Abstract
During puberty, humans develop a later chronotype, exhibiting a phase-delayed daily rest/activity rhythm. The purpose of this study was to determine: 1) whether similar changes in chronotype occur during puberty in a laboratory rodent species, 2) whether these changes are due to pubertal hormones affecting the circadian timekeeping system. We tracked the phasing and distribution of wheel-running activity rhythms during post-weaning development in rats that were gonadectomized before puberty or left intact. We found that intact peripubertal rats had activity rhythms that were phase-delayed relative to adults. Young rats also exhibited a bimodal nocturnal activity distribution. As puberty progressed, bimodality diminished and late-night activity phase-advanced until it consolidated with early-night activity. By late puberty, intact rats showed a strong, unimodal rhythm that peaked at the beginning of the night. These pubertal changes in circadian phase were more pronounced in males than females. Increases in gonadal hormones during puberty partially accounted for these changes, as rats that were gonadectomized before puberty demonstrated smaller phase changes than intact rats and maintained ultradian rhythms into adulthood. We investigated the role of photic entrainment by comparing circadian development under constant and entrained conditions. We found that the period(τ) of free-running rhythms developed sex differences during puberty. These changes in τ did not account for pubertal changes in entrained circadian phase, as the consolidation of activity at the beginning of the subjective night persisted under constant conditions in both sexes. We conclude that the circadian system continues to develop in a hormone-sensitive manner during puberty.
Keywords: adolescent, steroid hormones, rodent, rat, phase, period, sex difference, chronotype
During adolescence, humans develop a propensity towards “night-owl” behavior or “evening chronotype”, exhibiting a later, or delayed, timing of many daily rhythms including rest and activity (Crowley et al., 2007; Roenneberg et al., 2004; Thorleifsdottir et al., 2002; Yang et al. 2005). These developmental changes in chronotype are likely to be partially rooted in hormonal influences on the body’s circadian timekeeping system, as they persist under controlled, laboratory conditions (Carskadon et al., 2004; Carskadon et al., 1997), exhibit pronounced sex differences in timing and magnitude (Roenneberg et al., 2004) and correlate with secondary-sex development, even after taking into account age and related social influences (Carskadon et al., 1993; Sadeh et al. 2009). The purpose of this study, as well as another study published in the same issue of Hormones and Behavior (Hagenauer et al., pp.TBA), is to determine whether similar changes in chronotype can be reliably observed during puberty in laboratory rodent species, and, if so, to elucidate their physiological mechanism.
Circadian rhythms in mammals are generated by an endogenous pacemaker (Ralph et al., 1990) that must be entrained by external time cues (or “zeitgebers”) such as light to maintain a stable phase relationship with the outside world (Moore-Ede et al., 1982). Under conditions in which there are no time cues from the outside world (also referred to as constant or “free-running” conditions), the circadian system continues to generate daily rhythms with a period (or day length, τ) that only approximates 24 hours (Moore-Ede et al., 1982). Under entrained conditions, the phase relationship between solar day and the circadian system’s output rhythms, such as daily rest/activity and hormonal cycles, is used to characterize an individual’s chronotype. Thus, changes in chronotype, such as those seen during adolescence in humans, can be caused by changes in the entrainment of the circadian pacemaker (due to altered photic sensitivity or endogenous period) or changes in the phase relationship between the circadian pacemaker and its output rhythms.
It is already well-documented that the circadian system is sensitive to gonadal hormones during early development and adulthood. The timing of daily rhythms is shifted during different stages of the menstrual cycle (Manber and Bootzin, 1997; Parry et al., 1994; Parry et al., 2000). In adult laboratory rodents, the gonadal hormones that affect the phase and period of circadian rhythms include estrogens, progestins, androgens, and non-traditional neuroactive steroids (Albers et al., 1981; Axelson et al., 1981; Kent et al., 1991; Li and Satinoff, 1996; Davis et al., 1983; Morin et. Al, 1977; de Tezanos Pinto and Golombek, 1999; Daan et al., 1975; Iwahana et al., 2008; Karatsoreos et al., 2007; Jechura et al., 2000; Labyak and Lee 1995). The sensitivity of the adult circadian system to these steroidal hormones is determined in some rodent species by the organizational effects of gonadal hormones during the perinatal period (rat: Albers, 1981; hamster: Zucker et al., 1980).
The influence of gonadal hormones on the circadian system during puberty is less understood, although it is reported that pubertal hormones can alter circadian phase (degu: Hummer et al., 2007) as well as produce organizational effects on the circadian system (hamster: Davis et al., 1983). Indeed, in some species there is a critical window of sensitivity to the organizational effects of gonadal hormones as late as young adulthood (degu: Hummer et al. 2006). There has been little attempt to determine how common pubertal changes in chronotype are across mammalian species or to elucidate their hormonal or neural bases using animal models (Hagenauer et al., 2009). Evidence from five species suggests that pubertal changes in circadian phase are not uniquely human (rhesus macaque: Golub and Takeuchi, 2002; laboratory mouse: Weinert et al., 1994; Weinert and Waterhouse, 1999; laboratory rat: McGinnis et al., 2007; Kittrell and Satinoff, 1986; Octodon degus (degu): Hummer et al., 2007; Tate et al., 2002; Psammomys obsesus: Neuman et al., 2005). However, only three of the studies (using the slow-developing, diurnal species of the macaque and degu) have attempted to thoroughly characterize the developmental progression of circadian phase change in relation to secondary-sex development (Golub & Takeuchi 2002, Tate, Richardson, and Carskadon 2002, Hummer et al. 2007), and only one study directly examined the role of pubertal hormones (Hummer et al. 2007). Similar to humans, the macaque and degu show a delayed circadian phase during puberty (around the time of first menarche in the rhesus macaque, and first vaginal or prepucial opening in the degu) that reverses by adulthood. These developmental changes do not occur following pre-pubertal gonadectomy (Hummer et al., 2007). Therefore, pubertal elevations in sex hormones are likely to drive circadian phase changes.
Another mechanism underlying pubertal changes in circadian phase may be an elongation of the endogenous period of the circadian pacemaker (τ; Carskadon et al., 2004). In support of this theory, τ appears to elongate during puberty in humans and shorten during adulthood in a manner that parallels changes in chronotype (Carskadon et al., 1999; Carskadon et al., 2004). In rodent studies, male rats had a longer τ during late puberty (postnatal age P47-59 days, τ=23.89 hrs) than during adulthood (age P105-P115, τ=23.75 hrs; McGinnis et al., 2007). This theory needs to be explored further using additional timepoints and a comparison of the sexes because data from the diurnal degu indicated that pubertal changes in circadian phase are not dependent on changes in τ (Hummer et al., 2007).
The rat is a useful animal model for examining the mechanism underlying circadian chronotype change during puberty because there is previous indication that male rats undergo a 3 hr magnitude phase change during puberty (McGinnis et al., 2007). Secondary-sex characteristics typically first appear around the postnatal age (P) of P30 in females and P45 in males, with mature sexual characteristics evident by around P60 (Ojeda and Urbanski, 1994). We hypothesized that pubertal rats would show a delay in circadian phase that peaks around mid-puberty, similar to what is observed in slow-developing mammalian species (the degu and macaque; Golub & Takeuchi, 2002; Hummer et al., 2007). We further hypothesized that, as in the degu, these changes in phase would be dependent on gonadal hormones but independent of pubertal changes in τ. Finally, we expected that the development of delayed phase during puberty might be accompanied by a decrease in the ultradian components prevalent in the rhythms of newly-weaned young rats (Cambras and Diez-Noguera, 1988; Castro and Andrade, 2005; Diez-Noguera and Cambras, 1990; Ibuka, 1984, Joutsinemi et al., 1991; Kittrell and Satinoff, 1986). To test these hypotheses, we first evaluated the correlation between changes in activity distribution and sexual maturation in both sexes, and then determined the dependency of changes in activity distribution on pubertal exposure to gonadal hormones (Experiment 1). We then examined the relationship between developmental changes in circadian phase and changes in the endogenous period of the circadian pacemaker under constant conditions (τ) in both sexes (Experiment 2).
Methods
All procedures were conducted in accordance with the guidelines established for the care and use of laboratory animals by the National Institute of Health and under approved by local animal use and care committees (IACUC). Sprague-Dawley rats were housed during testing in individual plastic cages (47 × 27 × 20 cm) with a Nalgene running wheel (9 × 34.5 cm, Mini Mitter, Bend, OR) and free access to food (5001 Rodent Diet, PMI Nutrition) and water. They were maintained under a 12:12 light-dark (LD) cycle at 20°C. During the lighted part of the LD cycle, the testing environment was dimly lit (40-60 lux measured at cage level, provided by fluorescent house light) to reduce photic inhibition of activity (masking). Running wheel activity data was collected in 10-min bins using VitalView software (Minimitter, Bend, OR) and analyzed using ActiView software (Minimitter, Bend, OR). To prevent circadian disruptions, routine procedures occurred at random times during the lighted period of the LD cycle.
Experiment 1: Pubertal Changes in Circadian Rhythms under Entrained Conditions
Animals
Four iterations of the same experiment were conducted with a total sample size of 62 rats. These rats were obtained from breeding colonies at the University of Michigan, comprising eight litters from eight dams and sires. The dams and sires were purchased from Charles-River Laboratories (Wilmington, MA). Litters were reduced to 8 rats by postnatal day 3 (P3) with roughly balanced sex ratio
The rats were placed in the testing environment before weaning. The pups used in iteration 1 were raised under a 14:10 LD cycle (lights on 05:00-19:00) until the age of P8-P14, when they were moved to the testing environment (12:12 LD, lights on 06:00-18:00). All other rats were raised and tested on the 12:12 LD cycle. Within the testing environment, cages were kept on tables to standardize light exposure. At weaning (age P19-P22), rats were placed in individual opaque plastic cages. Wheel-running data from the first two days after weaning was not used to avoid transient artifacts. Thus, all rats had been exposed to the testing environment for 5-16 days before their activity data was used for analysis (beginning P22-P24).
Surgery
Approximately one third of the rats underwent gonadectomy (GDX) or SHAM GDX surgery prior to puberty at age P12-P15 (conditions were roughly balanced within a litter). The remaining rats underwent no surgery. Any GDX animals that developed secondary-sex characteristics were removed from the analysis (n=3), as were two females (no surgery) that exhibited low wheel-running counts, leaving a final sample size of 58 rats (8 SHAM M, 8 SHAM F, 12 GDX M, 11 GDX F, 7 no-surgery M, and 15 no-surgery F).
During surgery, rats were anesthetized with 4% isoflurane. For ovariectomies, a 1-1.5 cm dorsal incision was made bilaterally below the ribs. For castrations, a single incision was made in the scrotal sac and the testes were visualized via palpation. The major blood vessels of the testes and ovaries were cauterized prior to organ removal. Then, the skin and the abdominal wall (in the case of the ovariectomies) were sutured closed. Post-operative care included subcutaneous saline injection and the application of Nolvasan antiseptic ointment (Fort Dodge Labs, Madison, NJ) on the incision. Sham operations included incisions and suturing similar to gonadectomy, but no organ removal. Animals were placed back in their cage with mother and siblings for at least 5 days of recovery before weaning.
Monitoring Pubertal Development
Two secondary-sex characteristics, the development of a vaginal opening in female rats and a prepucial opening in male rats, were monitored daily around the typical age of puberty. The androgen-dependent development of the prepucial opening is marked by the separation of the sheath from the glans of the penis (Ojeda and Urbanski, 1994). The development of vaginal opening is estrogen-dependent and one of the first indications of puberty in female rats (Ojeda and Urbanski, 1994). Body weight and testicular volume were also measured twice weekly. Testicular volume was calculated by multiplying the length and width (mm) as defined by existing parameters (Hummer, et al., 2006).
Circadian Data Collection and Analysis
Running wheel activity data was collected from an age just prior to the onset of puberty (P19-P23) until animals were post-pubertal (P60-P70). Circadian parameters were calculated for each day of activity recording. Daily mean activity (average wheel turns/10 min bin) and the daily acrophase (determined using a cosine fit) were calculated automatically using Actiview software. Other indicators of circadian phase (initial activity (1°) onset, onset of the second (2°) major activity bout, and final offset) were scored by researchers blinded to the sex and gonadal status of the animals. The “threshold” for all phase analyses was defined as each day’s mean activity so as to avoid the confounding effects of developmental and estrus-related changes in activity level. 1°activity onset and final activity offset were defined respectively as the first and last three consecutive bins of activity exceeding this threshold around the time of lights off (hours ZT12-18 in Zeitgeber Time, which is defined as hours relative to lights on), and lights on (between ZT18-24). Visual inspection revealed that young rats exhibited a bimodal activity pattern with a short, initial (1°) activity bout that began immediately following lights off and that rarely continued longer than 2 hours. Thus, to track changes in the second (2°) major activity bout we also tracked 2°onset, which was defined as the first three consecutive bins of activity to exceed threshold following ZT14 (two hours after lights off). On days where three consecutive bins of activity exceeding threshold did not occur during the designated time periods a circadian parameter could not be calculated. These null values were replaced by the latest 1°onset or earliest offset allowable (ZT18), or by the mean 2°onset for ages P24-P60. Null values accounted for 10% of daily activity 1°onset and final offset scores, and 2.4% of 2°onset scores.
To better visualize changes in bimodality, data were plotted as a percent of nightly activity so as to control for age-related changes in activity levels. To produce these figures, raw activity data from the dark period (ZT12-ZT24) was averaged by hour. Activity data from the light period was ignored because the activity counts were so minimal that it provided little information about bimodal activity distribution. The hourly bins were then divided by the overall average activity for the night to produce a percent of nightly activity. These hourly percentages were averaged over five four-day sampling periods that evenly spanned the developmental period (P25-28, P33-36, P41-44, P49-52, P57-60).
Statistics
Initially, all intact animals (SHAM and no-surgery) were pooled and circadian parameters were analyzed for normal developmental changes over time and sex differences using a mixed design ANOVA, in which a repeated measures model was used to examine the variable of age, and a between-subjects comparison was used for the variable of Sex. This analysis was followed up using a one-way ANOVA for each sex with individual post-hoc comparisons of individual time points (Tukey corrected).
Subsequently, all data were analyzed for the between subjects effects of gonadectomy surgery (GDX) and Sex (a 2×3 comparison) using a mixed design ANOVA with repeated measures by age. A Huynh-Feldt correction was used for most analyses because the assumption of sphericity was violated. For post-hoc analyses of the effect of surgery type (no surgery, SHAM, GDX) on age-related changes in circadian parameters, individual repeated measures comparisons were run by group followed by Bonferonni correction. Between-subjects effects were also examined using 3×2 ANOVA, and significant effects were followed-up using Dunnett’s T3 post-hoc comparisons. The results section only presents analyses using data pooled weekly (to reduce variance) although bi-daily data is presented graphically for comparison in the supplement.
Experiment 2: A Comparison of Pubertal Changes in Circadian Rhythms Under Entrained and Constant Conditions
Animals
Thirty-two juvenile rats (age P23, 16 M, 16 F, 45-50 g) were purchased from Charles River laboratories. Upon arrival, the rats were immediately placed in individual, transparent plastic cages (12:12 LD, lights on at 08:00-20:00). During the light phase of the LD cycle the mean light level was 59+/−7 lux as measured at cage bottom, provided by house fluorescent lights. Cages were arranged on open racks in the testing environment so that they alternated by sex.
Procedure
After four days of baseline 12:12 LD recording, half of the rats (8 M, 8 F) were placed in constant conditions (dim red light maintained at < 1 lux at cage level, abbreviated as DD) for six weeks (P29-P70). The red light was provided by three safelights in a light-tight room, each with a number one Kodak red monochromatic filter and a 2 W bulb. The other 16 rats were kept under the 12:12 LD condition for the same duration of time. The rats were weighed at the end of the experiment to ensure normal growth.
Circadian Analyses
For initial analyses, running wheel activity data from the baseline entrainment period (P24-P28) were binned; other data were averaged in two week bins: P29-P42, P43-56, P57-P70. Similar to Experiment 1, daily mean activity and acrophase was measured using automated analyses, as was another phase variable, light-dark activity (LD) ratio. A lower LD Ratio score indicated greater nocturnality. Period (τ) was also analyzed under both entrained and constant conditions using a chi-square periodogram analysis that tested a window of 22-26 hrs while sampling successive 1-min intervals for best fit.
In order to better compare developmental changes in the distribution of activity under entrained and constant conditions, the percent of daily activity occurring during each 10 min bin was quantified for several ages: P27-P28 (when all animals were still entrained to a 12:12 LD cycle), P29-P30 (the first two days in DD, useful for determining the influences of photic masking), P35-36, P43-P44, P51-P52, P67-68. Under constant conditions the daily rhythms began to free-run, which meant that the entire active period consistently drifted earlier or later each day depending on the animal’s endogenous circadian period (τ). In addition to investigating age-related changes in τ (as discussed above), we were interested in the reorganization of the active period under constant conditions independent of τ. Therefore, we subtracted out this daily drifting of the active period due to τ by aligning the onset of the active period for each sample for all DD animals, such that the average onset of the active period for that sample (e.g., P67-68) was made equivalent to that found under entrained conditions (P27-P28). The onset of the active period (CT12) was defined as the beginning of the 12 hrs during the day when the most activity occurred as identified by a moving window analysis. Acrophase was also measured relative to the onset of the active period under both DD and LD conditions.
Statistics
Circadian parameters specific to entrained conditions (acrophase, LD ratio) were analyzed for age-related change and sex differences using a mixed design, in which a repeated measures model was used to examine the variable of age, and a between-subjects comparison was used for the variable of sex. General circadian parameters characterizing entrained and constant conditions (mean activity and τ) were analyzed for age-related change and effects of sex and condition using a mixed design, in which a repeated measures model was used to examine the variable of age, and a between-subjects comparison was used for the variables of Sex and Condition. For post-hoc analyses, if there was no main effect of condition but a main effect of sex, then individual comparisons were run by sex for each time point using independent samples T-tests followed by a Bonferonni correction. If there was a main effect of both condition and sex, then individual 2×2 ANOVAs (sex × condition) were performed by time point followed by Bonferonni correction. A Huynh-Feldt correction was used for most repeated measures analyses because the assumption of sphericity was violated.
A separate analysis was used to analyze the effect of masking on the distribution of pre-pubertal activity in both sexes. This analysis examined the within-subjects change in the acrophase relative to the onset of the active period (CT12) during the last two days in LD conditions (P27-P28) and the first two days in DD (P29-30). These changes were compared to the control animals that remained in LD conditions at both ages using a mixed design ANOVA (sex × condition), with repeated measures at the two ages.
The progression of developmental changes in the distribution of activity under entrained (LD) or constant conditions was further analyzed statistically by comparing the acrophase relative to the onset of the active period in both sexes at 5 ages (P29-P30, P35-36, P43-P44, P51-P52, P67-68) using a mixed design ANOVA (sex × condition), with repeated measures at the five ages.
Results
Experiment 1: Pubertal Changes in Circadian Rhythms under Entrained Conditions
Developmental Changes in Circadian Phase in Intact Animals
The data from all intact animals (no-surgery and SHAM animals) were combined in order to characterize developmental changes in circadian phase under normal pubertal hormonal conditions. This combination was warranted because post-hoc tests revealed that SHAM surgery did not significantly affect any of the circadian phase parameters, including the parameters’ interactions with sex or age (p>0.05).
Overall, both male and female intact animals (no-surgery and SHAM) demonstrated a bimodal activity pattern during early puberty, with increased activity around both times of transitioning light. This bimodality disappeared by late to post-puberty (Figure 1) due to the later (2°) activity bout phase-advancing until it merged with the initial (1°) activity bout. This change was reflected in a phase advance in the 2°onset, final activity offset, and the acrophase as indicated using repeated measures ANOVA (2°onset: F(2.367, 78.098) =52.542, p<0.001; the acrophase: F(3.327, 123.099)=6.417, p<0.001; activity offset: F(3.331, 123.243)=10.642, p<0.001). These changes were particularly pronounced in no-surgery intact males, which showed a 4.03 hour phase advance in 2°onset across the pubertal period. In all animals, the 1°onset at the beginning of the dark period held constant across the developmental period (no main effect of age: F(4, 148)=1.228, p=0.302). Consequently, by the time of maturation the rats showed a unimodal activity rhythm that peaked during the early portion of the dark period.
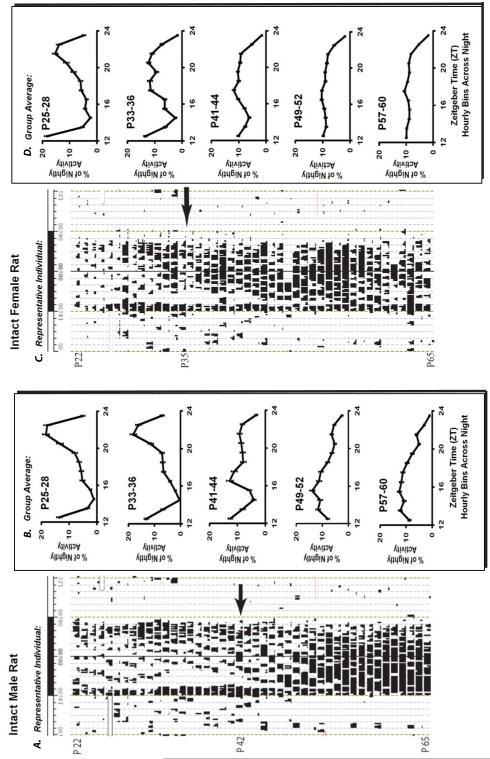
Figure 1. Intact rats show large changes in circadian phase and activity distribution during puberty in Experiment 1.
(A) Intact males have a bimodal activity pattern during pre-puberty that disappears by late puberty due to a phase advance of the later activity bout. Depicted is a sample actogram of wheel-running activity across pubertal development. The x-axis shows the clock time for one full day, with the light-dark bar illustrating the time of lights off and lights on. Each line on the y-axis represents one day’s worth of activity and has an upper threshold set at 200 wheel turns/10-min bin. The arrow indicates first day of prepucial opening, an androgen-dependent secondary-sex characteristic. (B) Average percent of nightly activity (+/− SE) for all intact male animals during pre puberty (P25-P28), pre/early puberty (P33-P36), early/mid puberty (P41-P44), mid/late puberty (P49-P52), and late/post-puberty (P57-P60) from zeitgeber time (ZT, which is defined as hours relative to lights on) 12 to 24 (the dark period). (C) Intact females show similar changes in activity distribution during puberty as males. In the sample actogram it is evident that intact females during puberty also begin to show a 4-day cycle of variation in activity levels due to the initiation of estrous cycles. The arrow indicates first day of vaginal opening, an estrogen-dependent secondary-sex characteristic. (D) Average percent of nightly activity (+/− SE) for all intact female animals at the same ages listed for males.
Sex Differences in Circadian Phase in Intact Animals
Similar to human adolescents, male rats demonstrated greater within-subjects change in circadian phase over development than females (Figure 2). This sex difference in the magnitude of age-related change reached significance for 2°onset (Age × Sex: F(3.392, 125.491)=3.021, p=0.027) and final activity offset (Age ° Sex: F(3.331, 123.243)=4.593, p=0.003). There was a between-subjects main effect of sex on 2°onset (F(1, 37)=4.835, p=0.034), with males showing an overall more delayed phase than females.
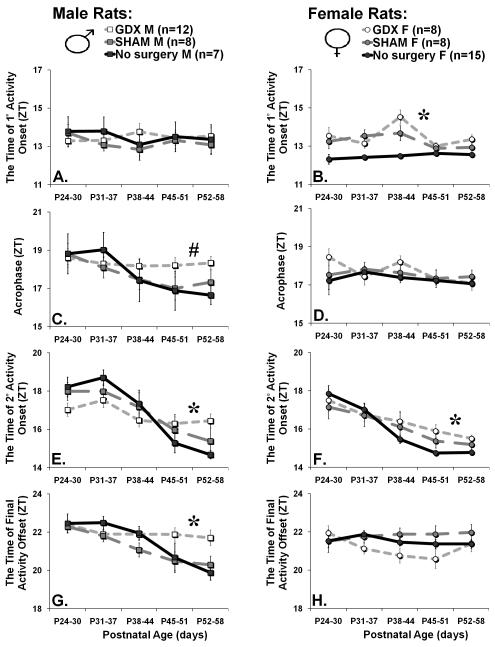
Figure 2. The effect of sex and pre-pubertal gonadectomy surgery on developmental changes in circadian phase parameters.
The left column of graphs illustrates age-related changes in circadian phase parameters in females, the right column of graphs illustrates males. The points on all graphs represent the mean for a six-day sample (+/− SE), with the age-range for the sample presented on the x-axis in postnatal-days. The line colors indicate gonadal status: Gonadectomized (GDX, light grey), SHAM gonadectomized (dark grey), or no-surgery (black). The y-axis for all graphs is given in zeitgeber time (ZT, lights off at ZT12). Asterisks indicates significant effects (p>0.05), whereas # indicates a non-significant trend (p<0.10). (A&B) The time of initial (1°) activity onset shows changes in SHAM and GDX females but not males or no-surgery females. (C&D) The onset of the second (2°) major activity bout phase advances across development in both males and females. These changes are larger in males, and smaller following gonadectomy surgery. (E&F) The acrophase phase advances across development in no-surgery and SHAM males but not in females or GDX males. (G&H) The time of final activity offset phase advances across development in no-surgery and SHAM males but not in females or GDX males.
There also appeared to be a sex difference in the developmental timing of circadian phase changes in intact animals that paralleled sex differences in the developmental timing of secondary-sex development. In males without surgery, the most delayed phase occurred between the ages of P31-P37, as indicated by 2°onset, acrophase, and final activity offset, whereas in females without surgery the most delayed phase occurred at ages P24-P30, as measured by 2°onset. Similarly, females showed 50% of their age-related change in 2°onset by P37, whereas males showed 50% of their age-related change in 2°onset by P41. Post-hoc tests supported these assertions, indicating that 2°onset and activity offset in males ages P24-P44 was phase delayed relative to maturity (P52-58), whereas in females 2°onset was phase delayed at ages P24-P37 relative to P38-58. However, within each sex, the relationship between circadian phase variables (2°onset, acrophase, and final activity offset in males, 2°onset in females) and developmental timing (first prepucial opening in male and first vaginal opening in females), was indistinguishable from the relationship between the circadian phase variables and postnatal age (Supplemental Figure 1), potentially due to lack of variation in developmental timing.
The Effect of Gonadectomy on Circadian Phase
The GDX animals exhibited a similar bimodal activity pattern as intact animals around the typical age of pre-puberty and early puberty. This bimodality lessened over time, but remnants still persisted into adulthood (Figure 3). GDX animals also demonstrated a phase advance in some of their circadian markers by late-to post- puberty, but again to a lesser extent than the intact animals (Figure 2). Thus, GDX surgery had a significant effect on within-subject change in 2°onset (F(5.860, 149.420)=4.451, p<0.001), final offset (F(6.926, 179.615)=2.682, p<0.001), and 1°onset (F(11.542, 201.157)=2.384, p=0.018). Overall, GDX animals exhibited a later 2°onset than intact animals, as indicated by a main effect of GDX between-subjects (F(1, 51)=5.619, p=0.022).
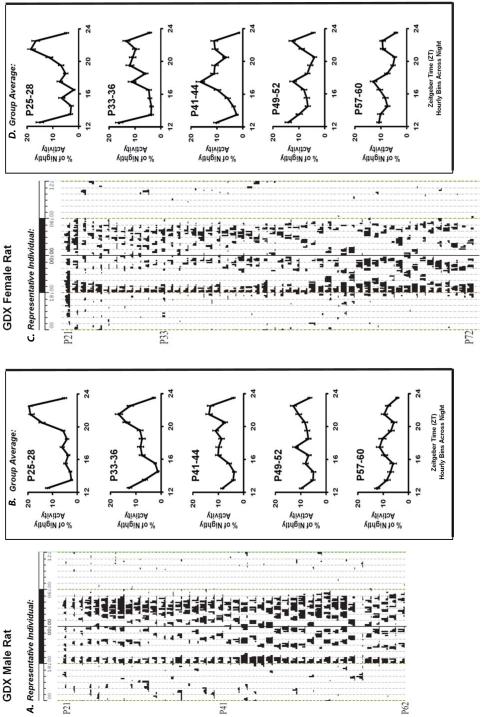
Figure 3. Rats gonadectomized (GDX) prior to puberty demonstrate an activity pattern that is bimodal during post-weaning and that remains dispersed into adulthood.
(A) In GDX males, the later activity bout diminishes and phase advances, but never fully consolidates with early evening activity. Following the conventions of Fig 1, depicted is an sample actogram and (B) the average percent of nightly activity (+/− SE) for all GDX males at multiple ages around the typical time of puberty. (C) GDX females exhibit similar activity patterns to GDX males, as shown in an sample actogram (upper threshold 150 turns/10 min) as well as in (D) the average percent of nightly activity (+/− SE) for all GDX females at multiple ages around the typical time of puberty.
The manner with which gonadectomy altered age-related change differed between the sexes: in males, gonadectomy consistently decreased the amount of age-related change in circadian phase parameters, whereas in females gonadectomy had variable effects that included: a) a decrease in age-related change (2°onset), b) little effect where change in intact animals was already minimal (final offset and acrophase), c) an increase in age-related change (1°onset). Therefore, several of the circadian parameters showed a significant interaction (or a trend towards an interaction) between the influence of sex and gonadectomy on within-subjects change (offset: F(6.926, 176.615)=3.086, p=0.004; acrophase: F(5.961, 145.132)=1.856, p=0.096; 1°onset: F(3.944, 201.157)=3.027, p=0.019) and offset showed a between-subjects interaction between the influences of sex and GDX (F(2, 51)=3.308, p=0.045).
Individual post-hoc comparisons indicated that there was a significant difference between GDX and no-surgery animals in within-subjects change in 1°onset, 2°onset, and final offset (p<0.05), as well as sexually diergic effects of gonadectomy on the acrophase, final offset (both p<0.05), and 2 onset (p=0.054*trend). However, SHAM and GDX groups only showed differences in within-subjects change in final activity offset, and this variable was influenced in a sex-specific manner (p<0.05, overall main effect of GDX: p=0.069*trend). As discussed above, when comparing no-surgery and SHAM animals there were no circadian phase parameters that were significantly affected by surgery.
Mean Activity
There were significant age-related changes in mean activity (F(1.848, 94.239)=64.708, p<0.001; Figure 4A&B). These age-related changes were affected by sex (Sex × Age: F(1.848, 94.239)=11.779, p<0.001), with females showing much greater increases in wheel running activity over development than males. Females generally had greater overall mean activity than males (F(1, 51)=8.891, p=0.004). These age-related changes were also affected by gonadectomy (GDX × Age: F(3.696, 94.239)=10.359, p<0.001). GDX animals maintained a relatively constant mean daily activity throughout development and had much lower overall mean activity levels (p<0.05). Individual post-hoc comparisons revealed that SHAM and no-surgery animals demonstrated a greater age-related increase in mean daily activity than GDX animals (post-hoc tests for GDX × Age: p<0.05). The manner with which gonadectomy affected age-related change depended on sex (GDX × Sex × Age: F(3.696, 94.239)=3.060, p=0.023), with females showing a much larger effect of GDX than males. Females also generally showed much larger effects of GDX on overall mean activity levels (between-subjects Sex × GDX: F(2,51)=3.537, p=0.036).
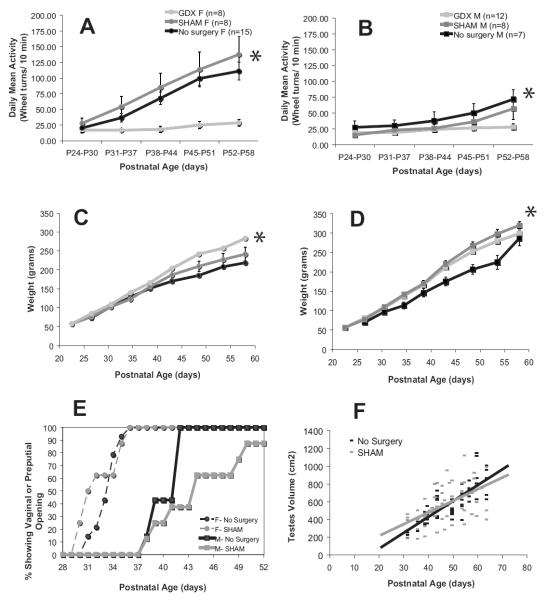
Figure 4. Indicators of health and maturation in Experiment 1.
Asterisks indicate significant group differences (p>0.05). The x-axis always shows postnatal age in days. The line colors indicate gonadal status as in Fig.4. (A&B) Daily mean activity increases across development for females (A) and males (B) as indicated by average daily wheel turns/10 min bin for a six-day sample (+/− SE). (C&D) Weight (g +/− SE) increases linearly over development for females (C) and males (D). (E) The timing of puberty as determined by the age of first prepucial opening for intact males (solid line) and by first vaginal opening for intact females (dashed line). The y-axis shows percent of total animals showing opening. (F) Testes volume (cm2) increases in a linear fashion across postnatal age in both no-surgery and SHAM males. Each point represents a measurement from a single animal.
Pubertal Development
The development of vaginal and prepucial openings around the time of mid-puberty provides us with a developmental marker for comparison with the circadian data. Similar to humans, female rats showed overt signs of puberty at an earlier age than males (Figure 4). The average day of first vaginal openings was P33.1 (+/−0.38 SE), with all females exhibiting vaginal openings by P36. The average day of first prepucial openings was P43.1 (+/− 1.42 SE), with all males exhibiting prepucial openings by P59. There was no significant difference in the timing of pubertal onset between SHAM and no-surgery animals, although SHAM males showed a trend towards developing prepucial openings later than no-surgery males (T(8.121)=−1.872, p=0.098). Testicular volume showed a linear increase across pubertal development (r2=0.487, p<0.001) in a manner that was similar in SHAM and no-surgery males (F(1, 126)=0.418, p=0.519 , Figure 4).
The timing of development was also monitored via weight gain (Figure 4). Measures of body weight for both males and females showed a linear within-subjects increase throughout development with little variability (F(2.173, 112.991)=1378.033, p<0.001). There was a significant effect of gonadectomy on within-subjects change in weight (F(4.346, 112.991)=5.683, p<0.001), with no-surgery animals showing a slower weight gain than SHAM or GDX animals. This effect of gonadectomy on weight gain differed by sex (F(4.346, 112.991)=5.590, p<0.001), with males showing more effect of surgery than females.
Experiment 2: A Comparison of Pubertal Changes in Circadian Rhythms Under Entrained and Constant Conditions
Circadian parameters under entrained conditions
The rats’ entrained activity rhythms showed a similar pattern of developmental change across puberty as those observed during Experiment 1 (Supplemental Figure 2). Thus, we found that the daily acrophase phase-advanced by approximately 3 hrs between ages P24-P70 (within-subjects change: F(1.735, 24.294)=33.890, p<0.001). These changes in circadian phase were accompanied by a relative decrease in daytime activity, as reflected by within-subjects change in the light/dark (LD) activity ratio during ages P24-P70 (F(1.599, 22.385)=6.693, p=0.008). Neither of these age-related changes differed by sex (p>0.26), nor was there an overall sex difference for either variable (p>0.10).
Circadian parameters under constant conditions
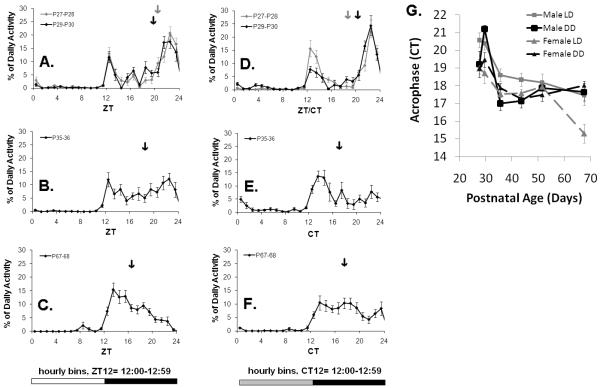
Immediately upon placement in constant conditions, the initial activity bout diminished slightly leading to a delay in acrophase (Figure 5&6). These changes did not occur when the animals were left in an LD cycle. This resulted in a significant main effect of lighting condition on within-subjects change in the acrophase relative to the onset of the active period (CT12, as defined by the beginning of the 12 hour period during the day which contained the highest mean activity, F(1, 28)=7.431, p=0.011). Then, under both entrained and constant conditions, the later (2°) activity bout phase advanced and consolidated into a unimodal rhythm, such that the acrophase grew closer to the onset of the active period (Figure 5&6; F(4, 112)=36.351, p<0.001). This phase advance occurred faster under constant conditions, leading to an overall effect of lighting condition (DD vs. LD) on within-subjects change in the acrophase relative to the onset of the active period (Age × Condition: F(4, 112)= 7.630, p<0.001). This phase advance was also smaller in females (Age × Sex: F(4, 112)=3.372, p=0.017), in a manner that depended on lighting condition (Age × Sex × Condition: F(4, 112)=2.672, p=0.036). Females in general showed an earlier acrophase relative to the onset of the active period than males (main effect of Sex: F(1, 28)=9.835, p=0.004) and this sex difference was exaggerated under entrained conditions (between-subjects effect of Sex × Condition: F(1, 28)=5.879, p=0.022).
Figure 5. Sex differences in free-running period develop during puberty under constant conditions.
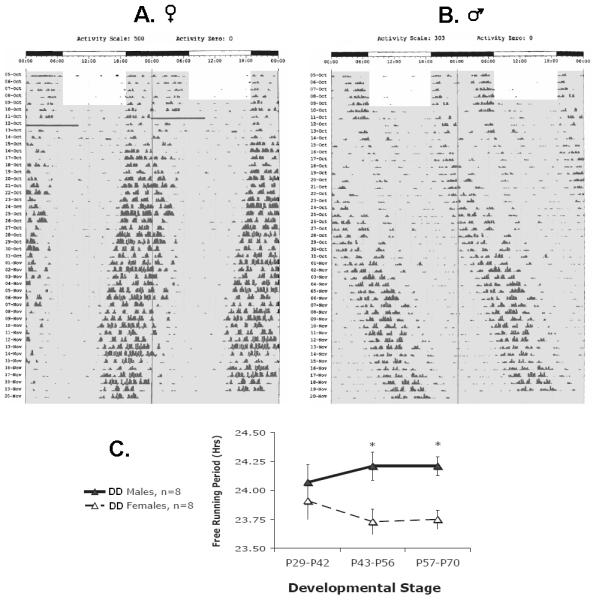
Actograms follow the same conventions as Fig 1, except the x-axis shows clock time for two full days (double-plotted) A. A representative actogram for a female rat under entrained conditions (ages P24-28) followed by placement in constant conditions (DD, ages P29-P70). The transition into DD is denoted by a grey background. The bimodal activity pattern is still evident following the transition into DD and diminishes with age. The small daily leftward shift in the onset of activity indicates that the rat’s free-running period (τ) is shorter than 24 hrs. The upper threshold for the y-axis each day is 500 turns/10 min. The solid dark bar on Day 7 was caused by an equipment failure. B. A representative actogram for a male rat under entrained (ages P24-28) and constant conditions (DD, ages P29-P70). The small daily rightward shift in the onset of activity indicates that the rat’s τ is longer than 24 hrs. The upper threshold for the y-axis each day is 303 turns/10 min. C. Average free-running period (+/−SE) changes in a sex-specific manner across pubertal development (males = filled triangles, females = open triangles, p=0.035). Asterisks indicate specific ages at which this sex difference reaches statistical significance (p<0.05).
Figure 6. Pubertal changes in the distribution of activity across the subjective night persists under constant lighting conditions.
A-C. The daily distribution of activity for male rats (n=8) kept under entrained conditions (LD) at four ages. Activity is plotted relative to zeitgeber time (ZT, ZT0=lights on). The light cycle is illustrated by the light-dark bar at the bottom of the column of graphs. Each point represents the average activity per hour (+/− SE) from a two-day sample (i.e. the point at ZT12= the average activity for 12:00-12.59). The lines illustrate four representative ages: A. pre-pubertal: P27-P28, P29-P30, B. early pubertal: P35-36, C. late/post-pubertal: P67-68. ZT0/24 are double-plotted. Arrows indicate the acrophase as measured via cosinor analysis. D-F. The daily distribution of activity for male rats (n=8) placed into constant conditions (DD) at age P29. Activity is plotted relative to circadian time (CT, CT12-24=subjective night) so that the onset of the active period is aligned with its previous timing under LD conditions. In the first graph (D) the grey line illustrates the activity of pre-pubertal animals under LD conditions (P27-P28), and the black line shows activity under DD (P29-30). All other graphs illustrate activity distribution under DD. Note that a bimodal activity distribution is present during pre-puberty and when the rats are placed in DD activity shifts from the initial (1°) activity bout to the later (2°) activity bout (p=0.011), demonstrating the influence of photic masking. Then activity phase-advances relative to the onset of the active period by P35-36 (F) and P67-68 (G). G. The acrophase phase-advances relative to the onset of the active period (CT12) under LD and DD conditions (p<0.001). This advance occurs faster under DD (p<0.001) and shows a sex difference (p=0.017). Each point represents the average of a two day sample (+/−SE). The first time point occurs under LD conditions for all groups.
Under constant conditions, free running-period (τ) changed over development in a manner that depended on sex (Figure 5). We also measured τ under entrained conditions to make sure that age-related phase changes were not biasing periodogram analyses, and τ was shown to hold steady around 24 hrs. Therefore, within-subjects change in τ was affected by an interaction between sex and lighting condition (F(2, 56)=3.549, p=0.035). Around the age of mid-puberty (P29-P42) both males and females had a τ that was close to 24 hrs in duration (24.07 +/− 0.15 SE and 23.91+/− 0.16 SE, respectively). Post-hoc comparisons indicated that τ at this age did not differ in either sex from τ under entrained conditions (Sex × Condition: p>0.05). As puberty progressed, females developed shorter τ’s, eventually plateauing at 23.75 hrs (+/− 0.08 SE) and males developed longer τ’s, eventually plateauing at 24.21 hrs (+/− 0.08 SE). Post-hoc comparisons indicated that a sex difference developed under constant conditions by ages P43-P56, and continued into ages P57-P70 (Sex × Condition: p<0.05). Overall, females had a shorter τ than males, as indicated by a main effect of sex (F(1, 28)=5.101, p=0.032; Sex × Condition: F(1, 28)=3.989, p=0.056*trend).
Activity level
Under both entrained and constant conditions activity levels increased over development (Supplemental Figure 2; within-subjects change: F(2.212, 61.946)=71.928, p<0.001). This within-subjects change varied by sex (F(2.212, 61.946)=9.940, p<0.001), with females showing greater age-related increases in mean activity than males. Overall, females were more active than males, as indicated by a main effect of sex (F(1, 28)=9.360, p=0.005).
Discussion
These data demonstrate that circadian activity continues to develop during post-weaning and pubertal development in the fast-developing nocturnal rat. Under both entrained and free-running conditions we observed a bimodal distribution of activity during early post-weaning, with the majority of activity occurring near the end of the active phase. As male rats progressed through puberty, this later bout of activity phase-advanced 4 hrs until it consolidated into a strong, unimodal rhythm that peaked near the beginning of the animal’s active phase. These pubertal changes in circadian phase were smaller in females, with female rats showing (at most) a 3.5 hr magnitude phase advance during puberty.
These results complement previous developmental sleep (n= 1 rat per age group, Ibuka, 1984) and activity data (Norton, 1975; Thiels et al.,1990; Joutsiniemi et al., 1991) in post-weaning rats. Those studies similarly implied that rats develop more consolidated activity and nocturnality across the post-weaning period (Ibuka, 1984; Norton, 1975; Thiels et al.,1990), although one low-resolution study suggested that rats were fully nocturnal even during the fourth week of life (P21-27, Joutsiniemi et al., 1991). Our previous developmental research on wheel-running rhythms in rats also suggested a more bimodal activity pattern in intact male pubertal rats (P32-46) which consolidated by adulthood, although this change was not explicitly quantified. Similar to our current study, this consolidation of activity was accompanied by an overall 3hr phase advance in the acrophase (McGinnis et al., 2007). It is possible that these age-related changes could be simply due to duration of running wheel exposure. However, other data from our laboratory show that rats raised in a similar manner and placed into running wheels for the first time after puberty exhibit unimodal, consolidated rhythms (Supplemental Figure 3). Taken collectively, this evidence strongly indicates that the processes governing the daily sleep and activity cycles of rats mature during the pubertal period.
These changes in circadian phase during puberty are dependent on gonadal hormones. We found that the reorganization of activity rhythms during puberty in the rat exhibited sex differences in timing and magnitude in a manner that strongly indicated a role for gonadal hormones in circadian development. Furthermore, GDX animals maintained a more dispersed activity distribution and delayed phase into adulthood that was reminiscent of the activity patterns of pre-pubertal intact animals.
The activity patterns of the GDX animals resembled those of rodents gonadectomized during adulthood in previous studies. Castrated male adults under entrained and free-running conditions had activity that was more dispersed across the active period (Morin et al., 1981), such that early night activity was less cohesive, diminished, lost, or delayed (Morin et al., 1981; Karatsoreos et al., 2007; Iwanahana et al. 2008, Daan et al. 1975; Davis et al. 1983). The administration of testosterone or di-hydrotestosterone was able to restore the original circadian activity patterns (Karatsoreos et al. 2007; Iwanahana et al. 2008, Daan et al. 1975; Morin et al. 1981). Thus, our observation that as male rats mature they develop increasingly unimodal rhythms that peak at the beginning of the active period may reflect the activational effects of increasing testosterone during puberty.
Female rats showed a similar, although smaller magnitude, circadian reorganization during puberty. It is already well known that female rodents demonstrate both a phase advance and increase in their wheel running activity on days of the estrous cycle when estrogen is relatively high (a cyclic pattern described as “scalloping” by Morin et al. 1977). This estrus-related activity disappears following ovariectomy (Morin et al. 1977, Davis, Darrow, and Menaker 1983; Labyak and Lee 1997; Iwanahana et al. 2008). The more dispersed activity distribution and delayed phase exhibited by our GDX and pre-pubertal intact females resembled that of ovariectomized adult female rats in previous studies (Wollnik and Döhler 1986; Thomas and Armstrong 1989). In those studies, estrogen treatment was able to reconsolidate rhythms in ovariectomized rats (Wollnik and Döhler 1986; Thomas and Armstrong 1989), hence it seems likely that the consolidation and phase-advance of activity rhythms that develops during puberty in female rats may be due to the activational effects of increasing estrogen.
Interestingly, GDX rats of both sexes still continued to show some circadian change at the typical age of puberty. This raised the possibility that a similar magnitude of change might still be occurring in GDX rats, but with a delayed developmental time course, perhaps due to the stress of surgery. To address this question, we examined the onset of the later (2°) activity bout in intact and GDX animals that we recorded for an additional ten days (up to P68). We found that in GDX animals the developmental changes in 2° activity bout plateaued by P49, and the circadian phase from P49-P68 was reliably more delayed than that of intact animals (Supplemental Figure 4). This indicated that the developmental changes in GDX animals were indeed smaller in magnitude than those found in intact animals, and that overall phase differences evident between the two groups at P49 were long-lasting.
The presence of some circadian change in GDX rats around the typical age of puberty contrasts with what we found in the slow-developing, precocial degu. In the degu, GDX males did not show any developmental changes in circadian organization or phase that resembled those of intact males (Hagenauer, Ku, and Lee, in revision). One possibility is that the hormone-independent circadian changes that we observe in the fast-developing rat are due to the gradual transition from maternal to photic entrainment mechanisms that accompanies weaning in altricial species (for review see Weinert, 2005). When young rats are allowed to remain with the dam they will continue to show nursing behavior until ages P28-P40 (Calhoun 1962; Cramer, Thiels, and Alberts 1990), which overlaps with the beginning of puberty. The dam nurses primarily during the day, and this nursing pattern is the primary influence on the phasing of activity rhythms in the pups (Shimoda et al. 1985; Sugishita et al. 1991; Thiels, Alberts, and Cramer 1990). The pups will exhibit a diurnal activity pattern until around age P18 when they begin to consume solid food (Bolles and Wood 1964; Thiels, Alberts, and Cramer 1990). Even after this point, the dam continues to serve as an effective zeitgeber until the 4th or 5th week of life (approximately P28-P35; Levin and Stern 1975; Takahashi, Hayafuji, and Murakami 1982). Thus, the earliest part of our sample (P21-P30) overlapped with the typical time of transition from maternal to photic entrainment, as well as from dependent, diurnal activity to independent, exploratory nocturnal activity. These changes would occur regardless of the hormonal condition of the animal. Similarly, the high metabolic demands on young, fast-growing animals would be likely to promote ultradian rhythms of sleep and activity that would diminish with age regardless of hormonal environment (Alfoldi et al. 1975).
Animals that received SHAM surgery showed significant pubertal circadian changes, but these changes were dampened compared to those of no-surgery animals. Males also showed a trend towards delayed secondary-sex development. It seems unlikely that these differences between SHAM and no-surgery rats were directly due to continued stress from the surgery, as both groups showed healthy weight gain throughout the recording period and almost identical activity levels. Perhaps instead there was a secondary effect of surgery on maternal care (Barnett & Burn 1967), or a sexually-diergic long-term effect of isoflurane anesthesia (Siegal and Dow-Edwards, 2009; McCann et al. 2009).
It is unclear what aspect of the circadian mechanism is affected by pubertal hormones to produce changes in circadian phase. Originally, we hypothesized that hormones altered the entrainment of the circadian system to the LD cycle to produce pubertal changes in daily activity rhythm phase and distribution. This altered entrainment could be produced by changes in the photic sensitivity of the circadian pacemaker or via an elongation of the endogenous free-running period (τ). In support of this hypothesis, we observed developmental changes in τ under constant conditions. However, these changes in τ seem unlikely to explain pubertal changes in the phasing and distribution of activity under entrained conditions for several reasons. First, both males and females showed similar changes in the phase of rhythms during puberty despite showing divergent developmental changes in τ. Second, the changes that we observed in τ during puberty in males in this study are actually the opposite of what we found in our earlier study (McGinnis et al. 2007). One possible explanation for this discrepancy is the differing duration of time the rats were placed in constant conditions. In an earlier study in pubertal degus, younger animals were found to maintain a free-running period close to 24 hrs for almost 2 weeks after placement in DD. These pronounced aftereffects disappeared as the animals matured (Hummer et al. 2007). Thus, it may be that the τ of 24 hrs that we observed in male and female rats at the earliest samples in both studies also reflects aftereffects of the photoperiod.
Our results also strongly resemble previous studies showing that gonadal hormones are capable of restoring vigorous activity to the beginning of the subjective night in GDX animals under constant conditions (e.g. Daan et al. 1975; Iwahana et al. 2008; Karatsoreos et al. 2007). Our pre-pubertal rats continued to exhibit a strongly bimodal activity rhythm with most activity at the end of their active period even after they entered constant conditions. Placement in constant conditions actually produced a small immediate decrease in the proportion of activity occurring during the activity bout at the beginning of the subjective night, suggesting that the LD cycle was masking a more delayed phase of pre-pubertal activity. This activity then consolidated into a unimodal rhythm that peaked near the beginning of the subjective night in a manner that resembled circadian development under entrained conditions. Similar consolidation of activity rhythms during post-weaning under constant conditions was previously observed in rats born and raised in conditions of constant light (Diez-Noguera and Cambras, 1990).
Thus, despite evidence that pubertal hormones might affect the τ and photic sensitivity of the circadian pacemaker (Carskadon et al. 2004; Hummer et al., 2007; Weinert et al., 1994; Weinert and Kompauerova, 1998), our data suggest that pubertal hormones in this species affect the phasing and distribution of activity rhythms in a manner that is independent from τ and photic entrainment, since a similar reorganization of the active period occurred while the animals were free-runnning under constant conditions in which there was no photic zeitgeber. Likewise, our data strongly indicate that changes in activity rhythms during the pubertal period are not due to the passive masking of rhythms by direct behavioral responses to the LD cycle; instead, masking may dampen the expression of these changes.
Our results add to growing evidence that circadian development during puberty is common across the mammalian kingdom (Hagenauer et al. 2009). The structure of these developmental changes differs by species. Diurnal primates (humans and rhesus macaques) show a relatively advanced circadian phase during pre-puberty, phase-delay during puberty, and then phase advance again during adulthood (Golub et al. 2002; Roenneberg et al., 2004; Thorleifsdottir et al., 2002). An earlier report also observed a more advanced activity rhythm phase during pre-puberty in degus (Hummer et al., 2007), as did preliminary data in degus and rats (Hagenauer et al., 2009). However, once the initial days (for the rat) or week (for the degu) of recording were removed from the analysis, this advanced phase during pre-puberty statistically disappeared (Hagenauer 2010). Thus, our current data suggest that a relatively advanced phase of activity during pre-puberty may instead be characteristic of species that develop slowly and progress through a pre-pubertal gonadal quiescent period, such as humans and macaques (Plant, 1994).
Supplementary Material
Supplemental Figure 1. Developmental changes in circadian phase parameters are equally accounted for by pubertal timing and postnatal age. Activity rhythm phase parameters for all intact rats (SHAM and No Surgery) were averaged bi-daily and then aligned based on the age of 1st vaginal opening (for females) or the age of 1st prepucial opening (for males). One intact male was excluded from the data set due to irregular prepucial opening. All females had data from 7 days prior to vaginal opening to 26 days after, whereas all males had data from 12 days prior to prepucial opening to 14 days after. This bi-daily data was then averaged by sex (+/−SE) and compared to bi-daily data similarly averaged by sex (+/−SE) from −7 to +26 days relative to the median postnatal age of 1st vaginal opening (P26-P59) and −12 to +14 days relative to the median postnatal age of 1st prepucial opening (P30-P57). Graphically, developmental changes in circadian phase in relationship to pubertal timing and postnatal age appeared identical: A. The time of secondary activity onset in males, B. Acrophase in males, C. The time of final activity offset in males, D. The time of secondary activity onset in females. E. The average amount of standard error associated with both model fits for all phase variables did not significantly differ (p>0.90).
Supplemental Figure 2. Developmental changes in circadian parameters in entrained and constant conditions. Error bars represent +/−SE. A. The acrophase phase-advances across pubertal development (P24-P70) under entrained conditions (12:12 LD, p<0.001). This change occurs similarly in both sexes (males = filled squares, females = open squares). The acrophase is represented on the y-axis in zeitgeber time (hrs, lights-off=ZT12). B. Nocturnality intensifies across pubertal development (p=0.008) as indicated by light-dark activity ratio. A lower score indicates greater nocturnality. Differences between the sexes did not reach significance. C. Daily mean activity increases across pubertal development under both entrained (squares) and constant (triangles) conditions (p<0.001). Overall activity levels differed by sex (p<0.001; the asterisk indicates the specific age at which the difference reached significance (p<0.05).
Supplemental Figure 3. Intact rats exhibit unimodal activity rhythms that peak at the beginning of the night immediately after placement in a wheel during late puberty. Male (n=11) and female (n=10) rats were raised in-house using an identical procedure to Experiment 1. They served as a control for another experiment, and thus received saline injections bi-daily for 5 days/week for 3 weeks during puberty, but they were not exposed to running wheels during this time. Immediately upon placement in a running wheel, A. males (average age: P60-63) and B. females (average age: P59-62) showed a unimodal rhythm that peaked near the beginning of the night. This general pattern held steady after another 10 days in the wheel. (Error bars = +/− SE)
Supplemental Figure 4. When considering additional data collected at later ages, gonadectomized animals (GDX: open circles, dashed lines) continue to lack typical agerelated changes in the timing of the onset of the second (2°) major activity bout. Any males and females that had data extending out to P68 were averaged to produce this figure. The intact group (closed circles, black line) includes both no-surgery and SHAM rats. Graphs depict mean 2°onset (+/− SE) for every two days across ages P22-P68 in terms of zeitgeber time (ZT, lights off at ZT12).
Acknowledgements
We would like to thank Dr. Megan Mahoney, Blair Sutton, Ana Kantarowski, Jennifer HeeYoung Ku, Jessica Koch, David Altshuler, Shuoqi Scott Wang, Dr. Bob Thompson, Dr. Jill Becker, Dr. Jimo Borjigin, and Dr. Daniel Forger for their technical support and advice. We would also acknowledge Kathy Gimson, Julie Stewlow, and Jim Donner for their excellent animal care. This research was supported by a laboratory grant from the National Science Foundation (TML, MHH - IBN-0212322) and a training grant awarded to the University of Michigan Reproductive Science Program from the National Heart, Lung, and Blood Institute (MHH - T32 HD07048).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241(1):R 62–66. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- 2.Axelson JF, Gerall AA, Albers HE. Effect of progesterone on the estrous activity cycle of the rat. Physiol Behav. 1981;26(4):631–635. doi: 10.1016/0031-9384(81)90137-2. [DOI] [PubMed] [Google Scholar]
- 3.Bolles RC, Woods PJ. The ontogeny of behavior in the albino rat. Anim Behav. 1964;12(4):427–441. [Google Scholar]
- 4.Cambras T, Diez-Noguera A. Changes in motor-activity during the development of the circadian rhythm in the rat. J Interdisciplinary Cyc Res. 1988;19(1):65–74. [Google Scholar]
- 5.Castro ECV, Andrade MMM. Longitudinal study of the spectral composition of behavioral rhythms in the rat. Biol Rhythm Res. 2005;36(1-2):131–140. [Google Scholar]
- 6.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 7.Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An Approach to Studying Circadian Rhythms in Adolescents. J Biol Rhythms. 1997;12:278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- 8.Carskadon MA, Labyak S,E, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci Lett. 1999;260:129–132. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- 9.Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: Implications for behavior. Ann NY Acad Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- 10.Cramer CP, Thiels E, Alberts JR. Weaning in Rats: Maternal Behavior. Develop Psychobio. 1990;23(6):479–493. doi: 10.1002/dev.420230604. [DOI] [PubMed] [Google Scholar]
- 11.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on the circadian pacemaker in mice. Proc Natl Acad Sci USA. 1975;72:3744–3747. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol. 1983;244(1):R93–105. doi: 10.1152/ajpregu.1983.244.1.R93. [DOI] [PubMed] [Google Scholar]
- 14.Diez-Noguera A, Cambras T. Sex differences in the development of the motor activity circadian rhythm in rats under constant light. Physiol Behav. 1990;47:889–894. doi: 10.1016/0031-9384(90)90014-u. [DOI] [PubMed] [Google Scholar]
- 15.Golub MS, Takeuchi PT, Hoban-Higgins TM. Nutrition and circadian activity offset in adolescent rhesus monkeys. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 50–68. [Google Scholar]
- 16.Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31(4):276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23(2):693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hummer DL, Jechura TJ, Mahoney MM, Lee TM. Gonadal hormone effects on entrained and free-running rhythms in the developing diurnal rodent, Octodon degus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R586–597. doi: 10.1152/ajpregu.00043.2006. [DOI] [PubMed] [Google Scholar]
- 19.Ibuka N. Ontogenesis of circadian sleep-wakefulness rhythms and developmental changes of sleep in the altricial rat and in the precocial guinea pig. Behav Brain Res. 1984;11:185–196. doi: 10.1016/0166-4328(84)90210-9. [DOI] [PubMed] [Google Scholar]
- 20.Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav. 2008;53(3):422–430. doi: 10.1016/j.yhbeh.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jechura TJ, Walsh JM, Lee TM. Testicular hormones modulate circadian rhythms of the diurnal rodent, Octodon degus. Horm Behav. 2000;38(4):243–249. doi: 10.1006/hbeh.2000.1624. [DOI] [PubMed] [Google Scholar]
- 22.Joutsiniemi SL, Leinonen L, Laakso ML. Continuous recording of locomotor activity in groups of rats: Postweaning maturation. Physiol Behav. 1991;50:649–654. doi: 10.1016/0031-9384(91)90562-3. [DOI] [PubMed] [Google Scholar]
- 23.Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148(11):5487–5495. doi: 10.1210/en.2007-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kent S, Hurd M, Satinoff E. Interactions between body temperature and wheel-running over the estrous cycle in the rat. Physiol Behav. 1991;49:1079–1084. doi: 10.1016/0031-9384(91)90334-k. [DOI] [PubMed] [Google Scholar]
- 25.Kittrell EMW, Satinoff E. Development of the circadian rhythm of body temperature in rats. Physiol Behav. 1986;38:99–104. doi: 10.1016/0031-9384(86)90138-1. [DOI] [PubMed] [Google Scholar]
- 26.Labyak SE, Lee TM. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol Behav. 1995;58(3):573–585. doi: 10.1016/0031-9384(95)00096-2. [DOI] [PubMed] [Google Scholar]
- 27.Levin R, Stern JM. Maternal influences on ontogeny of suckling and feeding rhythms in the rat. J Comp Physiol Psychol. 1975;89(7):711–721. doi: 10.1037/h0077038. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Satinoff E. Body temperature and sleep in intact and ovariectomized female rats. Am J Physiol: Reg, Integ, and Comp Physiol. 1996;271:1753–1758. doi: 10.1152/ajpregu.1996.271.6.R1753. [DOI] [PubMed] [Google Scholar]
- 29.Manber R, Bootzin RR. Sleep and the menstrual cycle. Health Psychol. 1997;16(3):209–214. doi: 10.1037//0278-6133.16.3.209. [DOI] [PubMed] [Google Scholar]
- 30.McCann ME, Bellinger DC, Davidson AJ, Soriano SG. Clinical research approaches to studying pediatric anesthetic neurotoxicity. Neurotoxicol. 2009;30:766–771. doi: 10.1016/j.neuro.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 31.McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol Behav. 2007;92:1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore-Ede MC, Sulzman FM, Fuller CA. Clocks That Time Us; Characteristics of Circadian Clocks. Harvard University Press; 1982. pp. 30–112. [Google Scholar]
- 33.Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196(4287):305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- 34.Neuman A, Gothilf Y, Haim A, Ben-Aharon G, Zisapel N. Nocturnal patterns and up-regulated excretion of the melatonin metabolite 6-sulfatoxymelatonin in the diurnal rodent Psammomys obesus post-weaning under a short photoperiod. Comp Biochem Physiol (A) 2005;142:297–307. doi: 10.1016/j.cbpa.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Norton S, Culver B, Mullenix P. Development of nocturnal behavior in albino rats. Behav Biol. 1975;15:317–331. doi: 10.1016/s0091-6773(75)91717-4. [DOI] [PubMed] [Google Scholar]
- 36.Ojeda SR, Urbanski HF. Puberty in the Rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven; 1994. pp. 363–409. [Google Scholar]
- 37.Parry BL, et al. Neuroendocrine effects of light therapy in late luteal phase dysphoric disorder. Biol Psychiatry. 1994;36(6):356–364. doi: 10.1016/0006-3223(94)91210-6. [DOI] [PubMed] [Google Scholar]
- 38.Parry BL, et al. Cortisol circadian rhythms during the menstrual cycle and with sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Biol Psychiatry. 2000;48(9):920–31. doi: 10.1016/s0006-3223(00)00876-3. [DOI] [PubMed] [Google Scholar]
- 39.De Tezanos Pinto FT, Golombek DA. Neuroactive steroids alter the circadian system of the Syrian hamster in a phase-dependent manner. Life Science. 1999;65(23):2497–2504. doi: 10.1016/s0024-3205(99)00516-0. [DOI] [PubMed] [Google Scholar]
- 40.Plant TM. Puberty in Primates. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven; 1994. pp. 453–485. [Google Scholar]
- 41.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 42.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr Biol. 2004;14(24):R1038–1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 43.Sadeh A, Dahl RE, Shahar G, et al. Sleep and the transition to adolescence: a longitudinal study. Sleep. 2009;32(12):1602–1609. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimoda K, Hanada K, Yamada N, Takahashi K, Takahashi S. Periodic exposure to mother is potent zeitgeber of rat pups’ rhythm. Physiol Behav. 1985;36:723–730. doi: 10.1016/0031-9384(86)90360-4. [DOI] [PubMed] [Google Scholar]
- 45.Siegal N, Dow-Edwards D. Isoflurane anesthesia interferes with the expression of cocaine-induced sensitization in female rats. Neurosci Letters. 2009;464:52–56. doi: 10.1016/j.neulet.2009.07.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugishita M, Takashima M, Takeuchi Y, Kato Y, Yamauchi T, Takahashi K. Periodic mother deprivation during the light period reversed the phase of serotonin N-acetyltransferase activity rhythm of the pineal gland in rat pups. Pharm, Biochem Behav. 1993;46:609–615. doi: 10.1016/0091-3057(93)90551-4. [DOI] [PubMed] [Google Scholar]
- 47.Tate BA, Richardson GS, Carskadon MA. Maturational changes in sleep-wake timing: longitudinal studies of circadian activity rhythm of a diurnal rodent. In: Carkadon MA, editor. Adolescent Sleep Patterns: Biological, Social and Psychological Influences. pp. 40–49. [Google Scholar]
- 48.Takahashi K, Hayafuji C, Murakami N. Foster mother rat entrains circadian adrenocortical rhythm in blinded pups. Am J Physiol Endocrinol Metab. 1982;243(6):E443–E449. doi: 10.1152/ajpendo.1982.243.6.E443. [DOI] [PubMed] [Google Scholar]
- 49.Thiels E, Alberts JR, Cramer CP. Weaning in rats: II pup behavior patterns. Develop Psychobiol. 1990;23(6):495–510. doi: 10.1002/dev.420230605. [DOI] [PubMed] [Google Scholar]
- 50.Thomas EMV, Armstrong SM. Effect of ovariectomy and estradiol on unity of female circadian rhythms. Am J Physiol. 1989;257(5):R1241–1250. doi: 10.1152/ajpregu.1989.257.5.R1241. [DOI] [PubMed] [Google Scholar]
- 51.Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason TH, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year Period. J Psychosom Res. 2002;53:529–537. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 52.Weinert D, Eimert H, Erkert HG, Schneyer U. Resynchronization of the circadian corticosterone rhythm after a light/dark shift in juvenile and adult mice. Chronobiol Int. 1994;11(4):222–231. doi: 10.3109/07420529409067791. [DOI] [PubMed] [Google Scholar]
- 53.Weinert D, Kompauerova V. Light induced phase and period responses of circadian activity rhythms in laboratory mice of different age. Zoology. 1998;101:45–52. [Google Scholar]
- 54.Weinert D, Waterhouse J. Daily activity and temperature rhythms do not change spontaneously with age in laboratory mice. Physiol Behav. 1999;66(4):605–612. doi: 10.1016/s0031-9384(98)00342-4. [DOI] [PubMed] [Google Scholar]
- 55.Weinert D. Ontogenetic development of the mammalian circadian system. Chronobiol Int. 2005;22(2):179–205. doi: 10.1081/cbi-200053473. [DOI] [PubMed] [Google Scholar]
- 56.Wollnik K, Döhler D. Effects of adult or perinatal hormonal environment on ultradian rhythms in locomotor activity of laboratory LEW/Ztm rats. Physiol. Behav. 1986;38:229–240. doi: 10.1016/0031-9384(86)90158-7. [DOI] [PubMed] [Google Scholar]
- 57.Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115(1 Suppl):250–260. doi: 10.1542/peds.2004-0815G. [DOI] [PubMed] [Google Scholar]
- 58.Zucker I, Fitzgerald KM, Morin LP. Sex differentiation of t-e circadian system in the golden hamster. A J Physiol. Reg, Integ, Comp Physiol. 1980;238:97–101. doi: 10.1152/ajpregu.1980.238.1.R97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Developmental changes in circadian phase parameters are equally accounted for by pubertal timing and postnatal age. Activity rhythm phase parameters for all intact rats (SHAM and No Surgery) were averaged bi-daily and then aligned based on the age of 1st vaginal opening (for females) or the age of 1st prepucial opening (for males). One intact male was excluded from the data set due to irregular prepucial opening. All females had data from 7 days prior to vaginal opening to 26 days after, whereas all males had data from 12 days prior to prepucial opening to 14 days after. This bi-daily data was then averaged by sex (+/−SE) and compared to bi-daily data similarly averaged by sex (+/−SE) from −7 to +26 days relative to the median postnatal age of 1st vaginal opening (P26-P59) and −12 to +14 days relative to the median postnatal age of 1st prepucial opening (P30-P57). Graphically, developmental changes in circadian phase in relationship to pubertal timing and postnatal age appeared identical: A. The time of secondary activity onset in males, B. Acrophase in males, C. The time of final activity offset in males, D. The time of secondary activity onset in females. E. The average amount of standard error associated with both model fits for all phase variables did not significantly differ (p>0.90).
Supplemental Figure 2. Developmental changes in circadian parameters in entrained and constant conditions. Error bars represent +/−SE. A. The acrophase phase-advances across pubertal development (P24-P70) under entrained conditions (12:12 LD, p<0.001). This change occurs similarly in both sexes (males = filled squares, females = open squares). The acrophase is represented on the y-axis in zeitgeber time (hrs, lights-off=ZT12). B. Nocturnality intensifies across pubertal development (p=0.008) as indicated by light-dark activity ratio. A lower score indicates greater nocturnality. Differences between the sexes did not reach significance. C. Daily mean activity increases across pubertal development under both entrained (squares) and constant (triangles) conditions (p<0.001). Overall activity levels differed by sex (p<0.001; the asterisk indicates the specific age at which the difference reached significance (p<0.05).
Supplemental Figure 3. Intact rats exhibit unimodal activity rhythms that peak at the beginning of the night immediately after placement in a wheel during late puberty. Male (n=11) and female (n=10) rats were raised in-house using an identical procedure to Experiment 1. They served as a control for another experiment, and thus received saline injections bi-daily for 5 days/week for 3 weeks during puberty, but they were not exposed to running wheels during this time. Immediately upon placement in a running wheel, A. males (average age: P60-63) and B. females (average age: P59-62) showed a unimodal rhythm that peaked near the beginning of the night. This general pattern held steady after another 10 days in the wheel. (Error bars = +/− SE)
Supplemental Figure 4. When considering additional data collected at later ages, gonadectomized animals (GDX: open circles, dashed lines) continue to lack typical agerelated changes in the timing of the onset of the second (2°) major activity bout. Any males and females that had data extending out to P68 were averaged to produce this figure. The intact group (closed circles, black line) includes both no-surgery and SHAM rats. Graphs depict mean 2°onset (+/− SE) for every two days across ages P22-P68 in terms of zeitgeber time (ZT, lights off at ZT12).