Abstract
Evidence suggests that women are more susceptible to stress-related disorders than men. Animal studies demonstrate a similar female sensitivity to stress and have been used to examine the underlying neurobiology of sex-specific effects of stress. Although our understanding of the sex-specific effects of chronic adolescent stress has grown in recent years, few studies have reported the effects of adolescent stress on depressive-like behavior. The purpose of this study was to determine if a chronic mixed modality stressor (consisting of isolation, restraint, and social defeat) during adolescence (PND37-49) resulted in differential and sustained changes in depressive-like behavior in male and female Wistar rats. Female rats exposed to chronic adolescent stress displayed decreased sucrose consumption, hyperactivity in the elevated plus maze, decreased activity in the forced swim test, and a blunted corticosterone response to an acute forced swim stress compared to controls during both adolescence (PND48-57) and adulthood (PND96-104). Male rats exposed to chronic adolescent stress did not manifest significant behavioral changes at either the end of adolescence or in adulthood. These data support the proposition that adolescence may be a stress sensitive period for females and exposure to stress during adolescence results in behavioral effects that persist in females. Studies investigating the sex-specific effects of chronic adolescent stress may lead to a better understanding of the sexually dimorphic incidence of depressive and anxiety disorders in humans and ultimately improve prevention and treatment strategies.
Introduction
Evidence suggests that women are more susceptible to stress-related disorders than men, and interactions between the hypothalamic-pituitary-gonadal (HPG) axis and the hypothalamic-pituitary-adrenal (HPA) axis have been hypothesized to underlie women’s vulnerability to clinical depression (Young, 1998). Some animal studies demonstrate a similar female sensitivity to stress and have been used to examine the underlying neurobiology of sex specific effects of stress.
In addition to sex-specific effects of stress exposure, the effects of stress can also be dependent on developmental stage. Laboratory animals exposed to stressful conditions during early postnatal development can manifest short and long-term abnormal behavior associated with alterations of the HPA axis. Although not as well characterized as early postnatal stress, the available literature on the long term behavioral effects of adolescent stress demonstrates that exposure to chronic stress during adolescence can be more detrimental than similar exposure in adulthood (Avital and Richter-Levin, 2005; Andersen and Teicher, 2008). In addition, stress during adolescence can reprogram the HPA axis to cause long-term effects on stress reactivity. HPA axis development continues during adolescence (Dhom, 1973; Ducharme et al., 1976; Pignatelli et al., 2006) and during this developmental stage, adolescent rats demonstrate slower corticosterone recovery than adult rats, and inefficient habituation to stressors (Doremus-Fitzwater et al., 2009). Compared to adult subjects, a reduced stress response in terms of corticosterone release has also been reported in mice during adolescence. These developmental differences in HPA axis physiology may predispose adolescent rats to the adverse effects of chronic stress. Furthermore, temporal differences in HPA axis development between males and females (Pignatelli et al., 2006; Sapolsky & Meaney, 1986) may account for prolonged sex-dependent effects induced by adolescent stress on physiology and behavior.
Although our understanding of the sex-specific effects of chronic adolescent stress has grown in recent years, these studies have largely focused on anxiety-like behavior, reward behaviors, and HPA axis physiology; few studies have reported the effects of adolescent stress on depressive-like behavior. The purpose of this study was to determine if chronic stress during adolescence resulted in sustained changes in depressive-like behavior in male and female rats. In addition, we assessed anxiety-like behaviors and HPA axis function to determine if a chronic mixed-modality stress paradigm produced effects similar to other adolescent stress models in the literature.
Materials and methods
Animals
Timed pregnant Wistar rats (Charles River, Wilmington, MA) arrived on gestational day 12 (n = 24). Pregnancy was determined at the Charles River facility by the presence of a vaginal plug. Rats were housed on a 14:10 reverse light:dark cycle in a facility controlled for humidity (60%) and temperature (20°C–23°C). Rodent diet 5001 chow (Purina Mills, Richmond, IN) and water were available ad libitum throughout the study. Three days after birth, rat pups were sexed and litters were culled to four male and four female pups. No more than two pups per litter were assigned to a group in order to prevent litter effects (Holson and Pearce, 1992). Each group was assigned between 8 and 12 pups. Pups were weighed and weaned on postnatal day (PND) 21 and housed two per cage in same sex groups. Estrous cycle was tracked throughout the study using vaginal lavage. All animal procedures were approved by Emory University’s IACUC and were carried out to minimize pain and suffering to the animal in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Identification of the Control Group
Typically in stress studies the control group is individually housed to provide a closely matched control group for the stress group. Because individual housing during adolescence can be detrimental, the first study determined whether standard pair housing or single housing during adolescence would serve as a more appropriate control when endpoints were assessed either at the end of adolescence or in adulthood. For this assessment, we measured behavioral and physiological endpoints for the following groups both at the end of adolescence and in adulthood (separate cohorts for each age group): 1) male pair housed, 2) male single housed (isolation initiated on PND 36), 3) female pair housed, and 4) female single housed (isolation initiated on PND 36). Data from this initial study, demonstrated that single housing during adolescence affected multiple behavioral endpoints. These data confirmed that individual housing was a stressor for adolescent rats. Therefore, pair housing was chosen for the adolescent control group and individual housing was considered part of the mixed modality stress paradigm.
Chronic Mixed-Modality Stress
Adolescent stress (Stress) was defined as individual housing beginning at PND 36 and continuing throughout the study combined with randomly alternating daily exposure to social defeat or restraint from PND 37–49. The cohort of rats tested as adolescents and the cohort tested as adults each consisted of the following groups: 1) pair housed males (Control male), 2) isolated males exposed to restraint and defeat during adolescence (Stress male), 3) pair housed females (Control female), and 4) isolated females exposed to restraint and defeat during adolescence (Stress female). The social defeat stress was adapted from previous studies. Resident Long-Evans rats (Charles River, Wilmington, MA) were trained to defeat an intruding rat (experimental subject) in their home cage and selected based on likelihood to pin an intruder. Rats in the stress group were paired with same sex residents, placed in the resident’s home cage with the two rats separated by a wire mesh screen. Male residents were adult Long Evans retired breeders and female residents were adult Long Evans ovariectomized females. Both male and female Long Evans rats demonstrate the territorial aggression necessary for the social defeat stressor and ovariectomy accentuates this behavior in females (DeBold and Miczek, 1984). On the first day of social defeat, after five minutes, the barrier was removed for five minutes or until the intruder was pinned five times. On the second day, the barrier was replaced after three pins or five minutes. All subsequent bouts of defeat interactions consisted of only one pin or a maximum of five minutes of interaction. At the conclusion of the physical interaction, the wire mesh screen was replaced. Intruders and residents remained separated by the wire mesh screen for an additional 30 minutes and subsequently the intruder was returned to its home cage. Intruder and resident pairings were randomly assigned each day to prevent stabilization of a dominance hierarchy. For the restraint portion of the mixed-modality stressor, animals were restrained for 60 min in acrylic rat restraints (BrainTree Scientific, Braintree, MA) which prevented head to tail turns but did not compress the rat. All stressors occurred during the light cycle to coincide with the nadir of the circadian cycle of corticosterone to maximally increase corticosterone. Adolescents received six total exposures to social defeat and six total exposures to restraint over a 12 day period in a randomly alternating pattern. The study was not designed to assess the specific effects of individual housing, restraint, or social defeat but was designed to use this combination of established stressors to induce chronic stress during adolescence. Adolescent control groups were pair housed throughout the study, and adult control groups were individually housed prior to behavioral testing in adulthood. Therefore, comparison of results between adolescent and adult subjects should take into account a possible influence of differential housing condition of the control groups.
Behavioral Testing
Behavioral testing for the adolescent cohort was conducted between PND 48 to PND 55 and testing for the adult cohort was conducted between PND 96 and PND 103. Behavioral testing for each cohort consisted sequentially of the sucrose consumption test, the elevated plus maze, the acoustic startle test, and the forced swim test. Animals were randomized during each test day to minimize any effect due to testing time. One behavioral test was conducted per day with the exception of the sucrose consumption test which was administered over two days. The elevated plus maze was conducted during the beginning of the dark cycle while all other tests were conducted in the light cycle. All animals during the adult portion of the study were single housed one week prior to behavioral testing.
Sucrose Consumption Test
The sucrose consumption test measures the rat’s consumption of a sucrose solution (0.8%) versus water as a measure of hedonic state. Rats subjected to chronic social defeat consume less sucrose than control rats and this behavior has been described as a depressive-like state. Rats were given free access to one bottle of tap water and one bottle of a 0.8% sucrose solution in tap water. In order to prevent any effect due to side bias, bottle location was reversed after 24 hours. After 24 and 48 hours, the bottles were weighed to determine sucrose and water consumption. Data reported are the average consumption of day 1 and day 2 of the sucrose consumption test and normalized to the number of animals per cage.
Elevated Plus Maze
As an index of anxiety-like behavior, animals were tested in the elevated plus maze. Rats were placed in the center of a plastic plus maze and recorded for five minutes. Testing took place two hours after lights out and was conducted under dim red light. Tapes were scored for number of entries and time spent in the closed or open arms. Percent of time spent in the open arm was calculated as time spent in the open arm divided by total testing time (300 seconds). To measure other exploratory behavior, rearing behavior was counted.
Acoustic Startle Reflex
The acoustic startle reflex test was performed as previously described. Briefly, during the light cycle, rats were placed in a ventilated startle chamber (San Diego Instruments, San Diego, CA) using an accelerometer to measure the startle reflex. After a white-noise (65 db) acclimatization period in the chamber lasting five minutes, individual rats were exposed to 16 trials consisting of a short pulse of noise (115 db). Between each trial, there was a period of white noise which lasted 2, 5, 25, or 60 seconds. The initial pulse trial was disregarded due to a lack of a habituation response.
Forced Swim Test
In order to assess motor activity in an inescapable environment, the forced swim test was employed. The forced swim test, while traditionally a screen for antidepressant drug efficacy, has been used to assess the tendency to respond actively or inactively to a challenge. Rats were placed in a clear acrylic beaker (40 cm high × 18 cm in diameter) filled with 30°C water for ten minutes during the light cycle. Following this training session, rats were tested the following day for five minutes. Two observers blind to treatment group scored the latency to the first float, time spent struggling, and the number of dives which previous studies have described as escape or active coping behavior. Latency to first float was defined as the rat’s limbs remaining motionless for at least two seconds. Struggling was defined as the rat breaking the surface of the water with all four limbs in motion. Diving was defined as the rat swimming below the surface of the water.
Endocrine and Tissue Weight Analyses
Rats were decapitated on PND 57 for adolescent endpoints or PND 104 for adult endpoints two hours prior to the dark cycle. Following a 30 minute restraint session used to stimulate the HPA axis, rats were rapidly decapitated without anesthesia and trunk blood was collected immediately in BD Vacutainer EDTA collection tubes (BD, Franklin Lakes, NJ). Blood was spun down at 1,800 rcf and the plasma fraction was collected. Plasma corticosterone was assayed using 10 μL of plasma with the ImmuChem 125I Corticosterone RIA Kit with an intra-assay variability of 5.55%, an inter-assay variability of 6.97% and a sensitivity of 1 ng/mL (MP Biomedicals, Solon, OH). Plasma testosterone was assayed using 50 μL of plasma with the DSL-4100 RIA Kit with an intra-assay variability of 6.31%, an inter-assay variability of 6.93% and a sensitivity of 0.05 ng/mL (Diagnostic Systems Laboratories, Webster, TX). Plasma estradiol was assayed using 50 μL of plasma with the ImmuChem 125I 17β-Estradiol RIA Kit with an intra-assay variability of 4.02%and a sensitivity of 10 pg/mL (MP Biomedicals, Solon, OH). All estradiol samples were run in one assay, therefore no inter-assay variability was calculated. Samples were run in duplicate for all endocrine assays. Reproductive tissues were collected and wet weights were assessed as an index of the degree of sexual maturity. Body mass was recorded throughout the study.
Statistical Analyses
SPSS was used to conduct statistical analyses. Two-way ANOVA was used for most statistical comparisons (age × stress). A Bonferroni post-hoc test was used to assess individual group differences. In the case of a comparison between only two groups, a Student’s t test was used. The alpha value was set to 0.05. Testicular and uterine masses were normalized to account for body mass discrepancies.
Results
Identification of Control Group
Housing condition did not impact adolescent weight gain, reproductive tissue mass, plasma testosterone (males), plasma estradiol (females), the corticosterone response to restraint, sucrose consumption, behavior in the forced swim test, or acoustic startle (p > 0.05, data not shown for this section). Housing condition during adolescence also did not alter total arm entries, percent of time in the open arms, or number of rears in the elevated plus maze for female adolescent rats (p > 0.05). However, single housing of male adolescent rats decreased the percent of time spent in the open arms of the elevated plus maze as compared to pair housed controls (U= 69, p < 0.05). Other metrics in the elevated plus maze were not altered by individual housing of male rats (p > 0.05). Single housing during adolescence did not alter body mass, reproductive tissue mass, testosterone (males), estradiol (females), the corticosterone response to restraint, or sucrose consumption for either adult male or female rats compared to those housed in pairs during adolescence (p > 0.05, data not shown for this section). Adult male rats that were housed individually during adolescence had an increased latency to float and spent more time struggling than did adult male rats that were pair housed during adolescence (p < 0.05), but there was no difference between the groups in the number of dives during the forced swim test (p > 0.05). Behavior of adult female rats in the forced swim test was unaltered by exposure to individual housing during adolescence as compared to adult female rats that were pair housed during adolescence (latency to float, time struggling, number of dives: p > 0.05). Housing condition during adolescence did not alter total arm entries or number of rears in the elevated plus maze for adult male rats (p > 0.05), but consistent with data from the adolescent endpoint, individual housing during adolescence resulted in reduced time in the open arms compared to rats housed in pair during adolescence (p < 0.05). Adult behavior of female rats in the elevated plus maze was not altered by adolescent housing condition (p > 0.05).
Body Mass
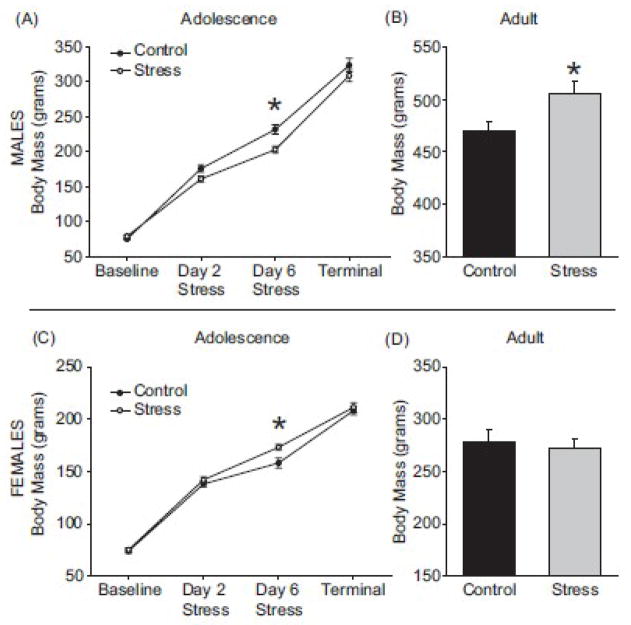
Adolescent males exposed to stress gained less body mass than control adolescent males (F(1,60) = 10.5; p < 0.05; Figure 1A), but all male rats gained body mass during adolescence (F(3,60) = 553; p < 0.05). Halfway through the chronic mixed-modality stress model, males exposed to adolescent stress weighed less than control males (t(15) = p < 0.05; Figure 1A). However, this effect was transient and the groups did not differ following behavioral testing at the terminal collection point. Although adolescent stress transiently reduced body mass in adolescence, the effect was reversed in adulthood such that adult male rats with a history of adolescent stress weighed more than controls (t(11) = 2.3; p < 0.05; Figure 1B).
Figure 1.
Animals exposed to stress during adolescence displayed abnormal weight gain. Males and females pair housed (Control) or exposed to the chronic mixed-modality stress model (Stress) were compared to determine changes in weight gain due to chronic adolescent stress. (A) Males exposed to stress during adolescence gained less body mass than controls. (B) At the adult terminal collection point, adult males exposed to stress weighed more than age-matched controls (* p < 0.05 Student’s t test). (C) Females exposed to stress during adolescence gained more weight than controls. (D) Adult females exposed to chronic adolescent stress had no changes in weight gain compared to age-matched controls. * p < 0.05 main effect due to stress, # p < 0.05 main effect due to age 2-way ANOVA. Data are presented as mean ± SEM, N = 5–9.
Regardless of stress exposure, all female rats gained body mass during adolescence (F(3,72) = 526.4; p < 0.05). Adolescent female rats exposed to stress weighed more than control females on the sixth day of stress exposure (F(1,72) = 5.5; p < 0.05; Figure 1C), but body mass normalized by the time of adolescent tissue collection. For female rats, weight in adulthood was not affected by exposure to chronic adolescent stress (t(11) = 0.47; p > 0.05; Figure 1D).
Reproductive Tissues and Sex Steroids
Chronic adolescent stress caused increased testicular mass at the end of adolescence but not in adulthood (t(15) = 4.2; p < 0.05; Table 1). Plasma testosterone concentrations were unchanged by exposure to chronic adolescent stress when measured either during adolescence or adulthood (F(1,22) = 1.1; p > 0.05). Plasma testosterone did not differ between adolescent and adult rats (F(1,22) = 0.32; p > 0.05).
Table 1.
Tissue weights and gonadal hormones. Reproductive tissues were removed post decapitation and wet weights were measured. Trunk blood was used to determine plasma values of gonadal hormones.
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Adolescence | Adult | Adolescence | Adult | |||||
| Control | Stress | Control | Stress | Control | Stress | Control | Stress | |
| Testosterone (ng/mL) | 4.01 ± 0.41 | 2.88 ± 0.42 | 3.17 ± 0.79 | 4.82 ± 0.90 | - | - | - | - |
| Testes weight (g) | 3.11 ± 0.10 | 3.99 ± 0.10* | 4.16 ± 0.29 | 3.72 ± 0.07 | - | - | - | - |
| Estradiol (pg/mL) | - | - | - | - | 50.80 ± 3.06 | 52.94 ± 5.56 | 49.23 ± 3.32 | 49.19 ± 2.45 |
| Uterine weight (mg) | - | - | - | - | 385.6 ± 34.3 | 412.3 ± 27.2 | 442.7 ± 35.4 # | 498.3 ± 32.5 # |
p < 0.05 main effect due to age 2-way ANOVA,
p < 0.05 Student’s t test. Data are presented as mean ± SEM, N = 5–9.
For female rats, uterine mass was not altered by stress compared to control females in either adolescence or adulthood (F(1,28) = 1.8; p > 0.05; Table 1). Adult females had an increased uterine mass compared to adolescent females (F(1,28) = 5.5; p < 0.05). Plasma estradiol did not differ due to stress (F(1,21) = 0.07; p > 0.05) or age (F(1,21) = 0.44; p < 0.05).
Corticosterone
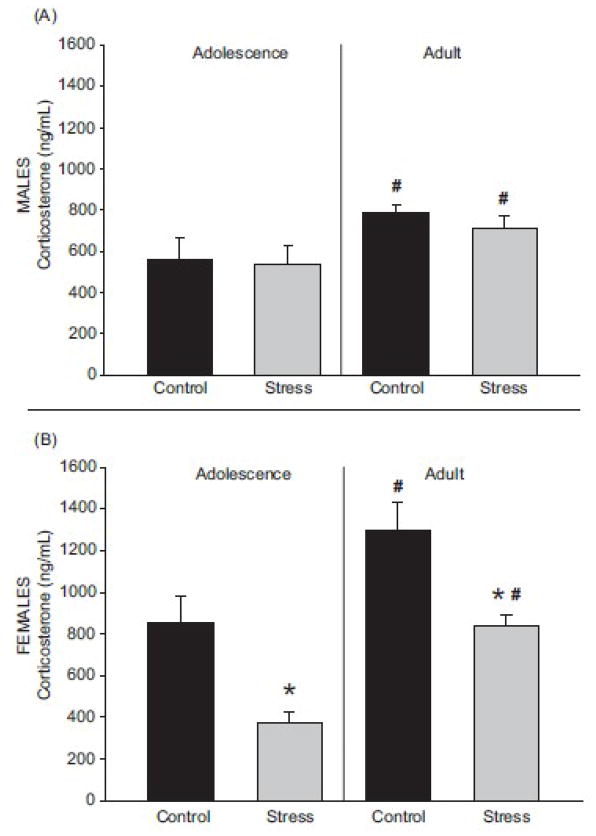
Plasma corticosterone concentrations following acute stress challenge were higher in adult male rats than adolescent males rats (F(1, 26) = 5.41; p < 0.05). Exposure to chronic adolescent stress did not alter the plasma corticosterone response to an acute stress challenge in either adolescent or adult male rats (Figure 2A; F(1, 26) = 0.31; p > 0.05).
Figure 2.
Rats were exposed to an acute stress challenge (30 minute restraint session) followed by rapid decapitation and plasma collection to determine the concentration of corticosterone. (A) Male rats exposed to the acute stress challenge showed plasma corticosterone concentrations indicative of a stress response, but no difference was observed between treatment groups (Control = pair housed controls; Stress = isolation, social defeat, and restraint). (B) In females, the acute stress challenge elicited a blunted corticosterone increase in rats that were previously exposed to chronic adolescent stress compared to the acute stress-induced corticosterone increase for controls. * p < 0.05 main effect due to stress, # p < 0.05 main effect due to age 2-way ANOVA. Data are presented as mean ± SEM, N = 5–11.
Age significantly affected plasma corticosterone, as adolescent females displayed lower plasma corticosterone compared to adult females (F(1, 25) = 23.56; p < 0.05). Females exposed to chronic adolescent stress displayed a blunted increase in plasma corticosterone compared to control females (Figure 2B; F(1, 26)= 25.53; p < 0.05). Blunted plasma corticosterone due to a history of chronic adolescent stress was observed in adolescence (t(16) = 4.16; p < 0.05) and in adulthood (t(9) = 3.14; p < 0.05).
Sucrose Consumption
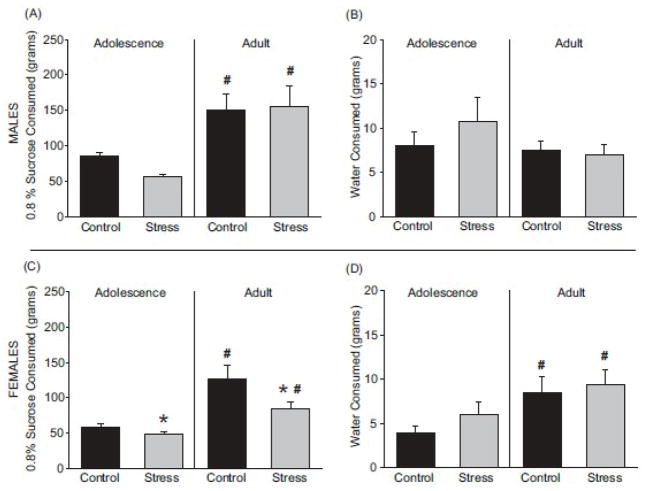
Adult male rats consumed more sucrose solution than adolescent male rats (Figure 3A; F(1, 28) = 26.1; p < 0.05) and the same effect was observed in female rats (Figure 3C; F(1, 24) = 29.44; p < 0.05). Chronic adolescent stress resulted in a reduced consumption of sucrose as compared to age-matched controls for female (Figure 3C; F(1, 24) = 7.69; p < 0.05) but not male (Figure 3A; F(1, 28) = 0.6; p > 0.05) rats. This effect was most apparent in adulthood (Figure 3C; t(10) = 2.91; p < 0.05). Water consumption during the sucrose consumption test was unchanged in males (Figure 3B; p > 0.05). Adult females consumed more water than adolescent females (Figure 3D; F(1, 24) = 7.75; p < 0.05), but chronic adolescent stress had no effect on water consumption in females (Figure 3D; F(1, 24) - 1.03; p > 0.05).
Figure 3.
The effects of chronic adolescent stress on sucrose consumption were determined using a 48 hour sucrose consumption test. Rats were given free access to identical bottles which contained either tap water or 0.8% sucrose in tap water. Consumption of each liquid was measured at 24 hours and 48 hours and the values averaged for each rat. Male rats exposed to chronic adolescent stress consumed similar amounts of sucrose (A) and water (B) compared to male controls. Female rats exposed to chronic adolescent stress consumed less sucrose than control rats during adolescence and adulthood (C). Water consumption in females exposed to chronic adolescent stress was unaltered compared to female controls (D). * p < 0.05 main effect due to stress, # p < 0.05 main effect due to age 2-way ANOVA. Data are presented as mean ± SEM, N = 5–11.
Acoustic Startle Response
Acoustic startle response was unaffected by chronic adolescent stress for both adolescent male and female rats as compared to same sex control groups. There were no differences in acoustic startle response or habituation to repeated startles (p > 0.05, data not shown). Similarly, acoustic startle response in adulthood was unaffected by a history of chronic adolescent stress for male or female rats compared to same sex control groups. There were no differences in acoustic startle response or habituation to repeated startles (p > 0.05, data not shown).
Elevated Plus Maze
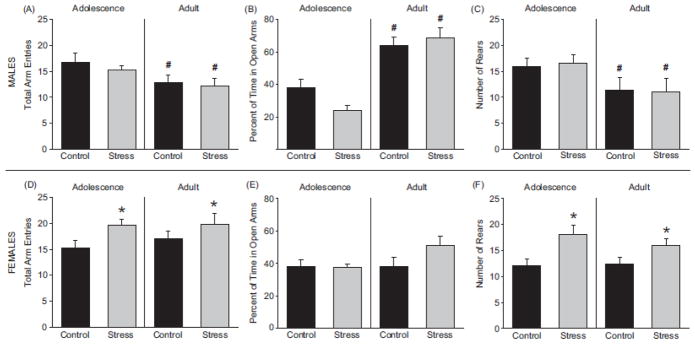
Adult male rats exhibited fewer total arm entries in the elevated plus maze compared to adolescent male rats (Figure 4A; F(1, 28) = 5.70; p < 0.05). Adult males spent more time exploring the open arm compared to adolescent males (Figure 4B; F(1, 20) = 49.77; p < 0.05). The number of rears was also reduced in adult males compared to adolescent males (Figure 4C; F(1, 20) = 5.91; p < 0.05). While age changed the behavior of males in the elevated plus maze, adolescent stress did not; males displayed no behavioral changes in the elevated plus maze due to stress in adolescence or adulthood, (total arm entries (F(1, 28) = 0.581; p > 0.05); exploration of the open arm (F(1, 20) = 0.77; p > 0.05); number of rears (F(1, 20) = 0.001; p > 0.05)).
Figure 4.
Stress altered behavior in the elevated plus maze for females. Total arm entries (A) percent time in the open arm (B) and number of rears (C) were not altered by stress in male adolescent rats compared to same sex controls (p > 0.05). Female rats exposed to stress engaged in more total arm entries (D). Percent of time in the open arm was unaffected by chronic adolescent stress in females (E). Females exposed to chronic adolescent stress demonstrated increased rearing behavior (F) compared to same sex controls. * p < 0.05 main effect due to stress, # p < 0.05 main effect due to age 2-way ANOVA. Data are presented as mean ± SEM, N = 5–12.
In females, chronic adolescent stress increased total locomotor activity as assessed by total arm entries compared to controls at both adolescent and adult testing points (Figure 4A; F(1, 28) = 5.00; p < 0.05); chronic adolescent stress did not affect exploration of the open arm at either time point (Figure 4B; F(1, 28) = 2.65; p > 0.05). Rearing behavior was increased due to adolescent stress compared to female controls (Figure 4C; F(1, 28) = 8.23; p < 0.05) and this effect was significant in adolescence (t(15) = 2.83; p < 0.05). In contrast to males, adolescent and adult females displayed similar behaviors in the elevated plus maze; total arm entries (F(1, 28) = 0.38; p > 0.05), time in the open arm (F(1, 28) = 3.08; p > 0.05), and the number of rears (Figure 4C; F(1, 28) = 0.31; p > 0.05) did not differ between adolescent and adult rats.
Forced Swim Test
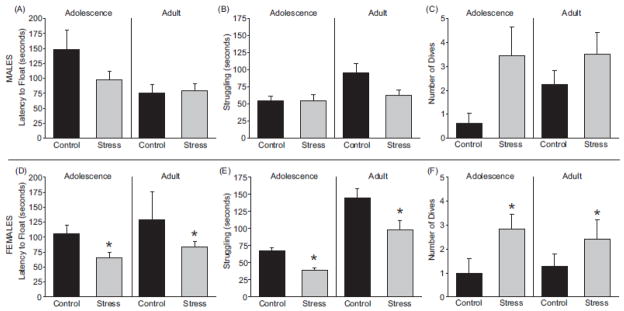
Neither exposure to chronic adolescent stress nor age altered latency to float (Figure 5A; p > 0.05), total time spent struggling (Figure 5B; p > 0.05), or the number of dives (Figure 5C; p > 0.05) among male groups. However, there was an interaction between stress and age to increase the number of dives (Figure 5C; F(1, 32) = 4.45; p < 0.05).
Figure 5.
The effects of chronic adolescent stress on behavior in the forced swim test were assessed and compared to control littermates. In adolescent and adult males, chronic adolescent stress did not alter the latency to float (A), time spent struggling (B), or diving behavior (C) compared to the control group. Females in the stress group exhibited a decreased latency to float (D) and decreased time spent struggling (E) compared to control females. Exposure to stress resulted in increased diving behavior compared to control females (F). These effects in females were observed regardless of age and the behavioral effects were sustained into adulthood for females. * p < 0.05 main effect due to stress, # p < 0.05 main effect due to age 2-way ANOVA. Data are presented as mean ± SEM, N = 5–12.
In females, chronic adolescent stress decreased the latency to float (Figure 5D; F(1, 25) = 5.90; p < 0.05) and decreased the time spent struggling compared to controls regardless of age (Figure 5E; F(1, 26) = 16.11; p < 0.05). Chronic adolescent stress also increased the number of dives in females compared to controls (Figure 5F; F(1, 31) = 7.04; p < 0.05). Age alone did not have an effect in females for any of the forced swim test endpoints (p > 0.05).
Discussion
These data demonstrate that chronic mixed-modality stress during adolescence produces persistent behavioral changes in female rats. Chronic mixed-modality stress caused decreased sucrose consumption in female adolescent rats and this behavioral state persisted into adulthood (Figure 3). Furthermore, female rats exposed to chronic adolescent stress demonstrated reduced struggling in the forced swim test both during adolescence and months later in adulthood (Figure 5). In addition to generating behavioral changes that have been classified as depressive-like behaviors, the chronic adolescent stress paradigm used in this study (isolation, restraint, and social defeat) produced anxiety-like behavior and HPA axis alterations (Figures 2 and 4). Collectively, these data indicate that female adolescent rats are more adversely affected by chronic adolescent stress than are male rats and the adverse behavioral effects persist into adulthood for female rats.
Behavioral Effects of Adolescent Stress are Sustained and Sexually Dimorphic
The rapid development of the HPA axis and other brain regions during adolescence has been reported to play a neurobiological role in behavioral alterations associated with this sensitive period of development. Although effects of chronic adolescent stress on anxiety-like behavior, cognitive behavior, and drug seeking behavior have been previously reported, this study demonstrates a sustained increase in depressive-like behaviors following chronic adolescent stress in female but not male rats. Previous studies have reported that chronic social housing stress at an earlier age (PND 30–45) tended to alter behavior in the forced swim test for adolescent female rats and altered corticosterone levels in an age and sex-specific manner. In the current study, female rats demonstrated decreased sucrose consumption immediately following chronic adolescent stress and this effect was sustained into adulthood. In addition, the chronic adolescent stress used in the current study caused an increase in passive coping behaviors of adolescent and adult female rats during the forced swim test. Although numeric differences in both the sucrose consumption test (Figure 3A) and forced swim test (Figure 5A) were apparent at the adolescent time point for chronically stressed male rats, these differences were not significant at this time point and not apparent at the adult time point, demonstrating that the impact of the mixed-modality adolescent stressor was more prominent in female rats. Instead of a strong behavioral phenotype, male rats stressed in adolescence manifested impaired weight gain in adolescence that reversed in adulthood such that adult male rats weighed more than pair-reared controls (Figure 1A and B). Previous work has demonstrated that prenatal stress exposure also results in increased weight gain in adults rats (Tamashiro and Moran, 2010). Collectively, these results suggest that developmental stress may cause long-term reprogramming of metabolism in male rats.
In addition to changes in depressive-like behaviors, exposure to chronic adolescent stress increased anxiety-like behavior at the end of adolescence. Female rats exposed to stress demonstrated increased locomotion in the elevated plus maze, whereas male rats exhibited differences due to age but not due to adolescent stress. A previous study in mice did not report an age-dependent increase in locomotor activity, suggesting that increased locomotor activity in adult males may be a species-specific event. Incongruent with our findings, other studies using social stressors during adolescence have found that female rodents have lower anxiety-like behavior in adolescence and both sexes have higher anxiety-like behavior in adulthood. These studies did not use the same timing or mixed stress (isolation, restraint, and social defeat) paradigm as in the current study and either of these variables could significantly impact the behavioral effects. Additional studies are necessary to isolate the key variables for this divergence in the effects of adolescent stress on behavior (Oldehinkel and Bouma, 2010), but the current data set indicates that chronic adolescent stress increases anxiety-like behavior in female rats at the end of adolescence.
Combined Stressors Provide More Robust Effects than Isolation Housing
Isolation is a potent stressor for adolescent rats; however, individually housed rodents are commonly used as the control condition for chronic stress studies because of the potential for group housing to buffer the effects of chronic stress. Isolation housing, in comparison to pair housing, altered behavior in the elevated plus maze and forced swim test in our study suggesting that isolation housing alone had the potential to induce anxiety-like and depressive-like behavior. This difference was most noticeable in males and may illustrate an important sex difference in the social buffering of behavioral responses that others have also observed. The control groups in the current study consisted of pair housed littermates and the condition of isolation housing during adolescence was considered a part of the mixed-modality chronic stressor. Although isolation alone has the potential to disrupt behavior, it does not appear that isolation alone is responsible for the persistent depressive-like behaviors documented in the female rats exposed to chronic adolescent stress because isolation alone did not reproduce these effects. The possibility that the isolation housing potentiated the effects of the chronic adolescent stress in the female rats remains and it is well documented that social buffering can prevent or reverse the effects of chronic stress. The precise experiment to determine if the adverse effects of chronic adolescent stress can be mitigated by pair housing extends beyond the scope of the current study but is an important question for future work aimed to address potential environmental interventions to prevent or reduce the effects of chronic adolescent stress.
Social defeat has been used by other groups to induce sexually dimorphic differences in behavior. The purpose of our study was not to examine the effect of a social stressor but rather to use a potent mixed-modality stress paradigm consisting of isolation, restraint, and social defeat to study the effect of stress itself during an important developmental window. Indeed, any one of these stressors may cause a sex-specific effect but the purpose of our study was to determine if chronic adolescent stress altered behavioral endpoints in a sex-specific manner. Sexually dimorphic differences due to adolescent stressors may be due to gonadal hormones which led us to examine these endpoints as possible mediators of behavioral effects.
HPA and HPG Axes as Possible Mediators of Behavioral Effects
Disrupted HPA axis regulation can lead to anxiety-like and depressive-like behavior, and could explain the female susceptibility to stress effects in our behavioral studies. In the postnatal period, male and female rat adrenal development progress at similar rates. However, beginning in adolescence, adrenal growth and secretion of corticosterone begin to increase and rapidly fluctuate in a sexually dimorphic manner providing a potential physiological sex difference to account for the sexually dimorphic behavioral effects of chronic adolescent stress. During adolescence, chronically stressed female rats in the current study had blunted plasma corticosterone in response to stress which persisted into adulthood and was not present in male littermates (Figure 2). This may indicate a dysfunction of the HPA axis in the female adolescent rats exposed to chronic adolescent stress; however, given that the stressor was restraint, this difference could represent a habituation of the stress response to restraint. Similar to our findings, other studies investigating social stressors report persistent sex-specific effects on the HPA axis; however, in those studies adolescent females displayed an exaggerated HPA response to a heterotypic stressor. The homotypic nature of the stressor involved in the current study could explain this divergence. While the blunted corticosterone response observed may be the result of habituation, this is a profound sex difference in the current study and indicates a sex-specific effect of chronic adolescent stress on the HPA axis.
Gonadarche is initiated by the HPG axis which is controlled by neurotransmitters, neuropeptides, and CRF. Additionally, it has been proposed that there is significant interplay between the HPA and HPG axes, and while stress may increase HPA axis activity, there is a corresponding decrease in HPG activity and heightened gonadal atrophy (Selye, 1946). In numerous studies, stress and CRF have been shown to suppress HPG axis activity. Female Wistar rats, the strain used in our study, are particularly sensitive to the effects of stress on HPG regulation. Despite these earlier accounts, in the current study only minor effects on the HPG axis of male and female rats were noted and there were no differences in the estrous cycle due to stress. We tracked the estrous cycle using daily vaginal lavage through the behavioral testing of adult female rats and there were no differences among the groups. While stress affected testicular mass, this effect may have been due to the social nature of the stress employed (Apter and Eriksson, 2006) and may not reflect effects of chronic adolescent stress in general. Although some effects of adolescent stress were documented in the current study, of the HPG metrics assessed, the differences were not profound or prolonged.
Conclusion
In conclusion, the current study demonstrates that female rats exposed to chronic adolescent stress exhibit decreased sucrose consumption, increased passive coping in the forced swim test, increased locomotion in the elevated plus maze, and a blunted corticosterone response to acute stress. These effects were persistent as adult females displayed a similar behavioral phenotype. Male littermates exposed to chronic adolescent stress did not manifest significant changes in behavior as adolescents or adults. Our findings support the proposition that adolescence is a sensitive period of development for females and stress during this period may have persistent effects into adulthood. Our study is congruent with human studies which indicate that women have a significantly higher risk of developing major depression compared to men. Studies investigating the sexually dimorphic effects of chronic adolescent stress may lead to a better understanding of the sexually dimorphic mechanisms of depressive and anxiety disorders in humans and ultimately improve prevention and treatment strategies.
Acknowledgments
This work was made possible through the generous support of Emory University’s Comprehensive Neuroscience Center Child and Adolescent Mood Program and a NIEHS Graduate Student Training in Toxicology Grant. Thanks to EE Hardy for animal care, behavioral testing, and behavioral analysis. Thanks to JC Ritchie and CH Ramsey for technical support. Special thanks to B Kinkead and SM Rogers for their assistance with animal handling and scoring and to CL Nemeth, L Pyter, J Weiss for comments on this manuscript.
Role of Funding Source
Funding for this study was provided by unrestricted funds provided by Emory University’s Comprehensive Neurosciences Center’s Child and Adolescent Mood Program and salary support for CH Bourke was provided by NIEHS Grant T32ES012870; neither funding source had a role in study design, data collection, analysis and interpretation of data, manuscript preparation, or the decision to submit the manuscript for publication.
GN Neigh receives grant funding from NIMH, AHA, NARSAD, GSK, and Emory University.
Footnotes
Conflicts of Interest
CH Bourke declares that he has no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–346. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Apter SJ, Eriksson CJP. The role of social isolation in the effects of alcohol on corticosterone and testosterone levels of alcohol-preferring and non-preferring rats. Alcohol Alcohol. 2006;41:33–38. doi: 10.1093/alcalc/agh220. [DOI] [PubMed] [Google Scholar]
- Avital A, Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int J Neuropsychopharmacol. 2005;8:163–173. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Bernal-Morales B, Contreras CM, Cueto-Escobedo J. Acute restraint stress produces behavioral despair in weanling rats in the forced swim test. Behav Processes. 2009;82:219–222. doi: 10.1016/j.beproc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Fitzgerald RE, Dijk DJ, Koolhaas JM. The corticomedial amygdala and learning in an agonistic situation in the rat. Physiol Behav. 1984;32:575–579. doi: 10.1016/0031-9384(84)90311-1. [DOI] [PubMed] [Google Scholar]
- Campbell T, Lin S, DeVries C, Lambert K. Coping strategies in male and female rats exposed to multiple stressors. Physiol Behav. 2003;78:495–504. doi: 10.1016/s0031-9384(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Leão RM, Marin MT, Planeta CS. Stress-induced reinstatement of amphetamine-conditioned place preference and changes in tyrosine hydroxylase in the nucleus accumbens in adolescent rats. Pharmacol Biochem Behav. 2010;96:160–165. doi: 10.1016/j.pbb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- DeBold JF, Miczek KA. Aggression persists after ovariectomy in female rats. Horm Behav. 1984;18:177–190. doi: 10.1016/0018-506x(84)90041-2. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG. Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int J Dev Neurosci. 2008;26:259–268. doi: 10.1016/j.ijdevneu.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Dhom G. The prepuberal and puberal growth of the adrenal (adrenarche) Beitr Pathol. 1973;150:357–377. doi: 10.1016/s0005-8165(73)80086-1. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme JR, Forest MG, De Peretti E, Sempé M, Collu R, Bertrand J. Plasma adrenal and gonadal sex steroids in human pubertal development. J Clin Endocrinol Metab. 1976;42:468–476. doi: 10.1210/jcem-42-3-468. [DOI] [PubMed] [Google Scholar]
- Faruzzi AN, Solomon MB, Demas GE, Huhman KL. Gonadal hormones modulate the display of submissive behavior in socially defeated female Syrian hamsters. Horm Behav. 2005;47:569–575. doi: 10.1016/j.yhbeh.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Bernardi F, Monteleone P, Luisi S, Luisi M. Neuropeptides, Neurotransmitters, Neurosteroids, and the Onset of Puberty. Ann N Y Acad Sci. 2000;900:1–9. doi: 10.1111/j.1749-6632.2000.tb06210.x. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav. 2003;79:471–478. doi: 10.1016/s0031-9384(03)00166-5. [DOI] [PubMed] [Google Scholar]
- Haller J, Fuchs E, Halász J, Makara GB. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res Bull. 1999;50:33–39. doi: 10.1016/s0361-9230(99)00087-8. [DOI] [PubMed] [Google Scholar]
- Holson R, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological. Psychoneuroendocrinology. 2009;34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kinkead B, Yan F, Owens MJ, Nemeroff CB. Endogenous neurotensin is involved in estrous cycle related alterations in prepulse inhibition of the acoustic startle reflex in female rats. Psychoneuroendocrinology. 2008;33:178–187. doi: 10.1016/j.psyneuen.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey-Jones JS, Li XF, Bowe JE, Lightman SL, O’Byrne KT. Corticotrophin-releasing factor type 2 receptor-mediated suppression of gonadotrophin-releasing hormone mRNA expression in GT1–7 cells. Stress. 2006;9:215–222. doi: 10.1080/10253890601040535. [DOI] [PubMed] [Google Scholar]
- Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci U S A. 2009;106:11324–11329. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Morley-Fletcher S, Terranova ML. Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: evidence of sex differences. Behav Brain Res. 2002;130:117–125. doi: 10.1016/s0166-4328(01)00420-x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macrì S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Li XF, Edward J, Mitchell JC, Shao B, Bowes JE, Coen CW, Lightman SL, O’Byrne KT. Differential effects of repeated restraint stress on pulsatile lutenizing hormone secretion in female Fischer, Lewis and Wistar rats. J Neuroendocrinol. 2004;16:620–627. doi: 10.1111/j.1365-2826.2004.01209.x. [DOI] [PubMed] [Google Scholar]
- Macrì S, Granstrem O, Shumilina M, Antunes Gomes dos Santos FJ, Berry A, Saso L, Laviola G. Resilience and vulnerability are dose-dependently related to neonatal stressors in mice. Horm Behav. 2009;56:391–398. doi: 10.1016/j.yhbeh.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Macrì S, Zoratto F, Laviola G. Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother-offspring hormonal transfer. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Pavlatou MG, Mizamtsidi M. The hypothalamic-pituitary-adrenal and the hypothalamic- pituitary-gonadal axes interplay. Pediatr Endocrinol Rev. 2006;3(Suppl 1):172–181. [PubMed] [Google Scholar]
- Mathews IZ, Wilton A, Styles A, McCormick CM. Increased depressive behaviour in females and heightened corticosterone release in males to swim stress after adolescent social stress in rats. Behav Brain Res. 2008;190:33–40. doi: 10.1016/j.bbr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Nakayasu T, Ishii K. Effects of pair-housing after social defeat experience on elevated plus-maze behavior in rats. Behav Processes. 2008;78:477–480. doi: 10.1016/j.beproc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Bouma EMC. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: A review of gender differences. Neurosci Biobehav Rev. 2010 doi: 10.1016/j.neubiorev.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File S, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pignatelli D, Xiao F, Gouveia AM, Ferreira JG, Vinson GP. Adrenarche in the rat. J Endocrinol. 2006;191:301–308. doi: 10.1677/joe.1.06972. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rivest S, Plotsky PM, Rivier C. CRF alters the infundibular LHRH secretory system from the medial preoptic area of female rats: possible involvement of opioid receptors. Neuroendocrinology. 1993;57:236–246. doi: 10.1159/000126365. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Haller J, Holmes A, Halasz J, Walton TJ, Brain PF. Corticosterone response to the plus-maze: high correlation with risk assessment in rats and mice. Physiol Behav. 1999;68:47–53. doi: 10.1016/s0031-9384(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Scholtens J, Roozen M, Mirmiran M, van de Poll NE. Role of noradrenaline in behavioral changes after defeat in male and female rats. Behav Brain Res. 1990;36:199–202. doi: 10.1016/0166-4328(90)90057-l. [DOI] [PubMed] [Google Scholar]
- Selye H. The general adaptation syndrome and the diseases of adaptation. J Clin Endocrinol Metab. 1946;6:117–230. doi: 10.1210/jcem-6-2-117. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Karom MC, Huhman KL. Sex and estrous cycle differences in the display of conditioned defeat in Syrian hamsters. Horm Behav. 2007;52:211–219. doi: 10.1016/j.yhbeh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Sterlemann V, Rammes G, Wolf M, Liebl C, Ganea K, Müller MB, Schmidt MV. Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus. 2010;20:540–549. doi: 10.1002/hipo.20655. [DOI] [PubMed] [Google Scholar]
- Tamashiro KLK, Moran TH. Perinatal environment and its influences on metabolic programming of offspring. Physiol Behav. 2010;100:560–566. doi: 10.1016/j.physbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova ML, Cirulli F, Laviola G. Behavioral and hormonal effects of partner familiarity in periadolescent rat pairs upon novelty exposure. Psychoneuroendocrinology. 1999;24:639–656. doi: 10.1016/s0306-4530(99)00019-0. [DOI] [PubMed] [Google Scholar]
- Tõnissaar M, Mällo T, Eller M, Häidkind R, Kõiv K, Harro J. Rat behavior after chronic variable stress and partial lesioning of 5-HT-ergic neurotransmission: effects of citalopram. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:164–177. doi: 10.1016/j.pnpbp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Lamberts RR, Wolterink G, Wiegant VM, Van Ree JM. Emotional and footshock stimuli induce differential long-lasting behavioural effects in rats; involvement of opioids. Brain Res. 1998;799:6–15. doi: 10.1016/s0006-8993(98)00397-7. [DOI] [PubMed] [Google Scholar]
- Vidal J, de Bie J, Granneman RA, Wallinga AE, Koolhaas JM, Buwalda B. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol Behav. 2007;92:824–830. doi: 10.1016/j.physbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Young EA. Sex differences and the HPA axis: implications for psychiatric disease. J Gend Specif Med. 1998;1:21–27. [PubMed] [Google Scholar]