Abstract
Malaria parasites harbour two organelles with bacteria-like metabolic processes that are the targets of many anti-bacterial drugs. One such drug is fusidic acid, which inhibits the translation component elongation factor G. The response of P. falciparum to fusidic acid was characterised using extended SYBR-Green based drug trials. This revealed that fusidic acid kills in vitro cultured P. falciparum parasites by immediately blocking parasite development. Two bacterial-type protein translation elongation factor G genes are identified as likely targets of fusidic acid. Sequence analysis suggests that these proteins function in the mitochondria and apicoplast and both should be sensitive to fusidic acid. Microscopic examination of protein-reporter fusions confirm the prediction that one elongation factor G is a component of parasite mitochondria whereas the second is a component of the relict plastid or apicoplast. The presence of two putative targets for a single inhibitory compound emphasizes the potential of elongation factor G as a drug target in malaria.
Introduction
Numerous anti-bacterials are also effective anti-malarials and are a part of current malaria management programs. For instance, doxycycline is widely prescribed for malaria chemoprophylaxis [1] and clindamycin and doxycycline are recommended for use in ACT therapy in the case of initial treatment failure [2]. Anti-bacterials typically work by blocking vital prokaryotic processes such as DNA replication, RNA transcription, protein translation, or peptidoglycan wall synthesis. The key to the success of anti-bacterials is that they specifically target the fundamental differences between these processes in bacteria and the equivalent processes in humans and other eukaryotes. How anti-bacterials kill eukaryotic malaria parasites is not well understood. Two malaria parasite organelles derived from endosymbiotic bacteria, the mitochondrion and the relict plastid (or apicoplast), are the likely targets of these compounds.
Mitochondria occur in most eukaryotes and derive from an alpha proteobacterium that entered into an endosymbiotic partnership with a host cell at the outset of eukaryotic evolution [3]. Though much reduced, mitochondria retain clear hallmarks of their bacterial ancestry, and their housekeeping and metabolic activities are essentially bacterial in nature. Plastids are typically the site of photosynthesis in algae and plants and derive ultimately from endosymbiotic cyanobacteria. A vestigial plastid that lacks the ability to photosynthesize was identified more than a decade ago in malaria parasites [4] and is indispensable for parasite survival [5]. It is now clear that non-photosynthetic plastids occur in almost all members of the Phylum Apicomplexa, where they are referred to as apicoplasts. Recently, a photosynthetic apicoplast was discovered in an apicomplexan symbiont of corals [6], confirming the hypothesis that the ancestors of apicomplexan parasites were photosynthetic autotrophs and converted to parasitism of animals early on in their evolutionary radiation. Apicoplasts are reminiscent of mitochondria in that they retain numerous hallmarks of their bacterial ancestry but their metabolic suite is markedly different, being derived from a different lineage of bacterial ancestors-the photosynthetic cyanobacteria.
Fusidic acid is a potent, narrow spectrum steroid anti-bacterial derived from the fungus Fusidium coccineum [7]. It is often used in conjunction with rifampicin to treat severe Gram-positive bacterial infections, such as methicillin-resistant Staphylococcus aureus (MRSA). Fusidic acid targets elongation factor G (EF-G), a GTPase critical to the translocation step of bacterial protein synthesis [8]. Fusidic acid binds to EF-G on the ribosome and prevents the EF-G:GDP complex from leaving the ribosome, effectively stalling protein synthesis by steric inhibition [9]. Fusidic acid exhibits anti-Plasmodium activity in vitro [10], but it has never been utilized as an anti-malarial and nothing is known about its mode of action.
Both the apicoplast and the mitochondrion apparently maintain bacterial-style translation machineries that are candidate targets for fusidic acid. Hard evidence for translation has only been obtained for the Plasmodium falciparum apicoplast courtesy of an antibody directed against elongation factor Tu that is encoded on the apicoplast genome [11]. No such evidence for translation in malaria parasite mitochondria exists, but assays of their predicted enzymatic activity suggest that the three proteins encoded by the Plasmodium mitochondrial genome are indeed manufactured within the mitochondria [12]. This evidence is supported by the presence of ribosomal RNAs [13] and a host of nucleus-encoded bacterial-like translation components that are targeted to the mitochondrion [14].
With a view to further defining the likely target of fusidic acid in malaria parasites, we explore whether either or both of these organellar translation systems utilize EF-G. Drug trials confirmed the anti-parasitic activity of fusidic acid but demonstrate different characteristics to other bacterial translation inhibitors. Searches of the P. falciparum genome for EF-G encoding genes identified two candidates. Bioinformatic analysis and protein sequence comparisons of the predicted protein sequence indicate that one candidate gene is of mitochondrial origin and the other is of a plastid origin. The two gene products localize to the predicted organelles, further confirming that protein translation is occurring in both the apicoplast and mitochondria and suggesting that one or both of these proteins is the target of fusidic acid.
Results
Fusidic acid kills P. falciparum in the first cycle
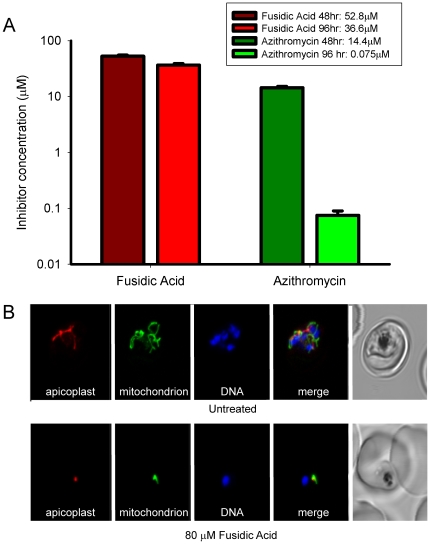
Most drugs targeting translation in the apicoplast produce a delayed effect in which the treated parasites grow and replicate normally for one life cycle following drug treatment, then die after invading a new blood cell [15], [16], [17]. To test for this drug response we determined the IC50 for fusidic acid in in vitro cultures of the P. falciparum line D10. The IC50 values after one life cycle (∼48 hours) are similar to those previously reported [10] and only slightly higher than the values found after two cycles (∼96 hours) (Fig. 1A) indicating that fusidic acid's effects are immediate. This contrasts with other translation-blocking anti-bacterials, such as azithromycin, clindamycin and tetracycline, which exhibit delayed death and have dramatically lower IC50 values at 96 hours compared to 48 hours (Fig. 1A, [15], [17]).
Figure 1. Fusidic acid kills malaria parasites within the first life cycle.
A. Parasiticidal activity of fusidic acid on in vitro cultured erythrocyte stages of Plasmodium falciparum. Fusidic acid kills parasites with an IC50 of 52.8 µM (S.E. 2.5, n = 3) after 48 hours (one life cycle). After drug treatment over two erythrocytic cycles, the IC50 of fusidic acid (36.6 µM S.E. 2.4, n = 3) is not significantly lower as compared the difference seen for azithromycin (14.4 µM (+/−0.85) v. 0.0750 µM (+/−.015), n = 3); death of malaria parasites is immediate and not delayed. B. Synchronised ring-stage parasites expressing RFP targeted to the apicoplast (red) and YFP targeted to the mitochondria (green) were exposed to i) 80 µM of fusidic acid (IC90) or left untreated ii) for 40 hours, the nuclei were stained with Hoescht 33342 (blue) and viewed by confocal laser microscopy.
Examination of parasite lines exposed to fusidic acid at concentrations equivalent to the 96 hour IC90 confirmed the immediate effect. Parasites treated with fusidic acid in early ring stages failed to progress beyond the early trophozoite stage. Consistent with this growth arrest is the lack of significant genome replication and a failure of the organelles to elongate (Fig. 1B). This contrasts with exposure to “delayed-death” antibiotics, where treatments with drug concentrations well in excess of the IC90 show no effect on parasite or organellar development during the first 48 hours of drug treatment [15], [17]. It was interesting to note that many of the fusidic acid treated parasites remained within the red blood cell after 48 hours of treatment but were no longer detectable by confocal microscopic examination after a further 48 hours and no nuclear division or merozoite formation was observed during this period. The progressive loss of drug-killed parasites may account for the slight reduction in IC50 values between 48 and 96 hours, as the assay used does not differentiate between live parasites and those dead and dying parasites remaining within the red blood cell.
P. falciparum encodes two EF-G proteins of bacterial ancestry
Searches of the P. falciparum genome identified two candidate EF-G encoding genes (PFL1590c and PFF0115c) both of which contain GTPase domains. PFL1590c encodes a protein of 803 amino acids that, excluding apicomplexan orthologues, is most similar to the EF-G encoding (fusA) gene of the delta proteobacterium Myxococcus xanthus (Genbank accession NC_008095.1) with which it shares 42 % amino acid identity. PFF0115c encodes a protein of 937 amino acids that, excluding apicomplexan orthologues, is most similar to the EF-G encoding (fusA) gene of the actinobacterium Nocardioides sp. (Genbank accession NC_008699.1), with which it shares 46 % amino acid identity. The presence of two bacterium-like EF-G proteins in P. falciparum is suggestive of an organellar localisation for these proteins and is consistent with the parasites sensitivity to fusidic acid.
Alignments of putative P. falciparum nucleus-encoded EF-G proteins reveals that the two proteins are distinct
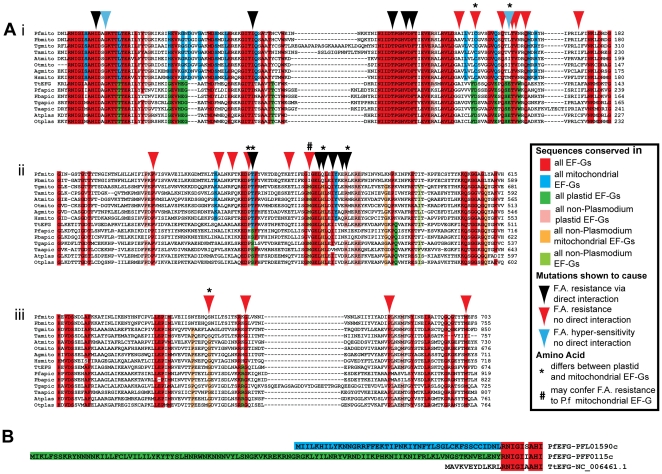
We aligned the two P. falciparum EF-G protein sequences with characterised and predicted EF-G sequences from other apicomplexans, algae, higher plants, insects and mammals. We also included the sequence of the previously crystallised Thermus thermophilus EF-G [18] as a reference for structural features. Accession numbers for protein sequences used in the alignment are listed in Table S1.
The alignment of the GTPase domains of these proteins defines two distinct groups, with a number of amino acids conserved within each group but differing between the two (Fig. 2Ai, blue and green boxes). The smaller of the putative P. falciparum EF-Gs (PFL1590c) and other predicted mitochondrial EF-Gs clearly group with eukaryotes that do not contain a plastid (i.e. human and Anopheles mosquito) and with the EF-G localised to the mitochondria in higher plants [19], [20]. The larger of the two PfEF-Gs (PFF0115c) groups with the plastid-localised EF-G from higher plants [21], [22] and the predicted plastid EF-Gs from other organisms (Fig. 2Ai). The grouping of the mitochondrial and plastid EF-Gs is also evident in alignments of other portions of the protein (Fig. 2A ii, iii), although the overall amino acid conservation in these regions is much lower making the associations less obvious. Indeed, in several instances, specific amino acids are conserved in all EF-Gs except the Plasmodium proteins. The grouping of EF-Gs in our alignment correlates with a recently published phylogenetic analysis of bacterial and organellar EF-Gs, which concluded that the phylogenetic signal for the apicomplexan EF-Gs is too weak to draw detailed conclusions about their relationships to other EF-Gs beyond the organellar origin. [23]
Figure 2. Bioinformatic analysis of P. falciparum EF-Gs.
A. Sequence alignment of Plasmodium falciparum EF-G proteins, selected eukaryotic EF-Gs and the previously crystallised EF-G from the bacterium Thermus thermophilus. Only the three regions known to carry fusidic acid resistance mutations are shown. Red boxes indicate amino acids conserved across all EF-Gs. Blue and dark green boxes highlight amino acids conserved within the mitochondrial or plastid EF-Gs, respectively. Light green, orange and pink boxes indicate amino acids conserved in all but the Plasmodium EF-Gs, Plasmodium mitochondrial EF-Gs and Plasmodium apicoplast EF-Gs, respectively. Arrows indicate amino acids known to be involved in fusidic acid activity. Black arrows indicate amino acids that directly interact with fusidic acid. Red and blue arrows indicate amino acids that do not interact directly with fusidic acid but whose mutation can cause resistance and hypersensitivity, respectively. Stars indicate residues that differ between mitochondrial and plastid EF-Gs. The pound sign indicates the only residue in P. falciparum mitochondrial EF-G in which the wild type amino acid may confer resistance to fusidic acid. Assignment of EF-Gs to cellular location is based on published studies for A.thaliana [20], [22], H. sapiens [41] and O. sativa [21], [23] and bioinformatic analysis of targeting sequences for the other proteins. B. Sequence alignment of the N-terminal region of the EF-Gs from P. falciparum and T. thermophilus. Red boxes indicate areas of homology between the three proteins. Blue and green boxes highlight the N-terminal extensions of the two P. falciparum proteins used to predict protein targeting.
To gain insight into the possible targets of fusidic acid in P. falciparum, we compared the conservation of amino acids known to confer fusidic acid resistance in various bacteria. These amino acids occur in three areas of the protein corresponding to Figure 2A i, ii and iii [24]. Amino acid residues identified as interacting with fusidic acid in crystallographic studies [25] are largely conserved in all EF-Gs examined, particularly in the GTPase domain (Fig. 2A i, ii black arrows). While several of the fusidic acid interacting amino acids in P. falciparum differ from the bacterial residues in the second region, none of the changes correspond to those conferring resistance. They do suggest differences between plastid-localised and mitochondrial EF-Gs (T437-starred) and unique amino acids in the Plasmodium EF-Gs (H458, R465-starred).
Other residues that have been correlated with resistance or hypersensitivity but do not interact directly with fusidic acid are highlighted with red and blue arrows, respectively. There is less conservation among these residues than those directly interacting with fusidic acid, but the pattern of conservation in these amino acids is similar to the fusidic acid interacting residues. There are several differences highlighting the separation of mitochondrial and plastid EF-Gs (D109 and E119, starred) and of the Plasmodium EF-Gs and all others (P436, M450, G617 – starred). Only one difference suggests enhanced resistance; the amino acid methionine 453 (black pound sign) is altered in the putative P. falciparum mitochondrial EF-G to an isoleucine residue that confers limited (4-fold) resistance in Staphylococcus aureus [26].
Bioinformatic analyses suggest that PFL1590c is a mitochondrial-targeted protein and that PFF0115c is an apicoplast-targeted protein
Nucleus encoded organelle-targeted proteins typically contain an N-terminal targeting peptide that directs them either to the P. falciparum mitochondrion [27] or apicoplast [28]. Alignment of the two putative EF-G proteins encoded by the P. falciparum genes PFL1590c and PFF0155c with EF-G from the bacterium T. thermophilus indicated that both malaria parasite proteins bear N-terminal extensions (Fig. 2B). The 44 amino acid N-terminal extension (Fig. 2B green box) of the PFL1590c protein was designated by the Plasmodium mitochondrial-targeting prediction program PlasMit [27] to be a likely mitochondrial targeting peptide (Table S2). The 103 amino acid N-terminal extension of PFF0115c (Fig. 2B blue box) is predicted to contain both a signal peptide and a transit peptide by the Plasmodium apicoplast targeting prediction program PlasmoAP [29], so PFF0115c is likely an apicoplast-targeted protein (Table S2).
PFL1590c is a nucleus-encoded mitochondrial-targeted EF-G protein involved in P. falciparum mitochondrial translation
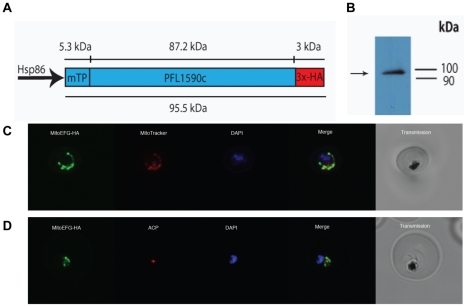
To provide insight into the localisation of PFL1590c, we transfected D10 parasite lines with constructs containing this putative EF-G fused to a 3x hemagglutinin (HA) tag at the C-terminus and driven by the 5′ region of PfHSP86, which we call MitoEFG-HA (Fig. 3A). Western blot analysis of the transgenic parasites confirmed expression of the recombinant protein. A single band of ∼96 kDa was detected, which matches the size of PFL1590c protein with its putative transit peptide and a 3x-HA tag (Fig. 3B). Transit peptides are usually removed after trafficking to the mitochondria and the slight mass anomaly may be due to small differences in predicted and observed molecular mass that are not unusual in polyacrylamide gel electrophoresis.
Figure 3. P. falciparum EF-G PFL1590c localises to the mitochondrion.
A. PFL1590c was fused to a 3x-HA tag to create the construct MitoEFG-HA. B. Western blot analysis of D10 parasites expressing MitoEFG-HA reveals a single band at ∼96 kDa, approximately the mass of the PFL1590c protein fused to a 3x-HA tag. C. An immunofluorescence assay on D10 parasites expressing MitoEFG-HA reveals that PFL1590c is localised to the P. falciparum mitochondrion. MitoEFG-HA (green) co-localises with MitoTracker labelling of the mitochondrion (red). DNA stained with Hoescht 33342 (blue). D. An immunofluorescence assay on the MitoEFG-HA expressing parasites counterstained with the ACP antibody as an apicoplast marker [29]. MitoEFG-HA protein localises to the typically elongated mitochondrion (green), but does not co-localise with the punctate apicoplast (red). DNA stained with Hoescht 33342 (blue).
Immunofluorescence analysis of parasites expressing this episome revealed that the MitoEFG-HA fusion protein is localised to the P. falciparum mitochondrion (Fig. 3C). MitoTracker labelling of the mitochondrion co-localised with staining of the MitoEFG-HA protein. Both MitoTracker labelling and antibody staining of the MitoEFG-HA protein reveal a large, crescent shaped organelle characteristic of the P. falciparum mitochondrion during the late trophozoite stage of the parasite's asexual cycle [22].
To confirm that the organelle containing PFL1590c is the mitochondrion, we compared the localisation of the MitoEFG-HA fusion protein with the apicoplast resident acyl carrier protein (ACP) [28]. The signal from the HA tagged protein clearly localises to an intercellular compartment distinct from the apicoplast (Fig. 3D), further confirming that PFL1590c is a nucleus-encoded EF-G protein targeted exclusively to the P. falciparum mitochondrion.
PFF0115c is a nucleus-encoded apicoplast-targeted EF-G protein involved in P. falciparum apicoplast translation
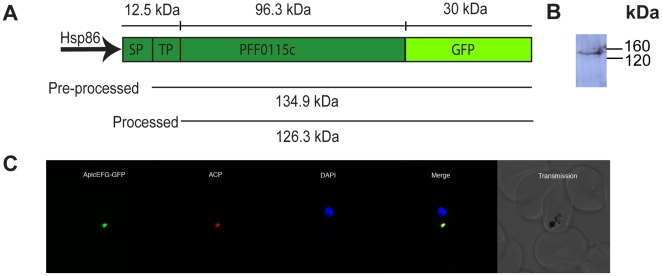
Bioinformatic analysis revealed that PFF0115c has all the characteristics of a nucleus-encoded apicoplast-targeted protein. To localise PFF0115c, we transfected constructs containing this putative EF-G fused to a C-terminal GFP in a construct driven by the 5′ region of PfHSP86, which we called ApicEFG-GFP (Fig. 4A). Western blot analysis of parasites expressing ApicEFG-GFP showed a band of between 120 and 160 kDa in mass. This corresponds reasonably well with the predicted molecular mass of the apicoplast EF-G protein fused to GFP (126.3 kDa). Some highly expressed apicoplast-targeted proteins show a second, larger product that corresponds to a preprocessed form of the protein that retains the apicoplast transit peptide while transiting to the apicoplast [30]. No such product was noted on the Western Blot (Fig. 4B). This is not unusual and likely results from the low levels of recombinant protein expression in this experiment.
Figure 4. P. falciparum EF-G PFF0115c localises to the apicoplast.
A. PFF0115c was fused to a C-terminal GFP tag to create the construct ApicEFG-GFP. B. Western blot analysis of D10 parasites expressing ApicEFG-GFP reveals a single band at approximately the predicted molecular mass of the PFF0115c protein attached to a GFP tag. C. An immunofluorescence assay on D10 parasites expressing ApicEFG-GFP reveals that PFF0115c is localised to the P. falciparum apicoplast. ApicEFG-GFP (green) co-localises with ACP labelling of the apicoplast (red), and shows the dot-like organelle typical of the P. falciparum apicoplast during the ring stage of the asexual cycle [23]. DNA stained with Hoescht 33342 (blue).
Immunofluorescence assays on parasites expressing the ApicEFG-GFP episome in conjunction with ACP as an apicoplast marker [28] reveal that PFF0115c is indeed localised to the P. falciparum apicoplast (Fig. 4C). Both ACP antibody labelling and ApicEFG-GFP fluorescence revealed a dot-like organelle in the parasite, characteristic of the apicoplast in ring stage parasites during the asexual cycle [31]. These data confirm that the gene annotated as PFF0115c is a nucleus-encoded EF-G protein targeted to the P. falciparum apicoplast.
Discussion
We have shown that fusidic acid, an anti-bacterial that stalls bacterial protein translation by binding to EF-G, kills malaria parasites with an IC50 of 52.8 µM. This inhibitory concentration falls within the range seen for other bacterial translation inhibitors when applied for only a single plasmodium life cycle [15], [17], although it is much higher than that seen after prolonged exposure of those compounds causing the delayed-death effect [15], [16], [17]. It is also too high for fusidic acid itself to be an effective drug. However, fusidic acid has only an immediate effect, suggesting that this compound kills Plasmodium via a different mechanism that the delayed-death anti-bacterials and may present an effective lead compound for drug development. The inhibitory concentration of any compound reflects many factors, including differences in their ability to come in contact with the target molecule, affinity for the site of action and the ability of the inhibitor to block target activity. All of these factors may be contributing to the IC50 of fusidic acid against P. falciparum and need to be optimised during further drug development. Determining the specific target of fusidic acid is an important first step in assessing these factors in a systematic way.
Two candidate EF-G proteins that may be fusidic acid targets were identified and localised (Fig. 2– 4). Bioinformatic analysis is consistent with these proteins having been introduced as endosymbiont-derived genes that are now located in the parasite nucleus. One EF-G is localised in the parasite mitochondrion, and the second is localised to the relict plastid or apicoplast (Fig. 3,4). Comparisons between primary protein structure of the apicoplast and mitochondrial EF-Gs and sensitive bacterial EF-G proteins suggests that the apicoplast localised EF-G is sensitive to fusidic acid while the mitochondrial EF-G carries a single amino acid residue that confers a weak resistance phenotype in S. aureus (Fig. 2,[26]). Although this finding implies that the P. falciparum mitochondrial EF-G may be resistant to fusidic acid, differences between the bacterial and Plasmodium mitochondrial EF-Gs at other conserved positions makes it difficult to draw specific conclusions about the sensitivity of the P. falciparum mitochondrial EF-G to fusidic acid from this single amino acid change without further investigation. A further possibility is that fusidic acid acts by targeting a mechanism unrelated to organellar protein synthesis, but the two EF-Gs identified here represent the most likely targets and require further investigation.
The identification of two nucleus-encoded, organelle localised EF-Gs that could be the target of the same inhibitor presents unique opportunities for the investigation of the effects of organelle specific drugs and in the development of novel anti-malarials. For almost all anti-bacterial compounds currently in use, the target (or predicted target) is encoded on the genome of at least one of the organelles [15], [17], [32] making them refractory to genetic manipulation and difficult to investigate. The presence of nucleus-encoded EF-Gs in both the mitochondrion and apicoplast provides the opportunity to use known bacterial resistance mutations to dissect the effects of specifically blocking protein synthesis in each organelle. It also presents the possibility of developing novel compounds that target EF-G in both organelles, thereby yielding a single anti-malarial compound that could have all the advantages of a multi-drug therapy in terms of avoiding drug resistance.
Materials and Methods
Fusidic acids drug trials
Drug trials on P. falciparum line D10, were performed as previously described [17]. Parasitemia was assessed using a modified version [17] of the SYBR-Green assay [33]. Inhibitory concentrations were calculated using the Hill-Slop method available in SigmaPlot (Systat software).
Bioinformatic analysis of EF-G in P. falciparum
Protein sequences of the putative mitochondrial EF-G, PFL1590c, and the putative apicoplast EF-G, PFF0115c, were obtained from the Plasmodium Genome Resource (www.plasmodb.org) as were sequences for putative homologs from Plasmodium berghei. Sequences for the putative Toxoplasma gondii EF-G proteins were retrieved using BLAST searches [34] of the Toxoplasma Genome Resource (www.toxodb.org). All other sequences were retrieved by text or BLAST searches of GenBank (www.ncbi.nlm.nih.gov) . Alignments were performed and visualised using ClustalX [35]. Accession numbers of EF-G sequences used are included in Table S1. Analysis of N-terminal targeting information was carried out using the Plasmodium targeting prediction programs Plasmit [27] and PlasmoAP [29].
Cloning of PFL1590c and PFF115c localisation constructs
Constructs were created using the MultiSite Gateway system™ (Invitrogen), as previously described [31]. The complete predicted open reading frames of PFL1590c and PFF0115c gene were amplified from P. falciparum genomic DNA using the primers shown in Table S3, with attB1 and attB2 sites underlined for the sense and antisense primer, respectively. The PCR products were recombined into the vector pDONR221 according to the manufacturer's instructions (Invitrogen) and fully sequenced. These vectors were then recombined via LR reaction (Invitrogen) to create a C-terminal GFP-tagged PFF0115c construct driven by the PfHSP86 5′ UTR (which we called ApicEFG-GFP) and a C-terminal 3x hemagglutinin (HA)-tagged PFL1590c construct driven by the PfHSP86 5′ UTR (which we called MitoEFG-HA). Both constructs carried a human DHFR expression cassette conferring resistance the drug WR99210 [36].
Parasite Lines and Transfection
Parasites were grown as previously described [37]. The D10 parasite line was used for all transfections. D10 parasites expressing apicoplast- targeted RFP and mitochondrial-targeted YFP [31] were used for microscopic analysis of drug treatment effects. Transfection of MitoEFG-HA and ApicEFG-GFP plasmids into P. falciparum was performed as previously described [38], [39]. Cultures were split 2∶1 14 and 28 days post transfection, yielding drug resistant parasites within 20–30 days.
Western Blotting
Western blots were performed as previously described [40]. Mouse anti-HA primary antibody was diluted 1/500, and anti-mouse HRP secondary antibody was diluted 1/5000. Rabbit anti-GFP primary antibody was diluted 1/500, and anti-rabbit HRP secondary antibody was diluted 1/5000.
Immunofluorescence assays
Immunofluorescence assays were performed as previously described [39]. For Mitotracker labelling of the P. falciparum mitochondrion, cells were incubated in 20 nM MitoTracker Red (Molecular Probes) diluted in 0/100 RPMI-HEPES medium for 15 mins at 37°C. Cells were washed in 0/100 medium, prior to fixation and labelling. Rat anti-HA primary antibody (Roche) was diluted 1/200. Anti-rat antibody conjugated to Alexafluor 488 (Molecular Probes) was diluted 1/1000. Rabbit anti-ACP antibody [30] was diluted 1/1000, as was anti-rabbit antibody conjugated to Alexafluor 546 (Molecular Probes).
Supporting Information
Primers used for amplication of PFL1590c and PFF0115c from P. falciparum gDNA.
(DOC)
Targeting predictions for leaders of PFL0159c and PFF0115c.
(DOC)
Accession numbers of protein sequences used in alignments.
(DOC)
Acknowledgments
The Australian Red Cross generously supplied human red blood cells.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Health and Medical Research Council (www.nhmrc.gov.au) Grant #637406, the Australian Research Council (www.arc.gov.au) Grant #FF0668705. RAJ was supported by a Fulbright Fellowship (fulbright.state.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bradley DJ, Bannister B. Guidelines for malaria prevention in travellers from the United Kingdom for 2001. Commun Dis Public Health. 2001;4:84–101. [PubMed] [Google Scholar]
- 2.WHO . Geneva: World Health Organization; 2010. Guidelines for the treatment of malaria.196. [PubMed] [Google Scholar]
- 3.Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 4.McFadden GI, Reith ME, Munholland J, Lang-Unnasch N. Plastid in human parasites. Nature. 1996;381:482. doi: 10.1038/381482a0. [DOI] [PubMed] [Google Scholar]
- 5.He CY, Shaw MK, Pletcher CH, Striepen B, Tilney LG, et al. A plastid segregation defect in the protozoan parasite Toxoplasma gondii. EMBO J. 2001;20:330–339. doi: 10.1093/emboj/20.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore RB, Obornik M, Janouskovec J, Chrudimsky T, Vancova M, et al. A photosynthetic alveolate closely related to apicomplexan parasites. Nature. 2008;451:959–963. doi: 10.1038/nature06635. [DOI] [PubMed] [Google Scholar]
- 7.Godtfredsen WO, Jahnsen S, Lorck H, Roholt K, Tybring L. Fusidic acid: a new antibiotic. Nature. 1962;193:987. doi: 10.1038/193987a0. [DOI] [PubMed] [Google Scholar]
- 8.Johanson U, Hughes D. Fusidic acid-resistant mutants define three regions in elongation factor G of Salmonella typhimurium. Gene. 1994;143:55–59. doi: 10.1016/0378-1119(94)90604-1. [DOI] [PubMed] [Google Scholar]
- 9.Besier S, Ludwig A, Brade V, Wichelhaus TA. Molecular analysis of fusidic acid resistance in Staphylococcus aureus. Mol Microbiol. 2003;47:463–469. doi: 10.1046/j.1365-2958.2003.03307.x. [DOI] [PubMed] [Google Scholar]
- 10.Black FT, Wildfang IL, Borgbjerg K. Activity of fusidic acid against Plasmodium falciparum in vitro. Lancet. 1985;1:578–579. doi: 10.1016/s0140-6736(85)91234-6. [DOI] [PubMed] [Google Scholar]
- 11.Chaubey S, Kumar A, Singh D, Habib S. The apicoplast of Plasmodium falciparum is translationally active. Mol Microbiol. 2005;56:81–89. doi: 10.1111/j.1365-2958.2005.04538.x. [DOI] [PubMed] [Google Scholar]
- 12.Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature. 2007;446:88–91. doi: 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- 13.Feagin JE, Werner E, Gardner MJ, Williamson DH, Wilson RJ. Homologies between the contiguous and fragmented rRNAs of the two Plasmodium falciparum extrachromosomal DNAs are limited to core sequences. Nucleic Acids Res. 1992;20:879–887. doi: 10.1093/nar/20.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Dooren GG, Stimmler LM, McFadden GI. Metabolic maps and functions of the Plasmodium mitochondrion. FEMS Microbiol Rev. 2006;30:596–630. doi: 10.1111/j.1574-6976.2006.00027.x. [DOI] [PubMed] [Google Scholar]
- 15.Dahl EL, Shock JL, Shenai BR, Gut J, DeRisi JL, et al. Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. Antimicrob Agents Chemother. 2006;50:3124–3131. doi: 10.1128/AAC.00394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fichera ME, Bhopale MK, Roos DS. In vitro assays elucidate peculiar kinetics of clindamycin action against Toxoplasma gondii. Antimicrob Agents Chemother. 1995;39:1530–1537. doi: 10.1128/aac.39.7.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman CD, Su V, McFadden GI. The effects of anti-bacterials on the malaria parasite Plasmodium falciparum. Mol Biochem Parasitol. 2007;152:181–191. doi: 10.1016/j.molbiopara.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 18.al-Karadaghi S, Aevarsson A, Garber M, Zheltonosova J, Liljas A. The structure of elongation factor G in complex with GDP: conformational flexibility and nucleotide exchange. Structure. 1996;4:555–565. doi: 10.1016/s0969-2126(96)00061-5. [DOI] [PubMed] [Google Scholar]
- 19.Millar AH, Sweetlove LJ, Giege P, Leaver CJ. Analysis of the Arabidopsis mitochondrial proteome. Plant Physiol. 2001;127:1711–1727. [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S, Taylor NL, Narsai R, Eubel H, Whelan J, et al. Experimental analysis of the rice mitochondrial proteome, its biogenesis, and heterogeneity. Plant Physiol. 2009;149:719–734. doi: 10.1104/pp.108.131300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrecht V, Ingenfeld A, Apel K. Characterization of the snowy cotyledon 1 mutant of Arabidopsis thaliana: the impact of chloroplast elongation factor G on chloroplast development and plant vitality. Plant Mol Biol. 2006;60:507–518. doi: 10.1007/s11103-005-4921-0. [DOI] [PubMed] [Google Scholar]
- 22.von Zychlinski A, Kleffmann T, Krishnamurthy N, Sjolander K, Baginsky S, et al. Proteome analysis of the rice etioplast: metabolic and regulatory networks and novel protein functions. Mol Cell Proteomics. 2005;4:1072–1084. doi: 10.1074/mcp.M500018-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Atkinson GC, Baldauf SL. Evolution of elongation factor G and the origins of mitochondrial and chloroplast forms. Mol Biol Evol. 2011;28:1281–1292. doi: 10.1093/molbev/msq316. [DOI] [PubMed] [Google Scholar]
- 24.Hansson S, Singh R, Gudkov AT, Liljas A, Logan DT. Structural insights into fusidic acid resistance and sensitivity in EF-G. J Mol Biol. 2005;348:939–949. doi: 10.1016/j.jmb.2005.02.066. [DOI] [PubMed] [Google Scholar]
- 25.Gao YG, Selmer M, Dunham CM, Weixlbaumer A, Kelley AC, et al. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science. 2009;326:694–699. doi: 10.1126/science.1179709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lannergard J, Norstrom T, Hughes D. Genetic determinants of resistance to fusidic acid among clinical bacteremia isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:2059–2065. doi: 10.1128/AAC.00871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender A, van Dooren GG, Ralph SA, McFadden GI, Schneider G. Properties and prediction of mitochondrial transit peptides from Plasmodium falciparum. Mol Biochem Parasitol. 2003;132:59–66. doi: 10.1016/j.molbiopara.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Waller RF, Keeling PJ, Donald RG, Striepen B, Handman E, et al. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc Natl Acad Sci U S A. 1998;95:12352–12357. doi: 10.1073/pnas.95.21.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foth BJ, Ralph SA, Tonkin CJ, Struck NS, Fraunholz M, et al. Dissecting apicoplast targeting in the malaria parasite Plasmodium falciparum. Science. 2003;299:705–708. doi: 10.1126/science.1078599. [DOI] [PubMed] [Google Scholar]
- 30.Waller RF, Reed MB, Cowman AF, McFadden GI. Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 2000;19:1794–1802. doi: 10.1093/emboj/19.8.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dooren GG, Marti M, Tonkin CJ, Stimmler LM, Cowman AF, et al. Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle of Plasmodium falciparum. Mol Microbiol. 2005;57:405–419. doi: 10.1111/j.1365-2958.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- 32.Sidhu AB, Sun Q, Nkrumah LJ, Dunne MW, Sacchettini JC, et al. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J Biol Chem. 2007;282:2494–2504. doi: 10.1074/jbc.M608615200. [DOI] [PubMed] [Google Scholar]
- 33.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 35.Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics Chapter. 2002;2:Unit 2 3. doi: 10.1002/0471250953.bi0203s00. [DOI] [PubMed] [Google Scholar]
- 36.Fidock DA, Wellems TE. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci U S A. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 38.Crabb BS, Cowman AF. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1996;93:7289–7294. doi: 10.1073/pnas.93.14.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonkin CJ, van Dooren GG, Spurck TP, Struck NS, Good RT, et al. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol Biochem Parasitol. 2004;137:13–21. doi: 10.1016/j.molbiopara.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Mullin KA, Lim L, Ralph SA, Spurck TP, Handman E, et al. Membrane transporters in the relict plastid of malaria parasites. Proc Natl Acad Sci U S A. 2006;103:9572–9577. doi: 10.1073/pnas.0602293103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used for amplication of PFL1590c and PFF0115c from P. falciparum gDNA.
(DOC)
Targeting predictions for leaders of PFL0159c and PFF0115c.
(DOC)
Accession numbers of protein sequences used in alignments.
(DOC)