Abstract
Roles of the sympathetic nervous system versus kidney salt transporters in hypertension are debated. A study in Nature Medicine (Mu et al., 2011) shows that dietary salt excess, coupled with beta-adrenergic stimulation, increases arterial pressure via glucocorticoid receptors and WNK4, suggesting interactions between these systems in the pathogenesis of hypertension.
Hypertension affects 1.5 billion people worldwide, and is the single largest risk factor for premature mortality (Lopez et al., 2006). Arterial pressure is, simply, the product of cardiac output and systemic vascular resistance, suggesting that causes of hypertension should be obvious. Still, two seemingly independent schools of thought dominate the current debate. Specifically, hypertension is often viewed as resulting from either over-activity of the sympathetic nervous system (Osborn et al., 2009) or from excessive kidney salt reabsorption (Montani and Van Vliet, 2009). In their recent study in Nature Medicine (Mu et al., 2011) Mu and colleagues provide evidence for a novel pathogenic model for human hypertension that synthesizes the effects of the renal and nervous systems.
High levels of salt (NaCl) consumption cause hypertension in some individuals (defining them as salt-sensitive), whereas other individuals are protected from such effects. Salt-sensitive individuals exhibit neurohormonal responses to dietary salt intake that differ from those who are resistant, but the underlying mechanisms remain unclear. In their recent study in Nature Medicine, Mu and colleagues (Mu et al., 2011) describe intriguing links between sympathetic over-activity, glucocorticoid receptor (GR) stimulation, WNK4 suppression, and thiazide-sensitive Na-Cl cotransporter (NCC) activation.
These investigators show that chronic stimulation of beta-adrenergic receptors, coupled with dietary salt excess, induces hypertension in mice; as shown schematically in Figure 1, they found that hypertension was associated with increases in the abundance and activity of NCC and with a decrease in the regulatory kinase, WNK4. Interestingly, the investigators also found that GR binding was essential for this effect, as treatment of mice with a GR antagonist, or adrenalectomy, abolished the effects of isoproterenol and salt loading on WNK4. Furthermore, genetic deletion of GR from the distal nephron prevented the hypertensive effects of salt loading and isoproterenol. This requirement for GR occupancy in the nephron resembles the well-recognized requirement of GR occupancy for sympathetic vasoconstriction, systemically.
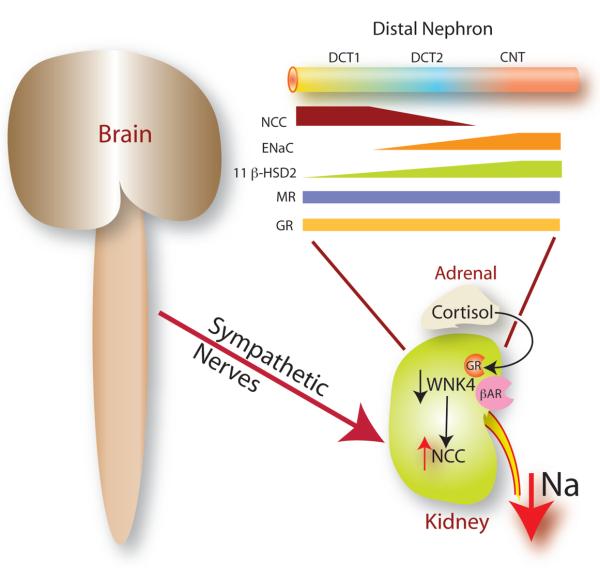
Figure 1. Interactions between the brain and kidney in hypertension.
Mu and colleagues (Mu et al., 2011) show that sympathetic nerve activity, acting through β adrenergic receptors (βAR), in cooperation with glucocorticoid receptors (GR), suppresses WNK4 abundance and activates the sodium chloride cotransporter (NCC) to raise arterial pressure. The inset shows the distal nephron, with distal convoluted tubule (DCT, segments 1 & 2) and connecting tubules (CNT) expressing the NCC, the epithelial Na channel (ENaC), mineralocorticoid receptors (MR) and 11 beta-hydroxysteroid dehydrogenase 2 (11 β-HSD). Note the gradation of 11 β-HSD abundance, conferring steroid specificity.
Their results suggest a key role for GR in mediating the effects of sympathetic activity on NCC. While aldosterone is well recognized for its role in regulating distal Na transport, glucocorticoids are not as widely recognized for playing an essential role. Yet, dexamethasone and aldosterone have been shown to stimulate NCC independently. A gradient of steroid hormone selectivity has been suggested to be present along the distal convoluted tubule (DCT) (Figure 1), with more proximal segments sensitive to glucocorticoids and more distal segments to aldosterone (Reilly and Ellison, 2000). The differential sensitivity results from the graded abundance of 11 β-OH steroid dehydrogenase 2 along the distal nephron (Reilly and Ellison, 2000); this enzyme metabolizes glucocorticoid hormones, leaving aldosterone to bind to mineralocorticoid receptors. The results of Mu and colleagues (Mu et al., 2011) suggest that sympathetic stimulation of NCC might occur predominantly along proximal, glucocorticoid-sensitive, segments of the DCT, whereas aldosterone stimulation would be predominantly distal.
This study also found that steroid hormones interact with WNK kinases to regulate NaCl transport (Mu et al., 2011). The investigators demonstrated that isoproterenol increased histone acetylation near glucocorticoid response elements (GREs) within the promoter of WNK4. Isoproterenol therefore reduced WNK4 transcription, an effect postulated to stimulate NCC. This work supports the view that, at least under some conditions, WNK4 inhibits NCC (Yang et al., 2003), and that sympathetic nerve activity raises arterial pressure partly by activating NCC. Yet, the precise role of WNK4 in activating NaCl transport in this study was not tested directly (Mu et al., 2011). WNK4 activity can switch from inhibitory to stimulatory during human disease or when activated by angiotensin II (McCormick and Ellison, 2010); thus, a clear view of WNK4's role in beta-adrenergic stimulation of NCC requires further work.
The results of this study raise other important questions. Beta-adrenergic receptor activation also increases plasma renin activity. Does isoproterenol infusion (in the context of a high salt diet) decrease WNK4 and increase NCC in part because of relative increases in renal angiotensin II? Although angiotensin receptor blockers (ARBs) did not prevent hypertension in this setting, plasma renin activity or renal renin/angiotensin II levels were not measured, and a role for angiotensin II was not excluded. In addition, while the focus of this study was on beta-adrenergic stimulation of renal NaCl reabsorption, how does their proposed effect of increased renal sympathetic nerve activity interact with the well-known actions of renal alpha-receptors to enhance salt retention (DiBona and Kopp, 1997)?
Nevertheless, while previous studies have hinted at a potential role for renal beta-adrenergic receptors in the control of NaCl reabsorption (DiBona and Kopp, 1997), this study (Mu et al., 2011) suggests this action of renal sympathetic nerves should receive greater attention. Beta-receptor antagonists have been used for decades to treat hypertension, yet their mechanism of action is incompletely understood. Is it possible that this previously unappreciated role of renal beta-adrenergic receptors on urinary sodium excretion, and therefore long-term control of blood pressure, contributes?
Finally, this study reiterates the view that sympathetic nervous stimulation and renal salt transport are usually stimulated concurrently and converge on common mechanisms (in this case, steroid hormones and WNK4). There is emerging interest in device-based approaches to resistant hypertension, some of which ablate renal sympathetic nerves (Krum et al., 2011). The results of the study by Mu and colleagues (Mu et al., 2011) suggest that such devises reduce blood pressure, in part, by activating WNK4 and inhibiting NCC. As NCC can be detected in urinary exosomes (Joo et al., 2007), it should be possible to test this hypothesis directly, in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Selected Reading
- DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- Joo KW, Lee JW, Jang HR, Heo NJ, Jeon US, Oh YK, Lim CS, Na KY, Kim J, Cheong HI, et al. Reduced urinary excretion of thiazide-sensitive Na-Cl cotransporter in Gitelman syndrome: preliminary data. Am J Kidney Dis. 2007;50:765–773. doi: 10.1053/j.ajkd.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Krum H, Sobotka P, Mahfoud F, Bohm M, Esler M, Schlaich M. Device-based antihypertensive therapy: therapeutic modulation of the autonomic nervous system. Circulation. 2011;123:209–215. doi: 10.1161/CIRCULATIONAHA.110.971580. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiological reviews. 2010 doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montani JP, Van Vliet BN. Understanding the contribution of Guyton's large circulatory model to long-term control of arterial pressure. Exp Physiol. 2009;94:382–388. doi: 10.1113/expphysiol.2008.043299. [DOI] [PubMed] [Google Scholar]
- Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, et al. Epigenetic modulation of the renal beta-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med. 2011;17:573–580. doi: 10.1038/nm.2337. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Averina VA, Fink GD. Current computational models do not reveal the importance of the nervous system in long-term control of arterial pressure. Exp Physiol. 2009;94:389–396. doi: 10.1113/expphysiol.2008.043281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev. 2000;80:277–313. doi: 10.1152/physrev.2000.80.1.277. [DOI] [PubMed] [Google Scholar]
- Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]