Abstract
During puberty, human adolescents develop a later chronotype, exhibiting a delay in the timing of rest and activity as well as other daily physiological rhythms. The purpose of this study was to determine whether similar changes in chronotype occur during puberty in a laboratory rodent species, and, if so, to determine whether they are due to pubertal hormones acting on the circadian timekeeping system. To test this hypothesis, we carefully tracked daily activity rhythms across puberty in the slow-developing rodent Octodon degus. We confirmed that male degus showed a large reorganization of activity rhythms that correlated with secondary sex development during puberty, including a loss of bimodality and 3–5 hr phase-advance. Similar to humans, this circadian reorganization showed distinct sex differences, with females showing little change during puberty in two separate experiments. Prepubertal gonadectomy (GDX) eliminated the changes, whereas SHAM gonadectomy had little impact. Therefore, gonadal hormones are likely to play a role in pubertal changes in chronotype in this rodent species. Using evidence from a variety of species, including our recent studies in the rat, we conclude that chronotype changes during puberty are a well-demonstrated phenomenon in mammals.
Keywords: adolescent, diurnal, phase, juvenile, bimodal, sex, crepuscular, circadian
Circadian rhythms in mammals are generated by an endogenous pacemaker (Ralph et al., 1990) that must be entrained by external time cues (or “zeitgebers”) such as light to maintain a stable phase relationship with the outside world (Moore-Ede et al., 1982). The phase relationship between the solar day and the circadian system’s output rhythms, such as daily rest/activity and hormonal cycles, is used to characterize an individual’s chronotype. In humans, we know that chronotype changes during adolescence, such that individuals develop a propensity towards “night-owl” behavior, exhibiting a later, or delayed, timing of many physiological rhythms including rest and activity (Crowley et al., 2007; Roenneberg et al., 2004; Thorleifsdottir et al., 2002; Yang et al. 2005). These developmental changes in chronotype are likely to be partially rooted in hormonal influences on the body’s circadian timekeeping system (Carskadon et al., 2004; Carskadon et al., 1997). Adolescent changes in chronotype correlate with secondary-sex development even after taking into account age and related social influences (Carskadon et al., 1993; Sadeh et al. 2009) and exhibit sex differences in timing and magnitude (Roenneberg et al., 2004). The purpose of this study, as well as another study published in the same issue of Hormones and Behavior (Hagenauer et al., pp. TBA), is to determine whether similar changes in chronotype can be reliably observed during puberty in laboratory rodent species, and, if so, to elucidate their physiological mechanism.
It is already well-documented that the circadian system is sensitive to gonadal hormones during early development and adulthood. The timing of daily rhythms is shifted during different stages of the menstrual cycle (Manber and Bootzin, 1997; Parry et al., 1994; Parry et al., 2000). In adult laboratory rodents, the gonadal hormones that affect the phase and period of circadian rhythms include estrogens, progestins, androgens, and non-traditional neuroactive steroids (e.g., Albers, Gerall, and Axelson 1981; Axelson, Gerall, and Albers, 1981; Davis, Darrow, and Menaker, 1983; Morin, Fitzgerald, and Zucker, 1977; de Tezanos Pinto and Golombek, 1999; Daan, Damassa, Pittendrigh, and Smith, 1975; Karatsoreos, Wang, Sasanian, Silver, 2007; Iwahana, Karatsoreos, Shibata, and Silver, 2008; Labyak and Lee, 1995; Jechura, Walsh, and Lee, 2000). During the perinatal period, gonadal hormones can produce organizational changes in the circadian system of fast-developing, altricial rodents, leading to adult sex differences in circadian sensitivity to gonadal hormones (Albers 1981; Zucker, Fitzgerald and Morin, 1980).
The influence of gonadal hormones on the circadian system during puberty is less understood, although it is known that pubertal hormones can alter activity rhythm phasing (Hummer et al., 2007) as well as produce organizational effects on the circadian system (Davis, Darrow, and Menaker, 1983). Indeed, in the degu, there is even a critical window of sensitivity to the organizational effects of gonadal hormones as late as young adulthood (Hummer et al., 2006). There has been little attempt to determine how common adolescent changes in chronotype are across mammalian species or to elucidate their hormonal or neural bases using animal models (Hagenauer et al., 2009). Evidence from five species suggests that pubertal changes in circadian phase are not uniquely human (rhesus macaque: Golub and Takeuchi 2002; mouse: Weinert et al., 1994; Weinert and Waterhouse, 1999; rat: McGinnis et al., 2007; Kittrell and Satinoff, 1986; degu: Tate, Richardson, and Carskadon, 2002; Hummer et al., 2007; Psammomys obsesus: Neuman et al., 2005). However, only three of the studies (using the slow-developing, diurnal species of the macaque and degu) have attempted to thoroughly characterize the full developmental progression of circadian phase change in relation to secondary-sex development (Golub and Takeuchi, 2002; Tate, Richardson, and Carskadon, 2002; Hummer et al., 2007), and only one study directly examined the role of pubertal hormones (Hummer et al., 2007). Similar to humans, the macaque and degu show a delayed circadian phase during puberty (around the time of first menarche in the rhesus macaque, and first vaginal or prepucial opening in the degu) that reverses by adulthood. This developmental change does not occur following pre-pubertal gonadectomy (Hummer et al., 2007). Therefore, pubertal elevations in sex hormones are likely to drive circadian phase changes.
This current study is designed to follow-up on previous work in the degu with more complete measurements of circadian rhythm phase and activity distribution across the pubertal period and additional experimental controls of circadian phase-influencing environmental variables (e.g., lighting intensity). The previous study regarding hormonal dependency only examined activity rhythm phase at one mid-pubertal time point (Hummer et al., 2007). We sampled more frequently across the pre-pubertal and pubertal periods to gain a better understanding of how pubertal changes in chronotype in degus compare to those observed in other well-characterized species (human; Roenneberg et al., 2004; Thorleifsdottir et al., 2002; macaque: Golub and Takeuchi, 2002). This also allowed for a careful examination of any sex differences present in the timing or magnitude of the phenomenon, such as those seen in humans (Roenneberg et al., 2004). Finally, we wished to confirm that the lack of developmental change observed in animals gonadectomized prior to puberty in the previous study (Hummer et al., 2007) was not merely due to a developmental delay, placing circadian phase changes outside of the sampling window.
The degu is a particularly useful animal model for examining the role of pubertal hormones in chronotype development for several reasons. Unlike other commonly-used laboratory rodents, degu females exhibit spontaneous, long reproductive cycles containing both follicular and luteal phases (Mahoney et al., in press) similar to that of human females. More importantly, degus are precocial, meaning that they are born with their eyes open, fully furred, and ambulatory, but they progress through puberty more slowly than most laboratory rodent species. Young degus consume solid food and emerge from their burrows within the first weeks of life (Reynolds and Wright, 1979; Jesseau 2004), but the pubertal period does not begin until after weaning at ~4–7 weeks of age (Reynolds and Wright, 1979; Kenagy, Place and Veloso, 1999). Males and females begin to exhibit secondary sex characteristics between 2–3.5m of age, with full maturity reached by 5–6m (Hummer et al., 2007; Kenagy, Place, and Veloso, 1999; Soto-Gamboa, 2004, as cited in Soto-Gamboa, 2005). Thus, the chronotype changes that occur during puberty in this species can be dissociated from other well-characterized developmental changes in the circadian system that have been described for juvenile altricial rodents gaining independence from the dam (Weinert et al., 2005).
Methods
General Methods
Subjects
The degus were obtained from a breeding colony at the University of Michigan. All animal handlings including cage changes were performed at random times during the lighted period of the day. All procedures for the housing and handling of the degus were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Breeding degus were kept in large (42.5 × 46 × 19.5 cm) transparent acrylic cages containing one sire, two dams, and their respective young. One side of each cage was kept on a heating pad at low heat in a room maintained at 20 +/− 1°C with a 12:12-h light-dark (LD) cycle (light intensity 250 lx). During breeding, pregnancy, and early development (0–90 days of age) the breeding animals and pups were provided ad libitum with Prolab Laboratory Animal Diet Product 5P06 and acidified water (2.5 × 10−5% HCl) to prevent bacterial digestive infections. They were also given generous handfuls of dried alfalfa 2 times weekly. This was reduced to once weekly for pups after weaning, which took place between ages P35-P64. At the age of 3 months (90 days), pups began receiving adult chow (5001 Rodent Diet, PMI Nutrition) and tap water.
Testing environment
The testing environment consisted of two light-tight environmental chambers. These chambers were maintained on a 12:12 LD cycle (at 20 +/− 1°C). The location of the cages was exposed (no shelving, cages maintained within 1m of the light source), and the relationship to the lights was recorded so as to maintain even light exposure. After weaning, the degus were moved to smaller opaque plastic cages (42.5 × 22 × 19 cm) equipped with Nalgene running wheels (9 × 34.5 cm) for individual daily activity recordings. In order to prevent abnormal development due to social isolation (e.g,. Ovtscharoff and Braun, 2001), on alternating weeks the degus were housed with a same-sex sibling. During these weeks, the animals were still kept in the testing environment with a running wheel but activity data were not recorded.
Monitoring activity rhythms
Activity rhythms were quantified using the number of running wheel turns per 10 min bin and stored using Vitalview Software (Mini mitter, Bend OR). These data were visualized as double-plotted actograms using Actiview Software (Mini mitter, Bend OR). Days with disruptions in data collection, while rare, were discarded from analysis. The distribution of daily wheel-running activity was characterized for each degu’s weekly recording session by averaging the activity counts for each of the ten minute bins (i.e., data from the 06:10–06:20 bin would be averaged across 7 days of recording). To control for between-subjects variation in overall activity level, these average bins were converted to a percentage of daily activity for the weekly recording session. In order to examine pubertal changes in the distribution of daily activity, we averaged these distributions for animals in each of the experimental groups using two-week age bins across pubertal development.
We also quantified several parameters indicative of circadian phase relative to the LD cycle. Activity rhythm acrophase, the ratio of activity during the light period to activity during the dark period (LD ratio), and the mean activity level for each degu’s weekly recording session were calculated automatically using Actiview Software (Mini mitter, Bend OR). Within this program, acrophase was defined as the peak of a cosine curve fit to the activity data. The time of activity onset and activity offset were calculated using two approaches, one of which was the traditional manner of scoring by two experimenters blind to the age and sex of the animals (Experiment 1) and the other was semi-automatic (Experiment 2). The two approaches produced similar results.
When scored by hand (Experiment 1), activity onset was defined as the first three consecutive bins of data which contained activity levels that exceeded the weekly mean activity level and that followed at least two hours of early morning inactivity (generally falling between ZT20-ZT23). Activity offset was defined as the last three consecutive bins of data which contained activity levels that exceeded the weekly mean activity level and preceded at least two hours of early morning inactivity. If a degu was found to have a strongly nocturnal activity pattern (LD Ratio <0.5) then it was scored as if it were a nocturnal animal, with onset defined as the first three consecutive bins surpassing the weekly mean activity level around the time of lights-off, and offset defined similarly but in proximity to lights-on. In the rare situations where the time of either onset of offset was ambiguous (containing two times that met the definition presented above), then both potential values were averaged for that day.
When scored semi-automatically (Experiment 2), the parameters were calculated using the activity distribution produced for each degu’s weekly recording session. Onset of the morning activity bout and offset of the evening activity bout were calculated similar to the method described above, but in a manner indifferent to the nocturnality of the animal. Instead, a third measure was used, in which the predominant active period was calculated using a moving-window analysis that identified the twelve-hour epoch in the daily activity distribution that contained the highest average activity. The first bin of this epoch was defined as the onset of the active period.
Statistical Analyses
All analyses were reviewed by the Center for Statistical Consulting (CSCAR) at University of Michigan to ensure proper interpretation. These analyses were performed using SPSS 17.0 software (SPSS, Chicago IL) with an alpha of 0.05. To determine if there was a relationship between pubertal development and circadian parameters a linear model was used because the variable of age was best treated as a continuous variable (containing individual variations in startpoint, endpoint, and full sampling duration). The linear fit was performed using a “Random Coefficients Model” so that each animal had its own random intercept and slope with unstructured covariance within the linear mixed model function of SPSS. The individual intercept term accounted for each degu’s individual variability in chronotype. It seemed most appropriate to control for this variability using circadian data from a relatively stable time period, thus the intercept was always centered around a post-pubertal age (P150). Depending on the experiment, intercept and slope terms were also included to provide group-related comparisons. For any particular model, if there was not sufficient variability in a term to run the model (for example, the individual intercept), that term was removed from the model and the conclusions for that term were treated as being clearly non-significant.
In the case of mean activity, the relationship with age did not appear to be strictly linear, thus a marginal model was used that defined mean activity as a repeated measure. For this model, the variable of age was treated as categorical and divided into two week sampling bins. To properly control for the correlated error terms produced by a repeated measures protocol, the appropriate covariance structure was determined by comparing the Akaike Information Criterion (AIC) for several commonly used model types: Diagonal, First-Order Autoregressive (AR(1)), and Compound Symmetry.
Experiment 1
This experiment tracked activity rhythms of male degus across pubertal development. It was intended to expand upon earlier experiments indicating pubertal changes in circadian phase (Hummer et al. 2007; Tate, Richardson, and Carskadon, 2002) using a greater number of sample points to better characterize the full developmental progression of circadian phase change in relation to secondary-sex development. Initial pilot work (Hagenauer 2010) indicated that pubertal changes might be diminished when the light phase of the LD cycle was of high intensity, therefore this experiment also explored two lighting intensities.
Subjects and Housing
Eight male Octodon degus (P61–P66) were selected from two litters the first week following weaning. To examine the effects of lighting condition on pubertal changes in circadian rhythms, degus were divided evenly between two light-sealed boxes that were maintained at different light intensities within a 12:12 LD cycle. Four degus were exposed to dimmer lighting levels (10 lux as measured from cage bottom) and kept in dark grey plastic cages. The other four degus were exposed to brighter lighting levels (60 lux as measured from cage bottom) and kept in white plastic cages. These lighting intensities were chosen because the pilot experiment (Hagenauer 2010) indicated that higher intensity (>250 lux) conditions could drive the wheel-running rhythms of young degus towards a nocturnal chronotype. To control for confounding genetic effects, we divided siblings from each litter in this experiment evenly between the two lighting conditions.
Monitoring pubertal development
Males were examined weekly for the growth of spikes on the glans of the penis (measured in mm), which are an androgen-dependent sign of pubertal development in male degus (Hummer et al., 2007).
Statistical Analyses
After confirming that all males had undergone puberty during our recording period, a linear model was used to examine the relationship between postnatal age and circadian parameters, as well as to test if the relationship varied with the intensity (lux) of the daily LD cycle. The variable of mean activity appeared to have a nonlinear relationship with age, so a marginal model was also run using all age bins.
Experiment 2
This experiment examined sex differences in pubertal changes in chronotype, as well as the role for pubertal gonadal hormones. In addition to the procedural controls used during Experiment 1, some of these animals were maintained in the testing environment with wheel exposure in a cage with dam and siblings before weaning in order to confirm that the changes observed during puberty did not reflect an overall adjustment to a new housing environment.
Subjects
This experiment examined the pubertal development and circadian rhythms of 23 male and 27 female Octodon degus (degus) from weaning (P39-50) until maturity (~P156-194) under different hormonal conditions. It was conducted in three waves (Jul–Oct, Jan–Apr, May–Sept), with each wave containing degus from 3–4 litters to ensure genetic diversity.
Gonadectomy surgery
To examine the influence of pubertal hormones on circadian parameters degus were either 1) gonadectomized (GDX) or 2) SHAM gonadectomized prior to weaning following procedures similar to those previously published (Hummer et al. 2007). These surgeries occurred before external evidence of pubertal onset (between ages P22-P47) during the light phase of the LD cycle. To control for genetic effects, same-sex siblings were evenly divided between GDX and SHAM groups when possible. During the procedure, degus were anesthetized with 2.5% isoflurane gas. Ovaries were accessed via two 1–2 cm long dorsal incisions whereas testes and epididymis were accessed via a single 1–2 cm long ventral incision. During SHAM surgeries incisions were made but the gonads were not removed. Surgical staples were removed 8–11 days after surgery.
Testing environment
During recovery from surgery, the degus were placed back in the home cage with siblings and dam and moved into the testing environment. The 12:12 LD cycle had a light intensity of ~100 lx at cage level. After at least six days of recovery from surgery, the degus were provided with running wheels. At weaning, the degus were moved to individual dark gray, opaque cages equipped with running wheels.
Monitoring Pubertal Development
All animals were weighed weekly to monitor their growth. The progress of puberty was monitored by examining secondary sex development using procedures similar to those previously published (Hummer et al. 2007). Briefly, males were examined weekly for the development of a separation between the prepuce and glans of the penis, called prepucial opening, and the growth of penile spikes, which are androgen-dependent secondary-sex characteristics. We monitored pubertal development in female degus by examining them three times weekly for the presence of vaginal opening, beginning between ages P25-58. Adult intact female degus maintain vaginal openings only during a few days during their three-week estrous cycles when they exhibit elevated estrogen and sexual receptivity (Mahoney et al. in press). We also blindly scored running wheel activity records for dates that contained the altered activity rhythms typical of estrus: persistent or elevated activity occurring for 1–2 days with an elongated or atypically-phased active period (estrus), followed by a day with decreased activity and delayed active phase (post-estrus; Labyak and Lee, 1995; Mahoney et al. in press). Circadian analyses were later examined statistically with data that both included and excluded dates with estrus-typical wheel-running that was predictive of vaginal opening.
Statistical Analysis
To determine if there was a relationship between pubertal development and circadian parameters, we first ran a separate linear model for the SHAM male and females in which the variable of Age was defined as either postnatal age or as pubertal stage (age relative to either first prepucial or vaginal opening). After confirming that circadian variables exhibited developmental changes in the intact animals, we ran a full linear model using all four groups (SHAM and GDX males and females) to determine the sex and gonadal dependency of age-related changes in circadian parameters. The variable of mean activity appeared to have a nonlinear relationship with age, so a marginal model was also run. Within the marginal model, due to limited sample size (<70% of n), data from the first and last age bins (before P55 and after P180, respectively) was removed from the analysis.
Results
Results from Experiments 1 & 2 have been integrated below because similar pubertal and chronotype development was observed in each experiment.
Pubertal Development: Experiments 1 & 2
Weight
Both sexes gained weight rapidly until around age P80. At this point, weight gain slowed, and finally plateaued by P155. Males weighed more than females but GDX and SHAM animals within each sex did not differ (Figure 1).
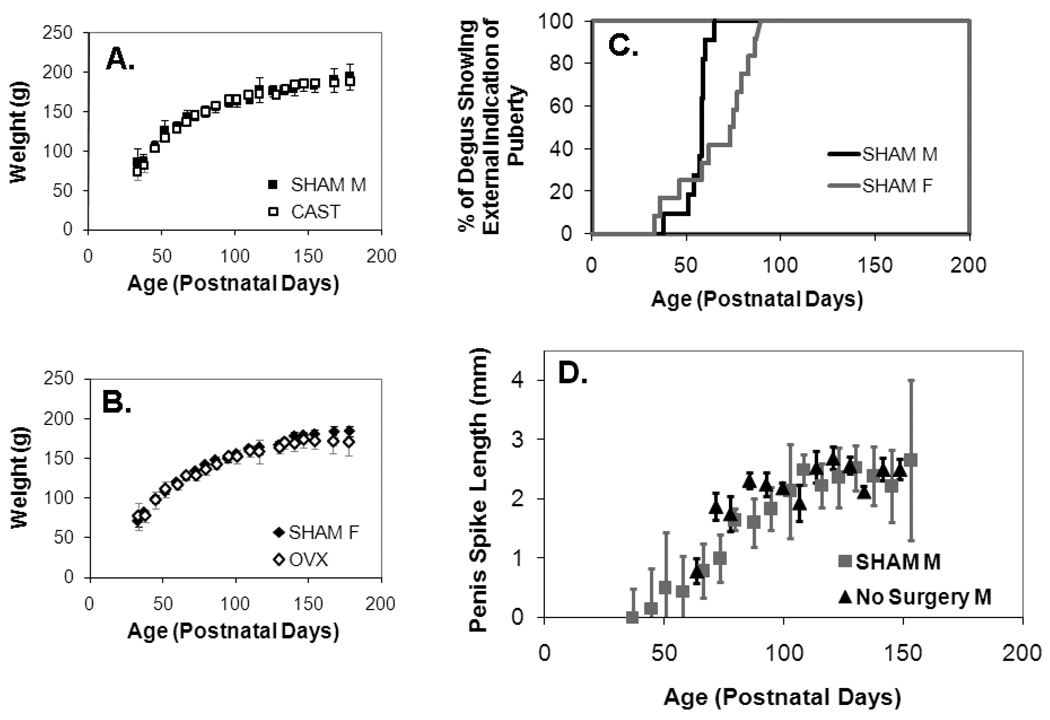
Figure 1. Pubertal development in the degu. (as measured in Experiments 1 & 2).
A. Both males and B. females showed the fastest weight gain before 100 days of age, although females overall weighed less than males. Gonadectomized male (CAST) and female (OVX) degus showed similar growth curves as intact animals (SHAM M and SHAM F). Error bars represent +/− SE. C. SHAM males and females both showed the first external indication of pubertal onset (first prepucial opening for males and first vaginal opening for females) between ages P33–P89. D. Spikes on the glans of the penis continued to grow (length in mm +/− SE) until age ~P100 for both naïve males (Exp. 1, black) and SHAM males (Exp. 2, dark grey).
Penile Maturation
All eight males without surgery (Experiment 1) and all eleven SHAM males (Experiment 2) developed both a full prepucial opening and complement of spikes on the glans of the penis. Eleven of the CAST males showed neither characteristic, although 10 temporarily showed a small or partial prepucial opening. One CAST male developed a full prepucial opening on the day of surgery and was subsequently removed from the data analysis.
All males without surgery (Experiment 1) had a full prepucial opening by the first time they were examined between age P61-66, and six of the eight already had their full complement of spikes. These spikes continued to grow at a linear rate until ~P90, at which point the growth plateaued, with final lengths reaching 2–3 mm (mean 2.6 mm +/−0.14 SE, Figure 1).
Full prepucial opening developed in the SHAM males between P38-65, with a median age of P58 (Figure 1). Within a week, spikes developed on the tip of the glans and grew at a linear rate (Figure 1). For 45% of the males, the spikes also increased in number, reaching a full complement of 4–6 spikes between ages P52-P95 (median age P73). Spike length plateaued between ages P100-P135, with final lengths reaching 1.8–3 mm (mean: 2.55 mm +/−0.51 SE).
Estrous Cycles
Twelve SHAM degus showed clear signs of estrous cyclicity as evidenced by both the cyclical presence of vaginal opening and estrus-typical wheel-running activity. The median age of first vaginal opening was P74 (ranging from P33-P89, Figure 1). Of the remaining two SHAM degus, one died following her first vaginal opening and the second showed two intermediate cycles (dimpling but no full opening), and then ceased to show any indications of cyclicity. Circadian data from these two degus were removed from the data set. Three of the OVX females eventually developed small vaginal openings and were removed from the data set. All other OVX females (n=10) showed no sign of vaginal opening or cyclic patterns of estrus-typical wheel running.
Pubertal Changes in Circadian Rhythms: Experiments 1 & 2
Intact Males
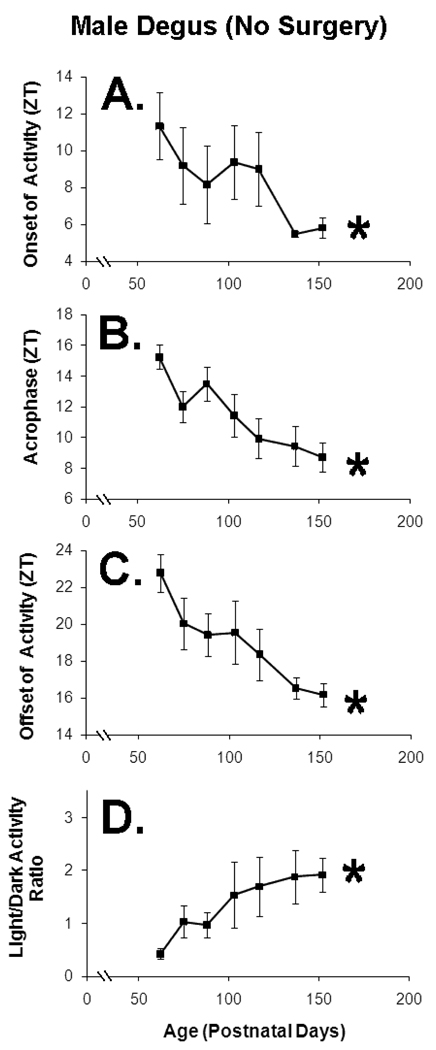
There was large reorganization of activity rhythms during puberty in the males that never received surgery (Experiment 1). At the youngest age, males showed a strongly crepuscular activity distribution, with a large percentage of activity occurring during the evening hours. As the males grew older, this evening activity bout diminished and daytime activity increased (Figure 2). Therefore, three activity rhythm phase parameters were found to advance with age: activity onset (F(1, 9.763)=4.889, p=0.05), acrophase (F(1, 17.549)=15.511, p<0.01), and activity offset (F(1, 10.470)=12.558, p<0.01). The light-dark activity (LD) ratio also increased with age (F(1, 19.196)=8.060, p=0.01), confirming that older males were more diurnal (Figure 3). Mean activity (daily mean wheel turns/10 min) peaked around mid-puberty and then decreased over the rest of the recording period. It did not show linear change with age (F(1, 12.714)=0.196, p=0.67), but a marginal model found significant overall within-subject variability across age (F(6, 35.461)=5.664, p<0.01). None of the activity rhythm phase variables were significantly affected by lighting intensity (p>0.10), nor did lighting intensity alter age-related changes in any of the variables (p>0.10).
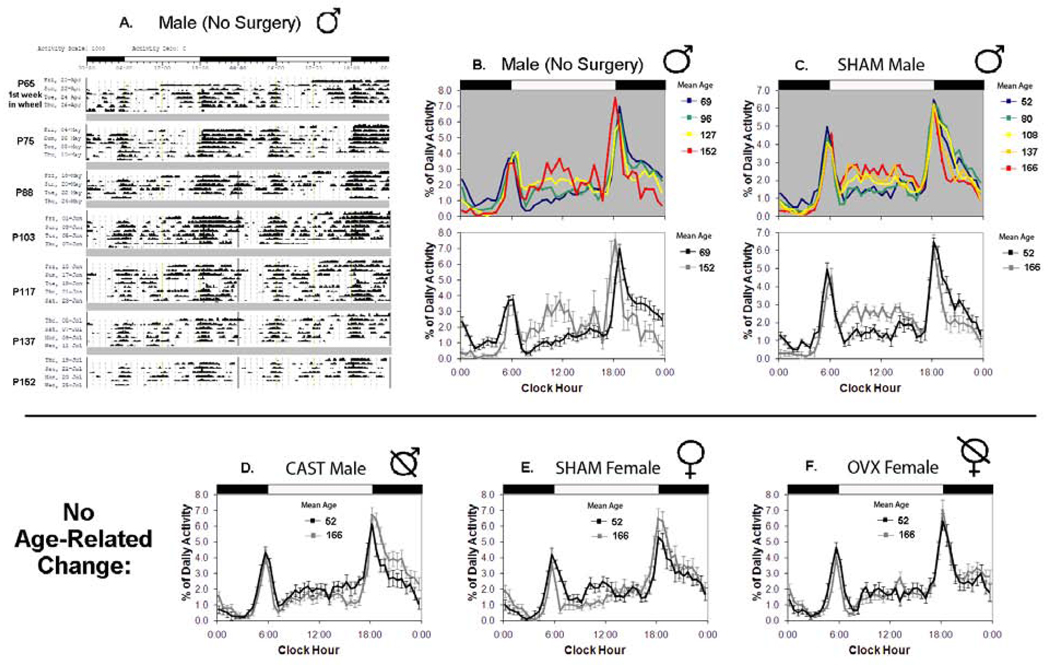
Figure 2. Intact male degus show a large reorganization of circadian activity during puberty that is not exhibited by females or castrated males. (as measured in Experiments 1 & 2).
A. A representative double-plotted actogram tracking wheel-running activity rhythms across pubertal development in one male. Each horizontal line sequentially represents 2 days of activity (# of wheel turns/ 10 min bin) and the LD cycles for both days are indicated by a bar at the top of the figure. Grey bars indicate week-long gaps in recording when the degu is living with a sibling. On the left is the average postnatal age for each recording session. Note that as the degu grew older his activity during the hours immediately following lights-off (evening) diminished and his daytime activity increased. B. The average percent of daily activity occurring during 30 min bins across the day for all males who did not receive surgery (n=8) at several ages during pubertal development (right). In the top panel, cool colors represent data from the youngest recording sessions (early pubertal), and warm colors represent the oldest (late-pubertal). Each data point represents the average of two recording sessions. The white and black bar illustrates the light-dark cycle. The bottom panel compares just the youngest (black) and oldest (grey) activity distribution (error bars: +/− SE). C. SHAM male degus (n=11) similarly showed a decrease in activity during the hours immediately following lights-off and an increase in daytime activity as they grew older. D, E, F. castrated (CAST) males (n=11), SHAM females (n=12), and ovariectomized (OVX) females (n=10) did not show the same developmental changes in activity distribution as intact males.
Figure 3. All activity rhythm phase parameters indicate that intact male degus phase-advance during pubertal development (Experiment 1, no surgery, n=8).
Activity rhythm phase is presented in terms of zeitgeber time (ZT), with ZT12 equal to the time of lights-off and ZT0 equal to the time of lights on. Error bars represent +/−SE. Asterisks indicate significant within-subject change (p<0.05). A. The onset of daily activity, as scored relative to the animal’s general active hemi-cycle (diurnal vs. nocturnal) B. Acrophase, as determined by a cosine fit. C. The offset of daily activity, relative to the animal’s general active hemi-cycle. D. Light/dark activity ratio, with greater values indicating a more diurnal activity distribution.
The reorganization of activity rhythms that occurred during puberty in SHAM males (Experiment 2) was identical to that found for males without surgery (Experiment 1; Figure 2). Therefore, three activity rhythm phase parameters were found to advance with age: the onset of the active period (F(1, 10.937)=4.795, p=0.05), acrophase (F(1, 10.028)=14.253, p<0.01), and the offset of the evening activity bout (F(1, 10.437)=4.873, p=0.05). The LD ratio also increased with age (F(1, 19.063)=10.339, p<0.01), confirming that older males were more diurnal (Figure 4). The onset of the morning bout remained relatively stable across pubertal development (F(1, 50.091)=1.788, p=0.19). Mean activity peaked around mid-puberty and then decreased over the rest of the recording period. It therefore did not show any linear change (F(1, 10.527)=1.678, p=0.22), but a marginal model did find significant overall within-subject variability across age (F(8, 73.108)=2.982, p<0.01).
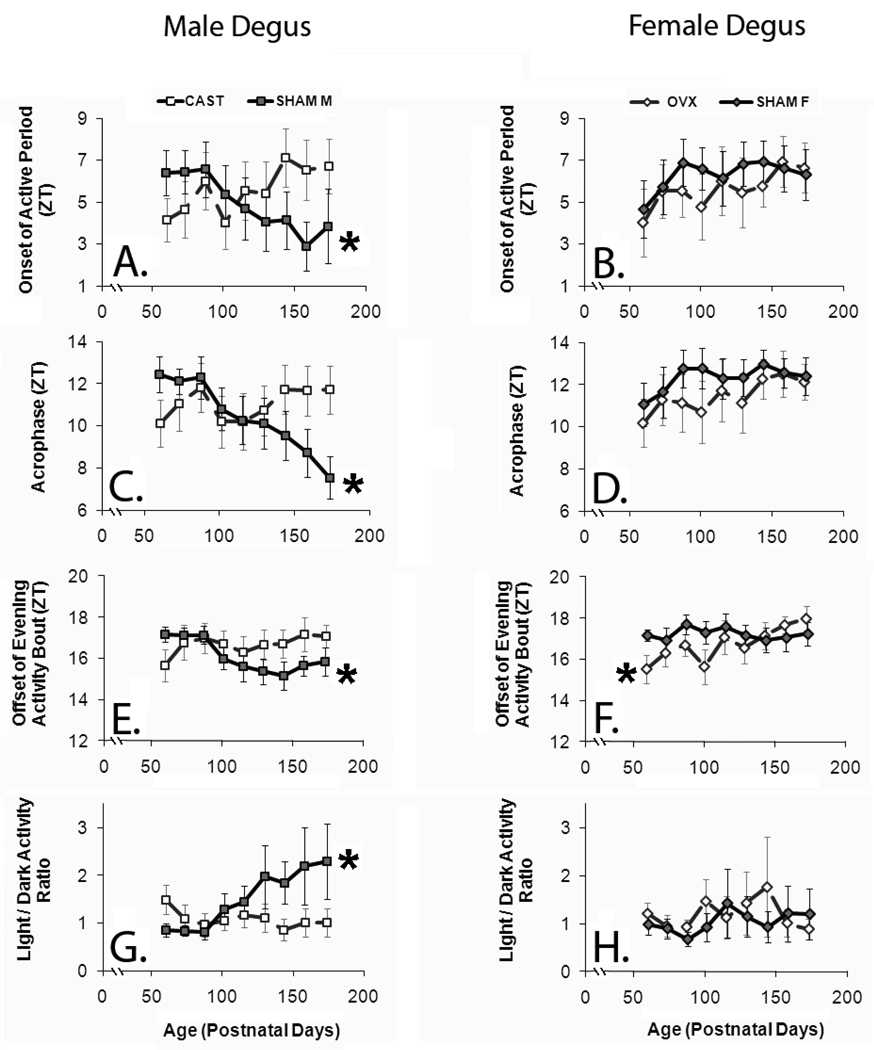
Figure 4. Activity rhythm phase parameters exhibit sex differences during puberty that depend on gonadal hormones.
Degus of both sexes that were gonadectomized prior to puberty (GDX) appear similar for almost all parameters. Activity rhythm phase is presented in terms of zeitgeber time (ZT). X- and Y-error bars represent +/−SE. Asterisks indicate significant within-subject change (p<0.05). A. Pre-pubertal GDX prevented males from developing a phase-advanced onset to their active period, which was defined as the 12 hrs of the day containing the highest mean activity. The onset of the morning activity bout did not significantly change with age in either group, and is not depicted. B. Neither SHAM nor GDX females showed significant changes in the onset of the active period. C. Pre-pubertal GDX prevented males from developing a phase-advanced acrophase. D. Neither SHAM nor GDX females showed significant changes in acrophase. E. Pre-pubertal GDX prevented males from developing a phase-advanced offset of the evening activity bout. F. SHAM females did not show age-related changes in offset of the evening activity bout, whereas GDX females showed a significant delay. G. Pre-pubertal gonadectomy prevented males from developing a more diurnal activity pattern as determined by LD ratio. H. Neither SHAM nor GDX females showed age-related changes in LD ratio.
It was ambiguous whether these developmental changes were specifically related to pubertal timing. To test this hypothesis, we compared developmental timing by using age adjusted to first prepucial opening in our statistical models. Most of the activity rhythm phase parameters which exhibited significant relationships with age also showed significant relationships with developmental timing (onset of the active period, acrophase, offset of the evening activity bout). However, developmental timing did not produce a better model fit than age using the criteria of p-value or model fit (determined by Akaike’s Information Criterion).
Intact Females
Similar age-related changes were not observed in the activity rhythms of SHAM Females, even after removing the day of estrus from the analyses (Figure 2). All variables showed no significant within-subject change (Figure 4). When pubertal timing was included in the model using age in relationship to first vaginal opening, females continued to lack significant maturation-related changes in circadian phase (p>0.10). Similar to males, mean activity did not show a linear change during puberty when examined in relationship to age nor to age in relationship to first vaginal opening (p>0.33), but the females also did not show withinsubject variability across age using the marginal model (p>0.13).
Hormonal Dependency
The activity rhythms of both males and females gonadectomized (GDX) prior to puberty followed developmental trends that resembled those of SHAM females. As the animals grew older, the rhythms were essentially stable, although slightly more crepuscular and evening type (Figure 2). There was no significant within-subject change in the GDX males for any of the activity rhythm phase variables (p>0.15; Figure 4). In GDX females, there was a significant delay in the offset of the evening activity bout (F(1, 8.328)=5.191, p=0.05) but no change in any of the other phase variables (p>0.16; Figure 4).
Therefore, a linear model including all four groups indicated that GDX significantly influenced age-related change in most activity rhythm phase variables. There was a main effect of GDX on age-related changes in the offset of the evening activity bout (F(1, 39.409)=8.085, p<0.01). There was also an interaction between the effects of GDX and sex on age-related changes in acrophase (F(1, 38.207)=7.162, p<0.01) and the onset of the active period (F(1, 38.414)=4.995, p=0.03). Surprisingly, age-related changes in mean activity were not affected by GDX (p>0.66) even after accounting for sex (p>0.58).
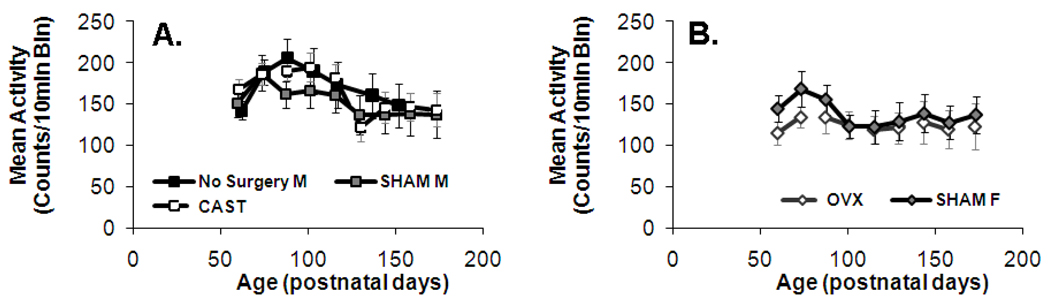
In addition to effects of GDX, a linear model fit including all groups (male and female, GDX and SHAM) also indicated that the effect of age on activity rhythm phase differed by sex, with males showing greater changes than females in acrophase (F(1, 38.207)=6.154, p=0.02). Age-related changes in other circadian phase variables were not found to show sex differences within the linear model (p>0.09). Sex differences were clear in overall mean activity. Mean activity peaked at mid-puberty and then decreased over the rest of the recording period in both sexes, but the duration of this pubertal period of elevated activity was longer in males, leading males to have overall higher activity during the recording period even though post-pubertal activity levels were almost identical between the sexes. Therefore, mean activity in all groups showed some overall linear age-related change (Figure 5; F(1, 39.617)=4.524, p=0.04), in a manner that did not differ by sex (p>0.49), but a marginal model found that sex influenced within-subject variability across age in activity levels (F(8, 281.579)=2.921, p<0.01).
Figure 5. Mean activity levels decrease across development (p=0.04) in a manner that differs by sex (p<0.01).
The daily mean activity (wheel turns/10 min bin) is illustrated for males that did not receive surgery, SHAM, and castrated males (A) and SHAM and ovariectomized females (B). X- and Y- error bars represent +/−SE.
Discussion
These experiments in the slow-developing degu indicate changes in daily activity distribution and circadian rhythm phase over the course of pubertal development that differed by sex. In four separate experiments (Experiments 1&2, two additional pilot experiments in Hagenauer 2010) we found that male degus exhibited an intensely crepuscular distribution of activity between post-weaning and pubertal onset, with the majority of activity occurring at the beginning of the dark period. As the males progressed through puberty, evening activity diminished and phase-advanced, and daytime activity increased. By the age of maturity the activity rhythms of male degus were strongly diurnal. Females, in contrast, showed a crepuscular rhythm during the post-weaning period that remained stable into reproductive maturity. Male and female animals gonadectomized (GDX) prior to puberty maintained a more delayed, crepuscular activity distribution across development similar to intact females. These results indicate that the pubertal rise in gonadal hormones drives chronotype changes in intact male degus.
These results closely parallel electrophysiological data regarding the development and sex differences in sleep in this species (Perryman, 2010). Those data showed a more crepuscular rhythm of NREM sleep in juvenile and female degus, such that the least NREM occurred around the time of dawn and dusk. Those observations were made on degus that were housed without running wheels. Thus, the developmental changes and sex differences in activity distribution that we observed in our experiments are unlikely to represent artifacts due to running wheel exposure (such as seen in Kas and Edgar, 1999). The sex differences that we observed are similarly unlikely to be a side-effect of surgery, as we have also observed them in animals that had not undergone surgery (Hagenauer 2010). The sex differences were also not due to estrus-induced wheel-running, as circadian analysis including and excluding the days of estrus produced similar results (data not shown).
The pubertal changes in activity distribution and activity rhythm phase exhibited by the degu strongly resemble our recent data in the developing nocturnal rat (Hagenauer et al. in revision). In that study, we found that rats showed a phase-delayed, bimodal activity distribution post-weaning that consolidated into a more phase-advanced, unimodal rhythm by maturity. These pubertal changes in the rat showed sex differences in magnitude, with males showing larger activity rhythm phase changes than females. However, females still continued to exhibit some developmental changes in activity rhythm phase. Pre-pubertal gonadectomy diminished these developmental changes in both sexes, causing a more distributed and delayed activity distribution (Hagenauer et al. in revision).
The similarity between the pubertal circadian rhythm development of these two species suggests that these changes are not limited to animals from a particular temporal niche or developmental trajectory. The activity rhythm changes observed during puberty in both species are remarkably similar to those observed in other laboratory rodents in response to differing levels of testosterone (mice: Daan et al., 1975; Iwahana et al., 2008; Karatsoreos et al., 2007; hamsters: Davis et al., 1983; Morin et al., 1981). In those studies, castrated adult males showed an altered distribution of activity under entrained and free-running conditions such that activity was dispersed across the active period and less cohesive at activity onset (Morin et al., 1981), or the initial activity bout was diminished, lost, or delayed (Karatsoreos et al., 2007; Iwanahana et al., 2008; Daan et al., 1975; Davis et al., 1983). The administration of testosterone or dihydrotestosterone was able to restore the adult castrates to their original circadian activity patterns (Karatsoreos et al., 2007; Iwanahana et al., 2008; Daan et al. 1975; hamsters: Morin et al. 1981). In adult male degus, castration caused different effects on activity rhythm phase (Jechura et al., 2000), in which activity onset advanced instead of delayed. Thus, the pubertal effects of male gonadal hormones on chronotype in degus appear specific to this developmental time period. Our current hormone replacement studies during puberty should distinguish between these possibilities.
Changes is chronotype, such as those seen in pubertal degus, can be caused by changes in the entrainment of the circadian pacemaker to environmental time cues or by downstream changes in the phase relationship between the circadian pacemaker and its output rhythms, such as the rest/activity cycle. For that reason, it is worth noting that degus are widely regarded to exhibit chronotype flexibility in response to environmental conditions that is independent of changes in the phasing of the circadian pacemaker (Otalara et al. 2010; Kas and Edgar, 1999). Wild degus are day-active (diurnal), although during the hot summer months they exhibit a more crepuscular pattern of activity when living in conditions that lack sufficient shade (Fulk, 1976; Kenagy et al., 2002; Bacigalupe et al., 2003). More exaggerated chronotype flexibility is reported in the laboratory due to housing and lighting variables (e.g,. Kas and Edgar, 1999; Ocampo-Garces, 2005; Hagenauer and Lee, 2008).
Although these variables were carefully controlled throughout this experiment, the possibility still exists that the sex differences that we observed in circadian development may represent sex differences in the sensitivity of degu chronotype to housing conditions (Hagenauer 2010). Indeed, the female degus in the current experiment were more crepuscular than is typically observed in previous studies in our lab or in the wild (for review see Hagenauer and Lee, 2008). Also, Hummer et al. (2007) observed a delayed phase during mid-puberty in both male and female degus, which disappeared upon reaching maturity. In that earlier study, the degus were housed on shelves which provided overhead cover, which we have found correlates with more diurnal activity (Hagenauer 2010). Similarly, in our recent study in the developing rat, we found that both males and females showed circadian phase changes during puberty, although these circadian changes exhibited sex differences in magnitude (Hagenauer et al., in revision).
We ran a preliminary test to see whether developmental changes in the chronotype of female degus were being masked by a passive suppression of activity rhythms in response to light (Vivanco, Rol, and Madrid, 2010). We examined the activity records of nine female degus that were placed in DD during mid-puberty or late/post-puberty (Hagenauer, 2010). The first two days after placement in DD, we observed an immediate increase in activity during the subjective day and a 1–3.5 hr phase-advance of circadian parameters, suggesting that rhythms were masked at both ages. However, a comparison of these “unmasked” rhythms gave little indication of pubertal change. Masking also seemed unlikely to account for the lack of circadian rhythm phase-advance that we observed during the development of GDX animals in the current study, as our previous studies in the degu under less exposed housing conditions (Hummer et al., 2007) as well as data in the rat (Hagenauer et al. in revision) similarly indicated that pre-pubertal gonadectomy inhibited activity rhythm phase changes during puberty.
Interestingly, an earlier report described a more advanced activity rhythm phase during pre-puberty in degus (Hummer et al., 2007), as did preliminary data in the degu and rat (Hagenauer et al., 2009), in a manner that better resembled the sleep patterns of children (e.g., Thorleifsdottir et al., 2002). However, once the initial days (for the rat) or week (for the degu) of recording were removed from the analysis, this advanced phase during pre-puberty statistically disappeared (Hagenauer 2010). In the current studies, where the degus had early exposure to the testing environment, we did not observe a more advanced activity rhythm phase during prepuberty. However, puberty now occurs earlier in our breeding colony due to improved husbandry and rapid growth. Thus, it is possible that the initial neuroendocrine changes associated with puberty may overlap with our earliest circadian samples. To distinguish between these two possibilities we may need to track the development of degu rhythms prior to weaning. Indeed, in the wild newly-emerged, pre-weaning juvenile degus (approximately 2–4 weeks old) exhibit rhythms of extra-burrow activity that are more unimodally diurnal and phase-advanced than those of their adult counterparts (Fulk, 1976).
These data, taken in tandem with evidence from a variety of species, support the assertion that chronotype changes during puberty are a well-demonstrated phenomenon in mammals. These chronotype changes are likely to arise from a gonadal-hormone sensitive circadian system. However, a full test of this hypothesis will require hormone-replacement during puberty in both males and females, as well as an examination of additional circadian rhythms besides wheel-running activity. Future experiments will also focus on which aspect of the circadian system is targeted, particularly whether gonadal hormones are acting at the level of the pacemaker itself or on downstream oscillators controlling output rhythms.
Acknowledgements
We would like to thank Dr. Megan Mahoney, Jennifer Agrusa, Myra Dimitrov, Blair Sutton, Ana Kantarowski, Andrea King, Ariel Haskins, Adam Colton, Jessica Koch, David Altshuler, Shuoqi Scott Wang, Dr. Bob Thompson, Dr. Jill Becker, Dr. Jimo Borjigin, and Dr. Daniel Forger for their technical support and advice. We also thank Kathy Welch from CSCAR for expert statistical consulting, and acknowledge Kathy Gimson, Julie Stewlow, and Jim Donner for their excellent animal care. This research was supported by a laboratory grant from the National Science Foundation (TML, MHH - IBN-0212322) and a training grant awarded to the University of Michigan Reproductive Science Program from the NICHD (MHH - T32 HD07048).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241(1):R 62–R 66. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- Axelson JF, Gerall AA, Albers HE. Effect of progesterone on the estrous activity cycle of the rat. Physiol Behav. 1981;26(4):631–635. doi: 10.1016/0031-9384(81)90137-2. [DOI] [PubMed] [Google Scholar]
- Bacigalupe LD, Resende EL, Kenagy GJ, Bozinovic F. Activity and space use in degus: A trade-off between thermal conditions and food availability? J Mammol. 2003;84(1):311–318. [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: Implications for behavior. Ann NY Acad Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An Approach to Studying Circadian Rhythms in Adolescents. J Biol Rhythms. 1997;12:278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on the circadian pacemaker in mice. Proc Natl Acad Sci USA. 1975;72:3744–3747. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol. 1983;244(1):R93–R105. doi: 10.1152/ajpregu.1983.244.1.R93. [DOI] [PubMed] [Google Scholar]
- Fulk GW. Notes on the activity, reproduction, and social behavior of Octodon degus. J Mammol. 1976;57(3):495–505. [Google Scholar]
- Golub MS, Takeuchi PT, Hoban-Higgins TM. Nutrition and circadian activity offset in adolescent rhesus monkeys. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 50–68. [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31(4):276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Lee TM. Circadian organization of the diurnal caviomorph rodent, Octodon degus. Biol Rhythm Res. 2008;39(3):269–289. [Google Scholar]
- Hagenauer MH, King AF, Possidente B, McGinnis MY, Lumia AR, Lee TM. Changes in Circadian Rhythms during Puberty in Rattus Norvegicus: Developmental Time Course and Gonadal Dependency. doi: 10.1016/j.yhbeh.2011.03.001. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH. Dissertation in fulfillment of a Doctor of Philosophy in Neuroscience. Ann Arbor: University of Michigan; 2010. Pubertal hormones alter circadian timekeeping: Evidence from two rodent models, Rattus norvegicus and Octodon degus. [Google Scholar]
- Hummer DL, Jechura TJ, Mahoney MM, Lee TM. Gonadal hormone effects on entrained and free-running rhythms in the developing diurnal rodent, Octodon degus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R586–R597. doi: 10.1152/ajpregu.00043.2006. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav. 2008;53(3):422–430. doi: 10.1016/j.yhbeh.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jechura TJ, Walsh JM, Lee TM. Testicular hormones modulate circadian rhythms of the diurnal rodent, Octodon degus. Horm Behav. 2000;38(4):243–249. doi: 10.1006/hbeh.2000.1624. [DOI] [PubMed] [Google Scholar]
- Jechura TJ. Dissertation in fulfillment of a Doctor of Philosophy degree in Psychology. University of Michigan; 2002. Sex differences in circadian rhythms: Effects of gonadal hormones in octodon degus. [Google Scholar]
- Jesseau SA. Dissertation in fulfillment of a Doctor of Philosophy degree in Psychology. Ann Arbor, Michigan: University of Michigan; 2004. Kin Discrimination and social behavior in communally-nesting degus (Octodon degus) [Google Scholar]
- Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148(11):5487–5495. doi: 10.1210/en.2007-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas MJH, Edgar DM. A non-photic stimulus inverts the diurnal-nocturnal phase-preference in Octodon degus. J Neurosci. 1999;19(1):328–333. doi: 10.1523/JNEUROSCI.19-01-00328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenagy GJ, Place NJ, Veloso C. Relation of Glucocorticosteroids and Testosterone to the Annual Cycle of Free-Living Degus in Semiarid Central Chile. Gen Comp Endocrinol. 1999;115:236–243. doi: 10.1006/gcen.1999.7307. [DOI] [PubMed] [Google Scholar]
- Kenagy GJ, Nespolo RF, Vasquez RA, Bozinovic F. Daily and seasonal limits of time and temperature to activity of degus. Rev Chil Hist Nat. 2002;75:567–581. [Google Scholar]
- Kent S, Hurd M, Satinoff E. Interactions between body temperature and wheel-running over the estrous cycle in the rat. Physiol Behav. 1991;49:1079–1084. doi: 10.1016/0031-9384(91)90334-k. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Prepucial Separation as an External Sign of Pubertal Development in the Male Rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Labyak SE, Lee TM. Estrus- and steroid-induced changes in circadian rhythms in a diurnal rodent, Octodon degus. Physiol Behav. 1995;58(3):573–585. doi: 10.1016/0031-9384(95)00096-2. [DOI] [PubMed] [Google Scholar]
- Li H, Satinoff E. Body temperature and sleep in intact and ovariectomized female rats. Am J Physiol: Reg, Integ, and Comp Physiol. 1996;271:1753–1758. doi: 10.1152/ajpregu.1996.271.6.R1753. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, Rossi BV, Hagenauer MH, Lee TM. Characterization of the Estrous Cycle in Octodon degus. Biol of Reprod. doi: 10.1095/biolreprod.110.087403. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, Bootzin RR. Sleep and the menstrual cycle. Health Psychol. 1997;16(3):209–214. doi: 10.1037//0278-6133.16.3.209. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol Behav. 2007;92:1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Ede MC, Sulzman FM, Fuller CA. Clocks That Time Us; Characteristics of Circadian Clocks. Harvard University Press; 1982. pp. 30–112. [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196(4287):305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Neuman A, Gothilf Y, Haim A, Ben-Aharon G, Zisapel N. Nocturnal patterns and up-regulated excretion of the melatonin metabolite 6-sulfatoxymelatonin in the diurnal rodent Psammomys obesus post-weaning under a short photoperiod. Comp Biochem Physiol (A) 2005;142:297–307. doi: 10.1016/j.cbpa.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Ocampo-Garces A, Hernandez F, Mena W, Palacios AG. Wheel-running and rest-activity pattern interaction in two octodontids (Octodon degus, Octodon bridgesi) Biol Res. 2005;38:299–305. doi: 10.4067/s0716-97602005000200019. [DOI] [PubMed] [Google Scholar]
- Otalara BB, Vivanco P, Madariaga AM, Madrid JA, Rol MA. Internal temporal order in the circadian system of a dual-phasing rodent, the Octodon degus. Chronobiol Int. 2010;27:1564–1579. doi: 10.3109/07420528.2010.503294. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Braun K. Maternal Separation and Social Isolation Modulate the Postnatal Development of Synaptic Composition in the Infralimbic Cortex of Octodon degus. Neuroscience. 2001;104:33–40. doi: 10.1016/s0306-4522(01)00059-8. [DOI] [PubMed] [Google Scholar]
- Parry BL, et al. Neuroendocrine effects of light therapy in late luteal phase dysphoric disorder. Biol Psychiatry. 1994;36(6):356–364. doi: 10.1016/0006-3223(94)91210-6. [DOI] [PubMed] [Google Scholar]
- Parry BL, et al. Cortisol circadian rhythms during the menstrual cycle and with sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Biol Psychiatry. 2000;48(9):920–931. doi: 10.1016/s0006-3223(00)00876-3. [DOI] [PubMed] [Google Scholar]
- Perryman JI. Dissertation in fulfillment of a Doctor of Philosophy in Neuroscience. Ann Arbor: University of Michigan; 2010. Circadian and homeostatic components of sleep across sex and development in a diurnal rodent, Octodon degus. [Google Scholar]
- Pinto FT, Golombek DA. Neuroactive steroids alter the circadian system of the Syrian hamster in a phase-dependent manner. Life Science. 1999;65(23):2497–2504. doi: 10.1016/s0024-3205(99)00516-0. [DOI] [PubMed] [Google Scholar]
- Reynolds TJ, Wright JW. Early postnatal physical and behavioural development of degus (Octodon degus) Laboratory animals. 1979;13:93–99. doi: 10.1258/002367779780943576. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr Biol. 2004;14(24):R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Dahl RE, Shahar G, et al. Sleep and the transition to adolescence: a longitudinal study. Sleep. 2009;32(12):1602–1609. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Gamboa M. Free and total testosterone levels in field males of Octodon degus (Rodentia, Octodontidae): Accuracy of the hormonal regulation of behavior. Revista Chilena de Historia Natural. 2005;78:229–238. [Google Scholar]
- Tate BA, Richardson GS, Carskadon MA. Maturational changes in sleep-wake timing: longitudinal studies of circadian activity rhythm of a diurnal rodent. In: Carkadon MA, editor. Adolescent Sleep Patterns: Biological, Social and Psychological Influences. pp. 40–49. [Google Scholar]
- Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason TH, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year Period. J Psychosom Res. 2002;53:529–537. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- Vivanco P, Rol MA, Madrid JA. Pacemaker phase control versus masking by light: Setting the circadian chronotype in dual Octodon degus. Chronobiol Int. 2010;27:1365–1379. doi: 10.3109/07420528.2010.502984. [DOI] [PubMed] [Google Scholar]
- Weinert D, Eimert H, Erkert HG, Schneyer U. Resynchronization of the circadian corticosterone rhythm after a light/dark shift in juvenile and adult mice. Chronobiol Int. 1994;11(4):222–231. doi: 10.3109/07420529409067791. [DOI] [PubMed] [Google Scholar]
- Weinert D, Kompauerova V. Light induced phase and period responses of circadian activity rhythms in laboratory mice of different age. Zoology. 1998;101:45–52. [Google Scholar]
- Weinert D, Waterhouse J. Daily activity and temperature rhythms do not change spontaneously with age in laboratory mice. Physiol Behav. 1999;66(4):605–612. doi: 10.1016/s0031-9384(98)00342-4. [DOI] [PubMed] [Google Scholar]
- Weinert D. Ontogenetic development of the mammalian circadian system. Chronobiol Int. 2005;22(2):179–205. doi: 10.1081/cbi-200053473. [DOI] [PubMed] [Google Scholar]
- Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;15(1 Suppl):250–260. doi: 10.1542/peds.2004-0815G. [DOI] [PubMed] [Google Scholar]
- Zucker I, Fitzgerald KM, Morin LP. Sex differentiation of t-e circadian system in the golden hamster. A J Physiol. Reg, Integ, Comp Physiol. 1980;238:97–110. doi: 10.1152/ajpregu.1980.238.1.R97. [DOI] [PubMed] [Google Scholar]