SUMMARY
The db/db mice serve as a good model for type 2 diabetes, characterized by hyperinsulinemia and progressive hyperglycemia. There are limited and conflicting data on the cardiovascular changes in this model. The aim was to characterize the cardiovascular/autonomic phenotype of male db/db mice and evaluate the role of angiotensin (Ang) AT1 receptors. Radiotelemetry was used to monitor 24 hr blood pressure (BP) in mice for 8 weeks. Parameters measured were mean arterial pressure (MAP), heart rate (HR) and their variabilities. MAP and BP circadian rhythms were not altered in 8 wk db/db while HR and locomotor activity were decreased. With aging, MAP gradually increased in db/db mice and the 12-h light values did not dip significantly from the 12-h dark periods. In 14 wk mice, MAP was increased during light (101 ± 1 vs. 117 ± 2 mmHg, p < 0.01; Control vs. db/db) and dark phases (110 ± 1.7 vs. 121 ± 3.1 mmHg, p<0.01; Control vs. db/db). This increase in BP was associated with significant increase in plasma ACE activity and Ang II levels. Chronic treatment with losartan (10 mg/kg/day) blocked the increase in MAP in db/db with no effect in controls. Spectral analysis was used to monitor autonomic cardiovascular function. The circadian rhythm observed in SAP variance and its LF component in control mice was absent in db/db. There were no changes in HR variability and spontaneous baroreflex sensitivity between control and db/db mice. Results document an age related increase in MAP in db/db reduced by antagonism of Ang AT1 receptors and alterations in autonomic balance and components of the renin angiotensin system.
Keywords: ACE, hypertension, renin, spectral analysis, losartan, insulin resistance
INTRODUCTION
The prevalence of type 2 diabetes is rising to epidemic levels worldwide. The pathology of diabetes is characterized not only by hyperglycemia, but also by hypertension, dyslipidemia, microalbuminuria and vascular inflammation. Diabetes is associated with a reduced lifespan and much of the cardiovascular disease (CVD) is attributed to hypertension. Thus, aggressive management of high blood pressure (BP) is essential for a reduction in risks of cardiovascular events (Sowers, 2004). Aggressive treatment of hyperglycemia is also an important aspect of the management of the long term complications of diabetes. Earlier clinical trials showed a correlation between strict glycemic control in patients with type 2 diabetes and a reduction in microvascular complications, but not macrovascular disease (UKPDS,1998). There are questions as to whether lowering blood glucose with currently available medications effectively reduces CVD. Recent clinical trials, such as ACCORD (Action to Control Cardiovascular Risk in Diabetes) (Gerstein et al., 2007) and ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) (Patel et al., 2008), were designed to determine the relationship between glycemic control and macrovascular events. The message from both clinical trials is that near normal glycemic control for a median of 3.5 to 5 years does not reduce macrovascular events (Dluhy & McMahon, 2008), suggesting that other factors are important in disease progression. However, the ADVANCE trial reconfirmed the predicted reduction in microvascular complications such as microalbuminuria and nephropathy (Dluhy & McMahon, 2008).
There are clinical data to document the effectiveness of ACE inhibitors (ACEi) and Ang receptor blockers (ARBs) in treatment of hypertensive diabetics (Gilbert et al., 2003). There are even data that show long term treatment with losartan lowers the risk of developing diabetes (Lindholm et al., 2002). A general role for RAS in diabetes-induced hypertension is supported by the depressor effects of ACEIs and ARBs in diabetic patients (Horio et al., 2004). In a recent clinical trial, candesartan prevented the development of diabetes in heart failure patients, further connecting cardiovascular disease, RAS and metabolic dysfunction. There are data in animals which show that ACEI or ARBs reduce the cardiovascular complications in animal models of type 1 and type 2 diabetes (Amazonas & Lopes De Faria, 2006; Nielsen et al., 2003). Our previous work documented the importance of Ang AT1 receptors in the development of cardiovascular dysfunction in chemical and dietary induced diabetic states (Farah et al., 2007a; Wichi et al., 2007). In the fructose loading model, the hypertension was reverted to a hypotension in mice lacking the Ang AT1a receptor (Farah et al., 2007a) and blocked in mice treated with losartan (Senador et al., 2006). The fructose diet itself produced glucose intolerance, increased Ang II levels and increased BP (Farah et al., 2006; Farah et al., 2007a). Recently, it has been shown that diet-induced obesity in mice is associated with resistance to the metabolic actions of leptin, but preservation of its BP raising effects (Rahmouni et al., 2005). In addition, leptin deficiency in murine model of diabetes, as in the ob/ob mouse, leads to decreased arterial pressure, despite severe obesity (Mark et al., 1999).
An important issue for the study of diabetic pathologies and the role of the RAS is the availability of an animal model that simulates the human condition. We chose the db/db genetic mouse model that has a point mutation in the diabetes (db) gene encoding the leptin receptor gene (Chen et al., 1996). At early ages, they serve as a good model for type 2 diabetes, characterized by hyperinsulinemia and progressive hyperglycemia (Coleman, 1983). With aging, there is a gradual loss of pancreatic function resulting in low insulin and extremely high glucose levels. The model bears similarities to the disease progression in humans in which the first stage is insulin resistance followed by a stage of pancreatic insufficiency (Coleman, 1978; Hummel et al., 1966). Although there are reports of in vitro vascular hyperactivity in db/db (Kanie & Kamata, 2000; Guo et al., 2005), there are limited and conflicting data on the in vivo cardiovascular changes in this model. Studies have employed the tail cuff measurement system and have found increased (Bagi et al., 2005; Brezniceanu et al., 2008), decreased (Moriyama et al., 2004) or no BP change in db/db mice (Guo et al., 2006; Kosugi et al., 2006).
We designed a study to characterize the metabolic and cardiovascular phenotypes of the db/db diabetic model and to determine the cardiovascular effects of chronic blockade of Ang AT1 receptors. We examined age related changes in glucose, insulin, body weight, BP, HR and cardiovascular autonomic function. A key aspect of the cardiovascular protocol was the use of radiotelemetry which allows for chronic BP measurements in freely moving mice. It is particularly useful for measurement of light/dark cardiovascular rhythms which are known to be altered in clinical and experimental and model of diabetes (Farah et al., 2006; Nakano et al., 1998).
EXPERIMENTAL PROCEDURES
Animals
Male BKS.Cgm +/+ Lepr db/J (db/db, n=38) diabetic mice and their age-matchednondiabetic littermates (db/m, n=38) were used for the experiments. The animals were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed at 22°C under a 12-hr light/12-hr dark cycle with ad libitum access to water and standard mouse chow. All experimental protocols were approved by the WSU Animal Care and Use Committee.
Measurement of Cardiovascular Parameters and Locomotor activity
Cardiovascular parameters were recorded by radiotelemetry. At 6–7 wks of age, mice were anesthetized with ketamine/xylazine (120:20 mg/kg, I.M.) and radiotelemetric catheters (model TA11PA-C10, Data Sciences International, St. Paul, MN) were inserted into the left common carotid artery. The transmitter body was positioned subcutaneously on the right flank (Farah et al., 2007a; Wichi et al., 2007). After 1 week recovery from surgery, continuous 24 h BP, HR and locomotor activity were monitored once per week, weekly for 8 weeks at 500 Hz. Cardiovascular parameters were analyzed and averaged over the entire 24 h period, the 12 h light phases (6:00 AM– 6:00 PM) and during the dark phases (6:00 PM to 6:00 AM). For spectral analysis, blood pressure (BP) was recorded at 5 kHz for 60 min during the light and dark phases. HR and pulse interval (PI) were calculated from the pressure signal. Data were stored and analyzed using Dataquest A.R.T. 4.0 software (Data Sciences International, St. Paul, MN).
Variability and Spectral Analysis
Peak interval (PI) and systolic arterial pressure (SAP) variabilities (HRV and BPV, respectively) were assessed in time and frequency domains using autoregressive spectral analysis, as described before (Farah et al., 2006). Briefly, PI and SAP series were divided in segments of 350 beats and overlapped by 50%. A spectrum was obtained for each of the segments via Levinson-Durbin recursion, with the model order chosen according to Akaike’s criterion, ranging between 10 and 14. The oscillatory components were quantified in the low (LF: 0.1 to 1 Hz) and high (HF: 1 to 5.0 Hz) frequency ranges (Farah et al., 2006). Spontaneous baroreflex sensitivity was calculated for each light/dark recording phase using sequence analysis technique(Farah et al., 2006).
Chronic treatment with losartan
To test the role of AT1 receptor, a separate group of db/db (n=6) and control mice (n=6) (6–7 wks of age) were given losartan in the drinking water (10 mg kg−1 day−1) for 8 wks. After 6 wks of administration of losartan, radiotelemetric probes were implanted. Mice were allowed to recover from surgery. At week 14, BP, HR and locomotor activity were sampled continuously day and night for a total period of 48 h at sampling rate of 500 Hz.
Blood glucose measurements and glucose tolerance test (GTT)
Blood samples were taken from a cut made on the tip of the tail and glucose was determined using an Accu-Check Advantage Blood Glucose Monitor (Roche Diagnostic Corporation, Indianapolis, IN). GTT was performed in control and db/db mice, untreated and treated with losartan (after 8 wks of treatment). Mice were fasted for 16 hours and blood samples were collected from a cut made at the tip of the tail at 0, 30, 60, 90 and 120 min after the glucose load (I.P. injection of glucose, 1.5 g/kg).
Plasma measurements: Insulin and Ang II
Plasma Ang II and insulin were measured in a separate group of 8 wk (33.9 ± 0.8 g) and 14 wk (35.8 ± 1.4 g) db/db mice and their age matched 8 wk (23.8 ± 0.7 g) and 14 wk (26.2 ± 0.4 g) controls (n=8). Mice were decapitated and trunk blood was collected in ice-chilled heparinized tubes. Plasma was immediately separated and stored frozen at −80°C. Plasma insulin levels were measured by radioimmunoassay in 20 μl aliquots of plasma using a commercial kit (Linco Research Inc., Missouri). For plasma Ang II, blood was collected as above in presence of 10 mM EDTA and 10 μM bestatin. Plasma Ang II concentrations were measured as described (Farah et al., 2006). Briefly, 50 μl plasma aliquots were extracted using phenylsilylsilica columns and Ang II was measured in duplicates using an RIA kit (ALPCO Diagnostics, Windham, NH).
Plasma ACE activity
Blood was collected as described above. ACE activity was measured using an assay kit purchased from ALPCO Diagnostic (Windham, NH, USA). Briefly, 10 μl plasma was incubated with 100 μl of HEPES buffer (pH 8) containing the synthetic substrate H3 hippuryl glycine glycine (H3 Hip-Gly-Gly) at 37° C. After 60 min incubation the reaction was terminated by adding 50 μl of 1 N hydrochloric acid. Liberated H3-Hippuric acid due to ACE activity in samples was separated from unreacted substrate by addition of 1.5 ml of scintillating fluid and measured in a beta counter. ACE activity is expressed in units/μg protein used as previously described (Neels et al., 1982).
Statistics
Values were expressed as means ± SEM. Data were analyzed using analysis of variance, two-way or three-way ANOVA when appropriate, followed by Tukey test for multiple comparisons. Differences were considered to be statistically significant at p<0.05.
RESULTS
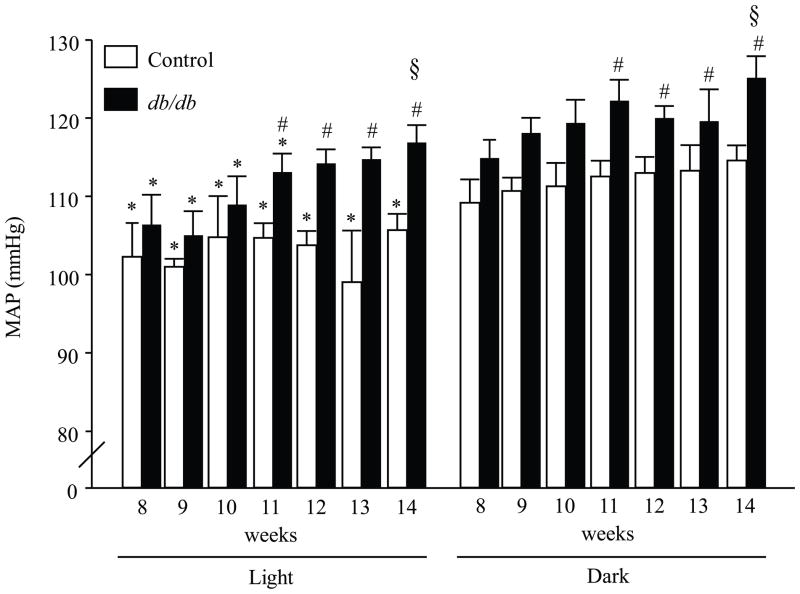
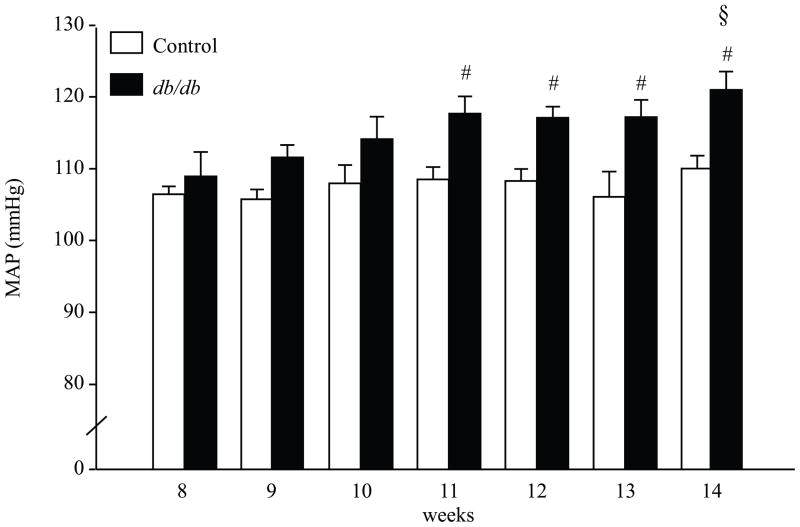
At the early age (8 wk), db/db mice showed hyperglycemia, hyperinsulinemia and increased body weight (Table 1). With aging, there was a gradual loss of pancreatic β-cells resulting in lower plasma insulin and severe hyperglycemia in 14 wk compared to 8 wk db/db mice (p<0.001, Table 1). To study the age-dependent changes in the cardiovascular parameters in db/db mice, MAP, HR, pulse rate and locomotor activities were recorded for 24 hours and analyzed during the light and dark phases once per week for 8 weeks. At the young age (8–10 wk) there was no difference in MAP between db/db and controls (Fig 1 and Fig 2). However, at 8 wk, db/db mice showed a significant decrease in HR during the light and dark phases (table 4, p < 0.05, control vs. db/db). This decrease in HR was observed in normotensive and hypertensive db/db mice. There was a gradual increase in BP and by 11 wks old, MAP was significantly higher in db/db mice compared to controls. This could be observed when BP measurements were averaged over the entire 24 h period (Fig 2, p < 0.005 control vs. db/db) and during 12 h light phases (6:00 AM– 6:00 PM) and dark phases (6:00 PM to 6:00 AM) (Fig 1, p < 0.05 control vs. db/db). In addition, there was a significant increase in MAP in 14 wk db/db mice compared to 8 wk db/db mice (Fig 1 and Fig 2, p < 0.05 8 wks vs. 14 wks). As expected, the circadian rhythm of BP was intact in control mice (Fig. 1). However, there was an age-dependent change in the circadian rhythm of BP in db/db mice. At early age (8–10 wk), when the db/db mice were normotensive there was a significant difference between the 12-h light and the 12-h dark MAP. However, in hypertensive db/db mice (12–14 wk) the 12-h light period did not significantly “dip” compared to the 12-h dark period (Fig. 1). Locomotor activity during the light and dark phase was significantly reduced in db/db mice (table 4, p < 0.05, control vs. db/db).
Table 1.
Body weight and metabolic parameters in control and db/db diabetic mice.
| Age | 8 weeks | 14 weeks | ||
|---|---|---|---|---|
| Strain | Control | db/db | Control | db/db |
| Weight (g) | 23.1 ± 0.3 | 33.5 ± 1.1* | 25.4 ± 0.3 | 35.5 ± 1.2* |
| Blood glucose (mg/mL) | 116.1 ± 0.3 | 315.1 ± 27.1* | 117.1± 0.5 | 550.1 ± 25.1*† |
| Insulin (ng/mL) | 0.9 ± 0.2 | 27.2 ± 2.1* | 1.2 ± 0.3 | 4.5 ± 0.8*† |
Body weight (g), blood glucose (mg/dL) and plasma insulin (ng/mL) in young (8 weeks) and older (14 weeks) db/db diabetic mice and their age matched controls.
p < 0.001 control vs. db/db;
p < 0.001 8 vs. 14 wk, n = 6.
Figure 1.
Mean arterial pressure (MAP) in db/db diabetic mice and their age matched controls. Blood pressure was recorded continously for 24 hours (500 Hz) once per week, and analyzed during light (□) and dark (■) phases. Three-way ANOVA showed a main effect of group [F (1.176) = 27.26, p < 0.0001] and time [F (1.176) = 29.92, p < 0.0001]. * p < 0.05 light vs. dark. # p < 0.05 control vs. db/db. § p < 0.05 14 vs. 8 wks, n = 8.
Figure 2.
24 hours mean arterial pressure (MAP) in db/db diabetic mice and their age matched controls. Blood pressure was recorded continously for 24 hours once per week at 500 Hz and averaged over entire 24 hours period. Two-way ANOVA showed a main effect of group [F (1.88) = 19.29, p < 0.0001]. # p < 0.005 control vs. db/db. p < 0.05 vs. 8 wks, n = 8.
Table 4.
Spontaneous baroreflex sensitivity (SBR), heart rate (HR), pulse pressure (PP) and locomotor activity in control and db/db diabetic mice.
| Strain | Control | db/db | |||
|---|---|---|---|---|---|
| Age | Light | Dark | Light | Dark | |
| SBR (ms/mmHg) | 8 wks | 2.8 ± 0.4 | 2.3 ± 0.4 | 2.7 ± 0.8 | 2.2 ± 0.4 |
| 14 wks | 2.4 ± 0.6 | 2.0 ± 0.3 | 2.1 ± 0.7 | 2.2 ± 0.4 | |
| HR (bpm) | 8 wks | 515 ± 35* | 570 ± 38 | 396 ± 29*# | 476 ± 41# |
| 14 wks | 501 ± 25* | 561 ± 16 | 422 ± 11*# | 490 ± 14# | |
| PP (mmHg) | 8 wks | 32 ± 1.7 | 33 ± 1.6 | 25 ± 4.7 | 25 ± 3.7 |
| 14 wks | 31 ± 2.6 | 30 ± 2.5 | 26 ± 2.1 | 27 ± 2.9 | |
| Activity (counts/min) | 8 wks | 6 ± 0.7* | 11 ± 1.4 | 3 ± 0.9 | 5 ± 1.3# |
| 14 wks | 5 ± 0.2* | 12 ± 1.1 | 4 ± 0.6 | 5 ± 0.5# | |
SBR (ms/mmHg), HR (bpm), PP (mmHg) and locomotor activity (counts/min) in young (8 wk) and older (14 wk) db/db diabetic mice and their age matched controls. There were no changes in SBR and PP. Three-way ANOVA showed main effect of group [F (1.29) = 33.2, p < 0.01] and time (light/dark) [F (1.29) = 16.8, p < 0.01] for HR. For activity, Three-way ANOVA showed main effect of group [F (1.43) = 40.2, p < 0.0001], time (light/dark) [F (1.43) = 27.8, p < 0.0001] and an interaction between group and time [F (1.43) = 43.6, p < 0.001].
p < 0.05 light vs. dark;
p < 0.05 control vs. db/db, n = 6.
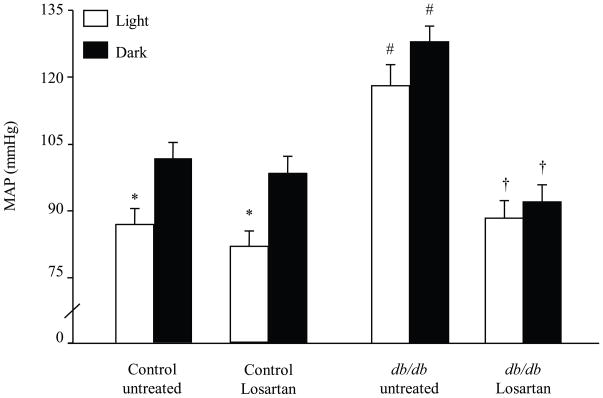
Chronic treatment with losartan (10 mg/kg/day in drinking water for 8 weeks) blocked the increase in MAP in 14 wk db/db mice (Fig 3, p < 0.01, untreated vs. losartan). However, treatment with losartan did not correct the disrupted BP circadian rhythms “nondipping” of the light period observed in the db/db mice. Losartan treatment had no significant effect on MAP in controls (Fig 3). HR and locomotor activity were not affected by losartan treatment in controls or db/db (data not shown). Losartan had no effect on blood glucose in control (124.6 ± 3.5 vs. 131.2 ± 4 mg/dl, untreated vs. losartan) or db/db mice (549.2 ± 8.6 vs. 550.2 ± 5.8 mg/dl, untreated vs. losartan). In regard to glucose handling, losartan had no effect on glucose tolerance test in control mice (Area under the curve for GTT was 179 ± 9 vs 186 ± 12 mg/dl/min, untreated vs losartan). In addition, losartan did not improve the impaired glucose tolerance in db/db mice (area under the curve for GTT was 756 ± 29 vs 788 ± 25 mg/dl/min, untreated vs losartan)
Figure 3.
Effect of chronic treatment (8 wks) with losartan (10 mg/kg/day in drinking water) on MAP in control and db/db mice. At 14 wks of age, blood pressure was recorded (500 Hz) continuously for 48 hours and analyzed during light (□) and dark (■) phases. Three-way ANOVA showed a main effect of treatment [F (1.40) = 42.81, p < 0.0001], group [F (1.40) = 7.48, p < 0.01] and time [F (1.40) = 17.89, p < 0.0001]. ANOVA also showed an interaction between treatment and group [F (1.40) = 7.61, p < 0.01]. * p < 0.05 light vs. dark. # p < 0.03 control vs. db/db. † p < 0.03 untreated vs. losartan, n = 8.
To study autonomic modulation of the heart and vasculature in the db/db model, we applied autoregressive spectral analysis of the telemetric blood pressure signal (Table 2 and 3). ANOVA showed an interaction between strain, age and time (light/dark) for SAP variance and LF components (p < 0.05). Post-hoc tests showed a significant light/dark difference in the 8 and 14 wk controls for SAP variance and LF as an evidence for a circadian rhythm (Table 2). The light/dark rhythm for SAP variance and LF (Table 2) were absent in 8 and 14 wk db/db mice.
Table 2.
SAP variability in time and frequency domains in control and db/db diabetic mice
| Strain | Control | db/db | |||
|---|---|---|---|---|---|
| Age | Light | Dark | Light | Dark | |
| 8 weeks | SAP var (mmHg2) | 12.8 ± 0.6* | 24.3 ± 1.3 | 22.2 ± 11.6 | 21.7 ± 4.2 |
| LF (mmHg2) | 9.0 ± 0.5* | 19.1 ± 1.0 | 7.0 ± 1.7* | 16.9 ± 3.5 | |
| 14 weeks | SAP var (mmHg2) | 16.6 ± 3.2* | 32.0 ± 4.8 | 28.3 ± 4.9 | 21.8 ± 3.4 |
| LF (mmHg2) | 9.1 ± 2.3* | 19.2 ± 1.9 | 12.8 ± 4.3* | 19.2 ± 4.2 | |
Systolic arterial pressure (SAP) variability in time (variance) and frequency domains (low frequency-LF) in young (8 wk) and older (14 wk) db/db diabetic mice and their age matched controls. BP and HR signals were recorded (5kHz) during the light and the dark phases and submitted to spectral analysis. Three-way ANOVA showed an interaction between strain and time (light/dark) for SAP variance [F (1.29) = 4.9, p < 0.05] and SAP-LF [F (1.29) = 5.4, p < 0.03].
p < 0.01 light vs. dark, n = 6.
Table 3.
PI variability in time and frequency domains in control and db/db diabetic mice
| Strain | Control | db/db | |||
|---|---|---|---|---|---|
| Age | Light | Dark | Light | Dark | |
| 8 weeks | PI var (ms2) | 57.1 ± 14.8 | 46.4 ± 10.1 | 53.9 ± 9.1 | 33.7 ± 2.9 |
| LF (ms2) | 36.1 ± 18.1 | 21.1 ± 10.6 | 34.2 ± 17.1 | 12.1 ± 6.1 | |
| HF (ms2) | 18.2 ± 9.1 | 20.3 ± 10.2 | 17.5 ± 8.7 | 13.9 ± 6.9 | |
| 14 weeks | PI var (ms2) | 67.6 ± 15.5 | 47.8 ± 3.5 | 24.3 ± 3.2 | 56.1 ± 7.1 |
| LF (ms2) | 20.6 ± 10.3 | 14.1 ± 7.2 | 5.2 ± 2.6 | 12.8 ± 6.4 | |
| HF (ms2) | 36.8 ± 18.4 | 24.9 ± 12.5 | 17.4 ± 8.7 | 37.9 ± 18.9 | |
Peak interval (PI) variability in time (variance) and frequency domains (low frequency-LF and high frequency-HF) in young (8 wk) and older (14 wk) db/db diabetic mice and their age matched controls. BP and HR signals were recorded (5kHz) during the light and the dark phases and submitted to spectral analysis. Three-way ANOVA showed no main effects for strain, age or time (light/dark) for PI variability. However, there was an interaction between the 3 factors for PI variance [F (1.29) = 8.6, p < 0.05], PI-LF [F (1.29) = 7.8, p < 0.05] and PI-HF [F (1.29) = 8.5, p < 0.05], n = 6.
For PI variability, ANOVA showed no main effects for strain, age or time (light/dark) and post-hoc analysis showed no significant differences in PI variability (Table 3). However, there was an interaction between the three factors for PI variance and the frequency components (p < 0.05). Spontaneous baroreflex sensitivity also showed no differences between control and db/db mice at either young or old ages (Table 4).
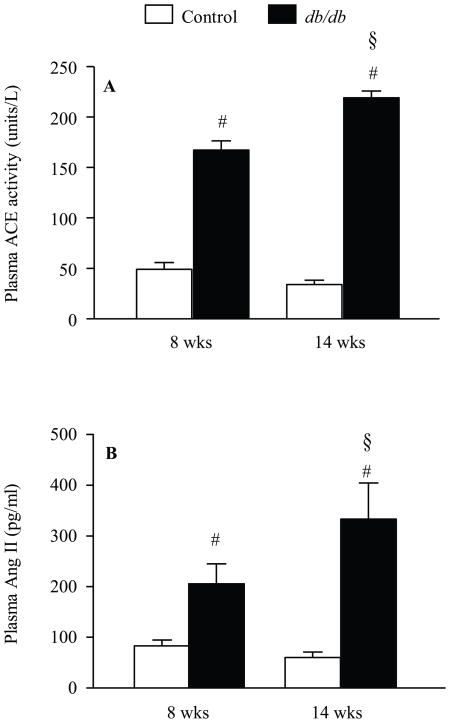
To further evaluate the RAS in this model, plasma Ang II and ACE activity were measured in normotensive (8 wk) and hypertensive (14 wk) db/db mice and their age matched controls. ANOVA showed a main effect of group and age on plasma ACE activity and Ang II (Fig. 4). Both plasma ACE activity and Ang II level were increased in db/db compared to controls (P<0.05, Fig. 4). There was a significant increase in plasma ACE activity and Ang II levels between 8wk and 14 wk db/db mice (Fig. 4, p<0.05).
Figure 4.
Plasma ACE activity (A) and plasma Ang II (B) in young (8 wk) and older (14 wk) db/db diabetic mice and their age matched controls. ACE activity was determined using the synthetic substrate H3 hippuryl glycine glycine purchased from ALPCO Diagnostic. Ang II was determined using RIA kit purchased from ALPCO Diagnostic. For ACE, two-way ANOVA showed a main effect of group [F (1.22) = 7.7, p < 0.03] and age [F (1.22) = 52.1, p < 0.0001] and an interaction between group and age [F (1.22) = 25.3, p < 0.0001]. For Ang II, two-way ANOVA showed a main effect of group [F (1.31) = 46.7, p < 0.0001] and an interaction between group and age [F (1.31) = 7.2, p < 0.01]. # p < 0.005 control vs. db/db. § p < 0.005 14 wks vs. 8 wks, n = 9.
DISCUSSION
Additional information is needed to clarify the cardiovascular phenotype of db/db diabetic mice and the role of RAS. The present study tested the hypothesis that there are developmental changes in BP in db/db mice, and these changes are mediated through Ang AT1 receptor. We used radiotelemetry to measure BP, allowing for study of day/night patterns. The key findings that young (8–10 wk) db/db mice were normotensive and old (11–14 wk) db/db mice were hypertensive compared to their age-matched controls. In addition, our data showed the light/dark circadian rhythm of BP was absent that in the hypertensive db/db mice. In addition, the spectral data from older db/db mice also showed disruption in SAP variance and its LF component circadian rhythms. This is the first report to demonstrate increased plasma Ang II and ACE in db/db mice. RAS appears to play a role in the development of hypertension in the db/db diabetic mice since the BP increase was blocked by chronic losartan treatment. The same low dose of losartan (10 mg/kg/day) had no effect on BP in normal mice, which agrees with previous studies in mice (Brochu et al., 2002) and rats (Iyer & Katovich, 1996).
Some of the discrepancies in the literature regarding BP changes in the db/db may be due to differences in age, time of experiment as well as methodology for BP measurement. Bagi et al reported increased BP in 12 wk mice (Bagi et al., 2005), while Kosugi et al. found no changes (Kosugi et al., 2006). Both studies used the tail cuff method which is not accurate for determining true baseline levels. Discrepancies can also be found in radiotelemetry studies of db/db mice. Reports documented either no change (Park et al., 2008; Belmadani et al., 2008), decreased (Bodary et al., 2007) or increased BP in db/db mice (Su et al., 2008). One study showed no difference in BP between 18 wk db/db and control when data was averaged over 24 hours or during light and dark phases (Park et al., 2008). Interestingly, in the same study BP measured between 12:00 PM and 3:00 PM was significantly higher in db/db mice compared to controls (Park et al., 2008). Our group previously reported that BP is increased in fructose-fed mice only during the dark phase (Farah et al., 2006; Farah et al., 2007a). Here for the first time, we show an age-dependent increase in BP which was seen when BP data were averaged over 24 h and during 12h light phase and dark phase. The present investigation used db/db micefrom Jackson Laboratories on a C57BL/BKS background, whereas Bodary et al. (Bodary et al., 2007) used Jackson db/db mice with a C57BL/6J background. Differences in background strain may account for this differencein db/db phenotype since the diabetic phenotype of db/db mice is markedly influencedby background strain (Coleman, 1983).
There is a growing consensus driven both by clinical and basic studies that Ang AT1 blockers improve β-cell function, glucose tolerance and delay onset of type 2 diabetes (Lindholm et al., 2002; Chu et al., 2006). Earlier clinical trials demonstrated that strict glycemic control in patients with type 2 diabetes correlates with a reduction in microvascular complications, but not macrovascular disease (UKPDS, 1998). However, many of the cardiovascular complications seen in type 2 diabetes cannot be completely prevented by lowering blood glucose. Epidemiological studies demonstrated that RAS blockade therapy reduces blood pressure and the onset of type 2 diabetes in patients with hypertension (Yusuf et al., 2005). The positive effects of ARBs and ACEi on metabolism are thought to be due to improvement in insulin sensitivity (Fogari et al., 1998; Moan et al., 1996). However, our results showed that chronic losartan treatment reduced BP in db/db mice without attenuating the state of diabetes and glucose tolerance. The lack of a blood glucose lowering effect of losartan agrees with previous studies in db/db diabetic mice that initiated treatment after glucose had started to rise (Mathew et al., 2005; Sugaru et al., 2007; Shao et al., 2006). The study by Chu et al. (Chu et al., 2006) reported a marked decrease in glycemia in db/db mice could be due to losartan treatment started at an earlier stage of diabetes, at 4 wks of age. In addition, studies in STZ diabetic rats and ob/ob mice also documented a lack of improvement in blood glucose after chronic treatment with losartan (Erbe et al., 2006; Raimondi et al., 2004). Therefore, it is unlikely that the BP lowering effect of losartan in db/db diabetic mice was mediated through lowering of blood glucose and insulin sensitization.
HR was reduced in 8 and 14 wks db/db mice without changes in spontaneous baroreflex. Isolated hearts from db/db mice showed cardiomyopathy, decrease in contractile function and altered metabolism (Aasum et al., 2003). Echocardiography in db/db mice showed reduced heart function (Kosugi et al., 2006), contractile function (Semeniuk et al., 2002), cardiac output and heart weight (Bagi et al., 2005). The changes in cardiac metabolism and contractile function observed previously in db/db mice are age-dependent (Aasum et al., 2003) and could explain the decreased HR in the present study. Although previous studies reported no change in HR; the data could be compromised because of the use of the tail-cuff method in which mice are restrained and stressed (Bagi et al., 2005; Kosugi et al., 2006). However, in agreement with our findings, a recent study using radiotelemetry demonstrated decreased HR in 18 wk db/db mice compared to controls (Park et al., 2008).
In the present study db/db mice showed an absence in the circadian oscillation in SAP variance and its LF component. It is the LF component of SAP variance which is indicative of sympathetic input to the vasculature (Stauss, 2007). The changes in the BP rhythms were observed in db/db diabetic mice prior to any changes in BP itself. This agrees with previous data showing changed BPV as a marker for cardiovascular risk in experimental and human diabetes (Tamura et al., 2007). Our data may suggest early occurrence of neuropathy in db/db mice as previously documented in these animals through nerve recording studies (Sullivan et al., 2007). Furthermore, changes in BPV were not associated with alterations in spontaneous baroreflex sensitivity, which agree with our previous data on animal models of type 1 diabetes and glucose intolerance (Farah et al., 2006; Wichi et al., 2007; Farah et al., 2007b). Decreased HRV is an early marker for diabetic neuropathy in humans (Vita et al., 1999; Schroeder et al., 2005). However, there is little evidence of decreased HRV in experimental models of type 1 diabetes (Wichi et al., 2007; Farah et al., 2007b) and high fructose fed mice (Farah et al., 2006). The results of the present study agree with these findings, no significant changes in HRV in db/db mice. In fact, there were some reports of increased HRV after long-lasting diabetes in rats (Balbinott et al., 2005).
There is much clinical interest in measurement of renin and ACE activity as biomarkers for disease states, such as hypertension and diabetes. In addition, there are considerable data to support the activation of plasma ACE levels in diabetes. However, in this emerging field of RAS there are few studies which address the activity of ACE in murine model of type 2 diabetes. Our results showed that db/db presented higher levels of plasma Ang II and ACE activity compared to their controls. To our knowledge, this is the first study to demonstrate increased plasma ACE activity in db/db diabetic mice. This increase in ACE activity and Ang II was significantly higher in 14 wk hypertensive mice compared to the 8 wks normotensive mice. The increase in plasma Ang II and ACE activity in 8 wk db/db mice was observed while these animals still have normal BP. Although, increases in ACE and/or Ang II levels are often associated with BP increases (Campbell et al., 1995), the modulation of the RAS and its components’ effects enclose a far more complex structure. For example, increased ACE activity in mice over expressing ACE gene copies is not associated with changes in BP (Krege et al., 1997; Senador et al., 2007). We have also shown previously increased renal ACE 2 activity in young normotensive db/db mice and decreased renal ACE 2 activity in older hypertensive db/db mice(Kanakamedala et al., 2007). The down regulation of ACE and up regulation of ACE2, in the kidney is an intrigue finding and could explain the normal blood pressure levels observed in 8wk db/db mice in the face of increased plasma ACE activity. This finding also agrees with recent reports of a renoprotective effect of increased renal ACE 2 and decreased renal ACE activity combination in 8wk db/db mice (Wysocki et al., 2006). It is tempting to speculate that there is an age-dependent change in the balance of ACE and ACE2 which could contribute to the regulation of BP in db/db mice.
In summary, the current study demonstrated that there was an age dependent increase in BP and disruption of its circadian rhythm during the progression of diabetes in db/db mice. In addition, our study documented a significant role of Ang AT1 in mediating hypertension in db/db mice since losartan blocked the progress in hypertension with no change in blood glucose. The spectral analysis of BP in db/db mice showed changes in BPV prior to changes in BP itself indicating a possible marker for cardiovascular risk and autonomic cardioneuropathy. The reduced HR observed in db/db mice could be related to contractile and metabolic dysfunctions of cardiac muscle since there were no changes in baroreflex function. The significant increase in plasma ACE activity and increased Ang II in normotensive diabetic mice indicate that other factors such as ACE2 could be involve in the regulation of BP in this model. The present findings provide evidence for cardiovascular dysfunction in db/db mice.
Acknowledgments
The authors acknowledge the financial support of the AHA Scientist Development Grant 0735112N (KME) and NIH R01HL69319 (MM). We gratefully acknowledge the help of Mary Key and Nathan Weir.
Reference List
- 1.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. . Lancet. 352:837–853. [PubMed] [Google Scholar]
- 2.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–441. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 3.Amazonas RB, Lopes De Faria JB. Effects of tight blood pressure control on glomerular hypertrophy in a model of genetic hypertension and experimental diabetes mellitus. Life Sci. 2006;79:2135–2143. doi: 10.1016/j.lfs.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, Kaley G. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol. 2005;25:1610–1616. doi: 10.1161/01.ATV.0000172688.26838.9f. [DOI] [PubMed] [Google Scholar]
- 5.Balbinott AW, Irigoyen MC, Brasileiro-Santos MS, Zottis B, De Lima NG, Passaglia J, Schaan BD. Dose-dependent autonomic dysfunction in chronic L-NAME-hypertensive diabetic rats. J Cardiovasc Pharmacol. 2005;46:563–569. doi: 10.1097/01.fjc.0000179433.80631.9f. [DOI] [PubMed] [Google Scholar]
- 6.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008;57:1629–1637. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodary PF, Shen Y, Ohman M, Bahrou KL, Vargas FB, Cudney SS, Wickenheiser KJ, Myers MG, Jr, Eitzman DT. Leptin regulates neointima formation after arterial injury through mechanisms independent of blood pressure and the leptin receptor/STAT3 signaling pathways involved in energy balance. Arterioscler Thromb Vasc Biol. 2007;27:70–76. doi: 10.1161/01.ATV.0000252068.89775.ee. [DOI] [PubMed] [Google Scholar]
- 8.Brezniceanu ML, Liu F, Wei CC, Chenier I, Godin N, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes. 2008;57:451–459. doi: 10.2337/db07-0013. [DOI] [PubMed] [Google Scholar]
- 9.Brochu I, Labonte J, Bkaily G, Orleans-Juste P. Role of endothelin receptors in the hypertensive state of kinin B(2) knockout mice subjected to a high-salt diet. Clin Sci (Lond) 2002;103Suppl 48:380S–384S. doi: 10.1042/CS103S380S. [DOI] [PubMed] [Google Scholar]
- 10.Campbell DJ, Rong P, Kladis A, Rees B, Ganten D, Skinner SL. Angiotensin and bradykinin peptides in the TGR (mRen-2)27 rat. Hypertension. 1995;25:1014–1020. doi: 10.1161/01.hyp.25.5.1014. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 12.Chu KY, Lau T, Carlsson PO, Leung PS. Angiotensin II type 1 receptor blockade improves beta-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes. 2006;55:367–374. doi: 10.2337/diabetes.55.02.06.db05-1022. [DOI] [PubMed] [Google Scholar]
- 13.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 14.Coleman DL. Lessons from studies with genetic forms of diabetes in the mouse. Metabolism. 1983;32:162–164. doi: 10.1016/s0026-0495(83)80031-6. [DOI] [PubMed] [Google Scholar]
- 15.Dluhy RG, Mcmahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358:2630–2633. doi: 10.1056/NEJMe0804182. [DOI] [PubMed] [Google Scholar]
- 16.Erbe DV, Gartrell K, Zhang YL, Suri V, Kirincich SJ, Will S, Perreault M, Wang S, Tobin JF. Molecular activation of PPARgamma by angiotensin II type 1-receptor antagonists. Vascul Pharmacol. 2006;45:154–162. doi: 10.1016/j.vph.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Farah V, Elased KM, Chen Y, Key MP, Cunha TS, Irigoyen MC, Morris M. Nocturnal hypertension in mice consuming a high fructose diet. Auton Neurosci. 2006;130:41–50. doi: 10.1016/j.autneu.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Farah V, Elased KM, Morris M. Genetic and dietary interactions: role of angiotensin AT1a receptors in response to a high-fructose diet. Am J Physiol Heart Circ Physiol. 2007a;293:H1083–H1089. doi: 10.1152/ajpheart.00106.2006. [DOI] [PubMed] [Google Scholar]
- 19.Farah VM, De AK, Joaquim LF, Candido GO, Bernardes N, Fazan R, Jr, Schaan BD, Irigoyen MC. Autonomic modulation of arterial pressure and heart rate variability in hypertensive diabetic rats. Clinics. 2007b;62:477–482. doi: 10.1590/s1807-59322007000400015. [DOI] [PubMed] [Google Scholar]
- 20.Fogari R, Zoppi A, Lazzari P, Preti P, Mugellini A, Corradi L, Lusardi P. ACE inhibition but not angiotensin II antagonism reduces plasma fibrinogen and insulin resistance in overweight hypertensive patients. J Cardiovasc Pharmacol. 1998;32:616–620. doi: 10.1097/00005344-199810000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Gerstein HC, Riddle MC, Kendall DM, Cohen RM, Goland R, Feinglos MN, Kirk JK, Hamilton BP, Ismail-Beigi F, Feeney P. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:34i–43i. doi: 10.1016/j.amjcard.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert RE, Krum H, Wilkinson-Berka J, Kelly DJ. The renin-angiotensin system and the long-term complications of diabetes: pathophysiological and therapeutic considerations. Diabet Med. 2003;20:607–621. doi: 10.1046/j.1464-5491.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- 23.Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, Kikuchi T, Lapointe N, Pojoga L, Williams GH, Ricchiuti V, Adler GK. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology. 2006;147:5363–5373. doi: 10.1210/en.2006-0944. [DOI] [PubMed] [Google Scholar]
- 24.Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, Gong MC. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res. 2005;67:723–735. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Horio N, Clermont AC, Abiko A, Abiko T, Shoelson BD, Bursell SE, Feener EP. Angiotensin AT(1) receptor antagonism normalizes retinal blood flow and acetylcholine-induced vasodilatation in normotensive diabetic rats. Diabetologia. 2004;47:113–123. doi: 10.1007/s00125-003-1262-x. [DOI] [PubMed] [Google Scholar]
- 26.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 27.Iyer SN, Katovich MJ. Effect of acute and chronic losartan treatment on glucose tolerance and insulin sensitivity in fructose-fed rats. American Journal of Hypertension. 1996;9:662–668. doi: 10.1016/0895-7061(96)00035-0. [DOI] [PubMed] [Google Scholar]
- 28.Kanakamedala K, Senador D, Zhang WF, Morris M, Elased KM. Increased ACE activity in young normotensive diabetic (db/db) mice. Hypertension. 2007;50:E134. [Google Scholar]
- 29.Kanie N, Kamata K. Contractile responses in spontaneously diabetic mice. I. Involvement of superoxide anion in enhanced contractile response of aorta to norepinephrine in C57BL/KsJ(db/db) mice. Gen Pharmacol. 2000;35:311–318. doi: 10.1016/s0306-3623(02)00115-5. [DOI] [PubMed] [Google Scholar]
- 30.Kosugi R, Shioi T, Watanabe-Maeda K, Yoshida Y, Takahashi K, Machida Y, Izumi T. Angiotensin II receptor antagonist attenuates expression of aging markers in diabetic mouse heart. Circ J. 2006;70:482–488. doi: 10.1253/circj.70.482. [DOI] [PubMed] [Google Scholar]
- 31.Krege JH, Kim HS, Moyer JS, Jennette JC, Peng L, Hiller SK, Smithies O. Angiotensin-converting enzyme gene mutations, blood pressures, and cardiovascular homeostasis. Hypertension. 1997;29:150–177. doi: 10.1161/01.hyp.29.1.150. [DOI] [PubMed] [Google Scholar]
- 32.Lindholm LH, Ibsen H, Borch-Johnsen K, Olsen MH, Wachtell K, Dahlof B, Devereux RB, Beevers G, De Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristianson K, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman JM, Snapinn S. Risk of new-onset diabetes in the Losartan Intervention For Endpoint reduction in hypertension study. J Hypertens. 2002;20:1879–1886. doi: 10.1097/00004872-200209000-00035. [DOI] [PubMed] [Google Scholar]
- 33.Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17:1949–1953. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- 34.Mathew R, Futterweit S, Valderrama E, Tarectecan AA, Bylander JE, Bond JS, Trachtman H. Meprin-alpha in chronic diabetic nephropathy: interaction with the renin-angiotensin axis. Am J Physiol Renal Physiol. 2005;289:F911–F921. doi: 10.1152/ajprenal.00037.2005. [DOI] [PubMed] [Google Scholar]
- 35.Moan A, Hoieggen A, Seljeflot I, Risanger T, Arnesen H, Kjeldsen SE. The effect of angiotensin II receptor antagonism with losartan on glucose metabolism and insulin sensitivity. J Hypertens. 1996;14:1093–1097. doi: 10.1097/00004872-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Moriyama T, Oka K, Ueda H, Imai E. Nilvadipine attenuates mesangial expansion and glomerular hypertrophy in diabetic db/db mice, a model for type 2 diabetes. Clin Exp Nephrol. 2004;8:230–236. doi: 10.1007/s10157-004-0303-1. [DOI] [PubMed] [Google Scholar]
- 37.Nakano S, Fukuda M, Hotta F, Ito T, Ishii T, Kitazawa M, Nishizawa M, Kigoshi T, Uchida K. Reversed circadian blood pressure rhythm is associated with occurrences of both fatal and nonfatal vascular events in NIDDM subjects. Diabetes. 1998;47:1501–1506. doi: 10.2337/diabetes.47.9.1501. [DOI] [PubMed] [Google Scholar]
- 38.Neels HM, Scharpe SL, Van Sande ME, Verkerk RM, Van Acker KJ. Improved micromethod for assay of serum angiotensin converting enzyme. Clin Chem. 1982;28:1352–1355. [PubMed] [Google Scholar]
- 39.Nielsen B, Gronbaek H, Osterby R, Flyvbjerg A. Effect of combination therapy with a calcium channel blocker and an angiotensin-converting enzyme inhibitor on renal hypertrophy and urinary albumin excretion in diabetic rats. Exp Diabesity Res. 2003;4:191–199. doi: 10.1155/EDR.2003.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S, Bivona BJ, Feng Y, Lazartigues E, Harrison-Bernard LM. Intact renal afferent arteriolar autoregulatory responsiveness in db/db mice. Am J Physiol Renal Physiol. 2008;295:F1504–F1511. doi: 10.1152/ajprenal.90417.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel A, Macmahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, De Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 42.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 43.Raimondi L, De PP, Mannucci E, Lonardo G, Sartiani L, Banchelli G, Pirisino R, Mugelli A, Cerbai E. Restoration of cardiomyocyte functional properties by angiotensin II receptor blockade in diabetic rats. Diabetes. 2004;53:1927–1933. doi: 10.2337/diabetes.53.7.1927. [DOI] [PubMed] [Google Scholar]
- 44.Schroeder EB, Chambless LE, Liao D, Prineas RJ, Evans GW, Rosamond WD, Heiss G. Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2005;28:668–674. doi: 10.2337/diacare.28.3.668. [DOI] [PubMed] [Google Scholar]
- 45.Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol. 2002;283:H976–H982. doi: 10.1152/ajpheart.00088.2002. [DOI] [PubMed] [Google Scholar]
- 46.Senador D, Farah V, Mariana M, Irigoyen MC. High fructose diet enhances Losartan’s hypotensive effect. Hypertension. 2006;48:E89–E90. [Google Scholar]
- 47.Senador D, Oroszi T, Key M, Elased KM, Morris M. Angiotensinergic control of blood pressure in mice overexpressing ACE. Hypertension. 2007;50:E143. [Google Scholar]
- 48.Shao J, Iwashita N, Ikeda F, Ogihara T, Uchida T, Shimizu T, Uchino H, Hirose T, Kawamori R, Watada H. Beneficial effects of candesartan, an angiotensin II type 1 receptor blocker, on beta-cell function and morphology in db/db mice. Biochemical & Biophysical Research Communications. 2006;344:1224–1233. doi: 10.1016/j.bbrc.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol. 2004;286:H1597–H1602. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- 50.Stauss HM. Power spectral analysis in mice: What are the appropriate frequency bands? Am J Physiol Regul Integr Comp Physiol. 2007;292:R902–R903. doi: 10.1152/ajpregu.00716.2006. [DOI] [PubMed] [Google Scholar]
- 51.Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC. Hypertension and disrupted blood pressure circadian rhythm in Type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol. 2008;295:H1634–H1641. doi: 10.1152/ajpheart.00257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugaru E, Nakagawa T, Ono-Kishino M, Nagamine J, Tokunaga T, Kitoh M, Hume WE, Nagata R, Taiji M. Amelioration of established diabetic nephropathy by combined treatment with SMP-534 (antifibrotic agent) and losartan in db/db mice. Nephron Exp Nephrol. 2007;105:e45–e52. doi: 10.1159/000097603. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su OS, Lentz SI, Brosius F, Iii, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamura K, Tsurumi Y, Sakai M, Tanaka Y, Okano Y, Yamauchi J, Ishigami T, Kihara M, Hirawa N, Toya Y, Yabana M, Tokita Y, Ohnishi T, Umemura S. A possible relationship of nocturnal blood pressure variability with coronary artery disease in diabetic nephropathy. Clin Exp Hypertens. 2007;29:31–42. doi: 10.1080/10641960601096760. [DOI] [PubMed] [Google Scholar]
- 55.Vita G, Bellinghieri G, Trusso A, Costantino G, Santoro D, Monteleone F, Messina C, Savica V. Uremic autonomic neuropathy studied by spectral analysis of heart rate. Kidney Int. 1999;56:232–237. doi: 10.1046/j.1523-1755.1999.00511.x. [DOI] [PubMed] [Google Scholar]
- 56.Wichi RB, Farah V, Chen Y, Irigoyen MC, Morris M. Deficiency in angiotensin AT1a receptors prevents diabetes-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1184–R1189. doi: 10.1152/ajpregu.00524.2006. [DOI] [PubMed] [Google Scholar]
- 57.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. ACE and ACE2 Activity in Diabetic Mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 58.Yusuf S, Ostergren JB, Gerstein HC, Pfeffer MA, Swedberg K, Granger CB, Olofsson B, Probstfield J, Mcmurray JV. Effects of candesartan on the development of a new diagnosis of diabetes mellitus in patients with heart failure. Circulation. 2005;112:48–53. doi: 10.1161/CIRCULATIONAHA.104.528166. [DOI] [PubMed] [Google Scholar]