression and to be activated by PI3K/Akt is mTOR. This transcription factor is activated through phosphorylation at serine 2448. Knocking out this transcription factor in mice also leads to thrombocytopenia because of fewer and smaller megakaryocytes.28 In the Meg-01 cell line, the addition of 1 μg/mL of Pam3CSK4 slightly increased the phosphorylation of mTOR at 30 minutes (103.1% ± 8.6%; Figure 1D). Other transcription factors cJun and cFos were not affected by Pam3CSK4 treatment (data not shown).
TLR2 increases ploidy, ROS, and adhesion in megakaryocytes
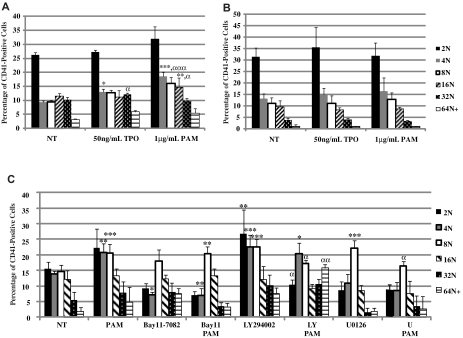
TLR2 binding leads to the up-regulation of transcription factors important for the maturation of megakaryocytes into platelets. As these cells mature, their DNA content increases from 2N to 128N. Bone marrow isolated from WT and TLR2−/− mice was used to determine whether TLR2 could increase the DNA content of the megakaryocytes in a manner similar to TPO, a known regulator of megakaryocyte maturation. WT megakaryocytes were treated daily with mTPO for 3 days, and there was an increase in the percentage of CD41-positive megakaryocytes at 4N and higher (Figure 2A). In the presence of 1 μg/mL of Pam3CSK4, there was also an increase in the number of CD41-positive WT megakaryocytes at 4N and higher (Figure 2A). Interestingly, treatment with Pam3CSK4 increased the percentage of megakaryocytes at 4N and 16N significantly more than TPO treatment (Figure 2A). There was also a small, nonsignificant increase in the 2N population with Pam3CSK4 treatment in WT bone marrow. It is possible that this treatment also increased the number of megakaryocytes in the bone marrow, which would increase the number of cells at each level of DNA content. It is also possible that in response to an increase in more mature cells, the bone marrow must replenish the less mature population. The addition of both TPO and Pam3CSK4 in culture did not increase ploidy any further than the addition of either reagent alone (data not shown). When TLR2 was not present, there was no effect of Pam3CSK4 on ploidy (Figure 2B). In addition, there was no effect of TPO on ploidy in the TLR2−/− megakaryocytes. However, Western blot analysis showed that STAT3, a known downstream signaling pathway of TPO, was activated (supplemental Figure 3), suggesting that inflammation and TLR2 may have a role in the response of megakaryocytes to TPO. Megakaryocyte maturation is further enhanced through TLR2 treatment, as seen by the increase in DNA content that was similar, if not better, than that after TPO treatment. WT bone marrow was also continuously treated with inhibitors to the NFκB, PI3K/Akt, and ERK-MAPK pathways. As seen in Figure 2C, Bay 11-7082, an inhibitor of the NFκB pathway, prevented the increase in 4N and higher by Pam3CSK4, as was seen with LY294002, an inhibitor of the PI3K/Akt pathway, and U0126, an inhibitor of the ERK-MAPK pathway. Any effects on ploidy were a result of the pharmacologic inhibitor. These results suggest that all 3 pathways are important for TLR2 to mediate the signal to increase megakaryocyte maturation.
Figure 2.
TLR2 increases polyploidization in mouse megakaryocytes. WT (A) and TLR2−/− (B) mouse bone marrow was treated for 3 days with 50 ng/mL mTPO or 1μg/mL Pam3CSK4 (PAM). (C) WT mouse bone marrow was treated for 3 days with 1 μg/mL of PAM, 50μM Bay 11-7082, 50μM LY294002, 50μM U0126, or DMSO. The percentage of mouse megakaryocytes at each level of DNA content was determined by flow cytometry (n = 3). *P < .05; **P < .01; ***P < .001 compared with NT; αP < .05, ααP < .01; and αααP < .001 comparing mTPO with PAM (A-B) or with inhibitor alone (C).
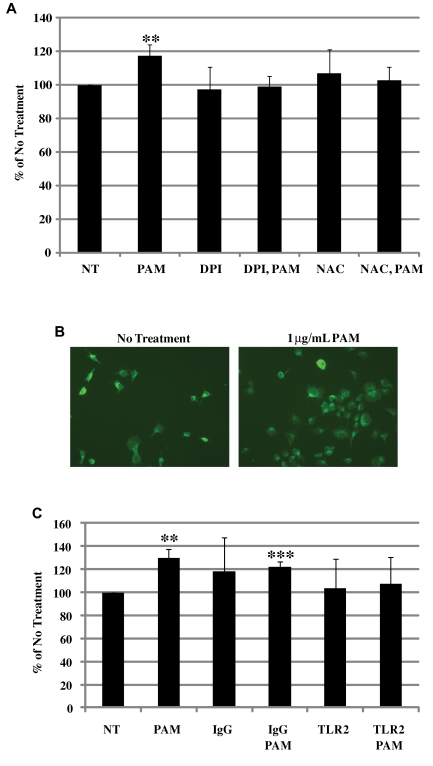
ROS production has been shown to drive megakaryocyte maturation and platelet production.29 To determine whether TLR2 also increases ROS to drive megakaryocyte maturation, Meg-01 cells were treated for 30 minutes with 1 μg/mL of Pam3CSK4, and the ROS level was measured by flow cytometry. As shown in Figure 3A, there was an increase in ROS by 117% in the presence of Pam3CSK4 over NT. TLR2-induced ROS generation was inhibited by the general antioxidant N-acetyl-L-cysteine and by the NADPH oxidase inhibitor diphenyliodonium (Figure 3A). Therefore, TLR2 also drives megakaryocyte maturation through ROS production by NADPH oxidase.
Figure 3.
TLR2 increases ROS production and adhesion to extracellular matrix proteins. (A) Meg-01 cells were stained with 10μM 5-(and-6)-carboxy-2′,7′-difluorodihydrofluorescein diacetate, and pretreated for 1 hour with 10μM N-acetyl-L-cysteine or diphenyliodonium. Cells were then treated with 1 μg/mL of Pam3CSK4 (PAM) and analyzed by flow cytometry (n = 6). **P < .01 compared with NT. (B) Meg-01 cells were treated for 3 hours with 1 μg/mL of PAM. Cells were then allowed to adhere to fibronectin-coated coverslips for 1 hour. Adherent cells were stained with FITC-conjugated anti-human CD41 antibody. Cells were counted in 5 separate 20× views. Representative photographs were taken of NT or PAM. (C) Meg-01 cells were treated with 25 μg/mL of IgG or TLR2 antibodies for 1 hour, treated with PAM as before, and allowed to adhere to fibrinogen-coated wells for 1 hour. Adherent cells were measured using MTT in serum free media. Levels were compared with NT (n = 8). **P < .01 and ***P < .001 compared with NT.
As megakaryocytes mature, they adhere to the extracellular matrix surrounding the extravascular space to release proplatelets into the circulation. To determine whether TLR2 promotes adhesion, Meg-01 cells treated with 1 μg/mL of Pam3CSK4 for 3 hours were allowed to adhere to glass coverslips coated with fibronectin for 1 hour. Cells that remained after washing were stained and 5-20× views were counted for the number of adherent CD41-positive Meg-01 cells. As shown in the representative photographs in Figure 3B, there was an increase in the number of adherent megakaryocytes after treatment with Pam3CSK4 (202.7% ± 13.7%; n = 3; P < .001) compared with NT.
For fibrinogen, Meg-01 cells were treated for 3 hours with 1 μg/mL of Pam3CSK4 and allowed to adhere to fibrinogen-coated wells for 1 hour. The number of adherent cells was quantified using an MTT assay. As shown in Figure 3C, Pam3CSK4 treatment increased Meg-01 cell adhesion to fibrinogen by approximately 130% compared with NT. Blocking TLR2 with antibodies returned adhesion back to NT levels (Figure 3C). Therefore, megakaryocyte adhesion to extracellular matrices is enhanced through TLR2 activation.
TLR2 alters gene and protein expression in megakaryocytes
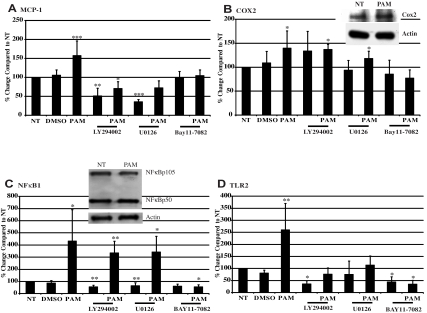
We hypothesized that mRNA and protein levels related to platelet markers and inflammation would be altered after the activation of signaling pathways and transcription factors. Known targets of the NFκB pathway, including monocyte chemotactic protein-1 (MCP-1), cyclooxygenase 2 (COX2), NFκB1, and TLR2, were measured through real-time PCR of Meg-01 cells exposed to 1 μg/mL of Pam3CSK4 for 3 hours. As shown in Figure 4A, MCP-1 mRNA levels were significantly increased over NT by approximately 57% with Pam3CSK4. MCP-1 protein levels in Meg-01–conditioned medium were also increased by 119.4% (supplemental Figure 4A). COX2 mRNA was significantly increased over NT by approximately 40% in the presence of Pam3CSK4 (Figure 4B). Western blot analysis verified that there was also an increase in COX2 protein levels (159.8% ± 47.2%; Figure 4B inset). The mRNA levels of NFκB1, also known as NFκB p105/p50, were by increased over 400% with Pam3CSK4 compared with NT (Figure 4C). In addition, Western blots showed that cells contained an increased amount of NFκB1 when treated with Pam3CSK4 (118.9% ± 6.3%; Figure 4C inset). Finally, real-time PCR results showed that TLR2 can up-regulate its own expression. Pam3CSK4 increased TLR2 mRNA levels by 260% over NT (Figure 4D). However, surface expression of TLR2 increased only by 105.5%, as determined by flow cytometry (supplemental Figure 4B).
Figure 4.
TLR2 ligand increases inflammatory-related gene and protein expressions. (A-D) Meg-01 cells were pretreated for 45 minutes with 50μM LY294002, U0126, BAY11-7082, or DMSO, and then treated for 3 hours with 1 μg/mL of Pam3CSK4 (PAM). MCP-1 (A), COX2 (B), NFκB1 (C), and TLR2 (D) gene expression was compared with NT, which was set at 100%. Insets in panels B and C show Meg-01 cells treated with 1 μg/mL of Pam3CSK4 for 3 hours. Western blots were probed for COX2 (B), NFκB105/50 (C), and actin (loading controls). Western blots are representative of 3 independent experiments. *P < .05, **P < .01, and ***P < .001 compared with NT (n = 12 in panel A; n = 9 in panel B; n = 8 in panel C; and n = 10 in panel D).
With the use of various pharmacologic inhibitors, we verified the role of the NFκB pathway in mediating the effects of TLR2 on gene expression and also confirmed the involvement of the PI3K/Akt and ERK-MAPK pathways. To investigate the role of the PI3K/Akt pathway, Meg-01 cells were pretreated with LY294002, a known specific PI3K inhibitor, before the addition of Pam3CSK4. LY294002 inhibited the up-regulation of MCP-1 (Figure 4A) and TLR2 (Figure 4D) by Pam3CSK4, but had no effect on COX2 or NFκB1 (Figure 4B and 4C, respectively). To investigate the ERK-MAPK pathway, U0126, a known inhibitor of MEK1/2, was shown to inhibit the effects of Pam3CSK4 on MCP-1 (Figure 4A) and TLR2 (Figure 4D) expression, but, again, had no effect on either COX2 or NFκB1 (Figure 4B and 4C, respectively). Finally, treatment with BAY11-7082, a compound that prevents the phosphorylation of the negative regulator of NFκB, IκB, inhibited the effects of Pam3CSK4 on the expression pattern of all 4 genes (Figure 4A-D). These results suggest that COX2 and NFkB1 expression is up-regulated by TLR2 through NFκB signaling, whereas the increased expression of MCP-1 and TLR2 by TLR2 involves all 3 pathways.
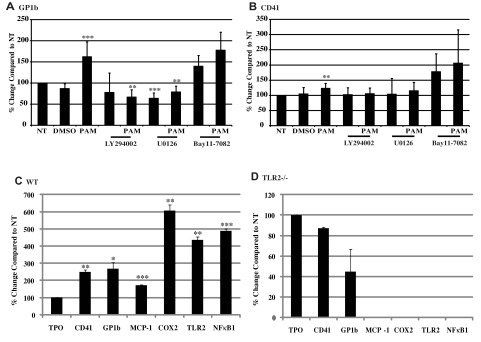
Two additional genes associated with thrombosis, GP1b and CD41, were also shown to be altered by TLR2 engagement of Pam3CSK4. GP1b is part of the GPIb-IX-V complex that binds VWF and thrombin. Its expression level was significantly increased by 162%, and its cell-surface expression, as determined by flow cytometry, was also increased by 114% with Pam3CSK4 treatment (Figure 5A and supplemental Figure 4C). CD41, also known as integrin αIIb, part of the αIIbβIII receptor that binds fibrinogen, was also significantly increased by 123% in the presence of Pam3CSK4 (Figure 5B). Surface expression of CD41 was increased by 125%, as shown by flow cytometry (supplemental Figure 4D). Pharmacologic inhibitors were used to study the signaling pathways activated by TLR2 to alter the expression levels of these genes. LY294002 inhibited the up-regulation of both GP1b and CD41 (Figure 5A and 5B, respectively). U0126 also prevented an increase in gene expression of both GP1b and CD41 (Figure 5A and 5B, respectively). BAY11-7082, however, did not hinder the effects of Pam3CSK4 on either GP1b or CD41 expression (Figure 5A and 5B, respectively). Therefore, we can conclude that TLR2 alters the expression of genes related to thrombosis through both PI3K/Akt and ERK-MAPK pathways independently of the NFκB pathway.
Figure 5.
TLR2 ligand increases GP1b and CD41 gene and protein expression, and expression is dependent on TLR2. (A-B) Meg-01 cells were pretreated for 45 minutes with 50μM LY294002, U0126, BAY11-7082, or DMSO, and then treated for 3 hours with 1 μg/mL of Pam3CSK4 (PAM). Gene expression was compared with NT. **P < .01 and ***P < .001 compared with NT (n = 13 in panel A and n = 10 in panel B). WT (C) and TLR2−/− (D) mouse bone marrow was cultured with 50 ng/mL of mTPO for 4 days, and then treated with 1 μg/mL of Pam3CSK4 (PAM) for 3 hours. Megakaryocytes were isolated by magnetic bead separation, and gene expression was measured for CD41, GP1b, MCP-1, COX2, TLR2, and NFκB1, all compared with NT (n = 3). *P < .05, **P < .01, and ***P < .001 compared with NT.
Finally, to verify that all of the changes in gene expression were dependent on TLR2, megakaryocytes from WT and TLR2−/− mouse bone marrow were treated for 3 hours with 1 μg/mL of Pam3CSK4, and gene expression for CD41, GP1b, MCP-1, COX2, TLR2, and NFκB1 was determined by real-time PCR. As seen in Figure 5C, WT megakaryocytes responded to the TLR2 ligand, and gene expression for the thrombotic markers CD41 and GP1b increased by 250% and 269%, respectively. MCP-1 increased by 172% over NT, whereas COX2, TLR2, and NFκB1 all increased over 400% (Figure 5C). When TLR2 was absent, there was no increase in gene expression even in the presence of Pam3CSK4 (Figure 5D). Therefore, the effects on gene expression with Pam3CSK4 were specific to TLR2.
TLR2 treatment leads to activation and sequestering of platelet and maturation of megakaryocytes
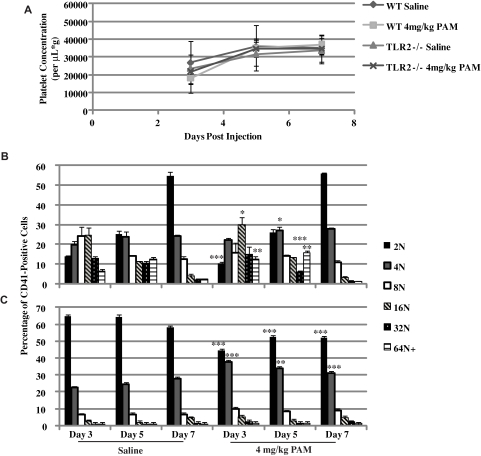
WT and TLR2−/− mice were treated with a single, intraperitoneal injection of saline or 4 mg/kg of Pam3CSK4. Mice were monitored for 1 week, taking blood and bone marrow samples at 3, 5, and 7 days after treatment. As seen in Figure 6A, platelet counts in WT mice decreased on day 3, returned to saline-treated levels on day 5, and start to increase slightly above saline on day 7. Corresponding to the decrease in circulating platelets, the spleens from these mice were significantly enlarged compared with saline-treated mice on day 3, but returned to normal on day 5. Megakaryocytes responded to the loss in platelets in the Pam3CSK4-treated WT mice, because there was an increase in 16N and 64N+ on day 3, and 4N and 64N+ on day 5 (Figure 6B). By day 7, Pam3CSK4-treated WT mice had the same megakaryocyte ploidy profile as saline-treated mice. In TLR2−/− mice, there was no response to Pam3CSK4 treatment. Platelet counts did not change between treatments (Figure 6A), nor were there any changes in ploidy in the megakaryocytes (Figure 6C). There was a difference in 2N and 4N between saline and Pam3CSK4 treatment in the TLR2−/− mice, but there was no difference at any other level of DNA content. These results suggest that TLR2 is needed by the platelets to respond to Pam3CSK4 in the circulation. The decrease in platelets resulted in the maturation of megakaryocytes to replenish this loss. However, there was a slight increase in platelet levels over saline treatment, suggesting that the megakaryocytes also respond to Pam3CSK4 in the circulation to produce a greater number of platelets.
Figure 6.
Pam3CSK4 treatment in WT mice affects platelet counts and megakaryocyte maturation. WT and TLR2−/− mice were injected once intraperitoneally with saline or 4 mg/kg of Pam3CSK4, and monitored for 1 week. (A) Platelet counts were done on blood samples from each mouse at days 3, 5, and 7 after the injection. Each point is the average ± SD of 3 mice. (B-C) Megakaryocyte ploidy determined on days 3, 5, and 7 (n = 3). *P < .05, **P < .01, and ***P < .001 compared with saline treatment on that day.
Discussion
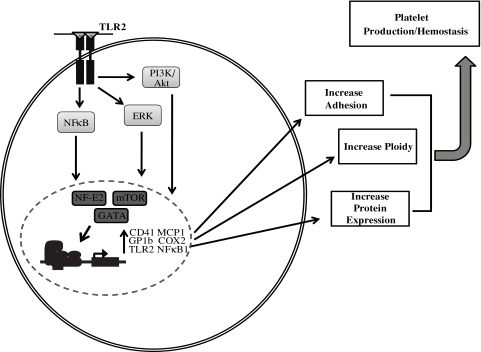
TLRs, known for their role in innate immunity to recognize PAMPs and to activate inflammatory processes, are expressed by megakaryocytes (Figure 7). Our previous studies and those of others have shown that TLRs are both expressed and functional on platelets. Specifically, TLR2 on platelets can react to bacterial PAMPs, which activates platelets through the PI3K/Akt pathway.15,20 TLR2 activation of platelets not only results in an increase in adhesion and platelet aggregate formation, but also in increased formation of platelet-neutrophil heterotypic aggregates,15 an effect that is greater than that seen with thrombin.20 It is hypothesized that platelets receive TLR mRNA and protein from megakaryocytes. Through flow cytometry of intact cells, megakaryocytes have been shown to express TLR1, TLR2, TLR4, and TLR6. Real-time PCR has also confirmed that megakaryocytes contain mRNA for TLR2. The levels of each TLR present in the Meg-01 cells may be lower than what has been shown in platelets. Previous findings suggested that the levels of TLR may increase with the maturation from a megakaryoblast to a platelet.30 However, the purpose of these receptors and the role of inflammation on megakaryocyte maturation and platelet development is not fully understood.
Figure 7.
Summary of the effects of TLR2 on megakaryocytes.
Binding of TLR2 by a specific PAMP in immune cells results in activation of the NFκB pathway.12–13 A TLR2-specific ligand, Pam3CSK4, increased the level of phosphorylation of the p65 subunit of NFκB. Other signaling pathways in immune cells have also been shown to be activated by the binding of TLR2 to its ligand, including the ERK-MAPK31–32 and PI3K/Akt pathways.33 These pathways were also shown in the present study to be important with Pam3CSK4 treatment of megakaryocytes. Therefore, PAMPs recognized by TLR2 on the surface of megakaryocytes can activate the NFκB, ERK-MAPK, and PI3K/Akt pathways (Figure 7). Potentially, any systemic infection that could enter into the bone marrow, including Porphyromonas gingivalis and Salmonella typhi,34 may be recognized by megakaryocytic TLR2 and activate these signaling pathways to alter cellular function.
After activation of these signaling pathways, factors that control megakaryocyte maturation may be up-regulated, including the transcription factors GATA-1, NF-E2, and mTOR. GATA-1 is a transcription factor that controls multiple steps of megakaryopoiesis and thrombopoiesis. Its expression has been shown to be regulated by both the ERK-MAPK35 and the PI3K/Akt pathways,36 both shown here to be activated by TLR2. NF-E2 regulates the later stages of megakaryopoiesis, including polyploidization and platelet production. GATA-1 has been shown to regulate NF-E2 expression,37 along with the JNK pathway.38 mTOR, a transcription factor involved in megakaryocyte polyploidization,39 is activated by the PI3K/Akt pathway.40 Up-regulation of these transcription factors all occurred in megakaryocytes after treatment with the TLR2 ligand Pam3CSK4. Further, with the up-regulation of these transcription factors, there were increases in megakaryocyte maturation marked by the up-regulation of the thrombotic-related genes GP1b and CD41, increases in DNA content and ROS production, and increased adhesion to extracellular matrices. After stimulation of the TLR2 receptor in megakaryocytes, it was shown here that all of these events occurred (Figure 7). Increased ROS production with Pam3CSK4 is another potential mechanism by which TLR2 affects megakaryocyte ploidy. Previous studies have shown that increased megakaryocyte ROS levels were responsible for increased platelet production.29
Activation of the NFκB pathway leads to an up-regulation of inflammatory-related genes, including MCP-1,41 COX2,42 NFκB1, and TLR2.43 In megakaryocytes, stimulation of the TLR2 receptor with Pam3CSK4 led to a significant up-regulation of all 4 genes. Only COX2 and NFκB1 were solely regulated by the NFκB pathway. Increases in the gene expression of MCP-1 and TLR2 was also affected by both the PI3K/Akt44–45 and the ERK-MAPK46–47 pathways in megakaryocytes, as has been shown in other cell types.
Up-regulation of these inflammatory-related genes affects not only megakaryocyte/platelet development, but also the differentiation of surrounding cells in the bone marrow. Increases in MCP-1 secretion by megakaryocytes could increase differentiation of immune-related cells. In addition, increases in MCP-1 secreted by the platelets could increase the immune response in the circulation. COX2 levels were shown to be up-regulated during megakaryocyte maturation48–49 and have an effect on this process.49 Increases in both TLR2 and NFκB1 further enhance the megakaryocyte TLR2 cell-surface recognition of PAMPs and increase the number of signaling proteins to respond to any activation of this receptor. In addition, an increase in TLR2 could lead to augmented TLR2 platelet expression, which is known to regulate activation.15,20
Up-regulation of the thrombotic markers GP1b and CD41 was also detected after stimulation of TLR2 on the megakaryocytes. Both genes are markers for mature megakaryocytes.2 TLR2 stimulation increased the expression of GP1b and CD41 through the PI3K/Akt and ERK-MAPK pathways, but not the NFκB pathway. It has been previously shown in megakaryocytes that the ERK-MAPK pathway,50 through GATA-1, regulates CD41 expression. Because GATA-1 is also regulated by PI3K/Akt pathway, it is hypothesized that CD41 is regulated by this pathway in a similar manner. GP1b has also been shown to be regulated by GATA-1,51 and therefore it is hypothesized that through this transcription factor, both the ERK-MAPK and the PI3K/Akt pathways can regulate its expression. The increased expression of both of these genes through TLR2 shows that megakaryocytes mature in the presence of a TLR2 agonist and that the platelets produced will have more GP1b and CD41. Further work is needed to determine the clinical significance of this finding. However, a previous study on inflammation and coronary artery bypass showed an increase in CD41 on platelets during surgery.52
Limitations to our study include the discrepancy between mRNA and protein levels. Because the Meg-01 cell line is a megakaryoblast cell line, the maturity of the cells caused mismatches in mRNA and protein levels within the cells and differences in the mRNA levels between the cell line and mouse megakaryocytes. Experiments done with mouse cells were completed after 4 days of continuous treatment with TPO, which resulted in more mature megakaryocytes that might respond differently than the less mature cell line. However, the overall effect was the same, in that TLR2 similarly affected megakaryocyte gene expression.
WT mice treated with Pam3CSK4, platelets reacted to TLR2 in the circulation, and because of their activation were sequestered in the spleen. Megakaryocytes responded to this loss and underwent maturation to form more platelets. By day 5, platelet counts had returned to normal; however, the megakaryocytes were still reacting to the Pam3CSK4 because there was still an increase in DNA content. As a result, on day 7, platelet levels had increased slightly over saline treatment. There was no effect of Pam3CSK4 in the TLR2−/− mice, however, there was a reoccurring deficiency in the response of these megakaryocytes to any stimulation. It is possible that there is a need for TLR2 to respond normally to TPO, as in the case of the ploidy studies shown in Figure 2. TLR2−/− mice had a reduced number of megakaryocytes at the 2N and 4N levels after Pam3CSK4 treatment. TLR1 was still present and functional in these cells. It is possible that TLR1 has effects on immature megakaryocytes, but that TLR2 is needed to affect the later stages of maturation.
Platelets have been shown previously to express functional TLR2, which enhances activation and heterotypic aggregate formation in the setting of inflammation and infection. However, the function of TLRs in megakaryocytes has not been studied. As summarized in Figure 7, the NFκB, PI3K/Akt, and ERK-MAPK pathways are activated through TLR2 on megakaryocytes, which leads to an increase in expression of the transcription factors GATA-1, NF-E2, and mTOR. These transcription factors regulate megakaryocyte maturation. In the present study, TLR2 on the megakaryocytes increased adhesion to fibrinogen and fibronectin, increased polyploidization to the extent of TPO, and increased both CD41 and GP1b mRNA and protein levels. Finally, TLR2 increased the inflammatory-related markers MCP-1, COX2, TLR2, and NFκB1. From these data, it can be surmised that one role of megakaryocytic TLR2 is to react to similar stimuli that contribute to the clearance of platelets via thrombosis and heterotypic aggregate formation in the setting of inflammation, infection, or sepsis. The results of this study suggest that inflammatory processes can increase megakaryocyte maturation and protein content through TLR2, which may affect platelet function and thrombosis.
Acknowledgments
This work was supported by National Institutes of Health–National Heart, Lung and Blood Institute grants P01 HL083801 (to J.E.F.) and T32 HL07224 (to L.M.B.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.M.B. designed and performed experiments, analyzed data, and edited the manuscript; E.L. performed experiments, analyzed data, and edited the manuscript; K.M.M. and K.T. designed experiments, analyzed data, and edited the manuscript; and J.E.F. designed experiments and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lea M. Beaulieu, Boston University School of Medicine, 700 Albany St, W533, Boston, MA 02118; e-mail: lmb@bu.edu.
References
- 1.de Sauvage FJ, Carver-Moore K, Luoh SM, et al. Physiological regulation of early and late stages of megakaryocytopoiesis by thrombopoietin. J Exp Med. 1996;183(2):651–656. doi: 10.1084/jem.183.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pang L, Weiss MJ, Poncz M. Megakaryocyte biology and related disorders. J Clin Invest. 2005;115(12):3332–3338. doi: 10.1172/JCI26720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111(3):981–986. doi: 10.1182/blood-2007-05-088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon MS, Hoffman R. Growth factors affecting human thrombocytopoiesis: potential agents for the treatment of thrombocytopenia. Blood. 1992;80(2):302–307. [PubMed] [Google Scholar]
- 5.Ishibashi T, Kimura H, Uchida T, et al. Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci U S A. 1989;86(15):5953–5957. doi: 10.1073/pnas.86.15.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majka M, Janowska-Wieczorek A, Ratajczak J, et al. Stromal-derived factor 1 and thrombopoietin regulate distinct aspects of human megakaryopoiesis. Blood. 2000;96(13):4142–4151. [PubMed] [Google Scholar]
- 7.Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115(12):3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaser A, Brandacher G, Steurer W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98(9):2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 9.Stohlawetz P, Folman CC, von dem Borne AE, et al. Effects of endotoxemia on thrombopoiesis in men. Thromb Haemost. 1999;81(4):613–617. [PubMed] [Google Scholar]
- 10.Baynes RD, Bothwell TH, Flax H, et al. Reactive thrombocytosis in pulmonary tuberculosis. J Clin Pathol. 1987;40(6):676–679. doi: 10.1136/jcp.40.6.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R, Preston-Hurlburt P, Kopp E, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2(2):253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Lin E, Freedman JE, Beaulieu LM. Innate immunity and toll-like receptor antagonists: a potential role in the treatment of cardiovascular diseases. Cardiovasc Ther. 2009;27(2):117–123. doi: 10.1111/j.1755-5922.2009.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair P, Rex S, Vitseva O, et al. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009;104(3):346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiraki R, Inoue N, Kawasaki S, et al. Expression of Toll-like receptors on human platelets. Thromb Res. 2004;113(6):379–385. doi: 10.1016/j.thromres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Ward JR, Bingle L, Judge HM, et al. Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost. 2005;94(4):831–838. [PubMed] [Google Scholar]
- 18.Aslam R, Speck ER, Kim M, et al. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107(2):637–641. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 19.Ståhl AL, Svensson M, Morgelin M, et al. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets through TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood. 2006;108(1):167–176. doi: 10.1182/blood-2005-08-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rex S, Beaulieu LM, Perlman DH, et al. Immune versus thrombotic stimulation of platelets differentially regulates signalling pathways, intracellular protein-protein interactions, and alpha-granule release. Thromb Haemost. 2009;102(1):97–110. doi: 10.1160/TH08-08-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson A, Zhang Y, Kamen D, et al. Deregulated expression of c-myc in megakaryocytes of transgenic mice increases megakaryopoiesis and decreases polyploidization. J Biol Chem. 1996;271(38):22976–22982. doi: 10.1074/jbc.271.38.22976. [DOI] [PubMed] [Google Scholar]
- 22.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 23.Arbibe L, Mira JP, Teusch N, et al. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1(6):533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 24.Lemarchandel V, Ghysdael J, Mignotte V, Rahuel C, Romeo PH. GATA and Ets cis-acting sequences mediate megakaryocyte-specific expression. Mol Cell Biol. 1993;13(1):668–676. doi: 10.1128/mcb.13.1.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vyas P, Ault K, Jackson CW, Orkin SH, Shivdasani RA. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood. 1999;93(9):2867–2875. [PubMed] [Google Scholar]
- 26.Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, et al. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81(5):695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- 27.Lecine P, Villeval JL, Vyas P, et al. Mice lacking transcription factor NF-E2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and show a platelet production defect that is intrinsic to megakaryocytes. Blood. 1998;92(5):1608–1616. [PubMed] [Google Scholar]
- 28.Raslova H, Baccini V, Loussaief L, et al. Mammalian target of rapamycin (mTOR) regulates both proliferation of megakaryocyte progenitors and late stages of megakaryocyte differentiation. Blood. 2006;107(6):2303–2310. doi: 10.1182/blood-2005-07-3005. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien JJ, Spinelli SL, Tober J, et al. 15-deoxy-Delta(12,14)-PGJ(2) enhances platelet production from megakaryocytes. Blood. 2008;112(10):4051–4060. doi: 10.1182/blood-2008-05-158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andonegui G, Kerfoot SM, McNagny K, et al. Platelets express functional Toll-like receptor-4. Blood. 2005;106(7):2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 31.Chen BC, Chang YS, Kang JC, et al. Peptidoglycan induces nuclear factor-kappaB activation and cyclooxygenase-2 expression via Ras, Raf-1, and ERK in RAW 264.7 macrophages. J Biol Chem. 2004;279(20):20889–20897. doi: 10.1074/jbc.M311279200. [DOI] [PubMed] [Google Scholar]
- 32.Pathak SK, Bhattacharyya A, Pathak S, et al. Toll-like receptor 2 and mitogen- and stress-activated kinase 1 are effectors of Mycobacterium avium-induced cyclooxygenase-2 expression in macrophages. J Biol Chem. 2004;279(53):55127–55136. doi: 10.1074/jbc.M409885200. [DOI] [PubMed] [Google Scholar]
- 33.Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull. 2007;30(9):1617–1623. doi: 10.1248/bpb.30.1617. [DOI] [PubMed] [Google Scholar]
- 34.Wain J, Pham VB, Ha V, et al. Quantitation of bacteria in bone marrow from patients with typhoid fever: relationship between counts and clinical features. J Clin Microbiol. 2001;39(4):1571–1576. doi: 10.1128/JCM.39.4.1571-1576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towatari M, Ciro M, Ottolenghi S, Tsuzuki S, Enver T. Involvement of mitogen-activated protein kinase in the cytokine-regulated phosphorylation of transcription factor GATA-1. Hematol J. 2004;5(3):262–272. doi: 10.1038/sj.thj.6200345. [DOI] [PubMed] [Google Scholar]
- 36.Kadri Z, Maouche-Chretien L, Rooke HM, et al. Phosphatidylinositol 3-kinase/Akt induced by erythropoietin renders the erythroid differentiation factor GATA-1 competent for TIMP-1 gene transactivation. Mol Cell Biol. 2005;25(17):7412–7422. doi: 10.1128/MCB.25.17.7412-7422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsang AP, Visvader JE, Turner CA, et al. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell. 1997;90(1):109–119. doi: 10.1016/s0092-8674(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 38.Lee TL, Shyu YC, Hsu PH, et al. JNK-mediated turnover and stabilization of the transcription factor p45/NF-E2 during differentiation of murine erythroleukemia cells. Proc Natl Acad Sci U S A. 2010;107(1):52–57. doi: 10.1073/pnas.0909153107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerriero R, Parolini I, Testa U, et al. Inhibition of TPO-induced MEK or mTOR activity induces opposite effects on the ploidy of human differentiating megakaryocytes. J Cell Sci. 2006;119(Pt 4):744–752. doi: 10.1242/jcs.02784. [DOI] [PubMed] [Google Scholar]
- 40.Sekulić A, Hudson CC, Homme JL, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60(13):3504–3513. [PubMed] [Google Scholar]
- 41.Wang Y, Rangan GK, Tay YC, Harris DC. Induction of monocyte chemoattractant protein-1 by albumin is mediated by nuclear factor kappaB in proximal tubule cells. J Am Soc Nephrol. 1999;10(6):1204–1213. doi: 10.1681/ASN.V1061204. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Zhao M, Rao R, Inoue H, Hao CM. C/EBP{beta} and its binding element are required for NF{kappa}B-induced COX2 expression following hypertonic stress. J Biol Chem. 2005;280(16):16354–16359. doi: 10.1074/jbc.M411134200. [DOI] [PubMed] [Google Scholar]
- 43.Wang T, Lafuse WP, Zwilling BS. NFkappaB and Sp1 elements are necessary for maximal transcription of toll-like receptor 2 induced by Mycobacterium avium. J Immunol. 2001;167(12):6924–6932. doi: 10.4049/jimmunol.167.12.6924. [DOI] [PubMed] [Google Scholar]
- 44.Bian ZM, Elner SG, Yoshida A, Elner VM. Differential involvement of phosphoinositide 3-kinase/Akt in human RPE MCP-1 and IL-8 expression. Invest Ophthalmol Vis Sci. 2004;45(6):1887–1896. doi: 10.1167/iovs.03-0608. [DOI] [PubMed] [Google Scholar]
- 45.Dos Santos S, Delattre AI, De Longueville F, Bult H, Raes M. Gene expression profiling of LPS-stimulated murine macrophages and role of the NF-kappaB and PI3K/mTOR signaling pathways. Ann N Y Acad Sci. 2007;1096:70–77. doi: 10.1196/annals.1397.071. [DOI] [PubMed] [Google Scholar]
- 46.Ni CW, Wang DL, Lien SC, et al. Activation of PKC-epsilon and ERK1/2 participates in shear-induced endothelial MCP-1 expression that is repressed by nitric oxide. J Cell Physiol. 2003;195(3):428–344. doi: 10.1002/jcp.10259. [DOI] [PubMed] [Google Scholar]
- 47.Jang BC, Jung TY, Paik JH, et al. Tetradecanoyl phorbol acetate induces expression of Toll-like receptor 2 in U937 cells: involvement of PKC, ERK, and NF-kappaB. Biochem Biophys Res Commun. 2005;328(1):70–77. doi: 10.1016/j.bbrc.2004.12.144. [DOI] [PubMed] [Google Scholar]
- 48.Rocca B, Secchiero P, Ciabattoni G, et al. Cyclooxygenase-2 expression is induced during human megakaryopoiesis and characterizes newly formed platelets. Proc Natl Acad Sci U S A. 2002;99(11):7634–7639. doi: 10.1073/pnas.112202999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka N, Sato T, Fujita H, Morita I. Constitutive expression and involvement of cyclooxygenase-2 in human megakaryocytopoiesis. Arterioscler Thromb Vasc Biol. 2004;24(3):607–612. doi: 10.1161/01.ATV.0000117181.68309.10. [DOI] [PubMed] [Google Scholar]
- 50.Sevinsky JR, Whalen AM, Ahn NG. Extracellular signal-regulated kinase induces the megakaryocyte GPIIb/CD41 gene through MafB/Kreisler. Mol Cell Biol. 2004;24(10):4534–4545. doi: 10.1128/MCB.24.10.4534-4545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludlow LB, Schick BP, Budarf ML, et al. Identification of a mutation in a GATA binding site of the platelet glycoprotein Ibbeta promoter resulting in the Bernard-Soulier syndrome. J Biol Chem. 1996;271(36):22076–22080. doi: 10.1074/jbc.271.36.22076. [DOI] [PubMed] [Google Scholar]
- 52.Massoudy P, Zahler S, Becker BF, et al. Evidence for inflammatory responses of the lungs during coronary artery bypass grafting with cardiopulmonary bypass. Chest. 2001;119(1):31–36. doi: 10.1378/chest.119.1.31. [DOI] [PubMed] [Google Scholar]