Abstract
Dystrophin absence in Duchenne muscular dystrophy (DMD) causes severe muscle degeneration. We describe that, as consequence of fibre damage, specific muscle-miRNAs are released in to the bloodstream of DMD patients and their levels correlate with the severity of the disease. The same miRNAs are abundant also in the blood of mdx mice and recover to wild-type levels in animals ‘cured’ through exon skipping. Even though creatine kinase (CK) blood levels have been utilized as diagnostic markers of several neuromuscular diseases, including DMD, we demonstrate that they correlate less well with the disease severity. Although the analysis of a larger number of patients should allow to obtain more refined correlations with the different stages of disease progression, we propose that miR-1, miR-133, and miR-206 are new and valuable biomarkers for the diagnosis of DMD and possibly also for monitoring the outcomes of therapeutic interventions in humans. Despite many different DMD therapeutic approaches are now entering clinical trials, a unifying method for assessing the benefit of different treatments is still lacking.
Keywords: biomarkers, diagnosis, Duchenne Muscular Dystrophy, miRNA, therapy
INTRODUCTION
Duchenne muscular dystrophy (DMD) is one of the most severe neuromuscular diseases and affects 1:3500 live males. DMD is a monogenic disorder caused by mutations in the 2.5 Mb-long dystrophin gene (DMD). Deletions/duplications and point mutations in this gene cause either the severe Duchenne phenotype (DMD) or the milder Becker muscular dystrophy (BMD), depending on whether the translational reading frame is lost or maintained (Hoffman et al, 1988).
Dystrophin (DYS) is localized on the inner face of the muscle fibre plasma membrane, linking cytoskeletal actin with a complex of proteins localized on the sarcolemma, named DYS-associated protein complex (DAPC). In the absence of DYS, muscle fibres become more sensitive to mechanical damage leading to muscle degeneration, chronic inflammation and increase in fibrosis, exacerbating the dystrophic phenotype. All these traits are clearly attenuated in Becker patients with a mild myopathic phenotype (Monaco et al, 1988).
In boys under 5 years, diagnostic investigation is usually triggered by the presence of abnormal muscle function manifested by Gowers' sign, calf hypertrophy, toe walking, and waddling gait. When DMD is suspected, the first assessment is the measurement of serum creatine kinase (CK) levels, which are markedly increased in early stages of the disease (10–100 fold higher than healthy controls; Ebashi et al, 1959). Genetic diagnosis is currently assessed by different diagnostic procedures: multiplex polymerase chain reaction (PCR) assay for the identifications of deletions that account for 65% of DMD patients (Chamberlain et al, 1988); multiplex ligation-dependent probe amplification that also allows identification of insertions (Schouten et al, 2002); Southern blotting (Stockley et al, 2006) or multiplex amplifiable probe hybridization (Dent et al, 2005). If the mutation remains unidentified, DMD sequencing is required to find point mutations or small insertions/deletions (Stockley et al, 2006).
Several strategies have been recently set up in order to rescue DYS synthesis in animal models of DMD (Aartsma-Rus and van Ommen, 2010; Cossu & Sampaolesi, 2007) and some of them have now entered clinical trials (Kinali et al, 2009; van Deutekom et al, 2007).
One of the major problems in comparing the benefit of different therapeutic treatments is to find common outcome measurements. The serum CK values are not reliable since they do not correlate with clinical assessments measured through magnetic resonance imaging (MRI; Kim et al, 2010). Moreover, CK levels are influenced by the age of the child (Zatz et al, 1991), by pharmacological treatments that do not have any effect on functional status and are increased by physical activity (Malm et al, 2000).
In this work, we showed that microRNAs, specifically expressed in muscle cells, were released into the blood of DMD patients as a consequence of muscle degeneration and their amount paralleled the severity of the disease. Notably, increased serum levels of muscle-specific miRNAs were also found in the animal model of DMD, the mdx mice, and these levels were lowered to almost wild-type (WT) values in animals treated with exon skipping, known to restore DYS synthesis and fibre integrity (Denti et al, 2006a, 2006b, 2008). Comparison between miRNA and CK measurements, both in DMD patients and mdx mice, indicated that miRNAs better correlate with DMD severity and suggest that they can be possibly utilized for measuring the outcomes of different therapeutic interventions.
RESULTS
Muscle miRNAs are enriched in the serum of DMD patients
Serum samples from healthy, Becker, and Duchenne children, ranging between 1- and 16-years-old, were collected under informed consensus. Prior to RNA extraction, a cocktail of artificial spiked miRNAs (ath-miR-159a, cel-lin-2, and cel-lin-4) was added to each sample in order to normalize raw data for qRT-PCR efficiency and for variability in volume handling. qRT-PCR was performed with probes for the muscle-specific miR-1, miR-206, and miR-133 as well as for the spikes. For mir-133, a single probe recognizing both miR-133a and miR-133b isoforms was utilized. Quantification of the granulocyte-specific miR-223 was utilized as control (Fazi et al, 2005). Figure 1A shows that all three miRNAs are almost undetectable in the serum of healthy controls (less than 10^3 copies/ml). A moderate enrichment (between 10^3 and 10^4 copies/ml) was detected in BMD individuals while a considerable increase was found in DMD patients (up to 10^6 copies/ml). In the different samples, no significant differences in the levels of miR-223 were observed. The same results were also obtained from whole blood RNA preparations (not shown). The statistical significance of these measurements was confirmed by receiver operating characteristics (ROC) analysis (Fig 1B) obtained by plotting the rate of true positive (sensitivity) versus false positive (1-specificity). These results show that miR-206 is able to discriminate DMD from WT (healthy) and BMD cases with almost absolute specificity (AUC always >0.94, p < 0.001) but also miR-1 and miR-133 display a very good statistical score (AUC always >0.84 and >0.76, respectively, p < 0.01).
Figure 1. Differential amounts of muscle miRNAs in the sera of healthy versus dystrophic children.
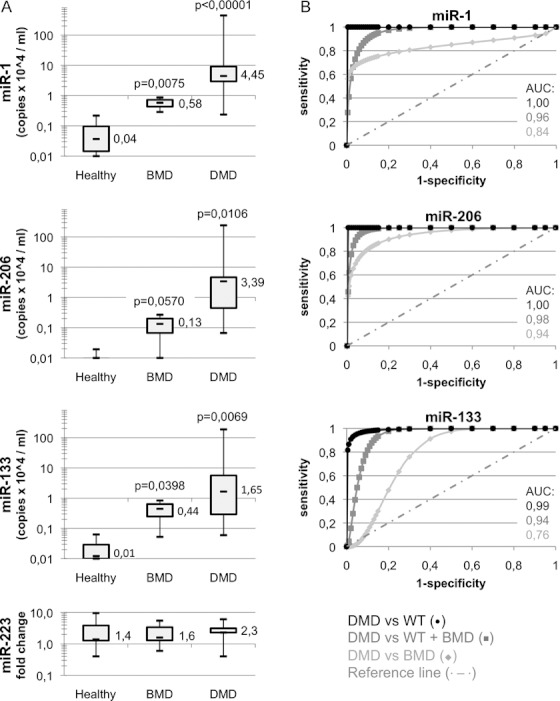
- Box plots comparing microRNA levels in the sera of 7 healthy, 26 Duchenne (DMD), and 5 Becker (BMD) patients (patient information is listed in supplementary Table 1). All data were normalized for three spiked miRNAs (ath-miR-159a, cel-lin-2, and cel-lin-4). miR-1, miR-206, and miR-133 values are shown as copy number per ml of serum. Control miR-223 levels are also shown as fold change values. All values are shown in logarithmic scale. p-values are derived from the comparison of miRNA levels in DMD or BMD versus healthy patients.
- ROC curves regarding diagnostic power to distinguish healthy from dystrophic cases. Area under the curve (AUC) values are also shown.
Serum miRNA levels correlate with clinical assessments
To study whether miR-1, miR-133, and miR-206 could represent bona fide markers for DMD, a case-by-case correlation analysis between miRNA levels and North Star Ambulatory Assessment (NSAA, Mazzone et al, 2010) was performed in 10 ambulating young DMD children (age range between 3 and 6 years) at the time of the molecular diagnosis. NSAA is one of the actual ‘gold standard’ functional tests to score DMD motor ability (from value 34 – normal ambulation, to value 0 – absence of ambulation). Figure 2A shows an inverse correlation between miRNA levels and NSAA score indicating that a progressive decrease in NSAA is associated with a gradual increase in miRNA serological levels. Figure 2B shows Spearman correlation between miRNA and NSAA, which is significant for all three miRNAs (the shown p-values are always <0.05). In addition, a direct correlation between miRNA levels and age is observed as well as a correlation between age and NSAA, as previously reported (Mazzone et al, 2010). Interestingly, patient DMD10, coetaneous with DMD9 but with worst motor ability (NSAA 16 and 25, respectively), displays a 26-fold increase of miRNA levels in comparison to DMD9 (right panel of Fig 2A). This specific case is quite interesting since it suggests that, independently from age, the increase in miRNA levels correlates with the severity of muscle damage. In contrast to miRNAs, which show a very clear inverse correlation with NSAA scores, the CK values show fluctuations unrelated to the severity of the disease (Fig 2A, lower panel).
Figure 2. Correlation between clinical assessment and serum miRNAs.
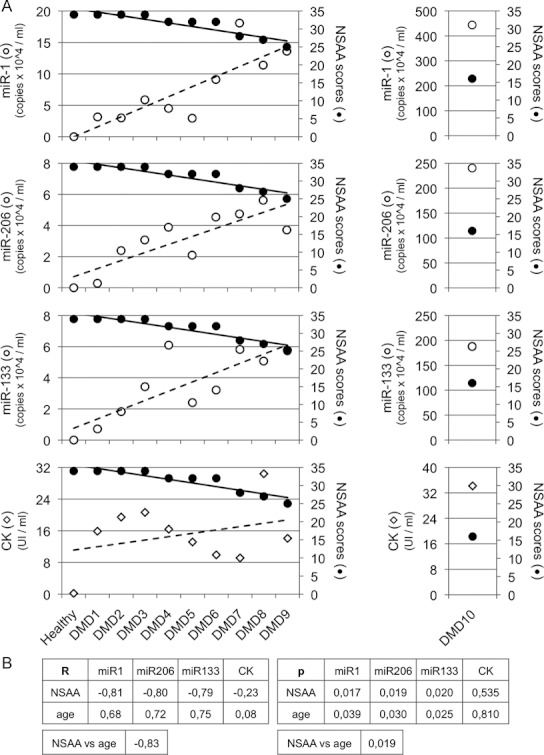
- Case-by-case comparison of miRNA levels and CK values with NSAA in 10 DMD children between 3 and 6 years of age (patient information is listed in supplementary Table 1). In each graph, miRNA levels are shown as white dots (copy numbers/ml of serum), NSAA scores as black dots, and CK values (units/ml of serum) as diamonds; regression lines are also displayed. Patients DMD 1–3, due to their age (3 years), have a non-collaborative behaviour to perform the entire NSAA; however, all of them had normal muscle strength at neurological assessment and they are indeed indicated with maximum NSAA score according to normal ambulation. For the outlier patient DMD10, different scales were used (graphs on the right).
- Spearman analysis correlating miRNA or CK levels with age or NSAA scores in DMD children. In the two tables, Spearman coefficients (R) and p-values (p) are displayed.
The situation in older, not ambulant patients, is more complicated due to heterogeneous clinical conditions and to the progressive loss of muscle mass. This can have strong effects on the amount of muscle cellular material released into the blood, as previously described for CK (Zatz et al, 1991). In agreement with this, in older patients, we observed a decrease of serum miRNA levels, even though they were still higher than samples from healthy controls (supplementary Fig 1).
miRNAs as biomarkers for therapeutic outcome measurements
Six-week-old mdx animals were tail vein injected with a recombinant adeno-associated viral vector carrying a U1-chimeric antisense construct (AAV#23, Fig 3A), previously reported to induce the skipping of the mutated exon 23 and to restore DYS synthesis and morpho-functional benefit in a body-wide manner (Denti et al, 2006a). After 1 month, mdx and AAV#23-treated mdx siblings were sacrificed in parallel with WT isogenic/aged matched animals. Different muscular districts [gastrocnemius (GAS), diaphragm (DIA), and heart (HEA)] were collected together with sera. Western blot revealed that through the exon skipping treatment, rescue of DYS was obtained (representative muscle districts are shown in Fig 3B), confirming the previously described body-wide activity of the AAV#23 virus. Moreover, the AAV#23 administration resulted in morphological amelioration of muscle fibres (Fig 3C) in agreement with our previous findings (Denti et al, 2006a, 2008). qRT-PCR for miR-1 and miR-206 in serum indicated that these miRNAs are 20/40-fold enriched in mdx with respect to WT mice (Fig 3D). Notably, the levels of these miRNAs are rescued to almost WT levels in AAV#23-treated mdx mice. In comparison to miRNA quantifications, CK titration reveals a mild increase in mdx mice, with respect to WT, without relevant statistical significance (Fig 3D, right panel). Altogether, these results indicate that the recovery of muscle integrity as a consequence of exon skipping is accompanied by a drastic decrease in miRNA release in the bloodstream. The conspicuous release of miR-1 and miR-206, observed in mdx conditions was also confirmed in WT animals treated with cardiotoxin, a drug normally used to induce acute muscle damage (not shown).
Figure 3. Muscle miRNAs (dystromiR) as diagnostic markers for DMD gene therapy.
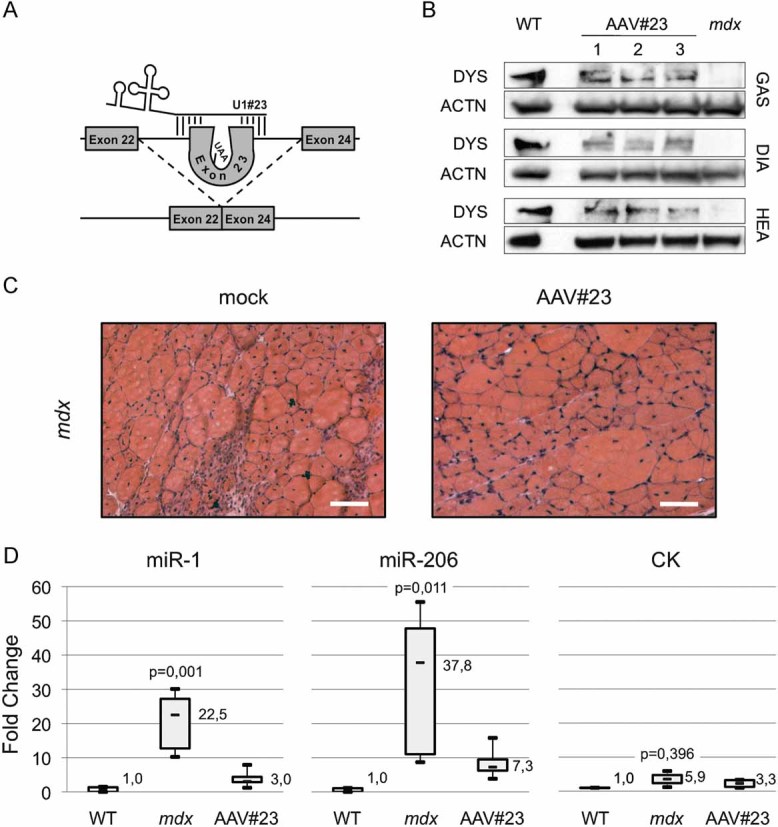
- Schematic representation of the exon skipping strategy in mdx mice (Denti et al, 2006a).
- Western blot for dystrophin (DYS) and actinin (ACTN) perfomed with 50 µg of muscle extracts (GAS, gastrocnemius; HEA, heart; DIA, diaphragm) of WT, mdx and AAV-U1#23 treated mdx (AAV#23). In control lane (wild-type; WT), 5 µg of WT protein extract were mixed with 45 µg of mdx proteins in order to avoid signal saturation.
- Mdx gastrocnemius sections from untreated (mock) or exon skipping-treated (AAV#23) animals were analysed by Hematoxylin/Eosin staining. Original magnification 20×. Scale bar: 100 µm.
- Box plots comparing microRNA and CK levels in the serum of WT, mdx, and AAV#23-treated mdx mice. miRNA values were normalized for three spiked miRNAs (ath-miR-159a, cel-lin-2, and cel-lin-4). All data are shown as fold change values with respect to WT set to a value of 1. p-values are derived from the comparison between miRNA or CK levels in mdx versus WT animals.
DISCUSSION
It has been recently reported that miRNAs expressed in specific body compartments can be released in to the blood as a consequence of different types of injuries and that they can represent sensitive biomarkers for several diseases, including cancer (Schöler et al, 2010). The surprisingly high stability of miRNAs in the serum was explained by the observation that miRNAs could be part of exosomal particles (Mitchell et al, 2008).
Since intense degeneration occurrs in many neuromuscular disorders, including DMD, we tested whether muscle miRNAs could be released in the serum, representing sensitive markers of muscle degeneration.
Several miRNAs have been shown to be specifically expressed in muscle cells and to play a very crucial role in the control of proper muscle development and function (Crist & Buckingham, 2009, 2010; van Rooij et al, 2008a). Moreover, altered levels of miRNAs were found in several muscular disorders such as myocardial infarction (van Rooij et al, 2008b), DMD, and other myopathies (Eisenberg et al, 2007). In particular, miR-1 and miR-133, highly abundant in mature muscle fibres, were found at reduced levels (two-fold) both in human and mouse dystrophic myoblasts (Cacchiarelli et al, 2010). In this study, we show that miR-1 and miR-133 are highly abundant in the bloodstream of DMD patients despite their reduced synthesis in dystrophic muscles: almost 100-fold enriched in comparison to samples from healthy controls, and 5–10 fold enriched with respect to BMD individuals.
At variance with miR-1 and miR-133, the levels of miR-206 are doubled in dystrophic versus WT muscles (Cacchiarelli et al, 2010). This was shown to be due to its specific expression in activated satellite cells and proliferating myoblasts, more numerous in dystrophic muscles where intense regeneration occurs. However, the 30- and 100-fold increase in respect to control, found, respectively, in the serum of BMD and DMD patients, cannot simply reflect the increase of miR-206 in DMD fibres. Overall, these results allowed us to conclude that the differences in serum levels of these miRNAs derive from the intensive degeneration occurring in DMD muscles.
In agreement with previous observations in dystrophic young dogs (Cozzi et al, 2001), intense degeneration occurs not only in mature fibres, but also in miR-206-enriched immature fibres determining the observed miR-206 release in the bloodstream.
Interestingly, when comparing the levels of serum miRNAs with the functional performances of DMD patients, we observed a very good inverse correlation: high levels of miRNAs corresponded to low ambulant activity. Notably, miRNA quantification was a better diagnostic marker than CK activity, which displays fluctuations unrelated to the severity of the disease. A further advantage of miRNA detection relates to the fact that CK activity is very sensitive to stress conditions and has to be measured in fresh serum preparations, while the serum stability of miRNA is very long lasting (Mitchell et al, 2008). Our analyses were performed on young ambulant patients (<6 years) since a very well established and reliable clinical assessment for the quantification of disease severity (NSAA) is available (Mazzone et al, 2010).
For non-ambulating patients, whose NSAA reaches the lowest score, there are indicators like muscle measurements, fat and fibrous tissue deposition measured by MRI (Kim et al, 2010). Unfortunately, no uniform functional, non-invasive tests to evaluate disease progression are available. In future work, we plan to extend our analysis to a large number of such ‘old’ cases in order to define whether we can establish reference values for testing: (i) whether miR-206 levels (specifically expressed in precursor muscle cells) can be utilized to establish the regenerating potentiality of the patients and (ii) whether miR-1 and miR-133 levels represent indicators of the residual muscle mass.
The results obtained from patients were confirmed in the animal model of DMD, the mdx mouse. In particular, in 2-month-old animals, both miR-1 and miR-206 were more abundant (38 and 23-fold, respectively) in the serum of mdx in comparison to WT controls. The mdx model allowed us to study whether muscle miRNAs can be also exploited as biomarkers for studying the recovery of muscle integrity upon therapeutic interventions. Nowadays, several strategies have been developed for the cure of DMD (Aartsma-Rus & van Ommen, 2010; Cossu & Sampaolesi, 2007) and among them, exon skipping has proved to be very promising. Indeed, two clinical trials of phase I have been recently concluded using different kinds of modified-antisense oligonucleotides (Kinali et al, 2009; van Deutekom et al, 2007). Exon skipping is effective in the mdx mice where, through the use of AAV vectors expressing U1-snRNA antisense molecules, efficient rescue of DYS was obtained together with recovery of correct tissue morphology and integrity (this work and Denti et al, 2006a, 2006b, 2008). In these mice, recovery of DYS paralleled the decrease of serum muscle miRNAs to almost WT levels. Notably, a partial recovery of DYS (<10%; Cacchiarelli et al, 2010; Denti et al, 2006a, 2008) was able to produce amelioration of morpho-functional parameters and to reduce serum levels of muscle miRNAs. Also in mouse sera, CK titration failed to accurately discriminate WT from mdx mice.
Even though a larger collection of data from DMD patients will be required in order to establish a precise correlation between miRNA levels, extent of muscle degeneration and stage of the disease (age, ambulating conditions, and time since wheel chair use), our studies indicate that the quantification of specific miRNAs (dystromiR), or even of only one of them, in serum, can be utilized as a biomarker tool to reveal DMD conditions in humans. In consideration of the results with mdx mice ‘cured’ through exon skipping, we propose to test whether this method can be applied to measure the outcomes and effectiveness of different therapeutic interventions. This seems particularly useful since, even though several therapeutic approaches are now entering clinical trials, a unifying method for assessing the benefit of the different treatments is still lacking.
The paper explained
PROBLEM
Duchenne muscular dystrophy (DMD) is a severe genetic disease caused by the lack of functional dystrophin (DYS). Nowadays, several strategies have been developed for the cure of DMD and among them, exon skipping has proved to be very promising. Indeed, two clinical phase I trials have recently been concluded using different kinds of modified-antisense oligonucleotides. In addition, exon skipping has proved to be very effective as a gene therapy treatment: in the mdx mice, efficient rescue of DYS was obtained together with recovery of correct tissue morphology and integrity through the use of AAV vectors expressing U1-snRNA antisense molecules. Such molecules have recently obtained the Orphan Drug designation from EMEA and FDA.
RESULTS
DYS absence in DMD causes intense muscle degeneration. CK blood levels have been utilized as diagnostic markers of several neuromuscular diseases, including DMD. However, CK levels are not reliable since they vary considerably upon several independent stress conditions, not necessarily associated with disease. In this work, we describe a new method formeasuring the onset and progression of DMD: we identified three muscle miRNAs that, as a consequence of muscle damage, are released into the bloodstream. Moreover, their abundance was described to correlate with the severity of the disease.
IMPACT
Since in mdx mice cured with exon skipping, the levels of serum miRNA recovered to WT levels, we propose that these miRNAs represent valuable biomarkers not only for the diagnosis of DMD onset and progression but also for monitoring the outcomes of therapeutic interventions on humans. Even though many different DMD therapeutic approaches are now entering clinical trials, a unifying method for assessing the benefit of different treatments is still lacking.
MATERIALS AND METHODS
Patient inclusion criteria
The study was approved by the local Ethical Committee and sera were obtained under informative consensus. Patient inclusion criteria were: genetically proven DMD or BMD diagnosis, patient still ambulant without any help (only for children younger than 6-years-old), no severe or moderate learning difficulties or behavioural problems.
Animal treatments and analyses
Six-week-old mdx mice were tail vein injected with 0.5–1 × 10^12 genome copies of the AAV-U1#23 virus (AAV#23). After 1 month, mdx and AAV#23-treated mdx siblings were sacrificed in parallel with WT isogenic/aged matched animals. The above procedures including H&E staining and Western blot analyses are fully described in Denti et al (Denti et al, 2008).
RNA preparation and analysis
Serum was harvested from patients or mice by centrifugation of blood in serological tubes. Prior to RNA extraction, 1 fmole of three spiked artificial miRNAs (ath-miR-159a, cel-lin-2 and cel-lin-4) was added each 150 µl of serum (300 µl for patients – 50 µl for mice). Small RNAs were extracted by miRNeasy (Qiagen), following manufacturer specifications for liquid samples. qRT-PCR were performed using miScript System (Qiagen). Copy number analyses were performed by using standard curves from artificial templates normalized for absolute quantifications obtained from miRNA-spiked.
Statistical analyses
Each data shown in qRT-PCR analysis is the result of at least five independent experiments performed on at least five different samples of the same group. Box Plots are produced indicating median (–, inside the box) first and third quartiles (box borders), minimum and maximum (–, outside the box). Statistical significance of differences between distributions was assessed by two-tailed t-test and a p < 0.05 was considered significant. In Fig 1A the Welch t-test was applied. ROC curves are produced by plotting sensitivity versus 1-specificity for 28 different thresholds (Søreide, 2009). Relationship between variables was calculated using the Spearman correlation coefficient (indicated as R in the text). A correlation >0.75 was considered as an acceptable relationship and a p < 0.05 was considered significant.
Acknowledgments
We thank Prof. A. Musarò and C. Nicoletti for animal facilities. We also thank M. Cesana for qRT-PCR support, O. Sthandier, T. Santini and T. Incitti for microscopic analysis and Parent Project ONLUS for access to the Italian DMD Patients Registry. DC is a recipient of a Microsoft research PhD fellowship. This work was partially supported by grants from: Telethon (GGP07049), Parent Project Italia, EU project SIROCCO (LSHG-CT-2006-037900), IIT ‘SEED’, PRIN and BEMM.
Supporting information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
CD designed experiments, set up and performed RNA procedures and qRT-PCR; LI performed qRT-PCR and statistical analyses; MJ and CV performed mice treatments and protein analyses; DA and BE collected human sera and performed clinical assessments; BI designed experiments and wrote the paper.
For more information
Parent association of DMD/BMD affected young
boys
Fund raising foundation for DMD and rare diseases
EU consortium created to investigate siRNA/miRNA functions
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Aartsma-Rus A, van Ommen GJ. Progress in therapeutic antisense applications for neuromuscular disorders. Eur J Hum Genet. 2010;18:146–153. doi: 10.1038/ejhg.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacchiarelli D, Martone J, Girardi E, Cesana M, Incitti T, Morlando M, Nicoletti C, Santini T, Sthandier O, Barberi L, et al. MicroRNAs involved in molecular circuitries relevant for the Duchenne muscular dystrophy pathogenesis are controlled by the dystrophin/nNOS pathway. Cell Metab. 2010;12:341–351. doi: 10.1016/j.cmet.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Chamberlain JS, Gibbs RA, Ranier JE, Nguyen PN, Caskey CT. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988;16:11141–11156. doi: 10.1093/nar/16.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G, Sampaolesi M. New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med. 2007;13:520–526. doi: 10.1016/j.molmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Cozzi F, Cerletti M, Luvoni GC, Lombardo R, Brambilla PG, Faverzani S, Blasevich F, Cornelio F, Pozza O, Mora M. Development of muscle pathology in canine X-linked muscular dystrophy. Quantitative characterization of histopathological progression during postnatal skeletal muscle development. Acta Neuropathol. 2001;101:469–478. doi: 10.1007/s004010000308. [DOI] [PubMed] [Google Scholar]
- Crist CG, Buckingham M. MicroRNAs gain magnitude in muscle. Cell Cycle. 2009;8:3627–3628. doi: 10.4161/cc.8.22.9960. [DOI] [PubMed] [Google Scholar]
- Crist CG, Buckingham M. Megarole for microRNA in muscle disease. Cell Metab. 2010;12:425–426. doi: 10.1016/j.cmet.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Dent KM, Dunn DM, von Niederhausern AC, Aoyagi AT, Kerr L, Bromberg MB, Hart KJ, Tuohy T, White S, den Dunnen JT, et al. Improved molecular diagnosis of dystrophinopathies in an unselected clinical cohort. Am J Med Genet. 2005;134:295–298. doi: 10.1002/ajmg.a.30617. [DOI] [PubMed] [Google Scholar]
- Denti MA, Rosa A, D'Antona G, Sthandier O, De Angelis FG, Nicoletti C, Allocca M, Pansarasa O, Parente V, Musarò A, et al. Body-wide gene therapy of Duchenne muscular dystrophy in the mdx mouse model. Proc Natl Acad Sci USA. 2006a;103:3758–3763. doi: 10.1073/pnas.0508917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denti MA, Rosa A, D'Antona G, Sthandier O, De Angelis FG, Nicoletti C, Allocca M, Pansarasa O, Parente V, Musarò A, et al. Chimeric adeno-associated virus/antisense U1 small nuclear RNA effectively rescues dystrophin synthesis and muscle function by local treatment of mdx mice. Hum Gene Ther. 2006b;17:565–574. doi: 10.1089/hum.2006.17.565. [DOI] [PubMed] [Google Scholar]
- Denti MA, Incitti T, Sthandier O, Nicoletti C, De Angelis FG, Rizzuto E, Auricchio A, Musarò A, Bozzoni I. Long-term benefit of adeno-associated virus/antisense-mediated exon skipping in dystrophic mice. Hum Gene Ther. 2008;19:601–608. doi: 10.1089/hum.2008.012. [DOI] [PubMed] [Google Scholar]
- Ebashi S, Toyokuma Y, Momoi H, Sugita H. High creatine phosphokinase activity of sera of progressive muscular dystrophy. J Biochem. 1959;46:103–104. [Google Scholar]
- Eisenberg I, Eran A, Nishino I, Moggio M, Lamperti C, Amato AA, Lidov HG, Kang PB, North KN, Mitrani-Rosenbaum S, et al. Distinctive patterns of microRNA expression in primary muscular disorders. Proc Natl Acad Sci USA. 2007;104:17016–17021. doi: 10.1073/pnas.0708115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, Bozzoni I. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell. 2005;123:819–831. doi: 10.1016/j.cell.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Fischbeck KH, Brown RH, Johnson M, Medori R, Loike JD, Harris JB, Waterston R, Brooke M, Specht L, et al. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N Engl J Med. 1988;318:1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- Kim HK, Laor T, Horn PS, Racadio JM, Wong B, Dardzinski BJ. T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology. 2010;255:899–908. doi: 10.1148/radiol.10091547. [DOI] [PubMed] [Google Scholar]
- Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, Guglieri M, Ashton E, Abbs S, Nihoyannopoulos P, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm C, Nyberg P, Engstrom M, Sjodin B, Lenkei R, Ekblom B, Lundberg I. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. 2000;529:243–262. doi: 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone E, Martinelli D, Berardinelli A, Messina S, D'Amico A, Vasco G, Main M, Doglio L, Politano L, Cavallaro F, et al. North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2010;20:712–716. doi: 10.1016/j.nmd.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Schöler N, Langer C, Döhner H, Buske C, Kuchenbauer F. Serum microRNAs as a novel class of biomarkers: a comprehensive review of the literature. Exp Hematol. 2010;38:1126–1130. doi: 10.1016/j.exphem.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søreide K. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J Clin Pathol. 2009;62:1–5. doi: 10.1136/jcp.2008.061010. [DOI] [PubMed] [Google Scholar]
- Stockley TL, Akber S, Bulgin N, Ray PN. Strategy for comprehensive molecular testing for Duchenne and Becker muscular dystrophies. Genet Test. 2006;10:229–243. doi: 10.1089/gte.2006.10.229. [DOI] [PubMed] [Google Scholar]
- van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, den Dunnen JT, Koop K, van der Kooi AJ, Goemans NM, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Liu N, Olson EN. MicroRNAs flex their muscles. Trends Genet. 2008a;24:159–166. doi: 10.1016/j.tig.2008.01.007. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008b;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz M, Rapaport D, Vainzof M, Passos-Bueno MR, Bortolini ER, Pavanello Rde C, Peres CA. Serum creatine-kinase (CK) and pyruvate-kinase (PK) activities in Duchenne (DMD) as compared with Becker (BMD) muscular dystrophy. J Neurol Sci. 1991;102:190–196. doi: 10.1016/0022-510x(91)90068-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


