Abstract
Alzheimer’s disease (AD) is associated with neuronal degeneration, synaptic loss, and deficits in multiple neurotransmitter systems. Alterations in the serotonin 1A (5-HT1A) receptor can contribute to impaired cognitive function in AD, and both in vitro binding and PET imaging studies have demonstrated that 5-HT1A receptors in the hippocampus/medial temporal cortex are affected early in AD. This neuropathological study examined the localization and immunoreaction intensity of 5-HT1A receptor protein in AD hippocampus with the goal to determine whether neuronal receptor levels are influenced by the severity of neurofibrillary tangles (NFT) severity defined by Braaks’ pathological staging and to provide immunohistochemical confirmation of the binding assays and PET imaging studies. Subjects included AD patients and non-AD controls (NC) stratified into three Braaks’ stages (Braak 0–II, NC; Braak III/IV and V/VI, AD). In the Braak 0–II group, 5-HT1A-immunoreactivity (ir) was prominent in the neuropil of the CA1 and subiculum, moderate in the dentate gyrus molecular layer (DGml), and low in the CA3 and CA4. No changes in 5-HT1A-ir were observed in the hippocampus of AD subjects in the Braak III/IV group. Hippocampal 5-HT1A-ir intensity was markedly decreased in the CA1 region in 6 out of 11 (54.5%) subjects in the Braak V/VI group. Across all three groups combined, there was a statistically significant association between reduced 5HT1A-ir and neuronal loss in the CA1, but not in the CA3. The present data demonstrate that hippocampal 5-HT1A receptors are mainly preserved until the end-stage of NFT progression in AD. Thus, the utility of PET imaging using a 5-HT1A specific radiolabeled probe as a marker of hippocampal neuronal loss may be limited to the CA1 field in advanced stage AD cases.
Keywords: Alzheimer’s disease, hippocampus, immunohistochemistry, serotonin 1A receptor, neurofibrillary tangle
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by intra- and extra-cellular amyloid aggregates and dysfunction of multiple neurotransmitter systems. Loss of cortical and hippocampal projecting cholinergic fibers has been established as a structural basis of cognitive impairment in AD, however the fate of other neurotransmitters is less understood. Serotonin containing neuronal processes originate in the brainstem dorsal and medial raphe nuclei and disseminate via widely projecting neuronal network involved in arousal, cognition, and other aspects of cerebral function. 5-hydroxytryptamine (serotonin) 1A (5-HT1A) receptors are part of this complex neural network essential for cognitive function and neuronal plasticity.1, 2 5-HT1A receptors are densely distributed in the hippocampus and neocortex3 where they modulate cholinergic and glutamatergic neurotransmission.4,5 Deficits in hippocampal and cortical serotonergic innervation are associated with aging, depression, and AD, and may explain some of cognitive and behavioral symptoms at advanced clinical stages of AD (for reviews see 6; 7). Several studies reported loss of 5-HT1A and 5-HT2 receptor binding in postmortem AD brain8–12 and reduced 5-HT1A radiotracer levels by in vivo PET imaging.13–16 Collectively, these reports suggest a decrease in hippocampal and medial temporal cortical 5-HT1A receptor concentration that may parallel impaired cognitive function and more advanced AD pathology.14 However, the potential association between 5-HT1A receptor changes and neurofibrillary tangle (NFT) pathology has not been examined; histopathological methods are needed to study this relationship and to validate the findings of radioligand binding studies at a higher anatomical resolution. The current immunohistochemical study evaluated 5-HT1A protein specific immunoreactivity in the hippocampus harvested postmortem from non-AD controls and AD subjects with a range of NFT pathology.
Methods
Subjects
Hippocampus tissue was harvested postmortem and dissected in the coronal plane at the level of lateral geniculate nucleus. Samples were obtained from 16 subjects with clinical and neuropathological diagnoses of AD (mean age 76.5 ± 10.2 years) and 4 age-matched control subjects with no clinical history of AD dementia (mean age 74.0 ± 11.5 years). Cases with neuropathological findings other than AD lesions were excluded from the study. All AD subjects were participants in the University of Pittsburgh’s Alzheimer’s Disease Research Center (ADRC). Clinical diagnosis of AD was based on a standardized ADRC evaluation at a Consensus Conference, utilizing DSM-IV17 and NINCDS/ADRDA18 criteria. Non-AD controls were cognitively normal hospice care subjects who came to autopsy at the University of Pittsburgh. Neuropathological diagnosis was determined by a board-certified neuropathologist; all AD subjects were assigned neuropathological diagnosis of “definite” AD, while controls were “not AD” according to CERAD criteria.19 NFT pathology was staged according to criteria by Braak and Braak20 (Table 1). Review of medical records revealed that three of the AD subjects had clinical history of depression, two in the Braak stage III/IV group and one in the Braak stage V/VI group.
Table 1.
Case demographics
| Braak stage 0/I/II | Braak stage III/IV | Braak stage V/VI | |
|---|---|---|---|
| Number of cases | |||
| total N | 4 | 5 | 11 |
| N per stage | 1/1/2 | 0/5 | 3/8 |
| Age (years) | |||
| mean±SD | 69.8+12.7 | 77.8+7.4 | 76.5+10.2 |
| range | 57–87 | 68–87 | 63–93 |
| Brain weight (grams) | |||
| mean+SD | 1367.5+175.8 | 1216+119.3 | 1102.7+132.2 |
| range | 1180–1600 | 1050–1340 | 880–1250 |
| PMI (hours) | |||
| mean+SD | 4.5+1.3 | 7+1.4 | 8.6+5.1 |
| range | 3–6 | 5–9 | 3–21 |
| Sex | |||
| Male/Female | 3/1 | 4/1 | 5/6 |
Tissue processing
Postmortem brain tissue was processed according to previously described procedures.21,22 Hippocampal tissue blocks were immersed in 4% paraformaldehyde (in 0.1 M phosphate buffer, PB, pH = 7.4) for 48 h at 4 °C and then cryoprotected in 30% sucrose (in PB). Forty μm-thick sections were cut on a sliding microtome, and processed for immunohistochemistry21,22 using a rabbit polyclonal antibody against 5-HT1A (Sc-10801, Santa Cruz, Lot# E0409, 1:250), generated against the epitope corresponding to amino acids 218–336 of human 5-HT1A. This antibody has been characterized previously and showed no cross-reactivity with other serotonin receptor subtypes.23 Sections from all subjects in the study were processed together. At least three sections from each case were immunolabeled with the 5-HT1A antibody, and additional three sections from each case were processed with cresyl violet to delineate the cytoarchitectural boundaries of the hippocampus as defined by Duvernoy, 24 and for neuron density measurements.
Quantitative measures
5-HT1A immunoreaction intensity was assessed by measuring the optical density of the immunoreaction product in the CA1 and CA3 hippocampal subfields. For each case, three images were taken in both subfields using an Olympus Vanox-T AH-2 microscope, equipped with a Sony DXC-950P CCD camera, at 10X magnification under constant illumination. Images were assessed for grayscale intensity using public domain image analysis software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2009). Assessment of neuronal density was performed using an Olympus BH-51 microscope equipped with an Olympus CX9000 digital camera and a motorized XYZ stage. CA1 and CA3 subfields were outlined at low magnification and in each subfield three 40X regions of interest (ROI) were chosen randomly using StereoInvestigator software (MBF Bioscience, Williston, VT). In each ROI, all neurons with a clearly defined nucleus and nucleolus were counted; neurons that touched the upper and left-hand side of the ROI frame were excluded. Section thickness was also determined at each sampling field and appropriate guard zones were calculated in the z-axis. Neuron density was then determined in each subfield in each case and expressed as # neurons/mm3.
Statistical analysis
Demographics, optical density values and neuronal densities were compared among the three Braak groups using a one way ANOVA with Tukey post-hoc testing. Correlation analyses of 5-HT1A immunoreaction intensity and neuronal densities in the CA1 and CA3 field were performed using Spearman rank order correlation. Statistical significance was set at p < 0.05.
Results
The mean age and postmortem interval were not significantly different among the three Braak groups (Table 1). The groups differed significantly by brain weight (p=0.01), with the Braak V–VI having a lower mean brain weight value than the Braak 0–II (p<0.05) but not the Braak III–IV group. All of the non-AD controls in the study were Braak stage 0–II cases (absent or scarce NFT in the hippocampus proper). In these subjects, the 5-HT1A-immunoreactivity (ir) was most prominent in the neuropil of CA1, subiculum and pre-subiculum (Figure 1A). In CA1, 5-HT1A immunoreaction intensity was greatest in the stratum pyramidale and lacunosum moleculare, while in the pyramidal layer it appeared as punctate labeling of neuronal processes and occasional cell bodies (Figure 2). 5HT1A-ir dendritic processes were easily distinguished from the surrounding neuropil as they spanned the stratum radiatum. The pattern of 5HT1A immunoreactivity in the stratum moleculare was similar to the dense network of 5HT1A-ir fibers in the stratum pyramidale. Compared to the CA1 field, 5HT1A-ir in CA2/3 and CA4 was significantly lighter and more punctate in the neuropil. Only a small number of multipolar CA4 neurons and a few small non-pyramidal neurons in the CA3 field were 5HT1A-immunoreactive (Figure 2).
Figure 1.
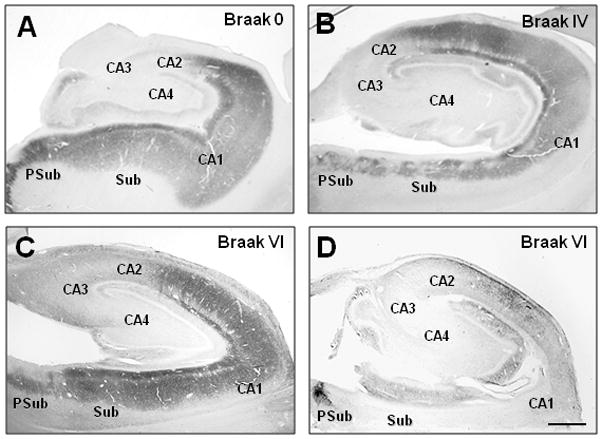
Low magnification photomicrographs of 5-HT1A staining immunostaining in the hippocampus of a control case (Braak stage 0; A), an AD case (Braak stage IV; B), and two advanced AD cases (Braak stage VI) with either preserved (C) or diminished (D) 5-HT1A immunoreactivity.
Figure 2.
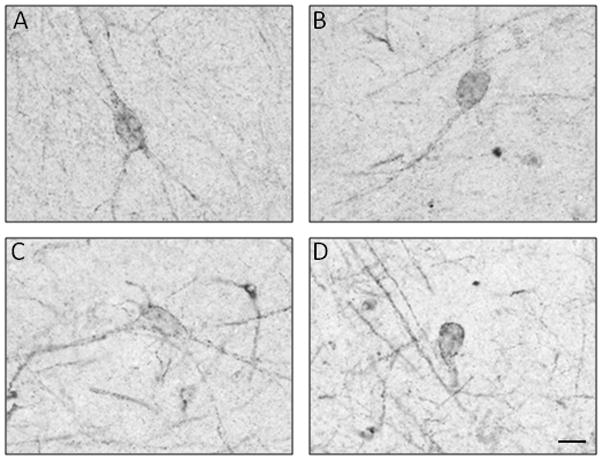
High-power photomicrographs of 5-HT1A-immunoreactive cells in the hippocampus of Braak 0–II cases. Cells with morphological characteristics of pyramidal (A), bipolar (B), or multipolar (C) neurons and interneurons (D) exhibit punctate 5-HT1A immunoreactivity at the level of cell membranes of soma and dendrites. Scale bar = 20 μm.
In AD subjects categorized as Braak stage III/IV (mild to moderate NFT in the hippocampus; Figure 1B) 5-HT1A immunoreactivity was indistinguishable from that observed in the hippocampus of Braak 0–II cases. AD cases categorized as Braak V/VI exhibited a range of 5HT1A immunoreaction intensity, with 5 out of 11 (45.5%) cases having 5HT1A-ir that was indistinguishable from the other two groups (Figure 1C). Six out of 11 cases (54.5%) displayed a marked loss of 5-HT1A-ir in the CA1 and subiculum fields (Figure 1D). The latter cases were also distinguished by very light punctate labeling of blood vessels (not shown).
Optical density measurements of 5-HT1A-ir revealed that the three Braak groups were significantly different (p< 0.05, Figure 3) with Braak V–VI cases having significantly lower 5-HT1A-ir intensity in the CA1 field when compared to both Braak 0–II and III–IV groups. The groups were not different when compared for 5-HT1A-ir intensity in the CA3 field (Figure 3). The three AD cases with a clinical history of depression displayed 5-HT1A-ir intensity that was average for their groups (data not shown).
Figure 3.
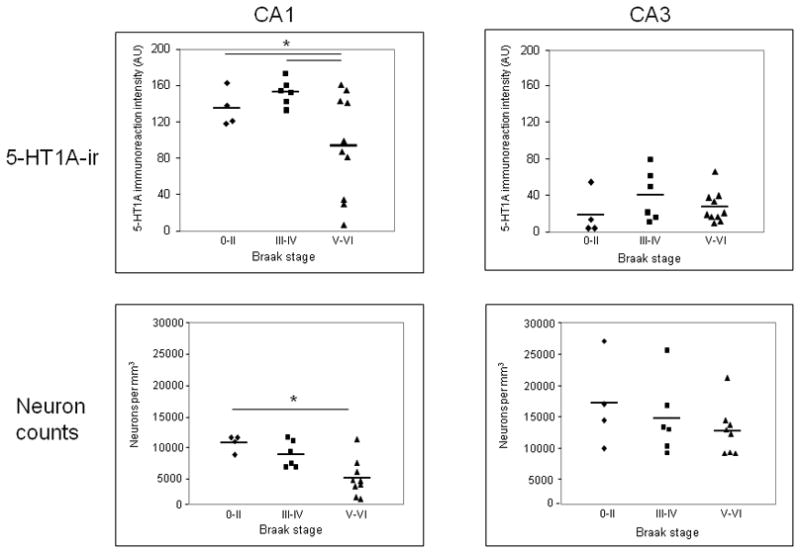
Scatter plots of 5-HT1A immunoreaction intensity values (top) and neuron density measurements (bottom) quantified in hippocampal CA1 and CA3 fields from Braak stage 0–II, III–IV, and V–VI cases. A subset of Braak V–VI cases show significant reductions in both measures in the CA1, but not CA3 region.
To examine whether the loss of 5-HT1A-ir in the CA1 of Braak V–VI cases was related to a reduced neuronal density in the same region, cell counts were performed in the CA1 and CA3 regions and compared across the three Braak stage groups. A significant decrease in neuronal density in CA1 was detected in the Braak stage V/VI group; neuron density did not differ among the three Braak groups in the CA3 region (Figure 3). There was a trend towards a reduction in CA1 neuronal density in the Braak V/VI cases with reduced 5HT1A-ir when compared to Braak V/VI cases with preserved 5HT1A-ir (not shown). Across all cases in the study, there was a trend between reduced 5HT1A immunoreactions intensity and lower neuronal density in the CA1 (r = 0.45, p = 0.09) but not in CA3 field (r = 0.054, p = 0.81; Figure 4).
Figure 4.
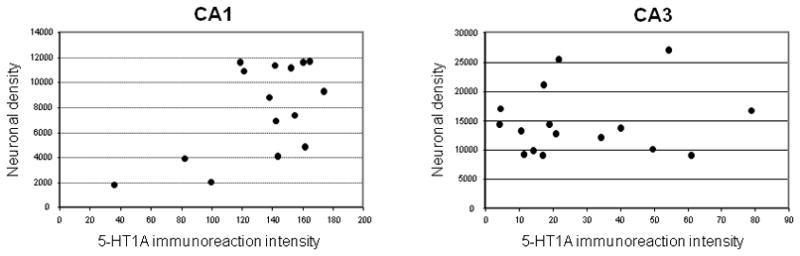
Correlation analysis of 5-HT1A immunoreaction intensity and neuronal density in CA1 and CA3 hippocampus across all cases in the study. The two variables correlated directly in CA1 (r = 0.59, p = 0.009) and not at all in CA3 (r = 0.10, p = 0.35).
Discussion
This is the first immunohistochemical study of 5-HT1A receptors in AD hippocampus. Previous studies using radiolabeled ligands in postmortem human brain demonstrated that 5-HT1A receptors are distributed densely in the hippocampus, especially in the CA1 subfield and the subiculum.3,25,26 The results of the present immunohistochemical study are in accord with these observations and extend them by demonstrating that in aged human hippocampus, 5-HT1A receptors are localized primarily to dendrites of pyramidal cells in the CA1/subiculum and of granule cells in the dentate gyrus. In contrast, the density of 5-HT1A receptors is low in the CA3 and CA4 regions, where they are detected on both dendrites and cell bodies of isolated non-pyramidal cells, similar to what had been described in the rat hippocampus.27
5-HT1A receptor density and neuronal loss
Several studies hypothesized that in vitro autoradiography and in vivo PET measures of 5-HT1A receptor density may be a surrogate marker of pyramidal cell loss in the hippocampus.14,28 This was supported further by the observation that reduced 5-HT1A receptor binding in the hippocampus of kainic acid-lesioned rats reflects CA1 neuronal loss.29 A recent PET imaging study14 found reduced 5-HT1A receptor density in the hippocampus of subjects with early AD and mild cognitive impairment, when hippocampal atrophy is already detectable. In contrast, an autoradiography study by Jansen et al25 reported that hippocampal serotonin 1 receptor ligand binding levels are not significantly different in AD compared to controls. The reason for these discrepancies is unclear, but likely reflects differences in methodology or subject populations. We observed no changes in hippocampal 5-HT1A immunoreactivity in AD cases with substantial hippocampal NFT pathology (diagnosed as Braak III–IV stage). Furthermore, only a subset (54.5%) of our AD subjects with neocortical NFT and severe hippocampal NFT pathology (Braak stage V–VI) displayed a markedly decreased 5-HT1A-ir in all hippocampal fields, with almost a complete loss in the CA1/subiculum. These observations argue that 5-HT1A receptors are lost only in end stages of the disease, and correlate with more extensive neuronal loss. Since 5-HT1A receptors in the hippocampal CA1 are mainly located on the dendrites of pyramidal cells (30, present study), loss of 5-HT1A in AD is likely related to pyramidal cell loss (14, 26, present study). Thus, even if the remaining neurons retain 5-HT1A receptors, the overall reduction in neuronal cell numbers will result in reduced total 5-HT1A binding in the hippocampus as determined by the PET and autoradiography studies. The present finding of a stability of hippocampal 5-HT1A receptors despite a substantial NFT pathology in AD hippocampus is likely due to the fact that tangle-bearing neurons remain viable and functional, albeit in diminished capacities, over prolonged periods of times.31
Functional relevance of 5-HT1A receptor changes in AD
The functional consequences of the 5-HT1A receptor preservation during the course of AD pathology progression, and of receptor loss in end-stage disease, are unclear. The highest densities of 5-HT1A receptors are in the hippocampus, and 5-HT function in this brain region involves, in part, modulation of other neurotransmitter input, including glutamatergic and cholinergic neurotransmission.5 Future studies examining potential changes in presynaptic 5-HT1A receptors will be important in this regard. Thus, loss of hippocampal 5-HT receptors may exacerbate memory and cognitive deficits in the more advanced disease stages both directly due to loss of postsynaptic receptors and indirectly by loss of modulation of glutamatergic, cholinergic and GABAergic activity. Changes in the 5-HT function are also postulated to contribute to non-cognitive functions including depression, anxiety, fear, irritability and aggression; these behavioral disturbances typically occur in more advanced stages of AD6 and result likely from a complex interaction of genetic and environmental factors, disease-specific and co-occurring pathologies, and patients’ medications. The relationship between the onset of these symptoms in AD patients and deficits in 5-HT receptors in the hippocampus and other brain regions including cerebral cortex remain to be determined.
Methodological considerations
The present study has some limitations. The number of examined subjects in this autopsy investigation was small, and although we applied stereological principles to perform unbiased counting of neurons in the hippocampus, the entire hippocampal structure was not available and our analysis was limited to the mid-portion of the hippocampus (at the level of lateral geniculate body). Whether the status of 5-HT1A receptors during the progression of NFT pathology is similar in the anterior and more posterior portions of the hippocampus remains to be determined in future studies. While Braak staging for NFT20 is a well established neuropathological diagnostic procedure, we did not count individual NFT in hippocampal fields. Future immunohistochemical studies performing dual labeling with 5-HT1A receptor and phospho-tau or Aβ antibodies will examine more closely the relationship between serotonin receptor changes and hallmark pathologies at different stages of AD progression.
Conclusion
In summary, the results of our immunohistochemical study suggest that cellular expression of the 5-HT1A receptor is preserved in majority of AD subjects, even in cases with considerable concomitant NFT pathology load. Contrary to the hypothesis that 5-HT1A receptor reductions precede the onset of clinical symptoms,14 our data indicate that these receptors are affected only in end-stage AD, where 5-HT1A loss reflects regional neuronal loss. Future studies are warranted to elucidate the mechanisms behind, and functional significance of, the 5-HT1A receptor changes in AD hippocampus.
Acknowledgments
We are indebted to the support of the participants in the ADRC at the University of Pittsburgh. This study was supported by NIH grants NIA AG05133 (University of Pittsburgh ADRC), and AG14449 (MDI), The Snee-Reinhardt Charitable Foundation (MDI), and by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (KM). Ms. Suganya Srinivasan, Ms. Natsuko Kato and Ms. Megumi Mitani provided expert technical assistance.
References
- 1.Cowen DS, Johnson-Farley NN, Travkina T. 5-HT receptors couple to activation of Akt, but not extracellular-regulated kinase (ERK), in cultured hippocampal neurons. J Neurochem. 2005;93:910–917. doi: 10.1111/j.1471-4159.2005.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci USA. 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall H, Lundkvist C, Halldin C, et al. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]way-100635. Brain Res. 1997;745:96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- 4.Lazaris A, Bertrand F, Lazarus C, et al. Baseline and 8-OH-DPAT-induced release of acetylcholine in the hippocampus of aged rats with different levels of cognitive dysfunction. Brain Res. 2003;967:181–190. doi: 10.1016/s0006-8993(02)04272-5. [DOI] [PubMed] [Google Scholar]
- 5.Schechter LE, Smith DL, Rosenzweig-Lipson S, et al. Lecozotan (SRA-333): a selective serotonin 1A receptor antagonist that enhances the stimulated release of glutamate and acetylcholine in the hippocampus and possesses cognitive-enhancing properties. J Pharmacol Exp Ther. 2005;314:1274–1289. doi: 10.1124/jpet.105.086363. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer CC, Smith G, DeKosky ST, et al. Serotonin in aging, late-life depression, and Alzheimer’s disease: the emerging role of functional imaging. Neuropsychopharmacology. 1999;21:321–322. doi: 10.1016/S0893-133X(97)00194-2. [DOI] [PubMed] [Google Scholar]
- 7.Borg J. Molecular imaging of the 5-HT(1A) receptor in relation to human cognition. Behav Brain Res. 2008;195:103–111. doi: 10.1016/j.bbr.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AV, Ferrier IN, Morris CM, et al. Cortical serotonin-S2 receptor binding in Lewy body dementia, Alzheimer’s and Parkinson’s diseases. J Neurol Sci. 1991;106:50–55. doi: 10.1016/0022-510x(91)90193-b. [DOI] [PubMed] [Google Scholar]
- 9.Cross AJ, Crow TJ, Johnson JA, et al. Studies on neurotransmitter receptor systems in neocortex and hippocampus in senile dementia of the Alzheimer-type. J Neurol Sci. 1984;64:109–117. doi: 10.1016/0022-510x(84)90029-7. [DOI] [PubMed] [Google Scholar]
- 10.Cross AJ, Crow TJ, Ferrier IN, Johnson JA, Bloom SR, Corsellis JAN. Serotonin receptor changes in dementia of the Alzheimer type. J Neurochem. 1984;43:1574–1581. doi: 10.1111/j.1471-4159.1984.tb06081.x. [DOI] [PubMed] [Google Scholar]
- 11.Cross AJ. Serotonin in Alzheimer-type dementia and other dementing illnesses. Ann NY Acad Sci. 1990;600:405–415. doi: 10.1111/j.1749-6632.1990.tb16897.x. [DOI] [PubMed] [Google Scholar]
- 12.Crow TJ, Cross AJ, Cooper SJ, et al. Neurotransmitter receptors and monoamine metabolites in the brains of patients with Alzheimer-type dementia and depression, and suicides. Neuropharmacology. 1984;23:1561–1569. doi: 10.1016/0028-3908(84)90100-x. [DOI] [PubMed] [Google Scholar]
- 13.Lai MK, Tsang SW, Francis PT, et al. Reduced serotonin 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer disease. Brain Res. 2003;974:82–87. doi: 10.1016/s0006-8993(03)02554-x. [DOI] [PubMed] [Google Scholar]
- 14.Kepe V, Barrio JR, Huang SC, et al. Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc Natl Acad Sci USA. 2006;103:702–707. doi: 10.1073/pnas.0510237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truchot L, Costes SN, Zimmer L, et al. Up-regulation of hippocampal serotonin metabolism in mild cognitive impairment. Neurology. 2007;69:1012–1017. doi: 10.1212/01.wnl.0000271377.52421.4a. [DOI] [PubMed] [Google Scholar]
- 16.Truchot L, Costes N, Zimmer L, et al. A distinct [18F]MPPF PET profile in amnestic mild cognitive impairment compared to mild Alzheimer’s disease. Neuroimage. 2008;40:1251–1256. doi: 10.1016/j.neuroimage.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychietric Association; 1994. [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Mirra SS, Heyman A, McKeel D, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 20.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 21.Ikonomovic MD, Mizukami K, Warde D, et al. Distribution of glutamate receptor subunit NMDAR1 in the hippocampus of normal elderly and patients with Alzheimer’s disease. Exp Neurol. 1999;160:194–204. doi: 10.1006/exnr.1999.7196. [DOI] [PubMed] [Google Scholar]
- 22.Mizukami K, Ikonomovic MD, Grayson DR, et al. Immunohistochemical study of GABAA receptor β2/3 subunits in the hippocampal formation of aged brains with Alzheimer-related neuropathologic changes. Exp Neurol. 1997;147:333–345. doi: 10.1006/exnr.1997.6591. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui EJ, Shabbir M, Mikhailidis DP, Thompson CS, Mumtaz FH. The role of serotonin (5-hydroxytryptamine1A and 1B) receptors in prostate cancer cell proliferation. J Urol. 2006;176:1648–1653. doi: 10.1016/j.juro.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 24.Duvernoy HM. The Human Hippocampus. 2. Berlin: Springer-Verlag; 1998. [Google Scholar]
- 25.Jansen KL, Faull RL, Dragunow M, Synek BL. Alzheimer’s disease: changes in hippocampal N-methyl-D-aspartate, quisqualate, neurotensin, adenosine, benzodiazepine, serotonin and opioid receptors - an autoradiographic study. Neuroscience. 1990;39:613–627. doi: 10.1016/0306-4522(90)90246-z. [DOI] [PubMed] [Google Scholar]
- 26.Palacios JM, Waeber C, Hoyer D, Mengod G. Distribution of serotonin receptors. Ann NY Acad Sci. 1990;600:36–52. doi: 10.1111/j.1749-6632.1990.tb16871.x. [DOI] [PubMed] [Google Scholar]
- 27.Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM. The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Res. 2003;959:58–67. doi: 10.1016/s0006-8993(02)03727-7. [DOI] [PubMed] [Google Scholar]
- 28.Salmon E. A review of the literature on neuroimaging of serotoninergic function in Alzheimer’s disease and related disorders. J Neural Transm. 2007;114:1179–1185. doi: 10.1007/s00702-007-0636-5. [DOI] [PubMed] [Google Scholar]
- 29.Van Bogaert P, De Tiège X, Vanderwinden JM, Damhaut P, Schiffmann SN, Goldman S. Comparative study of hippocampal neuronal loss and in vivo binding of 5-HT1a receptors in the KA model of limbic epilepsy in the rat. Epilepsy Res. 2001;47:127–139. doi: 10.1016/s0920-1211(01)00301-1. [DOI] [PubMed] [Google Scholar]
- 30.Kia HK, Brisorgueil MJ, Hamon M, Calas A, Vergé D. Ultrastructural localization of 5-hydroxytryptamine1A receptors in the rat brain. J Neurosci Res. 1996;46:697–708. doi: 10.1002/(SICI)1097-4547(19961215)46:6<697::AID-JNR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 31.Bobinski M, Wegiel J, Tarnawski M, de Leon MJ, Reisberg B, Miller DC, Wisniewski HM. Duration of neurofibrillary changes in the hippocampal pyramidal neurons. Brain Res. 1998;799:156–158. doi: 10.1016/s0006-8993(98)00441-7. [DOI] [PubMed] [Google Scholar]


