Abstract
Background
Maternal highly-active antiretroviral therapy (HAART) reduces mother-to-child HIV transmission (MTCT), but may increase the risk for infant anemia.
Methods
The incidence of first severe anemia (Grade 3 or 4, Division of AIDS 2004 Toxicity Table) was assessed among HIV-uninfected infants in the Mashi and Mma Bana MTCT prevention trials in Botswana. Severe anemia rates were compared between 3 groups: infants exposed to maternal HAART in utero and during breastfeeding and 1 month of postnatal zidovudine (HAART-BF); infants exposed to maternal zidovudine (ZDV) in utero, 6 months of postnatal ZDV, and breastfeeding (ZDV-BF); and infants exposed to maternal ZDV in utero, 1 month of postnatal ZDV, and formula-feeding (ZDV-FF).
Results
A total of 1719 infants were analyzed— 691 HAART-BF, 503 ZDV-BF, and 525 ZDV-FF. Severe anemia was detected in 118 infants (7.4%). By 6 months, 12.5% of HAART-BF infants experienced severe anemia, compared with 5.3% of ZDV-BF (P<0.001) and 2.5% of ZDV-FF infants (P<0.001). In adjusted analysis, HAART-BF infants were at greater risk of severe anemia than ZDV-BF or ZDV-FF infants (adjusted odds ratios 2.6 and 5.8, respectively; P < 0.001). Most anemias were asymptomatic and improved with iron/multivitamin supplementation and cessation of ZDV exposure. However, 11 infants (0.6% of all infants) required transfusion for symptomatic anemia. Microcytosis and hypochromia were common among infants with severe anemia.
Conclusions
Exposure to maternal HAART starting in utero was associated with severe infant anemia. Confirmation of this finding and possible strategies to mitigate hematologic toxicity warrant further study.
Trial Registration
ClinicalTrials.gov identifiers: NCT00197587 and NCT00270296.
Keywords: Mother-to-Child Transmission, Anemia, Antiretroviral Therapy, Fetal Drug Exposure, Human Immunodeficiency Virus, Infant
Background
Perinatal antiretroviral therapy dramatically reduces the risk of transmission of HIV to the 1.4 million infants born annually to HIV-infected mothers.1 Single-dose and single-drug strategies lead to important reductions in mother-to-child transmission (MTCT).2-5 However, the use of maternal highly active antiretroviral therapy (HAART) can further reduce the risk of MTCT during pregnancy and breastfeeding.6-8 This strategy is now recommended by the World Health Organization (WHO) for all pregnant women with a CD4+ cell count <350 cells/μL, and is a recommended option for MTCT prevention among women with higher CD4+ cell counts.9 Universal HAART among all pregnant women is being rolled out in several African and Asian countries and may be particularly important in settings where formula-feeding is unsafe or not feasible.10-15
Evaluation of infant toxicity from exposure to maternal HAART initiated in pregnancy has been limited. Of particular concern is the risk for hematologic toxicity. Several antiretrovirals, most importantly zidovudine and other nucleoside reverse transcriptase inhibitors (NRTIs), are known to cause anemia in adults and children.16-21 Observational studies from the United States and Europe suggest that exposure to in utero HAART is associated with an increase in mild, reversible anemia.22-25 However, with prevalent micronutrient deficiencies26-29 the incidence and severity of anemia may be greater for infants born in resource-limited settings. Additionally, limited access to blood transfusions and erythropoietin analogues may lead to greater clinical consequences from severe anemia in these settings.
We sought to evaluate the effect of maternal HAART started in pregnancy on the incidence of anemia among HIV-exposed, uninfected infants participating in two clinical trials in Botswana. Given the established benefits of HAART for preventing MTCT, we limited our analysis to severe and life-threatening anemia, as an increase in these events could be important to programmatic and clinical decisions.
Methods
Study design
We conducted a post-hoc analysis of pooled data from two randomized controlled trials of different strategies to prevent MTCT in Botswana. The Mashi study, in part 1, compared the efficacy of single-dose nevirapine versus placebo when added to maternal and infant zidovudine (ZDV) for the prevention of perinatal MTCT,30 and in part 2, compared the efficacy of breastfeeding plus 6 months of infant ZDV versus formula feeding plus 1 month of infant ZDV to prevent postnatal HIV.31 HAART became available to women with CD4 + cell counts < 200 cells/μL or AIDS illness (and to HIV-infected infants) 19 months into the study. The Mma Bana study evaluated the use of HAART for HIV-infected pregnant women opting to breastfeed for the prevention of perinatal and postpartum MTCT.8 Pregnant women with CD4+ cell counts ≥ 200 cells/μL were randomized to receive either abacavir/zidovudine/lamivudine or lopinavir/ritonavir/zidovudine/lamivudine. Women with CD4+ cell counts <200 cells/μL received the standard-of-care regimen of nevirapine/zidovudine/lamivudine. Women in the Mma Bana study were counseled to exclusively breastfeed to 6 months of age, and infants received 1 month of ZDV. There were no restrictions on CD4+ cell count for women entering either study.
Study subjects
HIV-infected, HAART-naïve pregnant women presenting for antenatal care were referred (for both studies) to the same 4 study clinics located in 1 city, 1 town, and 2 large villages in southern Botswana. The Mashi study enrolled women between March 2001 and October 2003, and the Mma Bana study enrolled women between July 2006 and May 2008. The trials had similar maternal eligibility criteria, including age of at least 18 years, confirmed HIV infection, a hemoglobin level ≥ 8g/dL, an absolute neutrophil count ≥ 1000 cells/μL, and limited ranges for alanine aminotransferase and aspartate aminotransferase (< 2.5 times and < 10 times the upper limit of normal for the Mashi and Mma Bana studies, respectively). Women in the Mashi study were enrolled between 33 and 35 weeks gestation, whereas women in the Mma Bana study were enrolled from 18 to 34 weeks gestation. Women who were not willing to breastfeed were not eligible for either study. HIV-uninfected infants did not receive cotrimoxazole prophylaxis in either trial. Immediate cord clamping was the standard obstetrical practice during both studies.
Eligible women who agreed to participate provided written informed consent. The studies were reviewed and approved by the Botswana Health Research Development Committee and the Harvard School of Public Health Human Subjects Committee.
Laboratory measurements
Peripheral blood for a full blood count with differential was taken via venipuncture at birth, 1 month, 4 months, and 7 months of age for formula-feeding infants in Mashi and monthly until 7 months for breastfeeding infants in Mashi. Infants in Mma Bana had measurements performed at birth, 1 month, 3 months, and 6 months of age. Both studies used the Sysmex XE 2100 automated flow cytometry analyzer (Sysmex Corporation, Kobe, Japan). Infant HIV testing was performed at similar intervals by qualitative polymerase chain reaction (PCR) DNA assay using the Amplicor HIV-1 test (Roche Diagnostic Systems, New Jersey). Total hemoglobin iron at birth was estimated using the method described by Saarinen and Siimes.32
Exposures and endpoints
Due to expected influences of both antiretroviral exposure and feeding method on rates of infant anemia, we summarized fetal and infant exposures into three categories (see Figure, Digital Supplemental Content 1). Mashi infants randomized to formula-feed (with 1 month infant ZDV) were included in the zidovudine, formula-feeding group (ZDV-FF). Mashi infants randomized to breastfeed (with 6 months infant ZDV) were placed in the zidovudine-breastfeeding group (ZDV-BF). Infants born to mothers in Mma Bana were included in the HAART, breastfeeding group (HAART-BF). For consistency, Mashi infants born to women taking HAART prior to delivery were excluded.
To reduce possibility for ascertainment bias given different frequencies of full blood count assessment in the study groups, only scheduled hemoglobin measurements at birth and 1 month, 3 months, and 6 months of age were included in the analysis. For infants in the ZDV-FF group without hemoglobin measurements at 3 and 6 months of age, we used values from scheduled 4 month and 7 month visits. Measurements within 5 days of birth and the 1-month visit were included, as were measurements within 10 days of later scheduled visits.
The primary study objective was to compare the cumulative incidence of severe or life-threatening (grade 3 or 4) anemia from birth to 6 months of age, as defined by the Division of AIDS (DAIDS) toxicity tables, 2004 revision,33 between the HAART-BF group and the two groups of infants not exposed to antenatal maternal HAART.
Statistical analysis
We restricted our analyses to singleton and first-born twin infants. To avoid confounding with HIV-related anemia,34 we also restricted our analysis to infants who remained uninfected with HIV through 7 months of follow-up. We used the chi-square test to assess for differences between exposure groups for the primary outcome of cumulative incidence of severe anemia. Subsequently, we used multiple logistic regression to adjust for differences between the exposure groups that could confound the association with severe anemia. The final model was determined using backwards variable selection, retaining only significant predictors.
We calculated standardized mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentrations (MCHC) using published age-specific normative means and standard deviations.35, 36 Analysis of variance was used to compare MCV, MCHC, and hemoglobin levels among exposure categories. When differences were detected, paired comparisons were explored using a Bonferroni-corrected Student's t-test. Statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC). All tests were two-tailed and P-values of less than 0.05 were considered statistically significant.
Results
Study infants
There were 1877 live singleton and first-born twin infants born to the 1930 HIV-infected pregnant women enrolled in the Mashi and Mma Bana studies (Figure, Digital Supplemental Content 2). A total of 90 infants became infected with HIV by 7 months of age and were excluded from further analyses. Within the Mashi study, 68 infants were exposed to antenatal HAART and were excluded. Of the remaining 1719 infants, there were 691 HAART-BF, 503 ZDV-BF, and 525 ZDV-FF infants. A total of 26 mothers (analyzed as part of the ZDV-BF group) initiated HAART during breastfeeding, at a median of 74 days after birth (interquartile range 41 to 132 days).
Table 1 shows the maternal enrollment and delivery characteristics. Although there were statistically significant differences in characteristics among the three exposure groups, with the exception of duration of antiretroviral treatment, these differences were modest in size. In addition to these maternal differences, a greater proportion of infants in the HAART-BF group were small for gestational age at birth than infants in the other exposure groups (P<0.001).
Table 1. Maternal characteristics.
| Exposure Category | ||||
|---|---|---|---|---|
| HAART-BF (N = 691) |
ZDV-BF (N = 503) |
ZDV-FF (N = 525) |
p-valuea | |
| Maternal Characteristics | ||||
| Enrollment site, no. (%) | ||||
| Molepolole (village) | 195 (28.2) | 144 (28.6) | 152 (29.0) | < 0.001 |
| Mochudi (village) | 126 (18.2) | 133 (26.4) | 140 (26.7) | |
| Lobatse (town) | 126 (18.2) | 113 (22.5) | 114 (21.7) | |
| Gaborone (city) | 244 (35.3) | 113 (22.5) | 119 (22.7) | |
| Education level, no. (%) | 0.002 | |||
| Primary or none | 146 (21.1) | 132 (26.6) | 156 (30.2) | |
| Secondary | 517 (74.8) | 349 (70.4) | 351 (68.0) | |
| University | 28 (4.1) | 15 (3.0) | 9 (1.7) | |
| Monthly personal incomea, no. (%) | <0.001 | |||
| None | 347 (50.3) | 310 (62.5) | 316 (61.2) | |
| ≤ $100 | 197 (28.6) | 90 (18.2) | 103 (20.2) | |
| > $100 | 146 (21.2) | 96 (19.4) | 97 (18.8) | |
| Age at delivery (years), median | 27.3 | 26.8 | 26.7 | 0.011 |
| Enrollment CD4+ cell count (cells/μL), median | 342 | 390 | 380 | <0.001 |
| Enrollment hemoglobin (mg/dL), median | 10.7 | 10.7 | 10.8 | 0.189 |
| Enrollment HIV-1 RNA (log10 copies/mL), median | 4.17 | 4.33 | 4.28 | 0.0085 |
| Postpartum body mass indexb (kg/m2), median | 22.9 | 23.4 | 23.3 | 0.034 |
| Duration of antenatal antiretroviral treatment (days), median | 81.0 | 39.0 | 39.0 | <0.001 |
| Gestational age at delivery (weeks), median | 39.3 | 39.4 | 39.3 | 0.034 |
Note. BF, breastfeeding; FF, formula-feeding; BMI body mass index.
Chi-square test used for categorical variables and Kruskal-Wallis test for continuous variables.
Inflation-adjusted, 2001 dollars.
Calculated using weight measured 1 month postpartum.
Retention and adherence to scheduled assessments was similar between groups with at least 3 hemoglobin measurements in 91.0%, 89.1%, and 88.8% of infants in the HAART-BF, ZDV-BF, and ZDV-FF groups, respectively.
Incidence of severe anemia (primary outcome measure)
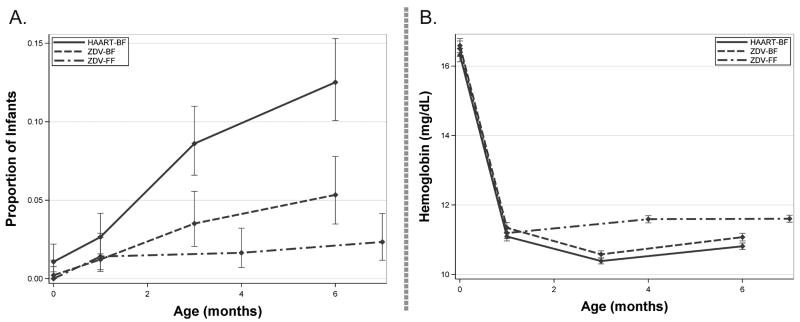
There were 118 (7.4%) infants overall with incident severe anemia from birth through 6 months of age (Table 2). Severe anemia occurred in 82 infants (12.5%, 71 grade 3, and 11 grade 4) in the HAART-BF group, 25 infants (5.3%, 23 grade 3, and 2 grade 4) in the ZDV-BF group, and 11 infants (2.5%, 10 grade 3, and 1 grade 4) in the ZDV-FF group. Severe anemia was more frequent among infants in the HAART-BF group than among infants in either the ZDV-BF group (P<0.001) or the ZDV-FF group (P<0.001). The incidence of severe anemia and the mean hemoglobin at scheduled measurements are plotted in Figure 1.
Table 2. Cumulative incidence of severe anemia.
| Cumulative Incidence of Grade 3 or 4 Anemiaa | Between Group Comparisons | ||||||
|---|---|---|---|---|---|---|---|
| HAART-BF | ZDV-BF | ZDV-FF | HAART-BF vs. (ZDV-BF and ZDV-FF) | ||||
| no. (%) | no. (%) | no. (%) | Odds Ratio (95% CI) |
p-valueb | |||
| Birth | 7/650 (1.1) | 1/466 (0.2) | 0/475 (0) | OR: 10.2 (1.26, 83.4) |
0.007 | ||
| HAART-BF vs. ZDV-BF | HAART-BF vs. ZDV-FF | ||||||
| Odds Ratio (95% CI) |
p-valueb | Odds Ratio (95% CI) |
p-valueb | ||||
| 1 month | 18/677 (2.7) | 6/489 (1.2) | 7/495 (1.4) | OR: 2.20 (0.87, 5.58) |
0.089 | OR: 1.90 (0.79, 4.59) |
0.145 |
| 3 monthsc | 58/674 (8.6) | 17/484 (3.5) | 8/486 (1.7) | OR: 2.59 (1.49, 4.50) |
<0.001 | OR: 5.63 (2.66, 11.9) |
<0.001 |
| 6 monthsc | 82/655 (12.5) | 25/468 (5.3) | 11/469 (2.5) | OR: 2.53 (1.59, 4.04) |
<0.001 | OR: 5.96 (3.14, 11.3) |
<0.001 |
Note. Infants with incident anemia and infants with hemoglobin measurement at scheduled time points are included in the denominator for incidence calculation. Only scheduled hemoglobin measurements within 5 days of birth and 1 month of age, and within 10 days of later time points are included. Hb, hemoglobin; OR, odds ratio.
Figure 1.
Pattern of severe anemia and mean hemoglobin from birth to 7 months of age, according to exposure group. Panel A shows the proportion of infants with severe anemia. Panel B shows the mean measured hemoglobin at scheduled visits. Mean hemoglobin at 6-7 months of age is significantly different by exposure category for all pairwise comparisons (student's t-test with Bonferroni adjustment). Connecting lines are added for interpretability, but measurements are only available for time points indicated by dots. Bars indicate 95% confidence intervals.
Different assessment frequencies between study groups (ZDV-BF had monthly hemoglobin measurements) could bias results if treatment was modified in response to anemia (supplementation, change in antiretroviral treatment, or transfusion) and this prevented subsequent detection at the next scheduled 1, 3-4, or 6-7 month visit. However, we found little evidence of potential bias, as there were no significant differences in the rate of treatment-modifying anemia detected between birth, 1, 3-4, or 6-7 month visits among the study groups (P=0.150)— 28 in HAART-BF (4.1%), 10 in ZDV-BF (2.0%), 17 in ZDV-FF (3.4%).
Risk factors for severe anemia
In addition to the effect of exposure group, there were significant positive associations between low maternal income, male sex and risk of severe infant anemia in univariate analysis. Gestational age at birth was negatively associated with the risk of severe anemia. No significant associations between severe anemia and other maternal or infant characteristics were found, including maternal HIV disease status (Table 3).
Table 3. Factors associated with severe incident anemia among ARV-exposed, HIV-uninfected infants.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P-valuea | aOR (95% CI) | P-valuea |
| Exposure category | ||||
| ZDV-FF | reference | <0.001 | reference | <0.001 |
| HAART-BF | 5.80 (3.05-11.0) | 0.037 | 5.78 (3.04-11.0) | 0.035 |
| ZDV-BF | 2.17 (1.05-4.52) | 2.20 (1.06-4.58) | ||
| Gestational Age (per week) | 0.87 (0.80-0.94) | <0.001 | 0.89 (0.82-0.96) | 0.005 |
| Male Sex | 1.49 (1.01-2.19) | 0.044 | 1.53 (1.03-2.27) | 0.035 |
| Maternal personal income < $100/month | 2.05 (1.13-3.70) | 0.018 | 2.04 (1.12-3.71) | 0.020 |
| Small for Gestational Age (< 10th percentile)b | 1.68 (1.00-2.82) | 0.052 | … | |
| Maternal body mass index (per kg/m2)c | 1.00 (0.95-1.05) | 0.953 | … | |
| Maternal CD4+ cell count (per 10 cells/μL) | 0.99 (0.98-1.00) | 0.232 | … | |
| Maternal HIV-1 viral load (per log10 copies/mL) | 1.03 (0.82-1.29) | 0.811 | … | |
| Maternal hemoglobin (per mg/dL) | 0.95 (0.80-1.11) | 0.500 | … | |
| Village residence | 1.06 (0.73-1.55) | 0.766 | … | |
| HAART regimend | ||||
| Nevirapine/ZDV/3TCe | reference | … | … | |
| Lopinavir/ritonavir/ZDV/3TC | 1.10 (0.53-1.92) | 0.979 | … | |
| Abacavir/ZDV/3TC | 0.74 (0.38-1.43) | 0.375 | … | |
| Duration of antenatal HAART (per week)f | 0.99 (0.92-1.06) | 0.748 | … | |
NOTE. Only significant factors (p<0.05) were retained in multivariate model. aOR, adjusted odds ratio; BF, breastfeeding; CI, confidence interval; FF, formula-feeding; HAART, highly-active antiretroviral therapy; HIV-1, human immunodeficiency virus, type I; ZDV, zidovudine; 3TC, lamivudine.
Wald chi-square.
Botswana normative tables [Botswana Harvard AIDS Institute Partnership, unpublished data]
Calculated using maternal weight measured one-month postpartum.
Analysis for maternal HAART is restricted to only HAART-BF group.
Includes one infant who mother took stavudine, lamivudine, and nevirapine for antenatal HAART.
To avoid confounding effect of prematurity, analysis for duration of antenatal HAART is restricted to term infants in the HAART-BF group.
In multivariate analysis, antiretroviral and feeding exposure group was identified as the strongest risk factor for severe infant anemia from birth to 6 months of age. Infants in the HAART-BF group had 2.6-fold greater odds of severe anemia as similarly breastfed but HAART-unexposed infants taking 6 months of postnatal zidovudine in the ZDV-BF group. HAART-BF infants had 5.8-fold greater odds of developing severe anemia compared with ZDV-FF infants. Decreased gestational age at birth, and male sex were also significantly associated with severe anemia (Table 3).
Outcomes of incident severe anemia
The majority of the episodes of severe anemia were asymptomatic and resolved with multivitamin and iron supplementation, or cessation of zidovudine exposure. Median time to improvement to less than grade 3 was 33 days (interquartile range, 16 to 62 days). Severe anemia persisted for greater than 90 days despite interventions for 18 (17.0%) anemic infants. In addition, 11 infants required transfusion (all in HAART-BF group). None of the transfused infants died prior to 6 months of age.
Severe anemia may have contributed to the death of 6 infants (0.3%)— 1 HAART-BF infant, 2 ZDV-BF infants, and 3 ZDV-FF infants. Severe anemia was reported as the cause of death for 3 of these infants. Alternative causes of death were reported for the remaining 3 infants (2 gastroenteritis, 1 unknown), but unresolved severe anemia may have contributed. Two infants were lost to follow-up prior to resolution of severe anemia and their vital status could not be determined.
Red blood cell morphology
The MCV was similar between the exposure groups at 1 month of age, however the change from 1 month to 6 months differed (P<0.001). Consistent with the observed relationship between zidovudine and macrocytosis,37-39 infants exposed to 6 months of postnatal zidovudine (ZDV-BF) experienced a mean gain in their standardized MCV (z-score) of +2.25 (P<0.001). In contrast, infants in the HAART-BF group with more limited zidovudine exposure progressed towards microcytosis with mean change in their MCV z-score of -0.60 (P<0.001). Infants in the ZDV-FF group, receiving iron-fortified infant formula, had a modest increase in their mean MCV z-score of +0.27 from 1 to 7 months of age (P<0.001).
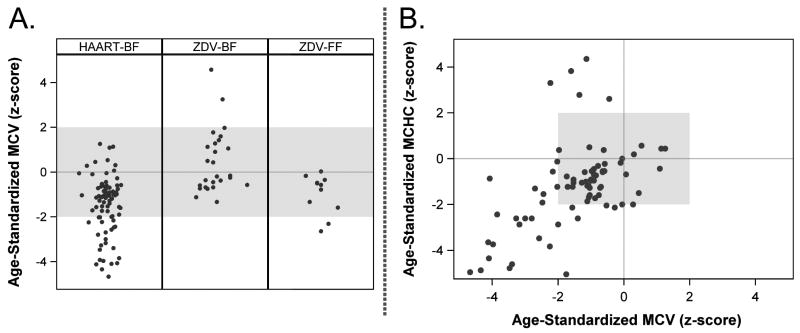
At the time of severe anemia, microcytosis was more common than macrocytosis, observed in 21.2% and 2.5% of infants, respectively. Similarly hypochromia was present in 29.3% of incident anemias with available MCHC measurement (see Figure 2). At the time of incident anemia the mean MCV z-score was -0.91 (95% CI -1.25 to -0.59) and the mean MCHC z-score was -1.31 (95% CI -1.77 to -0.84).
Figure 2.
Standardized mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) at the time of incident severe anemia. Panel A shows distribution of age-standardized MCV measurements by exposure group. Panel B displays a plot age-standardized MCV against age-standardized MCHC for incident anemias in the HAART-BF group. Shaded regions in both panels represent normal values. Age-specific normative data 35, 36 was used to calculate standardized MCV and MCHC (z-score) for incident cases of anemia.
Estimated hemoglobin iron at birth, the largest iron compartment of total body iron,40 was lower for HAART-BF, than for ZDV-BF, and ZDV-FF infants— 133g, 143g, and 142g, respectively (P<0.001).
Discussion
In this study of infants born to HIV-infected mothers in Botswana, exposure to maternal HAART was associated with a significantly higher risk of severe anemia during the first 6 months of life compared with exposure to maternal zidovudine alone. Among breastfeeding infants, assignment to in utero and breast milk HAART exposure (HAART-BF) was associated with an estimated 2.6-fold increased odds of severe anemia compared with infants assigned to in utero and 6 months of postnatal zidovudine exposure (ZDV-BF). Formula-fed infants assigned to in utero and 1 month of zidovudine (ZDV-FF) experienced the lowest risk of anemia, 5.8-fold and 2.2-fold decreased odds compared with HAART-BF and ZDV-BF, respectively.
The results of this study are in agreement with the findings of observational studies of largely formula-feeding cohorts in the United States and Europe that have demonstrated lower hemoglobin concentrations among infants exposed to in utero HAART compared with infants exposed to monotherapy or no in utero antiretrovirals.22-24, 41 Calculating from the incidence of anemia reported for a cohort of 215 German infants born in a German,41 HAART-exposed infants had 3.9-fold increased odds of severe anemia compared with infants exposed to mono- or dual-antiretroviral therapy, similar to the findings in our study. Our study, utilizing a controlled design, confirms findings reported in observational studies and extends the findings to African and breastfeeding populations.
An earlier analysis by Bae et al.42 comparing toxicity in a subset of Mashi study infants exposed and unexposed to maternal HAART did not identify a signal for increased risk of severe anemia with HAART exposure, and recommended larger studies. The apparent contradiction with our present findings can be understood by considering differences in sample size, inclusion criteria, and toxicity definitions. The Bae et al.analysis included only 178 infants (compared with 1719 infants in the present analysis), and had consequently had reduced power to detect differences. The earlier (but not the current) analysis included HIV-infected infants, who are at increased risk for severe anemia.34 Maternal HAART potently prevented HIV-infection in Mashi, potentially biasing results towards null of no effect of maternal HAART on severe infant anemia. Most importantly, the Bae et al. analysis used the prior 1994 version of the DAIDS toxicity table43, 44 which did not incorporate the expected hemoglobin recovery from the physiologic nadir of infancy. Re-grading the 178 infants in the Bae et al. cohort using the 2004 DAIDS toxicity table33 and excluding HIV-infected infants, exposes a pattern similar to the present analysis— 11.9% of HAART-exposed infants and 5.9% of ZDV-monotherapy-exposed infants developed severe anemia through 7 months of age (P=0.185).
The mechanism for the observed increase in severe anemia due to maternal HAART exposure is uncertain. Infants in the HAART-BF and ZDV-BF groups appeared to experience a deeper and more prolonged physiologic nadir in hemoglobin. Infants in the HAART-BF group were exposed to in utero zidovudine for a median of 11.5 weeks compared to 5.6 weeks in the ZDV-FF and ZDV-BF groups, but direct infant ZDV receipt occurred for a longer period in the ZDV-BF group. If zidovudine were the principal cause of severe anemia, we would have expected the greatest incidence of severe anemia in the ZDV-BF group exposed to 6 months of postnatal zidovudine, particularly given minimal exposure to zidovudine via breast milk in the HAART-BF group.45 The fact that the HAART-BF group had more severe anemias suggests that either the timing of exposure or the combination of ZDV with other antiretrovirals may be important considerations. In a randomized trial in Malawi of postnatal maternal HAART, rates of severe anemia were similar between infants with exposure to HAART via breast milk and those without exposure to antiretrovirals in breast milk,15 suggesting that it is in utero exposure that increases risk for anemia. The addition of lamivudine may also promote the hematologic toxicity of ZDV as suggested by several case reports46-48 and one controlled trial49 in adults. However, other larger controlled trials have not reported this effect.50, 51
The type of infant anemias that we observed is noteworthy. Despite universal exposure to zidovudine, which promotes macrocytosis, microcytic (and hypochromic) anemia were common, particularly among HAART-exposed infants, suggesting a role of iron-deficiency. Mothers in both the Mashi and Mma Bana studies were provided iron supplementation as part of antenatal care. It is possible that HAART-exposure could increase vulnerability of hematopoiesis to nutrient deficiencies, infection, or other insults. Additionally, HAART could impair the transfer of iron from mother to fetus as suggested by decreased total hemoglobin iron estimated in HAART-BF infants. The observed morphology of infant red blood cells and the decreased hemoglobin iron suggest that infant iron supplementation should be considered.
This study is subject to several limitations. The assigned exposures of the Mashi and Mma Bana trials preclude separation of antiretroviral and feeding effects, since almost all HAART-exposed infants were breastfed. A comparator group of HIV-exposed infants without exposure to antiretroviral treatment would be informative but unethical. Our analysis cannot separate contributions of antenatal and post-natal antiretroviral exposure towards anemia. Another potential limitation of our analysis was the difference in study timing— HAART-BF infants were from the Mma Bana study whereas the ZDV-BF and ZDV-FF groups were from the earlier Mashi study. However, mothers assigned to HAART were more likely to have higher incomes, more education, and live in urban areas, reflecting the economic development in the years between Mashi and Mma Bana. Consequently, the effect of measured or unmeasured socioeconomic and nutritional factors would be expected to be favorable to HAART-BF infants, decreasing their incidence of severe anemia. Additionally, measured differences in maternal or infant characteristics between comparator groups were controlled for in multivariate analyses, and indeed some of these factors (such as small for gestational age and prematurity) may be associated with in utero HAART exposure.52-54 For these reasons, our findings may underestimate the impact of maternal HAART on the incidence of severe early infant anemia. Nevertheless, our study design cannot conclusively establish causality between in utero HAART exposure and infant anemia, and further study of this important question is warranted.
The clinical implications of our findings also require further study. The majority of infants with severe anemia in the Mashi and Mma Bana trials did not have symptoms reported. Observational studies in Africa suggest that severe anemia is associated with increased mortality, however these studies have typically used a much lower hemoglobin threshold (<5.0 mg/dL) to define severe anemia28 than was used in this study. Our analysis used the DAIDS toxicity table definitions for severe anemia— less than 10 mg/dL at birth, 8 mg/dL at 1 month, and 9 mg/dL at 3 and 6 months.33 Data to describe the impact of less severe anemia are scant. In Tanzania, using a similar hemoglobin threshold of < 8.5 mg/dL, HIV-exposed infants with iron-deficiency anemia were approximately twice as likely to die prior to 2 years of age.55 Anemia may have contributed to 6 infant deaths in our study, but only one of these infants was HAART-exposed in utero. It is possible that routine screening and access to transfusion prevented additional deaths, particularly among the HAART-exposed infants.
While our analysis focused on severe anemia, HAART-BF infants also had lower hemoglobin measurements at 3 and 6 months and were more likely to have any grade of anemia. Mild or moderate anemia due to iron deficiency is of significant public health importance due to its association with impaired child development.56 It remains uncertain whether anemia in the absence of iron deficiency impairs child development,57 however the possibility of an adverse effect of maternal HAART, mediated via anemia, on subsequent infant development should be explored.
In summary, we have shown that maternal HAART started in pregnancy and continued during breastfeeding was associated with increased severe infant anemia. Confirmation of this finding and the etiology of anemia in HAART-exposed infants deserve further study. Given the established benefits of HAART for the prevention of MTCT11-14, 58 and promotion of maternal health,59 the clinical implications of laboratory-detected toxicity should be considered carefully. Mitigating strategies such as iron-supplementation to HIV-exposed breastfed infants or alternative antiretrovirals should be evaluated to maximize the benefits of maternal HAART while minimizing potential risks.
Supplementary Material
Figure A, Digital Supplemental Content 1. Schematic of maternal and infant exposures in the Mashi and Mma Bana studies forming the study groups. ZDV zidovudine, sdNVP single-dose nevirapine, 3TC lamivudine, rtv ritonavir, NVP nevirapine, BF breastfeeding. FF formula feeding, HAART, highly-active antiretroviral therapy.
a HAART became available through a national program in October 2002 (19 months into study), subsequently women in the Mashi study with CD4 count less than 200 cells/mm3 (or AIDS-defining illness) were offered HAART. Infants born to these women are excluded from the analysis.
b Mothers received supplemental zidovudine during labor and delivery.
c Infants were randomized to either sdNVP or placebo until August 2002 (17 months into study), after which protocol was modified to provide sdNVP to all infants.
Figure B, Digital Supplemental Content 2. Enrollment, exposure assignment, and follow-up of study population. ZDV zidovudine, FF formula-feeding, BF breastfeeding, HAART highly-active antiretroviral therapy.
a A second-born twin whose first-born sibling was stillborn is included in the analysis
b Does not include a second-born, HIV-infected twin in the Mma Bana study.
c Includes two HIV-infected infants exposed to antenatal HAART.
Acknowledgments
We are indebted to the participating patients and staff of the Mashi and Mma Bana studies, to the support of the Botswana Ministry of Health, and to our collaborating partners in the clinics and hospitals of Molepolole, Lobatse, Gaborone, and Mochudi. In addition, we thank the comments and suggestions offered by Drs. Parth Mehta, Kenneth McIntosh, and Heather Ribaudo and the programming assistance of Jean Leidner.
Financial Support: Work supported by grants from the Fogarty International Center (R24 TW007988), National Institute of Allergy and Infectious Diseases (UO1 AI066454), Eunice Kennedy Shriver National Institute of Child Health and Human Development (RO1 HD37793), Harvard Institute for Global Health (grant to S.D.P and K.P.).
Footnotes
Presented in part: 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, February 16-19, 2010. Fogarty International Clinical Research Scholars and Fellows and Doris Duke Charitable Foundation Alumni Symposium, Potomac, MD, September 24-26, 2010.
Potential conflicts of interest: M.D.H. is a paid member of Data and Safety Monitoring Boards for Boehringer Ingelheim, Medicines Development, Pfizer and Tibotec. The remaining authors declare that they do not have commercial or other association that might pose a conflict of interest.
References
- 1.Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report, November 2009. Geneva: World Health Organization, UNAIDS, UNICEF; 2009. [Google Scholar]
- 2.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994 Nov 3;331(18):1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 3.Achievements in public health Reduction in perinatal transmission of HIV infection--United States, 1985-2005. MMWR Morb Mortal Wkly Rep. 2006 Jun 2;55(21):592–597. [PubMed] [Google Scholar]
- 4.Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004 Jul 15;351(3):217–228. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 5.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999 Sep 4;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 6.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002 Apr 15;29(5):484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Dorenbaum A, Cunningham CK, Gelber RD, et al. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. Jama. 2002 Jul 10;288(2):189–198. doi: 10.1001/jama.288.2.189. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010 Jun 17;362(24):2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO . Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants - 2010 version. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 10.Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. Aids. 2007 Jul;21 4:S65–71. doi: 10.1097/01.aids.0000279708.09180.f5. [DOI] [PubMed] [Google Scholar]
- 11.Kilewo C, Karlsson K, Ngarina M, et al. MITRA Plus: Prevention of mother-to-child transmission for HIV-1 through breastfeeding by treating mothers prophylactically with triple antiretroviral therapy in Dar es Salaam, Tanzania. Paper presented at: 4th IAS Conference on HIV Pathogenesis, Treatment, and Prevention2007; Sydney, Australia. [Google Scholar]
- 12.Arendt V, Ndimubanzi P, Vyankandonera J, et al. AMATA study: effectiveness of antiretroviral therapy in breastfeeding mothers to prevent post-natal vertical transmissionin Rwanda. Paper presented at: IAS2007; Sydney, Australia. [Google Scholar]
- 13.Thomas T, Masaba R, Ndivo R, et al. Prevention of mother-to-child transmission of HIV-1 among breastfeeding mothers using HAART: The Kisumu Breastfeeding Study, Kisumu, Kenya, 2003–2007. Paper presented at: 15th Conference on Retroviruses and Opportunistic Infections2008; Boston. [Google Scholar]
- 14.de Vincenzi I, Kesho Bora Study Group Triple-antiretroviral (ARV) prophylaxis during pregnancy and breastfeeding compared to short-ARV prophylaxis to prevent mother-to-child transmission of HIV-1 (MTCT): the Kesho Bora randomized controlled clinical trial in five sites in Burkina Faso, Kenya, and South Africa. Paper presented at: Fifth International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention2009; Cape Town, South Africa. [Google Scholar]
- 15.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010 Jun 17;362(24):2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moh R, Danel C, Sorho S, et al. Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with cotrimoxazole in Cote d'Ivoire. Antivir Ther. 2005;10(5):615–624. doi: 10.1177/135965350501000510. [DOI] [PubMed] [Google Scholar]
- 17.Lahoz R, Noguera A, Rovira N, et al. Antiretroviral-Related Hematologic Short-term Toxicity in Healthy Infants: Implications of the New Neonatal 4-Week Zidovudine Regimen. Pediatr Infect Dis J. 2009 Nov 25; doi: 10.1097/INF.0b013e3181c81fd4. [DOI] [PubMed] [Google Scholar]
- 18.Majluf-Cruz A, Luna-Castanos G, Trevino-Perez S, Santoscoy M, Nieto-Cisneros L. Lamivudine-induced pure red cell aplasia. Am J Hematol. 2000 Nov;65(3):189–191. doi: 10.1002/1096-8652(200011)65:3<189::aid-ajh2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.John MA, Rhemtula YA, Menezes CN, Grobusch MP. Lamivudine-induced red cell aplasia. J Med Microbiol. 2008 Aug;57(Pt 8):1032–1035. doi: 10.1099/jmm.0.47782-0. [DOI] [PubMed] [Google Scholar]
- 20.McKinney RE, Jr, Maha MA, Connor EM, et al. A multicenter trial of oral zidovudine in children with advanced human immunodeficiency virus disease. The Protocol 043 Study Group. N Engl J Med. 1991 Apr 11;324(15):1018–1025. doi: 10.1056/NEJM199104113241503. [DOI] [PubMed] [Google Scholar]
- 21.Prazuck T, Semaille C, Roques S. Fatal acute haemolysis in an AIDS patient treated with indinavir. Aids. 1998 Mar 26;12(5):531–533. [PubMed] [Google Scholar]
- 22.Fernandez Ibieta M, Ramos Amador JT, Gonzalez Tome MI, et al. Anaemia and neutropenia in a cohort of non-infected children of HIV-positive mothers. An Pediatr (Barc) 2008 Dec;69(6):533–543. doi: 10.1016/s1695-4033(08)75236-6. [DOI] [PubMed] [Google Scholar]
- 23.Le Chenadec J, Mayaux MJ, Guihenneuc-Jouyaux C, Blanche S. Perinatal antiretroviral treatment and hematopoiesis in HIV-uninfected infants. AIDS. 2003 Sep 26;17(14):2053–2061. doi: 10.1097/00002030-200309260-00006. [DOI] [PubMed] [Google Scholar]
- 24.Pacheco SE, McIntosh K, Lu M, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: An analysis of the women and infants transmission study. J Infect Dis. 2006 Oct 15;194(8):1089–1097. doi: 10.1086/507645. [DOI] [PubMed] [Google Scholar]
- 25.Bunders MJ, Bekker V, Scherpbier HJ, Boer K, Godfried M, Kuijpers TW. Haematological parameters of HIV-1-uninfected infants born to HIV-1-infected mothers. Acta Paediatr. 2005 Nov;94(11):1571–1577. doi: 10.1080/08035250510042951. [DOI] [PubMed] [Google Scholar]
- 26.Calis JC, Phiri KS, Faragher EB, et al. Severe anemia in Malawian children. N Engl J Med. 2008 Feb 28;358(9):888–899. doi: 10.1056/NEJMoa072727. [DOI] [PubMed] [Google Scholar]
- 27.Totin D, Ndugwa C, Mmiro F, Perry RT, Jackson JB, Semba RD. Iron deficiency anemia is highly prevalent among human immunodeficiency virus-infected and uninfected infants in Uganda. J Nutr. 2002 Mar;132(3):423–429. doi: 10.1093/jn/132.3.423. [DOI] [PubMed] [Google Scholar]
- 28.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001 Feb;131(2S-2):636S–645S. doi: 10.1093/jn/131.2.636S. discussion 646S-648S. [DOI] [PubMed] [Google Scholar]
- 29.Miller MF, Humphrey JH, Iliff PJ, Malaba LC, Mbuya NV, Stoltzfus RJ. Neonatal erythropoiesis and subsequent anemia in HIV-positive and HIV-negative Zimbabwean babies during the first year of life: a longitudinal study. BMC Infect Dis. 2006;6:1. doi: 10.1186/1471-2334-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro RL, Thior I, Gilbert PB, et al. Maternal single-dose nevirapine versus placebo as part of an antiretroviral strategy to prevent mother-to-child HIV transmission in Botswana. Aids. 2006 Jun 12;20(9):1281–1288. doi: 10.1097/01.aids.0000232236.26630.35. [DOI] [PubMed] [Google Scholar]
- 31.Thior I, Lockman S, Smeaton LM, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006 Aug 16;296(7):794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 32.Saarinen UM, Siimes MA. Iron absorption from breast milk, cow's milk, and iron-supplemented formula: an opportunistic use of changes in total body iron determined by hemoglobin, ferritin, and body weight in 132 infants. Pediatr Res. 1979 Mar;13(3):143–147. doi: 10.1203/00006450-197903000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0, December 2004. [November 19, 2010];2004 http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf.
- 34.Calis JC, van Hensbroek MB, de Haan RJ, Moons P, Brabin BJ, Bates I. HIV-associated anemia in children: a systematic review from a global perspective. Aids. 2008 Jun 19;22(10):1099–1112. doi: 10.1097/QAD.0b013e3282fa759f. [DOI] [PubMed] [Google Scholar]
- 35.Matoth Y, Zaizov R, Varsano I. Postnatal changes in some red cell parameters. Acta Paediatr Scand. 1971 May;60(3):317–323. doi: 10.1111/j.1651-2227.1971.tb06663.x. [DOI] [PubMed] [Google Scholar]
- 36.Saarinen UM, Siimes MA. Developmental changes in red blood cell counts and indices of infants after exclusion of iron deficiency by laboratory criteria and continuous iron supplementation. J Pediatr. 1978 Mar;92(3):412–416. doi: 10.1016/s0022-3476(78)80429-6. [DOI] [PubMed] [Google Scholar]
- 37.Ferrazin A, De Maria A, Gotta C, et al. Zidovudine therapy of HIV-1 infection during pregnancy: assessment of the effect on the newborns. J Acquir Immune Defic Syndr. 1993 Apr;6(4):376–379. [PubMed] [Google Scholar]
- 38.Richman DD, Fischl MA, Grieco MH, et al. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- 39.Romanelli F, Empey K, Pomeroy C. Macrocytosis as an indicator of medication (zidovudine) adherence in patients with HIV infection. AIDS Patient Care STDS. 2002 Sep;16(9):405–411. doi: 10.1089/108729102760330245. [DOI] [PubMed] [Google Scholar]
- 40.Miller MF, Stoltzfus RJ, Mbuya NV, Malaba LC, Iliff PJ, Humphrey JH. Total body iron in HIV-positive and HIV-negative Zimbabwean newborns strongly predicts anemia throughout infancy and is predicted by maternal hemoglobin concentration. J Nutr. 2003 Nov;133(11):3461–3468. doi: 10.1093/jn/133.11.3461. [DOI] [PubMed] [Google Scholar]
- 41.Feiterna-Sperling C, Weizsaecker K, Buhrer C, et al. Hematologic effects of maternal antiretroviral therapy and transmission prophylaxis in HIV-1-exposed uninfected newborn infants. J Acquir Immune Defic Syndr. 2007 May 1;45(1):43–51. doi: 10.1097/QAI.0b013e318042d5e3. [DOI] [PubMed] [Google Scholar]
- 42.Bae WH, Wester C, Smeaton LM, et al. Hematologic and hepatic toxicities associated with antenatal and postnatal exposure to maternal highly active antiretroviral therapy among infants. Aids. 2008 Aug 20;22(13):1633–1640. doi: 10.1097/QAD.0b013e328307a029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Divsion of AIDS (DAIDS) Table for Grading Severity of Pediatric (< 3 Months of Age) Adverse Experiences. [November 19, 2010];1994 http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Pediatric_AEs_Under3MonthsAge_v02.pdf.
- 44.Divsion of AIDS (DAIDS) Table for Grading Severity of Pediatric (> 3 Months of Age) Adverse Experiences. [November 19, 2010];1994 http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Pediatric_AEs_Over3MonthsAge_v03.pdf.
- 45.Mirochnick M, Thomas T, Capparelli E, et al. Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 2009 Mar;53(3):1170–1176. doi: 10.1128/AAC.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weitzel T, Plettenberg A, Albrecht D, Lorenzen T, Stoehr A. Severe anemia as a newly recognized side-effect caused by lamivudine. Aids. 1999 Nov 12;13(16):2309–2311. doi: 10.1097/00002030-199911120-00018. [DOI] [PubMed] [Google Scholar]
- 47.Hester EK, Peacock JE., Jr Profound and unanticipated anemia with lamivudine-zidovudine combination therapy in zidovudine-experienced patients with HIV infection. Aids. 1998 Mar 5;12(4):439–440. [PubMed] [Google Scholar]
- 48.Tseng A, Conly J, Fletcher D, Keystone D, Salit I, Walmsley S. Precipitous declines in hemoglobin levels associated with combination zidovudine and lamivudine therapy. Clin Infect Dis. 1998 Oct;27(4):908–909. doi: 10.1086/517169. [DOI] [PubMed] [Google Scholar]
- 49.Katlama C, Ingrand D, Loveday C, et al. Safety and efficacy of lamivudine-zidovudine combination therapy in antiretroviral-naive patients. A randomized controlled comparison with zidovudine monotherapy. Lamivudine European HIV Working Group. Jama. 1996 Jul 10;276(2):118–125. [PubMed] [Google Scholar]
- 50.Eron JJ, Benoit SL, Jemsek J, et al. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. North American HIV Working Party. N Engl J Med. 1995 Dec 21;333(25):1662–1669. doi: 10.1056/NEJM199512213332502. [DOI] [PubMed] [Google Scholar]
- 51.Staszewski S, Loveday C, Picazo JJ, et al. Safety and efficacy of lamivudine-zidovudine combination therapy in zidovudine-experienced patients. A randomized controlled comparison with zidovudine monotherapy. Lamivudine European HIV Working Group. Jama. 1996 Jul 10;276(2):111–117. [PubMed] [Google Scholar]
- 52.Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. Aids. 2004 Nov 19;18(17):2337–2339. doi: 10.1097/00002030-200411190-00019. [DOI] [PubMed] [Google Scholar]
- 53.Tuomala RE, Watts DH, Li D, et al. Improved obstetric outcomes and few maternal toxicities are associated with antiretroviral therapy, including highly active antiretroviral therapy during pregnancy. J Acquir Immune Defic Syndr. 2005 Apr 1;38(4):449–473. doi: 10.1097/01.qai.0000139398.38236.4d. [DOI] [PubMed] [Google Scholar]
- 54.Machado ES, Hofer CB, Costa TT, et al. Pregnancy outcome in women infected with HIV-1 receiving combination antiretroviral therapy before versus after conception. Sex Transm Infect. 2009 Apr;85(2):82–87. doi: 10.1136/sti.2008.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chatterjee A, Bosch RJ, Kupka R, Hunter DJ, Msamanga GI, Fawzi WW. Predictors and consequences of anaemia among antiretroviral-naive HIV-infected and HIV-uninfected children in Tanzania. Public Health Nutr. 2009 Aug 4;:1–8. doi: 10.1017/S1368980009990802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.WHO . Iron deficiency anaemia: Assessment, Prevention, and Control. Geneva: 2001. [Google Scholar]
- 57.Stoltzfus RJ. Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. Summary: implications for research and programs. J Nutr. 2001 Feb;131(2S-2):697S–700S. doi: 10.1093/jn/131.2.697S. discussion 700S-701S. [DOI] [PubMed] [Google Scholar]
- 58.Chasela C, Hudgens M, Jamieson D, et al. Both maternal HAART and daily infant nevirapine (NVP) are effective in reducing HIV-1 transmission during breastfeeding in a randomized trial in Malawi: 28 week results of the Breastfeeding, Antiretroviral and Nutrition (BAN) Study. Paper presented at: Fifth International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention2009; Cape Town, South Africa. [Google Scholar]
- 59.Marazzi MC, Palombi L, Nielsen-Saines K, et al. Favorable pregnancy outcomes with reduction of abortion, stillbirth, and prematurity rates in a large cohort of HIV+ woemn in Southern Africa receiving highly active antiretroviral therapy (HAART) for preventio of mother-child-transmission (PMTCT). 5th IAS Conference on HIV Treatment, Pathogenesis and Prevention; Cape Town, South Africa. 2009. Vol. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A, Digital Supplemental Content 1. Schematic of maternal and infant exposures in the Mashi and Mma Bana studies forming the study groups. ZDV zidovudine, sdNVP single-dose nevirapine, 3TC lamivudine, rtv ritonavir, NVP nevirapine, BF breastfeeding. FF formula feeding, HAART, highly-active antiretroviral therapy.
a HAART became available through a national program in October 2002 (19 months into study), subsequently women in the Mashi study with CD4 count less than 200 cells/mm3 (or AIDS-defining illness) were offered HAART. Infants born to these women are excluded from the analysis.
b Mothers received supplemental zidovudine during labor and delivery.
c Infants were randomized to either sdNVP or placebo until August 2002 (17 months into study), after which protocol was modified to provide sdNVP to all infants.
Figure B, Digital Supplemental Content 2. Enrollment, exposure assignment, and follow-up of study population. ZDV zidovudine, FF formula-feeding, BF breastfeeding, HAART highly-active antiretroviral therapy.
a A second-born twin whose first-born sibling was stillborn is included in the analysis
b Does not include a second-born, HIV-infected twin in the Mma Bana study.
c Includes two HIV-infected infants exposed to antenatal HAART.