Abstract
A variety of electrophiles (anhydrides, acid chlorides, carbonochloridates, sulfonyl chlorides, and alkyl bromides) react with 3-methoxy-2H-indazole (1a), benzoxazin[3,2-b]indazole (1d), and oxazolino[3,2-b]indazole (1e) – substrates available by the Davis-Beirut reaction – to yield a diverse set of N1,N2-disubstituted-1H-indazolones. With certain electrophiles, an AERORC (Addition of the Electrophile, Ring Opening, and Ring Closure) process on indazole 1d results in indazoloindazolone formation. An intriguing aspect of these N1,N2-disubstituted-1H-indazolones is that they are poised for diversification through, for example, azide-alkyne cycloaddition chemistry reported here.
The indazole and indazolone ring systems are privileged heterocycles1 known to exhibit analgesic, antitumor, anticancer, antiangiogenic, antiviral, and antiinflammatory activities. Of the two isomers, 2H-indazoles are less explored than 1H-indazoles.2 In previous reports,3 our laboratory has demonstrated the utility of the Davis-Beirut reaction – an effective N,N-bond forming heterocyclization reaction – to deliver 3-alkoxy-2H-indazoles, benzoxazin[3,2-b]-indazole, oxazolino[3,2-b]indazole, and a variety of other indazolo-fused hetero-cycles from 2-nitrobenzaldehyde or 1-(bromomethyl)-2-nitrobenzene.
We showed more recently that 3-alkoxy-2H-indazoles can be converted into N2-substituted-1H-indazolones by treatment with various nucleophiles.4 For example, reaction of indazole 1a with sodium ethanethiolate under microwave conditions (155 °C, 10 min) delivers, by demethylation, indazolone 2 in 62% yield (Scheme 1). This led to an investigation of the scope of nucleophilic ring-opening of indazoles 1b-d and established that a variety of nucleophiles can be employed to produce a diverse set of N2-substituted-1H-indazolones.
Scheme 1.
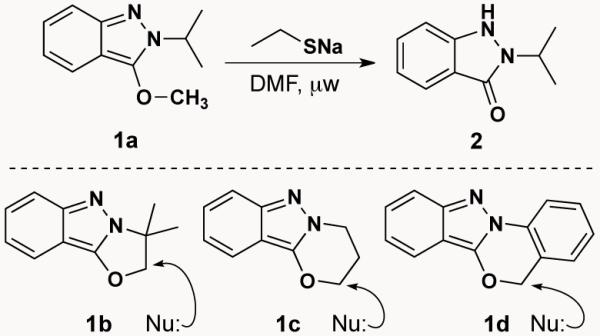
Nucleophilic ring-opening of 3-alkoxy-2H-indazoles
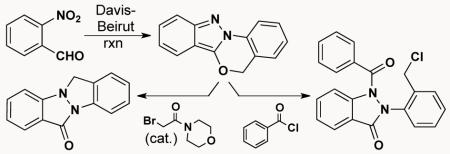
With these results as a backdrop, we speculated that 3-alkoxy-2H-indazoles, available by the Davis-Beirut reaction, might also react with an electrophile to give a positively charged N1 which would, in turn, drive a counter anion to attack giving net 1,6-electrophilic addition across the 2H-indazole. In fact, treating indazole 1a with refluxing HOAc affords indazolone 2 (Scheme 2), while heating with sodium acetate in DMF for the same period of time (118 °C, 17 h) gives no reaction.
Scheme 2.
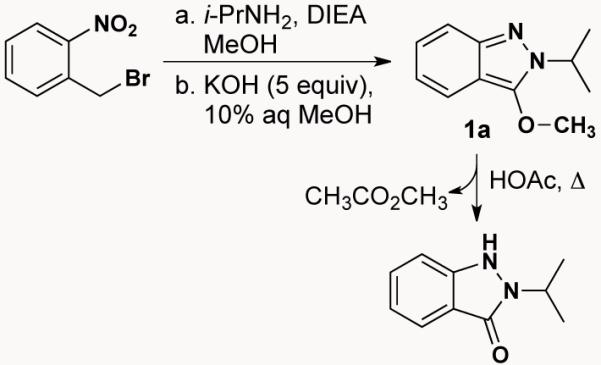
Davis-Beirut recation → 1a → 2
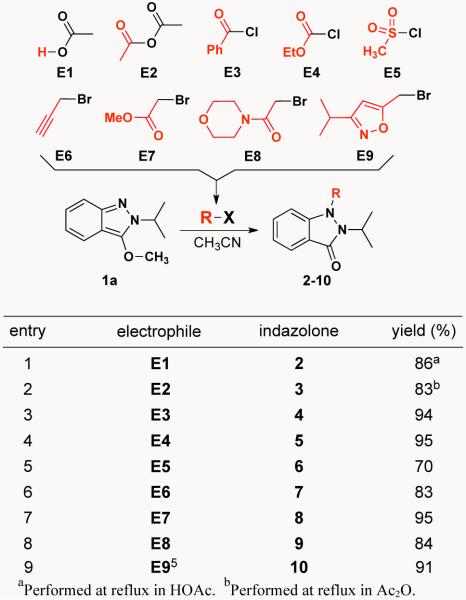
Building on this simple but encouraging result, we launched an investigation of the effectiveness of 1,6-electrophilic addition to indazole 1a using the diverse set of electrophiles shown in Scheme 3 (E1-E9). This study revealed that indazole 1a reacts with each of these various electrophiles to produce a diverse set of N1,N2-disubstituted-1H-indazolones. Reaction optimization established that thermal heating, although requiring a longer reaction time than microwave irradiation, results in higher yields. Additionally, for all electrophiles except E1 and E2 where reactions were performed in HOAc and Ac2O (respectively), solvent optimization showed that CH3CN led to higher yields than either DMF or DMSO.
Scheme 3.
1,6-Electrophilic addition to 3-methoxy-2H-indazole 1a
We next investigated the 1,6-electrophilic addition reactivity of oxazolino[3,2-b]indazole 1e. As presented in Scheme 4, indazole 1e reacts with all nine electrophiles (E1-E9) to produce a diverse set of N1,N2-disubstituted-1H-indazolones in excellent yield. It was also noted that electrophilic addition to 1e was generally much faster and higher yielding than addition to indazole 1a – most likely due to relief of strain in the 5-membered oxazolino ring.
Scheme 4.
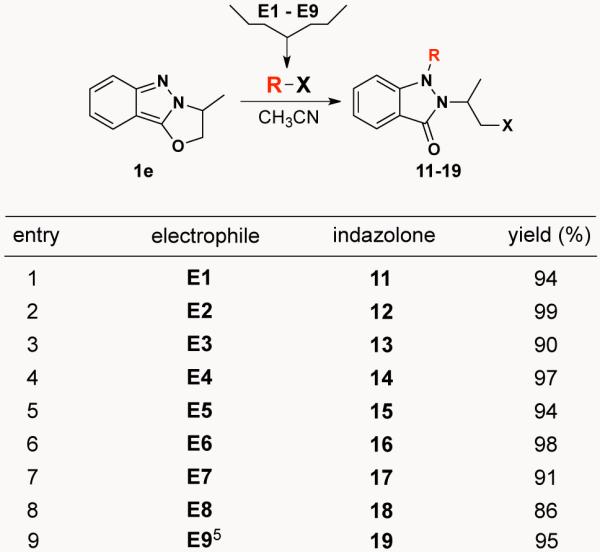
1,6-Electrophilic addition to oxazolino[3,2-b]-indazole 1e
An interesting turn of events occurred when we investigated the electrophilic addition to indazole 1d. While treatment of 1d with benzoyl chloride in acetronitrile at 60 °C delivered the anticipated indazolone 20 in 99% yield, treating it with 2-bromo-1-morpholinoethanone in acetonitrile at 82 °C gave indazolo[2,1-a]indazol-6(12H)-one 22 as the sole product (Scheme 5). LCMS monitoring of the reaction indicated that the originally anticipated indazolone 21 was indeed formed as a transient intermediate, but, under the conditions of the reaction, it quickly converted to indazoloindazolone 22. Based on the fact that 22 is not formed when N1 of the indazole is acylated (1d → 20), we speculate that indazoloindazolone formation occurs via an AERORC (Addition of the Electrophile, Ring Opening, and Ring Closure)6 process that we speculate transposes through the intermediacy of indazoloindazolium 21′. Indeed, this AERORC process (1d → 22) is competitive in rate with the alkylation/ring-opening reaction (1d → 21) and the only way to obtain appreciable amounts of 21 (28%) is to stop the reaction early (~57% conversion of 1d). It was also found that treating 1d with catalytic (10 mol%) 2-bromo-1-morpholinoethanone delivers 22 in high yield (82 °C, 7 d, 79% yield; μw, 150 °C, 5 h, 92 % yield).
Scheme 5.
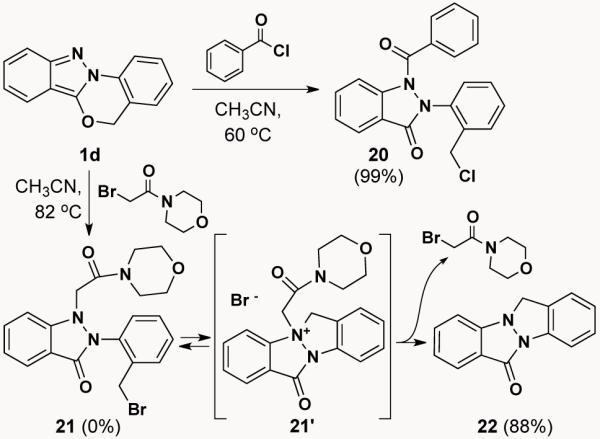
1,6-Electrophilic addition (→ 20) vs. AERORC (→ 22)
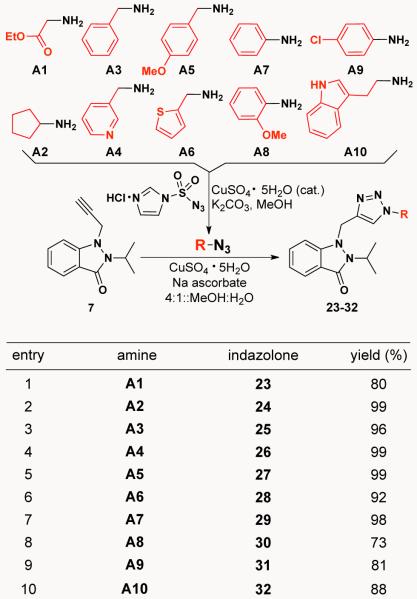
An intriguing aspect of many of the indazolones presented in Schemes 3 and 4 is that they are poised for further diverstification through, for example, azide-alkyne cycloaddition chemistry.7 Capitalizing on this opportunity, we next set out to synthesize a small library of twenty triazolyl-indazolones (23-32 and 33-42) as a part of our commitment to the NIH Molecular Libraries Small Molecule Repository for high-throughput biological screening. As illustrated in Scheme 6, indazolone 7 (entry 6, Scheme 3), containing a propynyl moiety, was used for part one of this click diversification study. In situ generated azides – prepared from amines A1-A10 by treatment with 1H-imidazole-1-sulfonyl azide8 and CuSO4 – were employed in these copper(I)-catalyzed cycloadditions to give indazolones 23-32 in high yields.
Scheme 6.
CuAAC reactions on indazolone 7
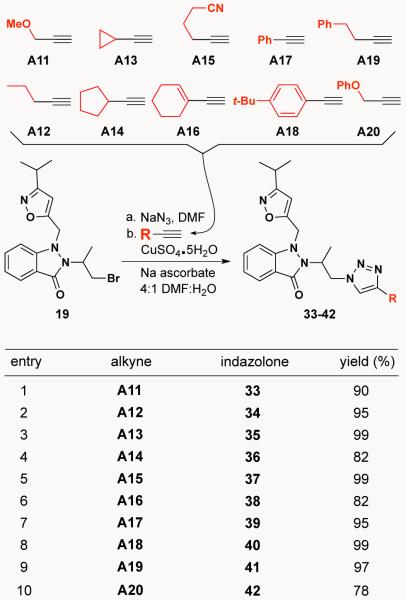
For part two of this click diversification study, we decided to prepare a collection of ten triazolyl-indazolones based on indazole 19 (entry 9, Scheme 4). We envisioned a one-pot reaction9 for this process wherein indazolone 19 was heated first with sodium azide, followed by the addition of copper (I) and the alkyne. To test the reliability of the first step (R-Br → R-N3), indazolone 19 was treated with sodium azide at 60 °C in DMF and the corresponding primary azide was cleanly obtained in 3 hours reaction time. We next sought to trap the azide with an alkyne in a one-pot, two-step reaction to give the corresponding triazole product. The results of ten such reactions are summarized in Scheme 7 and show that a variety of alkynes react to give 1,4-triazoles in good to excellent yields.
Schcme 7.
CuAAC reactions on indazolone 19
In summary, we have demonstrated that electrophilic addition to 3-methoxy-2H-indazole (1a), benzoxazin[3,2-b]indazole (1d), and oxazolino[3,2-b]indazole (1e) substrates can lead to novel N1,N2-disubstituted-1H-indazolones that are difficult to access by other methods. A rare example of a heterolytic AERORC reaction has been demonstrated with the rearrangement of benzoxazin-[2,3-b]indazole 1d to indazolonoindazole 22 via the intermediacy of indazolone 21. Finally, further diversification of two N1,N2-disubstituted-1H-indazolone products through azide-alkyne cycloaddition chemistry was demonstrated yielding a small library of novel triazoles.
Supplementary Material
Acknowledgment
We thank the National Science Foundation (CHE-0910870) and the National Institutes of Health (GM089153) for generous financial support. WEC acknowledges the United States Department of Education for a GAANN Fellowship.
Footnotes
Supporting Information Available: Full experimental details and characterization data (1H-NMR, 13C-NMR, IR, and LC/MS) for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).(a) Schmidt A, Beutler A, Snovydovych B. Eur. J. Org. Chem. 2008:4073. [Google Scholar]; (b) Fletcher SR, McIver E, Lewis S, Burkamp F, Leech C, Mason G, Boyce S, Morrison D, Richards G, Sutton K, Jones AB. Biorg. Med. Chem. Lett. 2006;16:2872. doi: 10.1016/j.bmcl.2006.03.004. [DOI] [PubMed] [Google Scholar]; (c) Kawanishi N, Sugimoto T, Shibata J, Nakamura K, Masutani K, Ikuta M, Hirai H. Biorg, Med. Chem. Lett. 2006;16:5122. doi: 10.1016/j.bmcl.2006.07.026. [DOI] [PubMed] [Google Scholar]; (d) Huang L-J, Shih M-L, Chen H-S, Pan S-L, Teng C-M, Lee F-Y, Kuo S-C. Biorg. Med. Chem. 2006;14:528. doi: 10.1016/j.bmc.2005.08.032. [DOI] [PubMed] [Google Scholar]; (e) Cerecetto H, Gerpe A, Gonzalez M, Aran VJ, Ochoa de Ocariz C. Mini-Rev, Med. Chem. 2005;5:869. doi: 10.2174/138955705774329564. [DOI] [PubMed] [Google Scholar]
- (2).(a) Halland N, Nazaré M, R’kyek O, Alonso J, Urmann M, Lindenschmidt A. Angew. Chem. Int. Ed. 2009;48:6879. doi: 10.1002/anie.200902323. [DOI] [PubMed] [Google Scholar]; (b) Viña D, del Olmo E, Lopez-Pérez JL, San Feliciano A. Org. Lett. 2007;9:525. doi: 10.1021/ol062890e. [DOI] [PubMed] [Google Scholar]; (c) Stadlbauer W. Science of Synthesis. 2002;12:227. [Google Scholar]; (d) Elguero I. In: Comprehensive Heterocyclic Chemistry. Katritzky AR, Rees CW, Scriven EFV, editors. Vol. 3. Pergamon; Oxford: 1996. pp. 1–75. [Google Scholar]
- (3).(a) Avila B, Solano DM, Haddadin MJ, Kurth MJ. Org. Lett. 2011;13:1060. doi: 10.1021/ol103108z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Solano DM, Butler JD, Haddadin MJ, Kurth M,J. Org. Synth. 2010;87:339. [Google Scholar]; (c) Butler JD, Solano DM, Robins LI, Haddadin MJ, Kurth MJ. J. Org. Chem. 2008;73:234. doi: 10.1021/jo702067z. [DOI] [PubMed] [Google Scholar]; (d) Mills AD, Maloney P, Hassanein E, Haddadin MJ, Kurth M,J. J. Comb. Chem. 2007;9:171. doi: 10.1021/cc060109o. [DOI] [PubMed] [Google Scholar]; (e) Mills AD, Nazer MZ, Haddadin MJ, Kurth MJ. J. Org. Chem. 2006;71:2687. doi: 10.1021/jo0524831. [DOI] [PubMed] [Google Scholar]; (f) Kurth MJ, Olmstead MM, Haddadin MJ. J. Org. Chem. 2005;70:1060. doi: 10.1021/jo048153i. [DOI] [PubMed] [Google Scholar]
- (4).(a) Oakdale JS, Solano DM, Fettinger JC, Haddadin MJ, Kurth MJ. Org. Lett. 2009;11:2760. doi: 10.1021/ol900891s. [DOI] [PubMed] [Google Scholar]; (b) Donald MB, Conrad WE, Oakdale JS, Butler JD, Haddadin MJ, Kurth MJ. Org. Lett. 2010;12:2524. doi: 10.1021/ol100751n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Liu Y, Cui Z, Liu B, Cai B, Li Y, Wang Q. J. Agric. Food Chem. 2010;58:2685. doi: 10.1021/jf902541w. [DOI] [PubMed] [Google Scholar]
- (6).(a) While the ANRORC6b-6d process is well documented, this is a rare example of a heterocyclic AERORC process. Van der Plas HC. Acc. Chem. Res. 1978;11:462. Van der Plas HC. Adv. Heterocycl. Chem. 1999;74:1. (d) See reference 8.
- (7).Rostovtsev VV, Green LG, Fokin VV, Sharpless BK. Angew. Chem., Int. Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. Tornoe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057. doi: 10.1021/jo011148j. For Reveiews of Click Chemistry, see: Meldal M, Tornøe W. Chem. Rev. 2008;108:2952. doi: 10.1021/cr0783479. Hein JE, Fokin VV. Chem. Soc. Rev. 2010;39:1302. doi: 10.1039/b904091a.
- (8).Goddard-Borger ED, Stick RV. Org. Lett. 2007;9:3797. doi: 10.1021/ol701581g. [DOI] [PubMed] [Google Scholar]
- (9).Feldman AK, Colasson B, Fokin VV. Org. Lett. 2004;6:3897. doi: 10.1021/ol048859z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.