Abstract
Drug resistance and brisk tumor initiation have traditionally been viewed as pre-existing phenotypes present in a small subpopulation of cancer cells which can expand under selective pressures. However, recent work in cancer cell lines has demonstrated that drug-resistant tumor initiating features can emerge de novo within fractionated subpopulations of cells initially lacking these phenotypes. In the present study, we asked whether such phenotypic plasticity exists broadly in unperturbed cancer cell lines and tumor xenografts growing spontaneously without interventions like drug selection or fractionation into subpopulations employed in prior studies. To address this question, we used side population (SP) analysis combined with fluorescence labeling to identify a drug-resistant highly-tumorigenic subpopulation and to track and analyze its interaction with the larger phenotypically negative population over time. Remarkably, we observed that SP size fluctuated in a cyclical manner: first contracting via differentiation into the non-SP (NSP) population, and then re-expanding via simultaneous direct conversion of numerous NSP cells back to the SP phenotype both in culture and in tumor xenografts. These findings demonstrate for the first time that adaptive, cancer-promoting traits like drug-resistance and brisk tumor initiation arise not only as solitary events under selective pressures, but also as highly orchestrated transitions occurring concurrently in large numbers of cells even without specifically-induced drug selection, ectopic gene expression, or fractionation into subpopulations. This high level of coordinated phenotypic plasticity bears consideration when using cancer cell lines as experimental models and also may have significant implications for therapeutic efforts targeting the drug-resistant tumor-initiating phenotype.
Keywords: Drug resistance, tumor initiation, side population
Introduction
The phenomena of drug resistance and tumor metastasis have long been recognized as central challenges in cancer therapy (1–2). Traditionally, these properties have been conceptualized as pre-existing molecular phenotypes randomly present in a small subset of cancer cells which – under the right environmental selection pressures (e.g. drug treatment or a new tumor site) – would be favored to expand (3–4). To study these adaptive responses, a variety of immortalized and transformed cell lines have been employed; while only offering an approximation of in vivo cancer behavior, these models have allowed for serial labeling, tracking, and characterization of phenotypically distinct subpopulations over time. In this manner, small subpopulations of cells marked by drug resistance and vigorous tumor formation have been repeatedly identified and expanded (5–8).
Recently, the established “unidirectional” model of a unique, pre-existing population selected to expand has been called into question by a number of high-impact studies: In several reports, cancer cells lacking the putative markers of drug resistance and brisk tumorigenicity were nonetheless capable of tumor formation under certain permissive conditions (9–11). In two recent reports, cells lacking drug-resistant and tumor initiating features were clonally isolated and shown to be capable of reconstituting heterogeneous populations comprised both of cells with drug-resistant and tumorigenic features as well as cells lacking those phenotypes (12–13). These findings have raised the intriguing possibility that a “pre-existing” seed population may not be necessary for the emergence of tumor-forming or drug-resistant phenotypes; rather, a subpopulation with these properties can arise de novo under certain conditions.
Given these observations, we wondered whether re-emergence of a drug-resistant highly-tumorigenic phenotype could be achieved only under selective growth conditions or through isolation from other subpopulations as done in previous reports, or was this a more universal and spontaneous phenomenon. Specifically, could phenotypic plasticity be readily observed even in cell lines propagated under standard conditions without extrinsic selective pressures and without separation into constituent subpopulations? Moreover, did such plasticity represent a clonal selection of one phenotype from the other over time, or did it represent a real-time conversion occurring rapidly in many cells at once?
To investigate this question, we analyzed cancer cell lines in vitro and in vivo over time using flow cytometry with Hoechst dye exclusion, a commonly-used method which yields a side population (SP) of cells with drug-resistant highly-tumorigenic properties in tumors and cell lines (14–18). Remarkably, when we coupled SP analysis with GFP labeling, we observed a dynamic two-way equilibrium between the SP and non-SP (NSP) subpopulations in cell culture and in tumor xenografts. Specifically, the SP subpopulation first became depleted by differentiation into NSP cells, and subsequently the SP subpopulation was reconstituted by direct conversion of numerous NSP cells simultaneously back to the SP phenotype; these transitions occurred spontaneously in the course of proliferation without exogenous selection pressures or separation into constituent subpopulations. Our findings demonstrate for the first time that intact cancer cell lines exhibit continuous, spontaneous plasticity whereby large numbers of cells lose and subsequently regain a drug-resistant highly-tumorigenic phenotype in a cyclical manner. These observations suggest that adaptive traits which confer a survival advantage may be acquired by cancer cell populations not only through clonal selection of pre-existing, solitary cells, but also through an ongoing, highly orchestrated process of phenotypic interconversion occurring simultaneously in large numbers of cells.
Methods
Cell culture and lentiviral infections
Cancer cell lines were obtained from collaborators at the University of California, San Francisco and the University of Southern California (see Acknowledgements) and were not re-authenticated prior to use in these experiments. Human bladder cancer cells (J82, RT4, UM-UC3), human breast cancer cells (MCF7) and rat glioma cells (C6) were maintained at 37°C, 5% CO2 in DMEM (Mediatech) supplemented with 10% of heat-inactivated fetal bovine serum (Omega), penicillin (100 units/ml, Invitrogen), and streptomycin (100μg/ml, Invitrogen). Human prostate cancer cells (PC3, Du145, LNCap, LNCap-C4-2B) and human lung cancer cell (H441) were maintained in RPMI medium (supplemented with 10% FBS, and antibiotics 100 units/ml penicillin and 100μg/ml streptomycin).
GFP labeling of cells: Lentivirus was generated as previously described to deliver either GFP under a CMV promoter or control empty vector (19). One day prior to infection 2X105 J82 cells were seeded in 10cm plates, and on the next morning media was replaced by 3 mL virus supernatant plus 7 mL media supplemented with 8 μg/ml polybrene. After 8 hr incubation at 37°C, the virus- containing media was replaced with fresh media. Cells were observed for 48 hr to ensure >90% GFP expression prior to fluorescence activated cell sorting (FACS) and side population studies.
Flow cytometry
Hoechst staining and FACS were conducted as described previously (20). Briefly, adherent cancer cells (1X106/mL) were trypsinized, counted, and resupended in prewarmed 10% FBS DMEM media. Hoechst 33342 (Sigma-Aldrich) was added at concentration of 5μg/mL, incubated for 2 hrs in 37°C water bath and gently inverted several times during the course of incubation. Parallel sample aliquots were prepared in the presence of 50μM verapamil (Sigma-Aldrich), an ATP-binding cassette transporter family inhibitor, at room temperature for 10min before adding the Hoechst 33342 dye. Cells were centrifuged at 1000rpm for 5 min after incubation and resuspended in ice-cold DMEM media. Propidium iodide (Sigma-Aldrich) was added to the cells at a final concentration of 2μg/mL. Samples were incubated for at least 5 min on ice before FACS analysis (FACSAria and FACSLSR-II, BD Biosciences, both equipped with UV lasers).
Drug resistance experiments
Cells were stained with Hoechst 33342, and FACS sorted into SP and NSP cells. Fractionated SP or NSP or unsorted WP (whole population) cells were seeded into 96 well plates at a concentration of 1×104 cells per well in the presence of Cisplatin (25 μM) or Docetaxel (0.4 mM for J82 cells and 0.1 mM for MCF7 cells respectively). MTS assay was performed after 24 hours according to manufacture’s protocol (Promega, Madison, WI).
Direct single cell isolation
Single cell suspension was made in DMEM media and stained by Hoechst 33342 for side population as described above. Side population and non-side population cells were sorted by FACSAria directly into 96-well plates at the concentration of one single cell per well (FACS 96 well sorting program), and single clone formation was confirmed visually on subsequent days.
Tumorigenicity assays
In vivo experiments were conducted under an approved protocol in accordance with the institutional guidelines for the use of laboratory animals. SP and NSP cells were suspended in 100 μL of media:matrigel=1:1 and inoculated subcutaneously into the right flank of male SCID mice (6–8 weeks old, NCI-Frederick). Time to onset of palpable tumor was recorded and tumor diameter was measured twice weekly. Mice were sacrificed and tumors were excised for wet weight measurement when the largest tumor diameters within each group exceeded 10 mm.
Analysis of excised tumors
In preparation for FACS, tumors were minced into small pieces (about 1 mm3) and immersed in digestion solution. (DMEM/F12 (50:50) supplemented with DNAse I (1 μg/mL ) and Liberase Blendzyme 3 (0.1 – 0.8 mg/mL)). Cells were incubated at 37 °C overnight, followed by trypsin incubation at 37 °C for 5 min the next morning, then trypsin neutralization with DMEM 10% FBS media. Cell suspension was filtered first by 100 μm strainer, then through 40 μm strainer (BD Falcon) prior to FACS.
Colony formation assays
1000 SP or NSP cells were seeded into 10 cm plates after FACS sorting. As a control for the presence of Hoechst dye, 1000 SP cells were treated with 50 μM verapamil for 10 min, and further stained by Hoechst33342 for another 2 hours, and then seeded into 10 cm plates. Cells were cultured at 37°C, 5% CO2. Plates were washed 3x with PBS and fixed with 100% methanol (Room temperature, 15 min), then stained for 1 hr at room temperature with 7 mL of Giemsa Stain (Sigma-Aldrich). After staining, cells were washed with water and air-dried overnight. The number of colonies with diameter of over 1mm was counted and photographed under bright-field microscopy (Nikon Eclipse TS100). All experiments were done in triplicate, and repeated at least twice.
Quantitative reverse transcription-PCR assays
Total RNA was isolated from FACS-sorted SP and NSP cells using manufacture’s RNA isolation protocol (RNA-Bee, Tel-Test). RNA was reverse transcribed using RETROscript kit (Ambion), and cDNA was then subjected to real time PCR amplification using gene specific primers and Quanta B-R Syber Green QPCR supermix (BioScience) with β-actin as a housekeeping gene loading control (primer sequences and conditions in Supplementary Table 1).
Statistical Analysis
Performed in collaboration with USC/Norris Biostatistics Core. All experiments were conducted in triplicate with error bars representing standard deviation around the mean. Student’s t-test was used to determine statistical significance when comparing mean values at one point in time (e.g. gene expression, tumor weights, SP%). Two-sided Wilcoxon matched-pairs signed rank test was performed by using Graphpad Prism5.0 software to compare statistical significance of tumor growth over time.
Results
The side population (SP) is enriched for drug-resistant highly-tumorigenic cells
We used Hoechst efflux and FACS analysis to identify side populations of cells with drug-resistant highly-tumorigenic properties as previously reported in tumors and cell lines (14–18). Using this technique, we found side populations in a variety of cancer cell lines, including prostate, breast, lung, bladder and glioma (Figure 1A and Supplementary Figure 1). Sorting of side population (SP) from non-side population (NSP) on a FACSAria flow cytometer (BD) yielded population purities of >91% and >97%, respectively as confirmed on LSR-II cell analyzer (BD) (Supplementary Figure 2). The J82 and MCF7 cancer cell lines yielded SPs which constituted ~22.3% and 6.1%, respectively, of the overall populations, and which diminished to 0.5% and 1.5%, respectively, after treatment with verapamil, which blocks the ABC transporter and prevents Hoechst efflux (Figure 1A). To confirm that SP cells were enriched for drug resistance, J82 bladder cancer and MCF7 breast cancer cell lines were fractionated into SP and NSP and exposed to cisplatin and docetaxel, two chemotherapeutic agents commonly used in bladder and breast cancer (Figure 1B). For both cell lines, SP cells demonstrated significantly greater survival than NSP or unfractionated whole population (WP); SP cells also had 2-fold mRNA expression of the ABCG2 transporter responsible for Hoechst and drug efflux (Supplementary Figure 3).
Figure 1. The side population (SP) is enriched for drug-resistant highly-tumorigenic cells.
(A) FACS plots gated for a Hoechst-negative side population (SP) of J82 bladder cancer and MCF7 breast cancer cells; treatment with Verapamil 50 μM drastically reduced the SPs. (B) Drug resistance of side population (SP), non-side population (NSP) or unfractionated whole population (WP) 24 hours after exposure to cisplatin (25 μM) or docetaxel (0.4 mM for J82, 0.1 mM for MCF7). (C) Colony forming assays comparing NSP to SP and to SP + verapamil (“vera”) + Hoechst33342. Left: Sample images of colony formation; Right: Colonies were quantified by counting those with radius >1mm 3 weeks after plating. (D) Xenograft tumor formation: Left: FACS-fractionated SP or NSP were inoculated subcutaneously into SCID mice at three dilutions (5×104, 1×104 and 1×103) and tumor volumes were measured twice weekly. Right: Wet weights of tumors: For each cell dilution, tumors were excised when the largest tumors exceeded 10mm in greatest diameter. All results are means of independent experiments conducted in triplicate.
Side populations derived from MCF7 cells have been extensively characterized and previously shown to have high clonogenicity and tumorigenicity (14, 21); therefore, we next confirmed the same to be true of the J82 cancer cell line using plating assays and SCID mouse inoculations. As expected, SP cells were significantly more clonogenic than NSP cells, generating ~3 times as many large colonies (>1mm diameter) at 5 weeks after seeding (Figure 1C). To ensure that these clonogenic differences were not spuriously caused by retention of Hoechst dye in NSP cells and absence of dye in SP cells, we repeated the clonogenic assays in the presence of Hoechst dye + verapamil. Even when SP cells contained Hoechst dye similarly to NSP cells, they still were significantly more clonogenic than NSP cells (Figure 1C). In vivo, SP cells formed tumors in all mice at all dilutions (5×104, 1×104, and 1×103). In contrast, NSP cells formed tumors in only 1 of 3 mice inoculated with 1×103 cells and in 2 of 3 mice inoculated with 1×104 cells (Figure 1D). Moreover, NSP-derived tumors formed only after a longer lag period and were smaller at excision than SP-derived tumors (Figure 1D). Thus, in summary, SP cells – relative to NSP cells – were found to be more drug-resistant, to form more colonies in vitro, and to form tumors more rapidly and efficiently in vivo, thus validating that SP cells indeed were enriched for a drug-resistant highly-tumorigenic phenotype. In a further analysis, J82-derived SP cells also possessed higher mRNA levels of several genes associated with self-renewal (Supplementary Figure 3), which was consistent with previous reports (14, 21–22). At the same time, J82-derived and MCF7-derived subpopulations expressed very low or non-discriminatory levels of CD133, ALDH1, and CD44+24low (Supplementary Figure 4), a finding that was not surprising, because these markers are known to be highly variable in different types of tumors and cell lines. In some cases they correlate with high tumorigenicity and drug resistance, while in other cases they are uniformly expressed or totally absent (23–24).
The NSP subpopulation generates SP and NSP subpopulations in vitro and in vivo
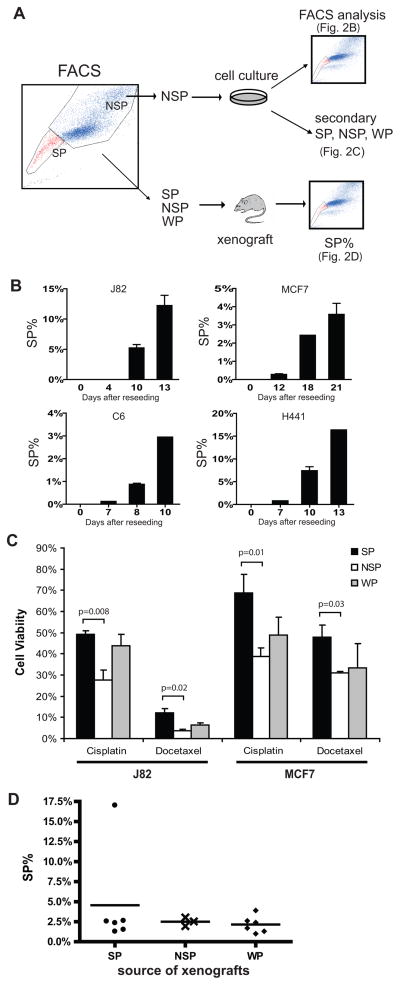
In the course of characterizing SP cells, we noted that NSP cells – though significantly less clonogenic and tumorigenic than SP cells – were nonetheless capable of forming some colonies in vitro and tumors in vivo (Figure 1C–D). We wondered whether this delayed, inefficient capacity of NSP cells was predicated upon reconstitution of an SP subpopulation, which in turn promoted colony and tumor formation. We tested this possibility by sorting J82 cells and several other cancer cell lines into SP and NSP subpopulations which were expanded and re-analyzed by FACS (Figure 2A). J82 NSP cells re-seeded in vitro reconstituted SP subpopulations that increased from undetectable at day 4, to ~6% at day 10, to ~12% at day 13 (Figure 2B). Notably, we observed a similar phenomenon (Figure 2B) in 3 other cancer cell lines previously shown to possess SP cells with drug-resistant highly-tumorigenic properties: MCF7 (breast cancer), C6 (rat glioma), and H441 (lung cancer) (14, 18, 21, 25). These NSP-derived subpopulations recapitulated the expected drug resistant phenotypes, with SP demonstrating significantly higher drug resistance than NSP (Figure 2C). In vivo we inoculated SCID mice with equal numbers of J82 SP, NSP, or unsorted whole population (WP), then excised the xenograft tumors when they reached 10mm in largest diameter and analyzed the cells by FACS LSR-II. Notably, although NSP cells required a longer lag period before generating tumor xenografts (Figure 1D), all the excised tumors – regardless of SP, NSP, or WP origin – contained similar (2–3%) SP subpopulations (Figure 2D). Thus, NSP subpopulations from several different cancer cell types successfully reconstituted SP subpopulations over time in vitro and in vivo.
Figure 2. The NSP subpopulation generates SP and NSP subpopulations in vitro and in vivo.
(A) Schematic of cancer cell subpopulation-based experiments. (B) SP composition over time of cultured cells derived from FACS-fractionated NSP using 4 different cancer cell lines. (C) Relative drug resistance of NSP-derived SP, NSP, and WP; (D) SP composition of mouse xenograft tumors derived from FACS-fractionated J82 SP, NSP, and whole population (WP); tumors were excised and analyzed when they reached 10mm in greatest diameter.
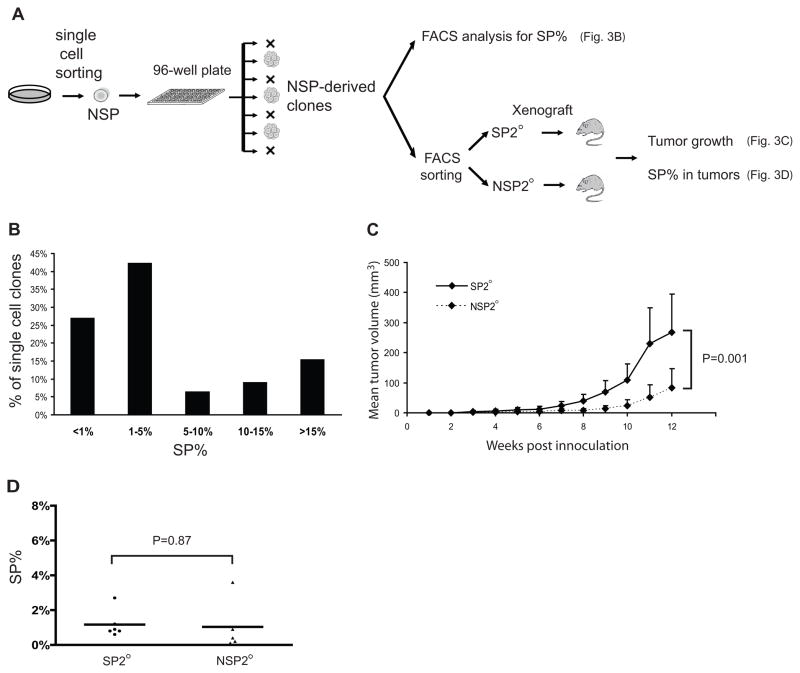
Reconstitution of SP and NSP subpopulations from isolated NSP cells in vitro and in vivo could have conceivably resulted from imperfect sorting (97% purity, Supplementary Figure 2) leading to unintentional inclusion of SP cells among the NSP population. To exclude this possible cross-contamination, we used FACS to sort single NSP cells directly into individual wells of 96-well plates and visually confirmed formation of a single clonal population in each well (Figure 3A). Significantly, NSP-derived single cells generated colonies in 78 of 600 wells (13%), a greater proportion than could have been caused by SP cross-contamination during FACSAria sorting which has only 3% inaccuracy. A large majority (~75%) of the NSP-derived colonies in turn generated a significant SP subpopulation, defined as SP%>1% (Figure 3B), thus confirming that NSP cells indeed gave rise to SP cells, and that this observation could not be explained by SP cross-contamination of NSP during sorting.
Figure 3. NSP single cell clones reconstitute SP and NSP subpopulations in vitro and in vivo.
(A) Schematic of single cell clone experiments: single NSP cells were sorted into 96-well plates, clonally expanded, and subjected to FACS analysis for SP% as well as FACS sorting into secondary SP and NSP (SP2° and NSP2°), which in turn were inoculated into SCID mice for in vivo analysis. (B) Distribution of SP% within cell clones derived from single NSP cells. (C) Xenograft tumor formation: 1×104 SP2° or NSP2° cells FACS-fractionated from NSP-derived clones were inoculated subcutaneously into SCID mice and tumor volumes were measured twice weekly. (D) SP% of xenograft tumors derived from SP2° or NSP2° inoculation.
We extended the single cell clone experiments in vivo to confirm that NSP-derived SP cells (termed secondary SP, or SP2°) reacquired the characteristic highly-tumorigenic phenotype displayed by “native” SP cells; that is, the ability to efficiently form new tumors comprised of heterogeneous cell subpopulations. To test this, NSP-derived clones were FACS sorted into secondary SP and NSP subpopulations (SP2° and NSP2°) and inoculated into SCID mice. While both SP2° cells and NSP2° cells were capable of forming tumors, SP2° cells were significantly more tumorigenic than NSP2° cells (Figure 3C). Subsequent xenograft excision and FACS analysis (Figure 3D) confirmed reconstitution of heterogeneous cell subpopulations in all tumors; both SP2° and NSP2° generated tumors with SP as well as NSP subpopulations, and there was no significant difference in SP% between the two groups. Collectively the single-cell clonal experiments confirmed both in vitro and in vivo that NSP cells were able to reconstitute SP subpopulations with a characteristic highly-tumorigenic phenotype.
The side population expands and contracts cyclically in the course of cell culture
Having observed that NSP cells were capable of reconstituting SP+NSP populations in vitro and in vivo, we sought to characterize the day-by-day dynamics of the two subpopulations growing in a “native” state (i.e. together in an intact cancer cell line). To accomplish this, we propagated unsorted J82 cells in cell culture and monitored daily the size of the SP, NSP, and whole population. Unexpectedly, we found that within one day of seeding J82 cells into a new plate, the SP subpopulation became rapidly depleted from a baseline of ~20% down to ~0.5% of the total population (Figure 4A). In subsequent days, the SP subpopulation experienced a gradual increase, punctuated by a sudden spike in SP subpopulation size on day 3 or 4 (Figure 4A, open arrows). The SP subpopulation continued to increase, approaching its original baseline percentage (20%) as the overall culture neared confluence. At that point, the cells were passaged into new plates and the cycle repeated. Interestingly, the sudden increase in SP percentage on day 3 or 4 was independent of the overall expansion rate of the cultured J82 cells; in fact, SP expansion at those inflection points (Figure 4B, open arrow) was ~4- to 7-fold more rapid than the expansion of the NSP subpopulation or of the entire population as a whole (WP).
Figure 4. SP subpopulation size fluctuates cyclically in the course of cell culture.
(A) SP composition during in vitro culture. 1×105 cells were seeded into 6-well plates at day 0 (passage 1) in triplicates and analyzed daily by Hoechst/FACS analysis. At day 6, 1×105 cells were reseeded into 6-well plates to regenerate the cycle (passage 2). (B) Relative daily change in size of SP, NSP and WP, calculated as {cell # at day (N+1)}/{(cell # at day (N)}. Open arrows indicate inflection points of sudden increase in SP size.
SP cells arise through simultaneous direct conversion of numerous NSP cells
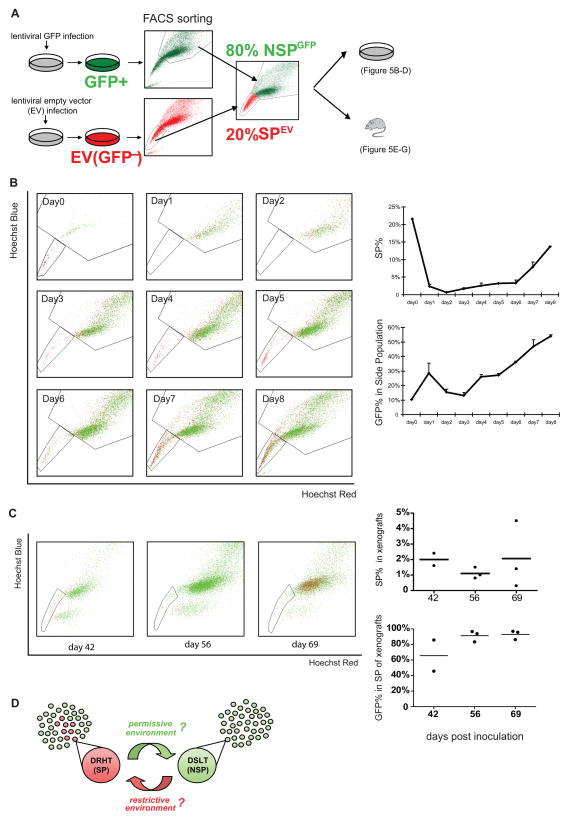
We observed that the SP subpopulation experienced a sudden, marked expansion at approximately mid-passage, a finding that could be explained one of two ways: According to the traditional model, drug-resistant highly-tumorigenic cells could arise only through expansion of pre-existing cells, implying that the observed sudden SP expansion was caused by an explosive increase in SP cell proliferation. An alternative explanation for the sudden SP expansion was that SP cells were arising by conversion of cells from the NSP subpopulation rather than by proliferation of pre-existing SP cells alone. To test this hypothesis, we generated a GFP-based model that enabled us to track the fates of the SP and NSP subpopulations (Figure 5A). Briefly, J82 cells were infected with lentivirus expressing either empty vector (EV) or GFP. J82GFP and J82EV were FACS sorted into their respective SP and NSP subpopulations. These were re-combined to create a J82 cell line consisting of 20% SPEV and 80% NSPGFP. This “hybrid” cell line then could be analyzed daily by FACS for Hoechst dye efflux and GFP status.
Figure 5. SP cells arise through conversion of NSP cells.
(A) Schematic of GFP experiments: FACS fractionated SPEV cells were mixed with NSPGFP cells at a 20:80 ratio at day 0; 1×105 of the mixed cells were cultured in 6-well plates for in vitro analysis or inoculated into SCID mice for in vivo xenograft tumor analysis. (B) In vitro analysis: Left: Daily FACS plots showing the relative proportions of GFP+ and GFP- cells in the SP and NSP gates over time. Right: Graphs plotting the relative size of SP and the GFP+ proportion within the SP over time. (C) In vivo analysis: Left: FACS plots showing the relative proportions of GFP+ and GFP- cells in the SP and NSP gates at 3 time points. Right: Graphs plotting the relative size of SP and the GFP+ proportion within the SP in xenograft tumors derived from the 20:80 SPEV:NSPGFP J82 cancer cell line. (D) Illustrated model summarizing the direct interconversion observed between the drug-resistant highly-tumorigenic phenotype (DRHT, SP) and the drug-sensitive low-tumorigenic phenotype (DSLT, NSP). This plasticity occurred cyclically in many cells at once, suggesting a possible adaptive response to environmental factors (italicized with question marks).
When analyzed daily by FACS during in vitro culture, the reconstituted J82 cells experienced the same initial (day 1–2) depletion of SP cells observed previously, from ~22% down to 0.7% (Figure 5B). During this early phase (day 1–2) the SP cells (red) migrated over to the NSP gate. Then, as the SP percentage gradually increased on days 3 to 6, there was a concomitant gradual increase in the GFP+ percentage (green cells) within the SP gate (Figure 5B). At the day 6 inflection point, the sudden sharp rise in SP subpopulation was mirrored by an equally sharp increase in GFP+ percentage within the SP gate. These findings confirmed that the rapid re-expansion of the SP subpopulation was not caused by intrinsic self-renewal and proliferation of pre-existing SP cells, but rather by conversion of NSP cells into the SP phenotype. Notably, this phenomenon was confirmed (and any confounding role of GFP protein expression was ruled out) with a reverse experiment wherein 20% SPGFP and 80% NSPEV were combined with similar results (Supplementary Figure 5). Hence, these experiments provide the first direct evidence that the drug-resistant highly-tumorigenic phenotype within an established cancer cell line is rapidly and simultaneously acquired by numerous cells previously lacking this phenotype.
The principle of phenotypic plasticity was borne out further in vivo. The “hybrid” J82 cell line consisting of 20% SPEV + 80% NSPGFP was inoculated into SCID mice, and the xenograft tumors were resected at 3 time points and analyzed by FACS for SP percentage and GFP percentage (tumor gating accuracy and persistence of GFP expression were confirmed in separate experiments, Supplementary Figure 6). Interestingly, the proliferating tumors recapitulated the early rapid proliferative phase observed in vitro; that is, the SP percentage declined from 20% in the “hybrid” J82 pre-inoculation down to ~1–3% in the xenograft tumors at subsequent time points (Figure 5C). Consistent with this trend, FACS plots of resected tumors showed that the original SPEV (red) cells migrated over to the NSP gate by day 69. At the same time, the GFP+ percentage within the SP gate increased from 30% GFP+ pre-inoculation to ~90% GFP+ in the xenograft-derived SP subpopulations, suggesting a progressive replacement of the original SPEV subpopulation (largely GFP-) by a new GFP+ SP subpopulation derived from the NSPGFP cells (Figure 5C). Hence, in tumor xenografts, as in vitro, drug-resistant highly-tumorigenic SP subpopulation was replenished by conversion of cells from the NSP subpopulation. As illustrated in the summary model (Figure 5D), the observed phenotypic inter-conversions were spontaneous but also cyclical and occurring simultaneously in large numbers of cells, a non-random pattern suggestive of a deliberate process, perhaps in response to some environmental factor or factors.
Discussion
We investigated whether emergence of drug-resistant highly-tumorigenic cells could be observed in cancer cell lines propagated in culture and as tumor xenografts. Specifically, we asked if phenotypic plasticity could be observed even in unfractionated, intact cell lines grown without specific selective pressures such as chemotherapy treatment. Moreover, could direct phenotypic conversion within groups of cells – rather than clonal selection over time – be documented as an adaptive mechanism employed by these cell lines. To study these questions, we used established cancer cell lines which contained a Hoechst-effluxing side population (SP) characterized by drug resistance and high tumorigenicity (Figure 1 and (5–6, 8, 14, 18, 21, 25–26)). We recognize that cancer cell lines may not perfectly represent the biology of spontaneous in vivo tumors; however, tumor tissues and primary tumor-derived cultures do not lend themselves to serial labeling and analyses of phenotypically distinct subpopulations over time. In contrast, cell lines do allow this type of characterization and therefore have been used extensively to explore how drug-resistant highly-tumorigenic phenotypes emerge (3, 7, 12–13, 27). Cancer cell subpopulations possessing these properties have sometimes been referred to as “cancer stem cells” or “tumor initiating cells” (28), because their typically high expression of self-renewal genes (also noted in our studies, Supplementary Figure 3) conjures a stem-like, progenitor or hierarchical association. However, as reported previously (24) and noted in our own studies (Supplementary Figure 4), particular markers can vary widely in different tumors and cell lines; therefore, for the purpose of these studies, we opted for terminology based strictly on functional, therapeutically relevant properties that were empirically demonstrated in our SP/NSP experiments: drug resistance and high tumorigenicity.
As expected, FACS-sorted SPs gave rise to entire heterogeneous populations (SP+NSP) in vitro and in vivo; however, NSPs from all of the cancer cell lines also were capable of reconstituting heterogeneity (SP+NSP) albeit less efficiently (Figures 1 and 2), a finding that we validated with single cell clone experiments (Figure 3). These findings were consistent with a recent study using embryonic stem cells where NSP was shown to give rise to SP (29), as well as with recent observations in cancer models where cells lacking the canonical surface markers for a highly-tumorigenic phenotype were nonetheless capable of tumor formation (9–11, 15–16). A host of explanations have been proposed to account for the observed tumorigenicity of marker-negative cells: limited discrimination of cell surface markers, cross-contamination during sorting, and variability of mouse models used to gauge tumorigenicity (9–11, 16, 18). However, another plausible explanation demonstrated in two recent papers (12–13) and supported by our own findings is that drug-resistant highly-tumorigenic subpopulations could arise directly from cells that initially lack these traits.
Having confirmed that fractionated subpopulations expanded in isolation could reconstitute phenotypic heterogeneity, next we wished to better-define the interaction of these subpopulations in an intact unfractionated cell line (J82) by tracking the SP and NSP cells in real time. Remarkably, we found that the SP subpopulation size fluctuated cyclically with each passage in cell culture (Figure 4). Fluctuations in SP size have been reported previously with exposure to radiation, hypoxia, or ectopic activation of specific signaling pathways and were attributed to intrinsic changes in SP proliferation in response to deliberate external stimuli (21, 30–33). In contrast, our experiments applied no external stimuli to the cultured cells; nevertheless, we unexpectedly observed cyclical SP fluctuations occurring spontaneously in two phases: First, the SP subpopulation contracted drastically after seeding, diminishing from ~20% to less than 1% in the first two days. There are three possible explanations for this: 1. SP cells died out in the first 2 days – this is unlikely as no such phenomenon was observed in our prior experiments using fractionated SP cells. 2. SP cells did not proliferate and therefore were relatively “swamped out” by the NSP cells – this also is unlikely because the overall population doubling time (~24 hr) could not possibly reduce the SP percentage 20-fold in 2 days. 3. SP cells rapidly “differentiated out” into NSP cells – this explanation is most consistent with the classic drug-resistant highly-tumorigenic phenotype, and is in fact borne out by the subsequent GFP labeling experiments, where GFP-negative cells were observed to migrate from the SP gate to the NSP gate in the first 2 days (Figure 5).
The second phase in the cyclical fluctuation of SP size was even more striking: At every passage, as the cell culture passed mid-passage and began to approach confluence, the SP subpopulation underwent a rapid expansion (Figure 4). When we GFP-labeled the NSP subpopulation (Figure 5), we observed that the rapid SP expansion derived not from intrinsic proliferation of pre-existing SP cells, but rather from direct conversion of NSP cells to the SP phenotype, a phenomenon that was recapitulated in vivo when labeled cells were xenografted into SCID mice. Thus, both in vitro and in vivo, the SP subpopulation was replenished by conversion of cells from the NSP subpopulation.
The results of our experiments suggest a higher order of population-wide plasticity than previously suspected. Recent reports showed that drug-resistant tumor-initiating traits could arise de novo within fractionated phenotypically negative subpopulations expanded in isolation or under selective pressures, suggesting that solitary cells or small numbers of cells were capable of altering their phenotypes under these ectopically induced conditions (12–13, 30–31, 33–34). Our current findings demonstrate for the first time that such plasticity is actually a robust phenomenon which can occur spontaneously in intact cell lines without the need for fractionation or selective pressures. Specifically, a drug-resistant highly-tumorigenic phenotype is lost and subsequently regained by subpopulations of cells during passaging in culture, and similar phenotypic interconversion is also observed in tumor xenografts.
Importantly, although the observed phenotypic inter-conversion was spontaneous (no ectopic drugs or gene expression), it was not entirely random. Rather, it was a cyclical, highly orchestrated process occurring simultaneously in large numbers of cells, suggesting a real-time adaptive capability, perhaps in response to environmental factors such availability of nutrients, oxygen or space (35–36). This type of rapid, population-wide adaptation constitutes an alternative to the traditional model wherein pre-existent traits that confer a survival advantage are expanded in a “blind”, passive manner under selective pressures. Instead, entire populations of cancer cells may possess innate, programmed adaptive responses that are activated by real-time sensing and response to environmental conditions. According to this hypothetical dynamic model (Figure 5D), when cancer cells are introduced into a new, permissive growth environment (e.g. culture dish or subcutaneous inoculation), the drug-resistant highly-tumorigenic subpopulation rapidly differentiates into the bulk phenotypically negative population and thus effectively populates the new environment. Conversely, as that environment becomes saturated and less hospitable – perhaps through depletion of critical resources (nutrients, space, oxygen) or accumulation of toxins – a portion of phenotypically negative cells respond by reverting to a drug-resistant highly-tumorigenic phenotype, which is more robust and better-able to sustain the cancer population until a new permissive environment (i.e. new culture plate or metastatic site) is encountered and the cycle repeats. Indeed, this type of highly-orchestrated direct conversion of phenotypically negative cells to a drug-resistant highly-tumorigenic phenotype may underlie some of the changes in subpopulation size previously-observed in response to deliberate manipulations (21, 30–34), as well as the observed capacity of phenotypically negative cells to form tumors (perhaps by first reconstituting a highly-tumorigenic subpopulation in vivo).
The environmental signals and cellular mechanisms governing these phenotypic inter-conversions are the subject of intense investigation by our group and are as yet to be fully elucidated. At the same time, the very presence of this phenomenon has important experimental and clinical implications. Experimentally, cancer cell lines – so commonly employed to study drug resistance and tumor formation – should be used with the recognition that phenotypic heterogeneity exists in subpopulations which are engaged in a continuous state of flux. Clinically, phenotypic plasticity – if confirmed in additional models – would have significant implications for cancer therapeutics. Current efforts to eradicate drug-resistant highly-tumorigenic subpopulations as distinct entities may meet with limited success, because this phenotype may simply be replenished by the phenotypically negative population. Hence, it may ultimately prove more productive to conceptualize drug resistance and high tumorigenicity as adaptive phenotypes assumed by a subset of the cancer population, rather than as discrete, static subpopulations of cells. Elucidating the signals and mechanisms that govern this dynamic, ongoing plasticity may ultimately lead to more effective therapeutic strategies aimed at disrupting the adaptive capabilities of cancer.
Supplementary Material
Acknowledgments
Financial Support: NIH/NCI K08 CA126983-01 (AG)
We thank Drs. Michael Kahn, Peter Laird and Parkash Gill (University of Southern California) for their critical reading of the manuscript, and Drs. Elizabeth Blackburn (University of California, San Francisco), Peter Jones, Gerhard Coetzee, Thomas Chen, Ite Laird-Offringa and Jacek Pinski (University of Southern California) for generously providing cancer cell lines used in this study.
Abbreviations
- FACS
fluorescence activated cell sorting
- SP
side population
- NSP
non side population
- WP
whole population
- GFP
green fluorescent protein
- SCID
severe combined immune deficient
Footnotes
Potential Conflicts: None
References
- 1.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 3.Tsuchida R, Das B, Yeger H, Koren G, Shibuya M, Thorner PS, et al. Cisplatin treatment increases survival and expansion of a highly tumorigenic side-population fraction by upregulating VEGF/Flt1 autocrine signaling. Oncogene. 2008;27:3923–34. doi: 10.1038/onc.2008.38. [DOI] [PubMed] [Google Scholar]
- 4.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu L, McArthur C, Jaffe RB. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br J Cancer. 2010;102:1276–83. doi: 10.1038/sj.bjc.6605626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh A, Wu H, Zhang P, Happel C, Ma J, Biswal S. Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer cells that confers side population and chemoresistance phenotype. Mol Cancer Ther. 2010;9:2365–76. doi: 10.1158/1535-7163.MCT-10-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukunaga-Kalabis M, Martinez G, Nguyen TK, Kim D, Santiago-Walker A, Roesch A, et al. Tenascin-C promotes melanoma progression by maintaining the ABCB5-positive side population. Oncogene. 2010 doi: 10.1038/onc.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steiniger SC, Coppinger JA, Kruger JA, Yates J, 3rd, Janda KD. Quantitative mass spectrometry identifies drug targets in cancer stem cell-containing side population. Stem Cells. 2008;26:3037–46. doi: 10.1634/stemcells.2008-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 10.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–20. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–94. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–19. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 15.Wu C, Wei Q, Utomo V, Nadesan P, Whetstone H, Kandel R, et al. Side population cells isolated from mesenchymal neoplasms have tumor initiating potential. Cancer Res. 2007;67:8216–22. doi: 10.1158/0008-5472.CAN-07-0999. [DOI] [PubMed] [Google Scholar]
- 16.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–35. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–33. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 19.Xu T, Xu Y, Liao CP, Lau R, Goldkorn A. Reprogramming murine telomerase rapidly inhibits the growth of mouse cancer cells in vitro and in vivo. Mol Cancer Ther. 2010;9:438–49. doi: 10.1158/1535-7163.MCT-09-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104:16158–63. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris MA, Yang H, Low BE, Mukherjee J, Guha A, Bronson RT, et al. Cancer stem cells are enriched in the side population cells in a mouse model of glioma. Cancer Res. 2008;68:10051–9. doi: 10.1158/0008-5472.CAN-08-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keysar SB, Jimeno A. More than markers: biological significance of cancer stem cell-defining molecules. Mol Cancer Ther. 2010;9:2450–7. doi: 10.1158/1535-7163.MCT-10-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuelten CH, Mertins SD, Busch JI, Gowens M, Scudiero DA, Burkett MW, et al. Complex display of putative tumor stem cell markers in the NCI60 tumor cell line panel. Stem Cells. 2010;28:649–60. doi: 10.1002/stem.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101:781–6. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salcido CD, Larochelle A, Taylor BJ, Dunbar CE, Varticovski L. Molecular characterisation of side population cells with cancer stem cell-like characteristics in small-cell lung cancer. Br J Cancer. 2010;102:1636–44. doi: 10.1038/sj.bjc.6605668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin T, Meng L, Li Y, Tsai RY. Tumor-initiating function of nucleostemin-enriched mammary tumor cells. Cancer Res. 2010;70:9444–52. doi: 10.1158/0008-5472.CAN-10-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 29.Vieyra DS, Rosen A, Goodell MA. Identification and characterization of side population cells in embryonic stem cell cultures. Stem Cells Dev. 2009;18:1155–66. doi: 10.1089/scd.2008.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang Y, Zhong Z, Huang Y, Deng W, Cao J, Tsao G, et al. Stem-like cancer cells are inducible by increasing genomic instability in cancer cells. J Biol Chem. 2010;285:4931–40. doi: 10.1074/jbc.M109.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 33.Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–30. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- 34.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–84. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 36.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–72. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.